Anti-Cancer and Medicinal Potentials of Moringa Isothiocyanate
Abstract
:1. Introduction
2. Research Progress in M. oleifera
2.1. Bioactive Compounds from M. oleifera
2.2. M. oleifera: A Promising Anti-Cancer Agent
3. The Introduction of Isothiocyanates
3.1. Basic Information of Isothiocyanates
3.2. The Extraction Methods of MIC-1 from M. oleifera
4. In Vivo and In Vitro Studies of MIC-1 from M. oleifera
4.1. Anti-Cancer
4.1.1. Neuroblastoma
4.1.2. Astrocytomas
4.1.3. Hepatocarcinoma
4.1.4. Skin Carcinoma
4.2. Anti-Inflammation
4.3. Anti-Chronic Diseases
4.3.1. Anti-Diabetic
4.3.2. Anti-Obesity
4.3.3. Anti-MS
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AITC | Allyl-isothiocyanate |
| BCL2 | B-cell lymphoma 2 |
| BITC | Benzylisothiocyanate |
| COX-2 | Cyclooxygenase-2 |
| GLs | Glucosinolates |
| IBD | Inflammatory bowel disease |
| iNOS | Inducible nitric oxide synthase |
| ITCs | Isothiocyanates |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinase |
| MS | Multiple sclerosis |
| MSE | M. oleifera seeds extraction |
| NBL | Neuroblastoma |
| NF-κB | Nuclear factor-kappa B |
| NO | Nitric oxide |
| PEITC | Phenethyl isothiocyanate |
| ROS | Reactive oxygen species |
| Se | Selenium |
| STAT | Signal transducer and activator of transcription |
| T2DM | Type-2 diabetes mellitus |
| UC | Ulcerative colitis |
References
- McGuire, S. World Cancer Report 2014. Geneva, Switzerland: World Health Organization, International Agency for Research on Cancer, WHO Press, 2015. Adv. Nutr. 2016, 7, 418–419. [Google Scholar] [CrossRef] [Green Version]
- Yasui, H. Safe Handling of Cancer Chemotherapy Drugs. Gan Kagaku Ryoho Cancer Chemother. 2016, 43, 503–508. [Google Scholar]
- Vogl, T.J.; Nour-Eldin, N.A.; Albrecht, M.H.; Kaltenbach, B.; Hohenforst-Schmidt, W.; Lin, H.; Panahi, B.; Eichler, K.; Gruber-Rouh, T.; Roman, A. Thermal Ablation of Lung Tumors: Focus on Microwave Ablation. RoFo: Fortschr. Auf Dem Geb. Der Rontgenstrahlen Und Der Nukl. 2017, 189, 828–843. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.I.; Chan, J.Y.W. Surgical Treatment of Advanced Staged Hypopharyngeal Cancer. Adv. Oto-Rhino-Laryngol. 2019, 83, 66–75. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Wang, Z.; Sarkar, F.H. Regulation of microRNAs by natural agents: An emerging field in chemoprevention and chemotherapy research. Pharm. Res. 2010, 27, 1027–1041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.; Wang, S.; Wu, X.; Zhang, J.; Chen, R.; Chen, M.; Wang, Y. Chinese herbal medicine-derived compounds for cancer therapy: A focus on hepatocellular carcinoma. J. Ethnopharmacol. 2013, 149, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Liu, C.; Li, T.; Lin, C.; Hao, Z.; Zhang, H.; Zhao, G.; Chen, Y.; Guo, A.; Hu, C. Gambogic acid alleviates inflammation and apoptosis and protects the blood-milk barrier in mastitis induced by LPS. Int. Immunopharmacol. 2020, 86, 106697. [Google Scholar] [CrossRef] [PubMed]
- Committee of Chinese Botany of CAS. Chinese Botany; Science Press: Beijing, China, 1984; Volume 34(1), p. 6. [Google Scholar]
- Popoola, J.O.; Obembe, O.O. Local knowledge, use pattern and geographical distribution of Moringa oleifera Lam. (Moringaceae) in Nigeria. J. Ethnopharmacol. 2013, 150, 682–691. [Google Scholar] [CrossRef]
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Moringa oleifera Seeds and Oil: Characteristics and Uses for Human Health. Int. J. Mol. Sci. 2016, 17, 2141. [Google Scholar] [CrossRef] [Green Version]
- Garcia, T.B.; Soares, A.A.; Costa, J.H.; Costa, H.P.S.; Neto, J.X.S.; Rocha-Bezerra, L.C.B.; Silva, F.D.A.; Arantes, M.R.; Sousa, D.O.B.; Vasconcelos, I.M.; et al. Gene expression and spatiotemporal localization of antifungal chitin-binding proteins during Moringa oleifera seed development and germination. Planta 2019, 249, 1503–1519. [Google Scholar] [CrossRef]
