Effects of Geomaterial-Originated Fillers on Microstructure and Mechanical/Physical Properties of α- and β-Chitosan-Based Films
Abstract
:1. Introduction
2. Results and Discussion
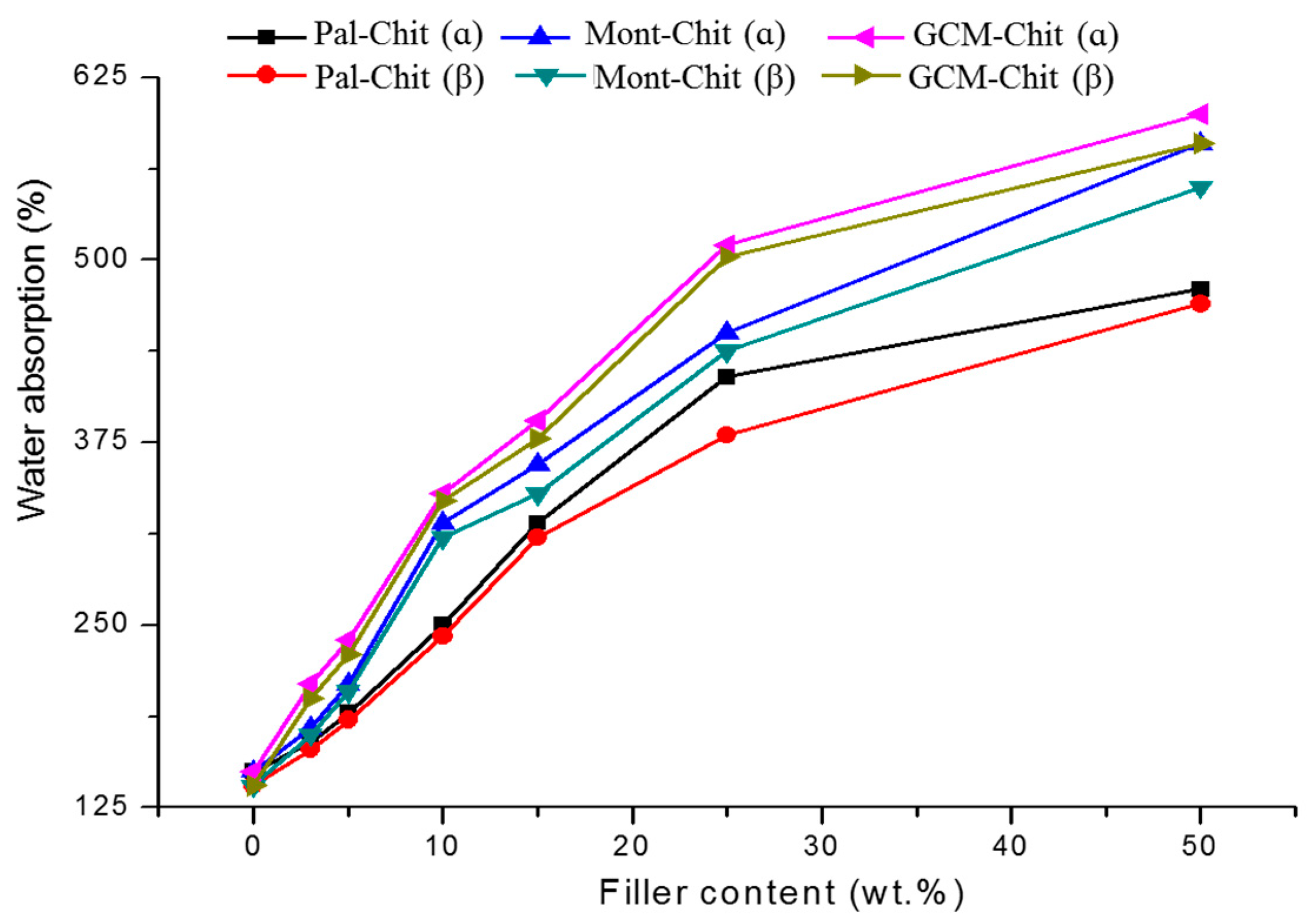
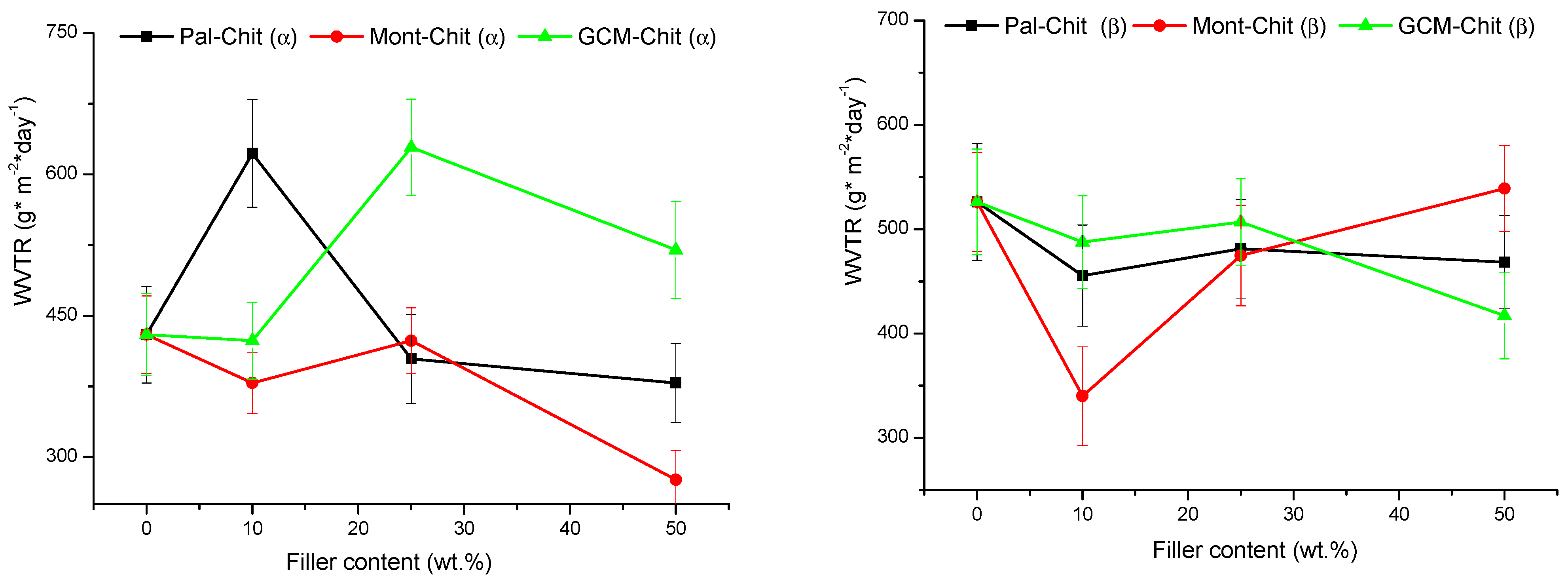
2.1. Mechanical and Physical Properties of the Composite Films
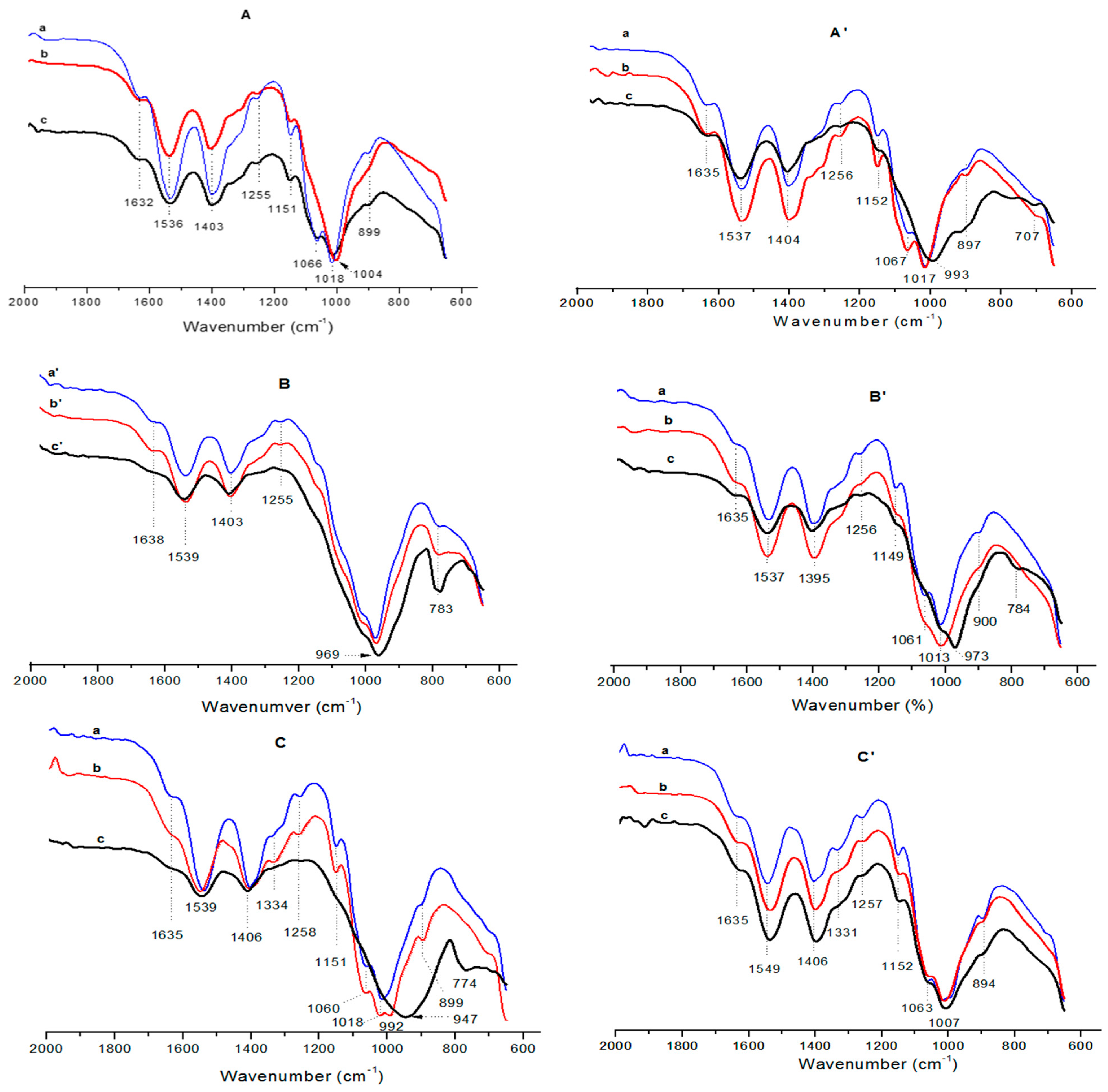
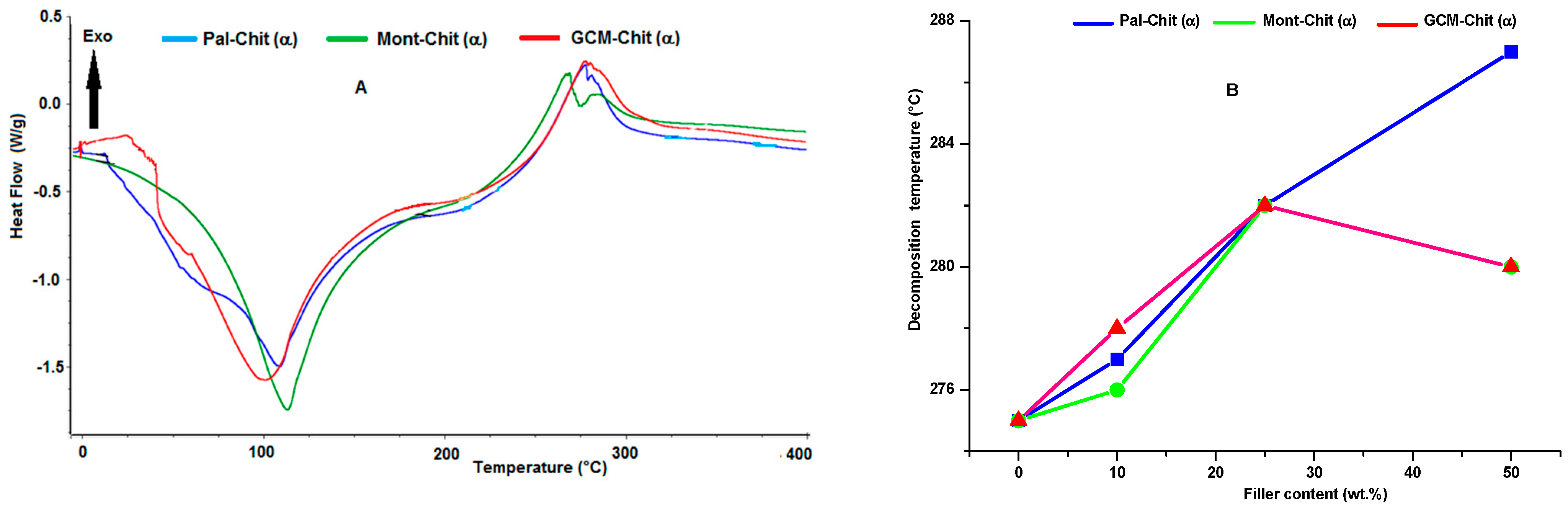
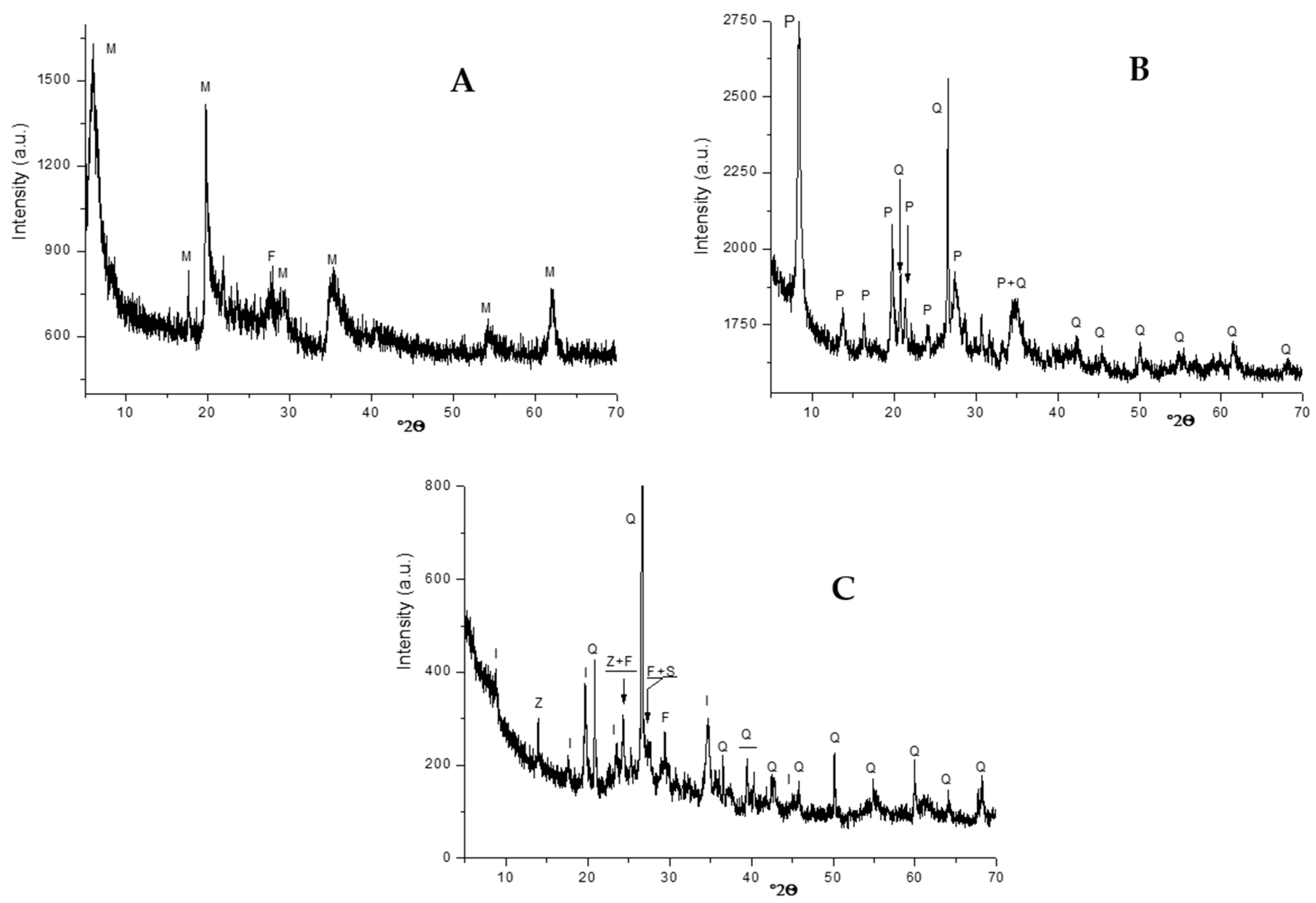
2.2. Microstructural Characterization of the Films
3. Materials and Methods
3.1. Fillers
3.2. Obtention of α- and β-Chitosans
3.3. Film Preparation
3.4. Measurements of Mechanical and Physical Properties
3.5. Characterization Techniques
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Tokiwa, Y.; Calabia, B.P.; Ugwu, C.U.; Aiba, S. Biodegradability of plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef]
- Coma, V.; Bartkowiak, A. Potential of chitosans in the development of edible food packaging. In Chitin and Chitosan: Properties and Applications, 1st ed.; van den Broek, L.A.M., Boeriu, C.G., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 349–369. [Google Scholar]
- Elsabee, M.Z.; Abdou, E.S. Chitosan based edible films and coatings: A review. Mater. Sci. Eng. C 2013, 33, 1819–1841. [Google Scholar] [CrossRef]