- Gupta, R.; Dubey, D.K.; Kannan, G.M.; Flora, S.J. Concomitant administration of Moringa oleifera seed powder in the remediation of arsenic-induced oxidative stress in mouse. Cell Biol. Int. 2007, 31, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Cultivation, Genetic, Ethnopharmacology, Phytochemistry and Pharmacology of Moringa oleifera Leaves: An Overview. Int. J. Mol. Sci. 2015, 16, 12791–12835. [Google Scholar] [CrossRef] [PubMed]
- Vergara-Jimenez, M.; Almatrafi, M.M.; Fernandez, M.L. Bioactive Components in Moringa oleifera Leaves Protect against Chronic Disease. Antioxidants 2017, 6, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atawodi, S.E.; Atawodi, J.C.; Idakwo, G.A.; Pfundstein, B.; Haubner, R.; Wurtele, G.; Bartsch, H.; Owen, R.W. Evaluation of the polyphenol content and antioxidant properties of methanol extracts of the leaves, stem, and root barks of Moringa oleifera Lam. J. Med. Food 2010, 13, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Madane, P.; Das, A.K.; Pateiro, M.; Nanda, P.K.; Bandyopadhyay, S.; Jagtap, P.; Barba, F.J.; Shewalkar, A.; Maity, B.; Lorenzo, J.M. Drumstick (Moringa oleifera) Flower as an Antioxidant Dietary Fibre in Chicken Meat Nuggets. Foods 2019, 8, 307. [Google Scholar] [CrossRef] [Green Version]
- Saini, R.K.; Sivanesan, I.; Keum, Y.S. Phytochemicals of Moringa oleifera: A review of their nutritional, therapeutic and industrial significance. 3 Biotech 2016, 6, 203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berkovich, L.; Earon, G.; Ron, I.; Rimmon, A.; Vexler, A.; Lev-Ari, S. Moringa oleifera aqueous leaf extract down-regulates nuclear factor-kappaB and increases cytotoxic effect of chemotherapy in pancreatic cancer cells. BMC Complementary Altern. Med. 2013, 13, 212. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Luo, F.X.; Shi, C.Y.; Jiang, W.W.; Qian, Y.Y.; Yang, M.R.; Song, S.; Dai, T.Y.; Peng, L.; Gao, X.Y.; et al. Moringa oleifera Alkaloids Inhibited PC3 Cells Growth and Migration Through the COX-2 Mediated Wnt/β-Catenin Signaling Pathway. Front. Pharmacol. 2020, 11, 523962. [Google Scholar] [CrossRef]
- Do, B.H.; Hoang, N.S.; Nguyen, T.P.T.; Ho, N.Q.C.; Le, T.L.; Doan, C.C. Phenolic Extraction of Moringa oleifera Leaves Induces Caspase-Dependent and Caspase-Independent Apoptosis through the Generation of Reactive Oxygen Species and the Activation of Intrinsic Mitochondrial Pathway in Human Melanoma Cells. Nutr. Cancer 2021, 73, 869–888. [Google Scholar] [CrossRef]
- Tan, H.W.; Mo, H.Y.; Lau, A.T.Y.; Xu, Y.M. Selenium Species: Current Status and Potentials in Cancer Prevention and Therapy. Int. J. Mol. Sci. 2018, 20, 75. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Zhou, Q.H.; Xu, K. Are isothiocyanates potential anti-cancer drugs? Acta Pharmacol. Sin. 2009, 30, 501–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Rodriguez, N.A.; Gaytán-Martínez, M.; de la Luz Reyes-Vega, M.; Loarca-Piña, G. Glucosinolates and Isothiocyanates from Moringa oleifera: Chemical and Biological Approaches. Plant Foods Hum. Nutr. 2020, 75, 447–457. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Liu, X.; Lin, Y.; Zheng, X.; Lu, Y. Hydrogen Sulfide (H2S) Releasing Capacity of Isothiocyanates from Moringa oleifera Lam. Molecules 2018, 23, 2809. [Google Scholar] [CrossRef] [Green Version]
- Jaja-Chimedza, A.; Zhang, L.; Wolff, K.; Graf, B.L.; Kuhn, P.; Moskal, K.; Carmouche, R.; Newman, S.; Salbaum, J.M.; Raskin, I. A dietary isothiocyanate-enriched moringa (Moringa oleifera) seed extract improves glucose tolerance in a high-fat-diet mouse model and modulates the gut microbiome. J. Funct. Foods 2018, 47, 376–385. [Google Scholar] [CrossRef] [PubMed]
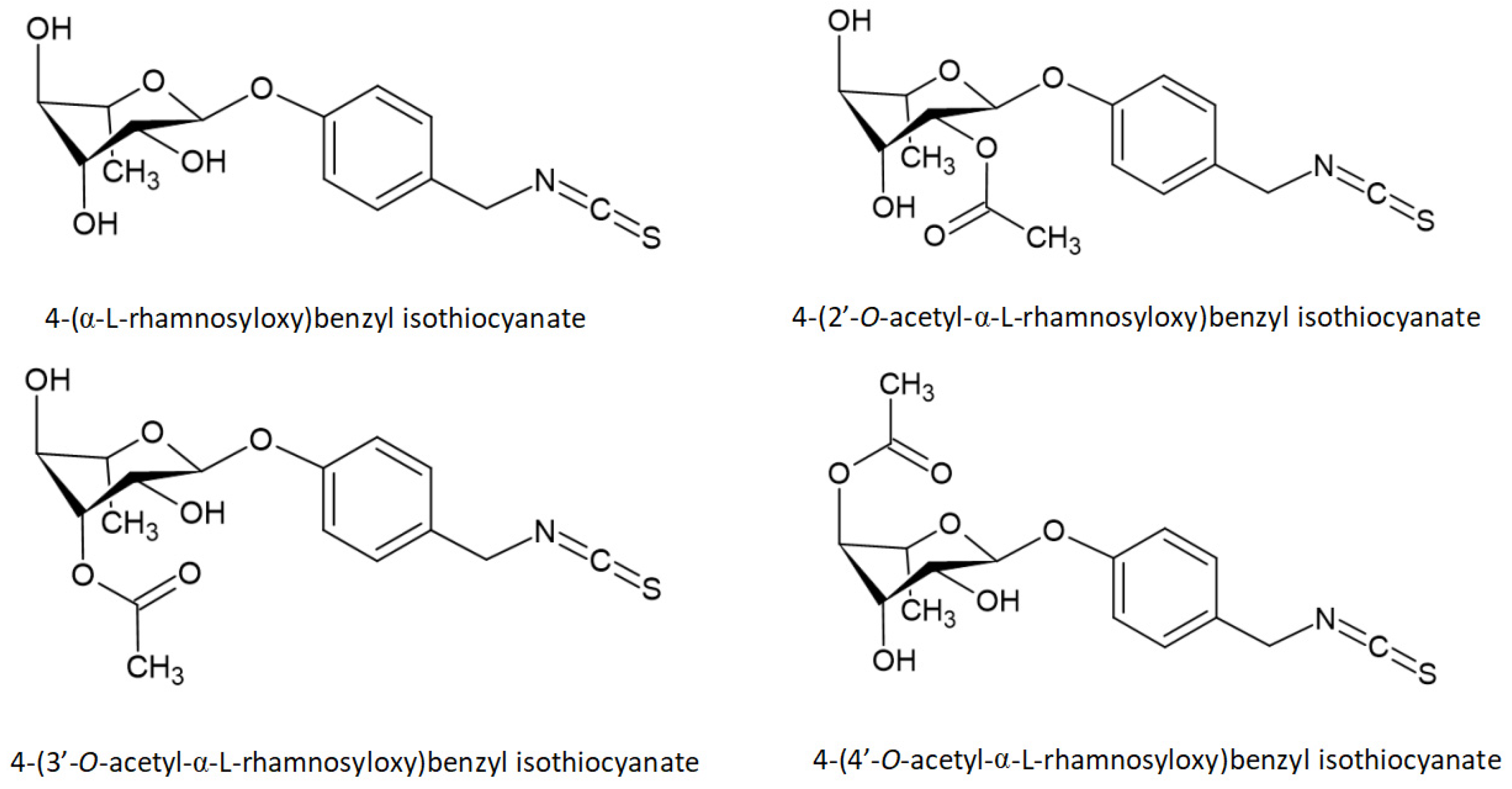
- Park, E.J.; Cheenpracha, S.; Chang, L.C.; Kondratyuk, T.P.; Pezzuto, J.M. Inhibition of lipopolysaccharide-induced cyclooxygenase-2 and inducible nitric oxide synthase expression by 4-[(2’-O-acetyl-α-L-rhamnosyloxy)benzyl]isothiocyanate from Moringa oleifera. Nutr. Cancer 2011, 63, 971–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Wang, X.; Pu, H.; Lin, Y.; Li, D.; Liu, S.Q.; Huang, D. Moringin and Its Structural Analogues as Slow H(2)S Donors: Their Mechanisms and Bioactivity. J. Agric. Food Chem. 2020, 68, 7235–7245. [Google Scholar] [CrossRef]