- Mujtaba, M.; Morsi, R.E.; Kerch, G.; Elsabee, M.Z.; Kaya, M.; Labidi, J.; Khawar, K.M. Current advancements in chitosan-based film production for food technology—A review. Int. J. Biol. Macromol. 2019, 121, 889–904. [Google Scholar] [CrossRef]
- Shahidi, F.; Abuzaytoun, R. Chitin, chitosan, and co-products: Chemistry, production, applications, and health effects. Adv. Food Nutr. Res. 2005, 49, 93–135. [Google Scholar] [PubMed]
- Casadidio, C.; Peregrina, D.V.; Gigliobianco, M.R.; Deng, S.; Censi, R.; Martino, P.D. Chitin and chitosans: Characteristics, eco-friendly processes, and applications in cosmetic science. Mar. Drugs 2019, 17, 369. [Google Scholar] [CrossRef] [Green Version]
- Kaya, M.; Mujtaba, M.; Ehrlich, H.; Salaberria, A.M.; Baran, T.; Amemiyaf, C.T.; Galli, R.; Akyuz, L.; Sargin, I.; Labidi, J. On chemistry of γ-chitin. Carbohydr. Polym. 2017, 176, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Rhim, J.W.; Hong, S.I.; Park, H.M.; Ng, P.K.W. Preparation and characterization of chitosan-based nanocomposite films with antimicrobial activity. J. Agric. Food Chem. 2006, 54, 5814–5822. [Google Scholar] [CrossRef] [PubMed]
- Galan, E. Properties and applications of palygorskite-sepiolite clays. Clay Miner. 1996, 31, 443–453. [Google Scholar] [CrossRef]