- Cheenpracha, S.; Park, E.J.; Rostama, B.; Pezzuto, J.M.; Chang, L.C. Inhibition of nitric oxide (NO) production in lipopolysaccharide (LPS)-activated murine macrophage RAW 264.7 cells by the norsesterterpene peroxide, epimuqubilin A. Mar. Drugs 2010, 8, 429–437. [Google Scholar] [CrossRef]
- Bower, J.E. Cancer-related fatigue--mechanisms, risk factors, and treatments. Nat. Rev. Clin. Oncol. 2014, 11, 597–609. [Google Scholar] [CrossRef]
- Chen, P.; Bonaldo, P. Role of macrophage polarization in tumor angiogenesis and vessel normalization: Implications for new anticancer therapies. Int. Rev. Cell Mol. Biol. 2013, 301, 1–35. [Google Scholar] [CrossRef]
- Saucedo-Pompa, S.; Torres-Castillo, J.A.; Castro-López, C.; Rojas, R.; Sánchez-Alejo, E.J.; Ngangyo-Heya, M.; Martínez-Ávila, G.C.G. Moringa plants: Bioactive compounds and promising applications in food products. Food Res. Int. 2018, 111, 438–450. [Google Scholar] [CrossRef]
- Devisetti, R.; Sreerama, Y.N.; Bhattacharya, S. Processing effects on bioactive components and functional properties of moringa leaves: Development of a snack and quality evaluation. J. Food Sci. Technol. 2016, 53, 649–657. [Google Scholar] [CrossRef] [Green Version]
- Prabakaran, M.; Kim, S.-H.; Sasireka, A.; Chandrasekaran, M.; Chung, I.-M. Polyphenol composition and antimicrobial activity of various solvent extracts from different plant parts of Moringa oleifera. Food Biosci. 2018, 26, 23–29. [Google Scholar] [CrossRef]
- Amaglo, N.K.; Bennett, R.N.; Lo Curto, R.B.; Rosa, E.A.S.; Lo Turco, V.; Giuffrida, A.; Curto, A.L.; Crea, F.; Timpo, G.M. Profiling selected phytochemicals and nutrients in different tissues of the multipurpose tree Moringa oleifera L., grown in Ghana. Food Chem. 2010, 122, 1047–1054. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, R.; Liu, J.; Huang, Q.; Liu, S.; Jiang, Y. Integrated Network Pharmacology Analysis and Experimental Validation to Reveal the Mechanism of Anti-Insulin Resistance Effects of Moringa oleifera Seeds. Drug Des. Dev. Ther. 2020, 14, 4069–4084. [Google Scholar] [CrossRef]
- Abdelsayed, E.M.; Medhat, D.; Mandour, Y.M.; Hanafi, R.S.; Motaal, A.A. Niazimicin: A thiocarbamate glycoside from Moringa oleifera Lam. seeds with a novel neuroprotective activity. J. Food Biochem. 2021, e13992. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yuan, C.; Wang, Y. Bioactivity-Guided Identification of Anti-Adipogenic Isothiocyanates in the Moringa (Moringa oleifera) Seed and Investigation of the Structure-Activity Relationship. Molecules 2020, 25, 2504. [Google Scholar] [CrossRef]
- Maldini, M.; Maksoud, S.A.; Natella, F.; Montoro, P.; Petretto, G.L.; Foddai, M.; De Nicola, G.R.; Chessa, M.; Pintore, G. Moringa oleifera: Study of phenolics and glucosinolates by mass spectrometry. J. Mass Spectrom. JMS 2014, 49, 900–910. [Google Scholar] [CrossRef]
- Muhammad, A.A.; Pauzi, N.A.; Arulselvan, P.; Abas, F.; Fakurazi, S. In vitro wound healing potential and identification of bioactive compounds from Moringa oleifera Lam. BioMed Res. Int. 2013, 2013, 974580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dou, Z.; Chen, C.; Fu, X. Bioaccessibility, antioxidant activity and modulation effect on gut microbiota of bioactive compounds from Moringa oleifera Lam. leaves during digestion and fermentation in vitro. Food Funct. 2019, 10, 5070–5079. [Google Scholar] [CrossRef]
- Vats, S.; Gupta, T. Evaluation of bioactive compounds and antioxidant potential of hydroethanolic extract of Moringa oleifera Lam. from Rajasthan, India. Physiol. Mol. Biol. Plants Int. J. Funct. Plant Biol. 2017, 23, 239–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barhoi, D.; Upadhaya, P.; Barbhuiya, S.N.; Giri, A.; Giri, S. Aqueous Extract of Moringa oleifera Exhibit Potential Anticancer Activity and can be Used as a Possible Cancer Therapeutic Agent: A Study Involving In Vitro and In Vivo Approach. J. Am. Coll. Nutr. 2021, 40, 70–85. [Google Scholar] [CrossRef]