- Grim, R.E.; Güven, N. Bentonites: Geology, Mineralogy, Properties and Uses, Development in Sedimentology; Elsevier scientific publishing company: Amsterdam, The Netherlands, 1978; p. 217. [Google Scholar]
- Singer, A.; Galan, E. Palygorskite-Sepiolite: Occurrence, Genesis and uses; Elsevier Scientific Publishing Co.: Amsterdam, The Netherlands, 1984; p. 276. [Google Scholar]
- Carretero, M.I.; Gomes, C.S.F.; Tateo, F. Clays and human health. In Handbook of Clay Science—Developments in Clay Science; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 1, pp. 717–741. [Google Scholar]
- Provis, J.L.; van Deventer, J.S.J. Geopolymers: Structure, Processing, Properties and Industrial Application, 1st ed.; Woodhead Publishing Limited: Cambridge, UK; CRC Press LLC: Cambridge, UK, 2009. [Google Scholar]
- Oudadesse, H.; Derrien, A.C.; Lefloch, M.; Davidovits, J. MAS-NMR studies of geopolymers heat-treated for applications in biomaterials field. J. Mater. Sci. 2007, 42, 3092–3098. [Google Scholar] [CrossRef]
- Cai, B.; Engqvist, H.; Bredenberg, S. Evaluation of the resistance of a geopolymer-based drug delivery system to tampering. Int. J. Pharm. 2014, 465, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Tjong, S.C. Structural and mechanical properties of polymer nanocomposites. Mater. Sci. Eng. R Rep. 2006, 53, 73–197. [Google Scholar] [CrossRef]