- Faizi, S.; Sumbul, S.; Versiani, M.A.; Saleem, R.; Sana, A.; Siddiqui, H. GC/GCMS analysis of the petroleum ether and dichloromethane extracts of Moringa oleifera roots. Asian Pac. J. Trop. Biomed. 2014, 4, 650–654. [Google Scholar] [CrossRef] [Green Version]
- Abd Rani, N.Z.; Kumolosasi, E.; Jasamai, M.; Jamal, J.A.; Lam, K.W.; Husain, K. In vitro anti-allergic activity of Moringa oleifera Lam. extracts and their isolated compounds. BMC Complementary Altern. Med. 2019, 19, 361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sreelatha, S.; Jeyachitra, A.; Padma, P.R. Antiproliferation and induction of apoptosis by Moringa oleifera leaf extract on human cancer cells. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2011, 49, 1270–1275. [Google Scholar] [CrossRef]
- Karim, N.A.; Ibrahim, M.D.; Kntayya, S.B.; Rukayadi, Y.; Hamid, H.A.; Razis, A.F. Moringa oleifera Lam: Targeting Chemoprevention. Asian Pac. J. Cancer Prev. APJCP 2016, 17, 3675–3686. [Google Scholar]
- Khan, F.; Pandey, P.; Ahmad, V.; Upadhyay, T.K. Moringa oleifera methanolic leaves extract induces apoptosis and G0/G1 cell cycle arrest via downregulation of Hedgehog Signaling Pathway in human prostate PC-3 cancer cells. J. Food Biochem. 2020, 44, e13338. [Google Scholar] [CrossRef]
- Luetragoon, T.; Pankla Sranujit, R.; Noysang, C.; Thongsri, Y.; Potup, P.; Suphrom, N.; Nuengchamnong, N.; Usuwanthim, K. Anti-Cancer Effect of 3-Hydroxy-β-Ionone Identified from Moringa oleifera Lam. Leaf on Human Squamous Cell Carcinoma 15 Cell Line. Molecules 2020, 25, 3563. [Google Scholar] [CrossRef]
- Tiloke, C.; Phulukdaree, A.; Gengan, R.M.; Chuturgoon, A.A. Moringa oleifera Aqueous Leaf Extract Induces Cell-Cycle Arrest and Apoptosis in Human Liver Hepatocellular Carcinoma Cells. Nutr. Cancer 2019, 71, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Caicedo-Lopez, L.H.; Cuellar-Nuñez, M.L.; Luzardo-Ocampo, I.; Campos-Vega, R.; Lóarca-Piña, G. Colonic metabolites from digested Moringa oleifera leaves induced HT-29 cell death via apoptosis, necrosis, and autophagy. Int. J. Food Sci. Nutr. 2021, 72, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Peng, L.J.; Yang, M.R.; Jiang, W.W.; Mao, J.Y.; Shi, C.Y.; Tian, Y.; Sheng, J. Alkaloid Extract of Moringa oleifera Lam. Exerts Antitumor Activity in Human Non-Small-Cell Lung Cancer via Modulation of the JAK2/STAT3 Signaling Pathway. Evid. -Based Complementary Altern. Med. Ecam 2021, 2021, 5591687. [Google Scholar] [CrossRef] [PubMed]
- Do, B.H.; Nguyen, T.P.T.; Ho, N.Q.C.; Le, T.L.; Hoang, N.S.; Doan, C.C. Mitochondria-mediated Caspase-dependent and Caspase-independent apoptosis induced by aqueous extract from Moringa oleifera leaves in human melanoma cells. Mol. Biol. Rep. 2020, 47, 3675–3689. [Google Scholar] [CrossRef]
- Abd-Rabou, A.A.; KhM, A.Z.; Kishta, M.S.; Shalby, A.B.; Ezzo, M.I. Nano-Micelle of Moringa oleifera Seed Oil Triggers Mitochondrial Cancer Cell Apoptosis. Asian Pac. J. Cancer Prev. APJCP 2016, 17, 49294–49933. [Google Scholar] [CrossRef]
- Potestà, M.; Minutolo, A.; Gismondi, A.; Canuti, L.; Kenzo, M.; Roglia, V.; Macchi, F.; Grelli, S.; Canini, A.; Colizzi, V.; et al. Cytotoxic and apoptotic effects of different extracts of Moringa oleifera Lam on lymphoid and monocytoid cells. Exp. Ther. Med. 2019, 18, 5–17. [Google Scholar] [CrossRef] [Green Version]
- Potestà, M.; Roglia, V.; Fanelli, M.; Pietrobono, E.; Gismondi, A.; Vumbaca, S.; Nguedia Tsangueu, R.G.; Canini, A.; Colizzi, V.; Grelli, S.; et al. Effect of microvesicles from Moringa oleifera containing miRNA on proliferation and apoptosis in tumor cell lines. Cell Death Discov. 2020, 6, 43. [Google Scholar] [CrossRef]