- Tan, B.; Thomas, N.L. A Review of the water barrier properties of polymer/clay and polymer/grapheme nanocomposites. J. Membr. Sci. 2016, 514, 595–612. [Google Scholar] [CrossRef] [Green Version]
- Chau, T.T.; Bruckard, W.J.; Koh, P.T.L.; Nguyen, A.V. A review of factors that affect contact angle and implications for flotation practice. Adv. Colloid Interface Sci. 2009, 150, 106–115. [Google Scholar] [CrossRef]
- Miller, J.D.; Veeramasuneni, S.; Drelich, J.; Yalamanchili, M.R.; Yamauchi, G. Effect of roughness as determined by atomic force microscopy on the wetting properties of PTFE thin films. Polym. Eng. Sci. 1996, 36, 1849–1855. [Google Scholar] [CrossRef]
- Bastarrachea, L.; Dhawan, S.; Sablani, S.S. Engineering properties of polymeric-based antimicrobial films for food packaging. Food Eng. Rev. 2011, 3, 79–93. [Google Scholar] [CrossRef]
- Han, J.H.; Cennadios, A. Edible films and coatings: A review. In Food Science and Technology, Innovations in Food Packaging, 2nd ed.; Han, J.H., Ed.; Academic Press: Cambridge, MA, USA, 2005; pp. 213–255. [Google Scholar]
- Aljawish, A.; Chevalot, I.; Jasniewski, J.; Scher, J.; Munigli, L. Enzymatic synthesis of chitosan derivatives and their potential applications. J. Mol. Catal. B Enzym. 2015, 112, 25–39. [Google Scholar] [CrossRef]
- Chen, P.; Xie, F.; Tang, F.; McNally, T. Influence of plasticizer type and nanoclay on the properties of chitosan-based materials. Eur. Polym. J. 2021, 144, 110225. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Du, W.X.; Avena-Bustillos, R.J.; Soares, N.F.F.; McHugh, T.H. Edible films from pectin: Physical-mechanical and antimicrobial properties—A review. Food Hydrocoll. 2014, 35, 287–296. [Google Scholar] [CrossRef]
- Lewandowska, K.; Sionkowska, A.; Kaczmarek, B.; Furtos, G. Characterization of chitosan composites with various clays. Int. J. Biol. Macromol. 2014, 65, 534–541. [Google Scholar] [CrossRef]
- Leszczyńska, A.; Njuguna, J.; Pielichowski, K.; Banerjee, J.R. Polymer/montmorillonite nanocomposites with improved thermal properties. Part II: Thermal stability of montmorillonite nanocomposites based on different polymeric matrixes. Thermochim. Acta 2007, 454, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Davidovits, J. Geopolymer Chemistry and Applications, 5th ed.; Institut Géopolymère: Saint-Quentin, France, 2020. [Google Scholar]