- Adebayo, I.A.; Arsad, H.; Samian, M.R. Antiproliferative effect on breast cancer (MCF7) of Moringa oleifera seed extracts. Afr. J. Tradit. Complementary Altern. Med. AJTCAM 2017, 14, 282–287. [Google Scholar] [CrossRef]
- Elsayed, E.A.; Sharaf-Eldin, M.A.; Wadaan, M. In vitro Evaluation of Cytotoxic Activities of Essential Oil from Moringa oleifera Seeds on HeLa, HepG2, MCF-7, CACO-2 and L929 Cell Lines. Asian Pac. J. Cancer Prev. APJCP 2015, 16, 4671–4675. [Google Scholar] [CrossRef] [Green Version]
- Patriota, L.L.S.; Ramos, D.B.M.; Dos Santos, A.; Silva, Y.A.; Gama, E.S.M.; Torres, D.J.L.; Procópio, T.F.; de Oliveira, A.M.; Coelho, L.; Pontual, E.V.; et al. Antitumor activity of Moringa oleifera (drumstick tree) flower trypsin inhibitor (MoFTI) in sarcoma 180-bearing mice. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2020, 145, 111691. [Google Scholar] [CrossRef]
- Al-Asmari, A.K.; Albalawi, S.M.; Athar, M.T.; Khan, A.Q.; Al-Shahrani, H.; Islam, M. Moringa oleifera as an Anti-Cancer Agent against Breast and Colorectal Cancer Cell Lines. PLoS ONE 2015, 10, e0135814. [Google Scholar] [CrossRef] [PubMed]
- Vasanth, K.; Ilango, K.; MohanKumar, R.; Agrawal, A.; Dubey, G.P. Anticancer activity of Moringa oleifera mediated silver nanoparticles on human cervical carcinoma cells by apoptosis induction. Colloids Surf. B Biointerfaces 2014, 117, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.; Upadhyay, S.; Ahmad, I.; Hussain, A.; Ahamed, M. Cytotoxicity of Moringa oleifera fruits on human liver cancer and molecular docking analysis of bioactive constituents against caspase-3 enzyme. J. Food Biochem. 2021, 45, e13720. [Google Scholar] [CrossRef]
- Bianchini, F.; Vainio, H. Isothiocyanates in cancer prevention. Drug Metab. Rev. 2004, 36, 655–667. [Google Scholar] [CrossRef]
- Romeo, L.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. Isothiocyanates: An Overview of Their Antimicrobial Activity against Human Infections. Molecules 2018, 23, 624. [Google Scholar] [CrossRef] [Green Version]
- Fimognari, C.; Turrini, E.; Ferruzzi, L.; Lenzi, M.; Hrelia, P. Natural isothiocyanates: Genotoxic potential versus chemoprevention. Mutat. Res. 2012, 750, 107–131. [Google Scholar] [CrossRef]
- Dufour, V.; Stahl, M.; Baysse, C. The antibacterial properties of isothiocyanates. Microbiology 2015, 161, 229–243. [Google Scholar] [CrossRef] [Green Version]
- Burčul, F.; Generalić Mekinić, I.; Radan, M.; Rollin, P.; Blažević, I. Isothiocyanates: Cholinesterase inhibiting, antioxidant, and anti-inflammatory activity. J. Enzym. Inhib. Med. Chem. 2018, 33, 577–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soundararajan, P.; Kim, J.S. Anti-Carcinogenic Glucosinolates in Cruciferous Vegetables and Their Antagonistic Effects on Prevention of Cancers. Molecules 2018, 23, 2983. [Google Scholar] [CrossRef] [Green Version]
- Senthilkumar, A.; Karuvantevida, N.; Rastrelli, L.; Kurup, S.S.; Cheruth, A.J. Traditional Uses, Pharmacological Efficacy, and Phytochemistry of Moringa peregrina (Forssk.) Fiori.—A Review. Front. Pharmacol. 2018, 9, 465. [Google Scholar] [CrossRef] [Green Version]
- Palliyaguru, D.L.; Yuan, J.M.; Kensler, T.W.; Fahey, J.W. Isothiocyanates: Translating the Power of Plants to People. Mol. Nutr. Food Res. 2018, 62, e1700965. [Google Scholar] [CrossRef]
- Zhang, Q.C.; Pan, Z.H.; Liu, B.N.; Meng, Z.W.; Wu, X.; Zhou, Q.H.; Xu, K. Benzyl isothiocyanate induces protective autophagy in human lung cancer cells through an endoplasmic reticulum stress-mediated mechanism. Acta Pharmacol. Sin. 2017, 38, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejski, D.; Koss-Mikołajczyk, I.; Abdin, A.Y.; Jacob, C.; Bartoszek, A. Chemical Aspects of Biological Activity of Isothiocyanates and Indoles, the Products of Glucosinolate Decomposition. Curr. Pharm. Des. 2019, 25, 1717–1728. [Google Scholar] [CrossRef] [PubMed]
- Waterman, C.; Cheng, D.M.; Rojas-Silva, P.; Poulev, A.; Dreifus, J.; Lila, M.A.; Raskin, I. Stable, water extractable isothiocyanates from Moringa oleifera leaves attenuate inflammation in vitro. Phytochemistry 2014, 103, 114–122. [Google Scholar] [CrossRef] [Green Version]
- Tian, S.; Liu, X.; Lei, P.; Zhang, X.; Shan, Y. Microbiota: A mediator to transform glucosinolate precursors in cruciferous vegetables to the active isothiocyanates. J. Sci. Food Agric. 2018, 98, 1255–1260. [Google Scholar] [CrossRef]
- Jaja-Chimedza, A.; Graf, B.L.; Simmler, C.; Kim, Y.; Kuhn, P.; Pauli, G.F.; Raskin, I. Biochemical characterization and anti-inflammatory properties of an isothiocyanate-enriched moringa (Moringa oleifera) seed extract. PLoS ONE 2017, 12, e0182658. [Google Scholar] [CrossRef] [Green Version]
- Brunelli, D.; Tavecchio, M.; Falcioni, C.; Frapolli, R.; Erba, E.; Iori, R.; Rollin, P.; Barillari, J.; Manzotti, C.; Morazzoni, P.; et al. The isothiocyanate produced from glucomoringin inhibits NF-kB and reduces myeloma growth in nude mice in vivo. Biochem. Pharmacol. 2010, 79, 1141–1148. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Li, W.; Wu, R.; Yin, R.; Sargsyan, D.; Raskin, I.; Kong, A.N. Epigenome and transcriptome study of moringa isothiocyanate in mouse kidney mesangial cells induced by high glucose, a potential model for diabetic-induced nephropathy. AAPS J. 2019, 22, 8. [Google Scholar] [CrossRef]
- Waterman, C.; Graham, J.L.; Arnold, C.D.; Stanhope, K.L.; Tong, J.H.; Jaja-Chimedza, A.; Havel, P.J. Moringa Isothiocyanate-rich Seed Extract Delays the Onset of Diabetes in UC Davis Type-2 Diabetes Mellitus Rats. Sci. Rep. 2020, 10, 8861. [Google Scholar] [CrossRef] [PubMed]
- Romeo, L.; Diomede, F.; Gugliandolo, A.; Scionti, D.; Lo Giudice, F.; Lanza Cariccio, V.; Iori, R.; Bramanti, P.; Trubiani, O.; Mazzon, E. Moringin Induces Neural Differentiation in the Stem Cell of the Human Periodontal Ligament. Sci. Rep. 2018, 8, 9153. [Google Scholar] [CrossRef]
- Müller, C.; van Loon, J.; Ruschioni, S.; De Nicola, G.R.; Olsen, C.E.; Iori, R.; Agerbirk, N. Taste detection of the non-volatile isothiocyanate moringin results in deterrence to glucosinolate-adapted insect larvae. Phytochemistry 2015, 118, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Chiricosta, L.; Gugliandolo, A.; Diomede, F.; Pizzicannella, J.; Trubiani, O.; Iori, R.; Tardiolo, G.; Guarnieri, S.; Bramanti, P.; Mazzon, E. Moringin Pretreatment Inhibits the Expression of Genes Involved in Mitophagy in the Stem Cell of the Human Periodontal Ligament. Molecules 2019, 24, 3217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giacoppo, S.; Iori, R.; Bramanti, P.; Mazzon, E. Topical moringin-cream relieves neuropathic pain by suppression of inflammatory pathway and voltage-gated ion channels in murine model of multiple sclerosis. Mol. Pain 2017, 13, 1744806917724318. [Google Scholar] [CrossRef]
- Giacoppo, S.; Rajan, T.S.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. The α-cyclodextrin complex of the Moringa isothiocyanate suppresses lipopolysaccharide-induced inflammation in RAW 264.7 macrophage cells through Akt and p38 inhibition. Inflamm. Res. 2017, 66, 487–503. [Google Scholar] [CrossRef]
- Romeo, L.; Lanza Cariccio, V.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. The α-Cyclodextrin/Moringin Complex: A New Promising Antimicrobial Agent against Staphylococcus aureus. Molecules 2018, 23, 2097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galuppo, M.; Giacoppo, S.; Iori, R.; De Nicola, G.R.; Bramanti, P.; Mazzon, E. Administration of 4-(α-L-rhamnosyloxy)-benzyl isothiocyanate delays disease phenotype in SOD1(G93A) rats: A transgenic model of amyotrophic lateral sclerosis. BioMed Res. Int. 2015, 2015, 259417. [Google Scholar] [CrossRef] [Green Version]
- Cirmi, S.; Ferlazzo, N.; Gugliandolo, A.; Musumeci, L.; Mazzon, E.; Bramanti, A.; Navarra, M. Moringin from Moringa oleifera Seeds Inhibits Growth, Arrests Cell-Cycle, and Induces Apoptosis of SH-SY5Y Human Neuroblastoma Cells through the Modulation of NF-κB and Apoptotic Related Factors. Int. J. Mol. Sci. 2019, 20, 1930. [Google Scholar] [CrossRef] [Green Version]
- Jaafaru, M.S.; Nordin, N.; Shaari, K.; Rosli, R.; Abdull Razis, A.F. Isothiocyanate from Moringa oleifera seeds mitigates hydrogen peroxide-induced cytotoxicity and preserved morphological features of human neuronal cells. PLoS ONE 2018, 13, e0196403. [Google Scholar] [CrossRef] [Green Version]
- Rajan, T.S.; De Nicola, G.R.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. Anticancer activity of glucomoringin isothiocyanate in human malignant astrocytoma cells. Fitoterapia 2016, 110, 1–7. [Google Scholar] [CrossRef]
- Michl, C.; Vivarelli, F.; Weigl, J.; De Nicola, G.R.; Canistro, D.; Paolini, M.; Iori, R.; Rascle, A. The Chemopreventive Phytochemical Moringin Isolated from Moringa oleifera Seeds Inhibits JAK/STAT Signaling. PLoS ONE 2016, 11, e0157430. [Google Scholar] [CrossRef]
- Waterman, C.; Rojas-Silva, P.; Tumer, T.B.; Kuhn, P.; Richard, A.J.; Wicks, S.; Stephens, J.M.; Wang, Z.; Mynatt, R.; Cefalu, W.; et al. Isothiocyanate-rich Moringa oleifera extract reduces weight gain, insulin resistance, and hepatic gluconeogenesis in mice. Mol. Nutr. Food Res. 2015, 59, 1013–1024. [Google Scholar] [CrossRef] [Green Version]
- Fahey, J.W.; Wade, K.L.; Stephenson, K.K.; Shi, Y.; Liu, H.; Panjwani, A.A.; Warrick, C.R.; Olson, M.E. A Strategy to Deliver Precise Oral Doses of the Glucosinolates or Isothiocyanates from Moringa oleifera Leaves for Use in Clinical Studies. Nutrients 2019, 11, 1547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Jaja-Chimedza, A.; Merrill, D.; Mendes, O.; Raskin, I. A 14-day repeated-dose oral toxicological evaluation of an isothiocyanate-enriched hydro-alcoholic extract from Moringa oleifera Lam. seeds in rats. Toxicol. Rep. 2018, 5, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Maiyo, F.C.; Moodley, R.; Singh, M. Cytotoxicity, Antioxidant and Apoptosis Studies of Quercetin-3-O Glucoside and 4-(β-D-Glucopyranosyl-1→4-α-L-Rhamnopyranosyloxy)-Benzyl Isothiocyanate from Moringa oleifera. Anti-Cancer Agents Med. Chem. 2016, 16, 648–656. [Google Scholar] [CrossRef]
- O’Shea, J.J.; Schwartz, D.M.; Villarino, A.V.; Gadina, M.; McInnes, I.B.; Laurence, A. The JAK-STAT pathway: Impact on human disease and therapeutic intervention. Annu. Rev. Med. 2015, 66, 311–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedram, M.; Vafaie, M.; Fekri, K.; Haghi, S.; Rashidi, I.; Pirooti, C. Cerebellar neuroblastoma in 2.5 years old child. Iran. J. Cancer Prev. 2013, 6, 174–176. [Google Scholar] [PubMed]
- Mei, H.; Wang, Y.; Lin, Z.; Tong, Q. The mTOR signaling pathway in pediatric neuroblastoma. Pediatric Hematol. Oncol. 2013, 30, 605–615. [Google Scholar] [CrossRef]
- Sui, X.; Kong, N.; Ye, L.; Han, W.; Zhou, J.; Zhang, Q.; He, C.; Pan, H. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett. 2014, 344, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Giacoppo, S.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. Moringa isothiocyanate complexed with α-cyclodextrin: A new perspective in neuroblastoma treatment. BMC Complementary Altern. Med. 2017, 17, 362. [Google Scholar] [CrossRef] [Green Version]
- Hirtz, A.; Rech, F.; Dubois-Pot-Schneider, H.; Dumond, H. Astrocytoma: A Hormone-Sensitive Tumor? Int. J. Mol. Sci. 2020, 21, 9114. [Google Scholar] [CrossRef]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef]
- Antonini, E.; Iori, R.; Ninfali, P.; Scarpa, E.S. A Combination of Moringin and Avenanthramide 2f Inhibits the Proliferation of Hep3B Liver Cancer Cells Inducing Intrinsic and Extrinsic Apoptosis. Nutr. Cancer 2018, 70, 1159–1165. [Google Scholar] [CrossRef]
- Goldstein, J.; Roth, E.; Roberts, N.; Zwick, R.; Lin, S.; Fletcher, S.; Tadeu, A.; Wu, C.; Beck, A.; Zeiss, C.; et al. Loss of endogenous Nfatc1 reduces the rate of DMBA/TPA-induced skin tumorigenesis. Mol. Biol. Cell 2015, 26, 3606–3614. [Google Scholar] [CrossRef]
- Wang, C.; Wu, R.; Sargsyan, D.; Zheng, M.; Li, S.; Yin, R.; Su, S.; Raskin, I.; Kong, A.N. CpG methyl-seq and RNA-seq epigenomic and transcriptomic studies on the preventive effects of Moringa isothiocyanate in mouse epidermal JB6 cells induced by the tumor promoter TPA. J. Nutr. Biochem. 2019, 68, 69–78. [Google Scholar] [CrossRef]
- Lu, Y.; Maria Vos, R.D.; Zhang, Y.; Zhang, M.; Liu, Y.; Fu, C.; Liu, S.Q.; Huang, D. The degradation kinetics and mechanism of moringin in aqueous solution and the cytotoxicity of degraded products. Food Chem. 2021, 364, 130424. [Google Scholar] [CrossRef] [PubMed]
- Opal, S.M. Endotoxins and other sepsis triggers. Contrib. Nephrol. 2010, 167, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Sailaja, B.S.; Aita, R.; Maledatu, S.; Ribnicky, D.; Verzi, M.P.; Raskin, I. Moringa isothiocyanate-1 regulates Nrf2 and NF-κB pathway in response to LPS-driven sepsis and inflammation. PLoS ONE 2021, 16, e0248691. [Google Scholar] [CrossRef]
- Kim, Y.; Wu, A.G.; Jaja-Chimedza, A.; Graf, B.L.; Waterman, C.; Verzi, M.P.; Raskin, I. Isothiocyanate-enriched moringa seed extract alleviates ulcerative colitis symptoms in mice. PLoS ONE 2017, 12, e0184709. [Google Scholar] [CrossRef]
- Boroujerdi, A.; Welser-Alves, J.V.; Milner, R. Extensive vascular remodeling in the spinal cord of pre-symptomatic experimental autoimmune encephalomyelitis mice; increased vessel expression of fibronectin and the α5β1 integrin. Exp. Neurol. 2013, 250, 43–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galuppo, M.; Giacoppo, S.; De Nicola, G.R.; Iori, R.; Navarra, M.; Lombardo, G.E.; Bramanti, P.; Mazzon, E. Antiinflammatory activity of glucomoringin isothiocyanate in a mouse model of experimental autoimmune encephalomyelitis. Fitoterapia 2014, 95, 160–174. [Google Scholar] [CrossRef]
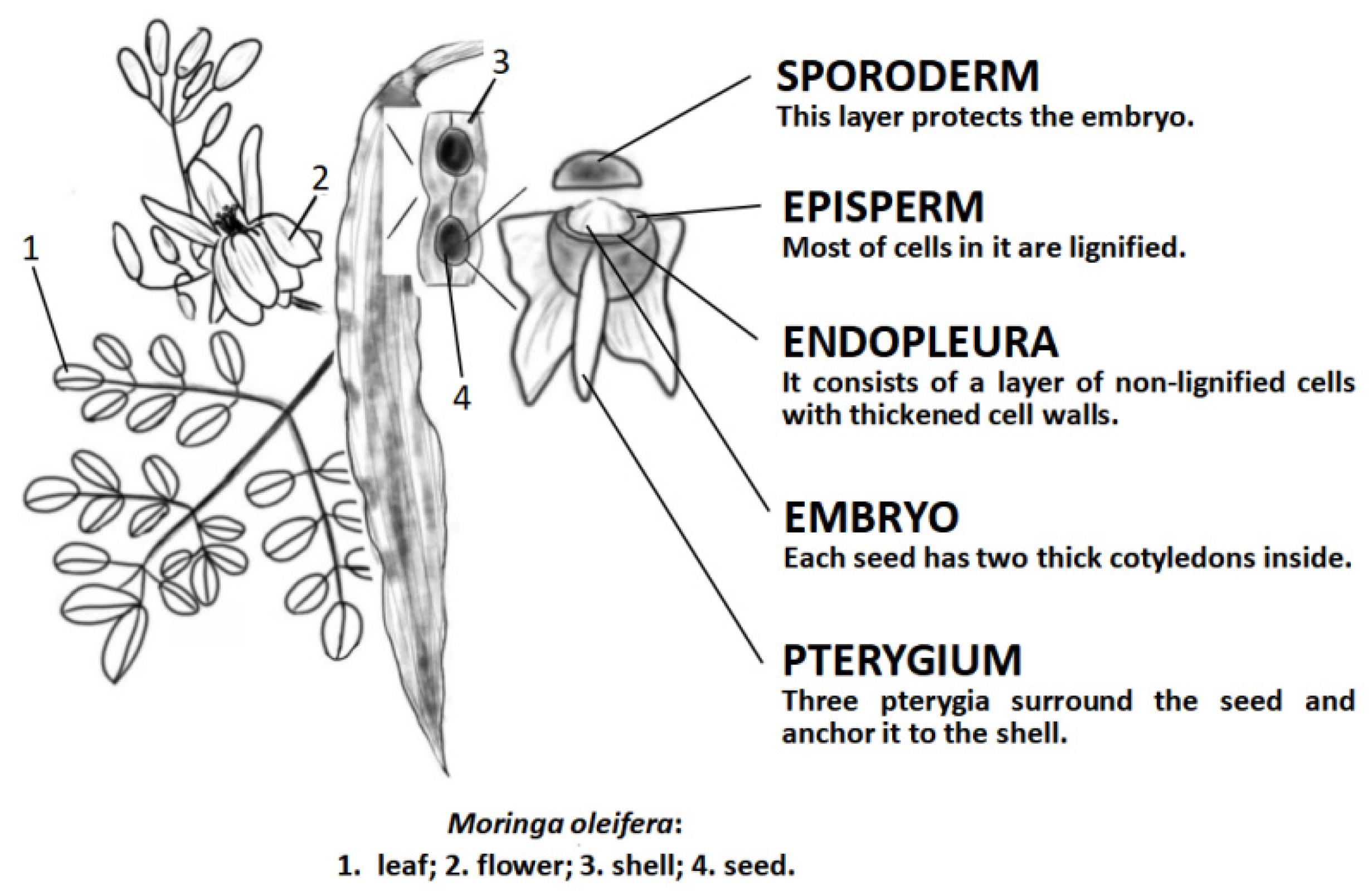

| Location | Bioactive Compounds | Reference |
|---|---|---|
| Seed | glycosidic benzylamines; niazimicin; isothiocyanates; phenolics; glucosinolates | [35,36,37,38] |
| Leaf | phytol; flavonoids; phenolics; β-carotene; lycopene; vicenin-2; quinic acid; octadecanoic acid; hexadecanoic acid (palmitic acid); α-tocopherol (vitamin-E); ɣ-sitosterol | [32,39,40,41,42] |
| Flower | β-sitosterol; flavonoids; anthocyanin | [41] |
| Root | nasimizinol; oleic acid; N-benzyl-N-(7-cyanato heptanamide; N-benzyl-N-(1-chlorononyl) amide; bis [3-benzyl prop-2-ene]-1-one; N, N-dibenzyl-2-ene pent-1,5-diamide | [43] |
| Shell | 3,5,6-trihydroxy-2-(2,3,4,5,6-pentahydroxyphenyl)-4H-chromen-4-one; β-sitosterol-3-O-glucoside; 2,3,4-trihydroxybenzaldehyde; stigmasterol | [44] |
| Bark | epiglobulol; flavonoids; anthocyanin | [41] |
| Location | Function | Extraction Method | Reference |
|---|---|---|---|
| Seed | anti-inflammation; anti-diabetic; counteracting ulcerative colitis | Jaja-Chimedza et al. | [25,74,76,77] |
| Seed | anti-cancer; counteracting neurodegeneration; anti-oxidation; counteracting neuropathic pain; anti-inflammation; antibiotics; counteracting amyotrophic lateral sclerosis | Brunelli et al. | [75,78,79,80,81,82,83,84,85,86,87,88] |
| Leaf | anti-inflammation; anti-obesity, anti-diabetic | Waterman et al. | [72,89] |
| Leaf | anti-inflammation | Fahey et al. | [90] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.-Y.; Xu, Y.-M.; Lau, A.T.Y. Anti-Cancer and Medicinal Potentials of Moringa Isothiocyanate. Molecules 2021, 26, 7512. https://doi.org/10.3390/molecules26247512
Wu Y-Y, Xu Y-M, Lau ATY. Anti-Cancer and Medicinal Potentials of Moringa Isothiocyanate. Molecules. 2021; 26(24):7512. https://doi.org/10.3390/molecules26247512
Chicago/Turabian StyleWu, Yu-Yao, Yan-Ming Xu, and Andy T. Y. Lau. 2021. "Anti-Cancer and Medicinal Potentials of Moringa Isothiocyanate" Molecules 26, no. 24: 7512. https://doi.org/10.3390/molecules26247512
APA StyleWu, Y.-Y., Xu, Y.-M., & Lau, A. T. Y. (2021). Anti-Cancer and Medicinal Potentials of Moringa Isothiocyanate. Molecules, 26(24), 7512. https://doi.org/10.3390/molecules26247512