- El Hafid, K.; Hajjaji, M. Geopolymerization of glass-and silicate-containing heated clay. Constr. Build. Mater. 2018, 159, 598–609. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.M.; Arguelles-Monal, W.; Desbrieres, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Tolaimate, A.; Desbrières, J.; Rhazi, M.; Alagui, A. Contribution to the preparation of chitins and chitosans with controlled physicochemical properties. Polymer 2003, 44, 7939–7952. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]






| α-Chitosan | Mont-α Chitosan | Pal-α Chitosan | GCM-α Chitosan | Assignment | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Montmorillonite Content (wt.%) | Palygorskite Content (wt.%) | GCM Content (wt.%) | ||||||||
| 3 | 5 | 25 | 15 | 25 | 50 | 3 | 5 | 25 | ||
| 1658 | 1632 | 1632 | 1632 | 1638 | 1640 | 1639 | 1635 | 1635 | - | νCO (amide I) |
| 1598 | 1536 | 1536 | 1536 | 1539 | 1541 | 1538 | 1539 | 1547 | 1547 | δ-NH3+ |
| 1428 | 1403 | 1403 | 1403 | 1403 | 1406 | 1411 | 1406 | 1406 | 1414 | δ CH2 (CH2OH) |
| 1383 | δ CH3 (NHCOCH3) | |||||||||
| 1335 | 1334 | 1332 | - | δ CH (pyranose ring) | ||||||
| 1255 | 1255 | 1255 | 1255 | 1255 | 1250 | - | 1258 | 1261 | - | NHCO group (amide III) |
| 1151 | 1151 | 1151 | 1151 | 1151 | 1151 | - | νCOC (glycosidic linkage) | |||
| 1094 | 1066 | - | 1066 | 1060 | 1063 | - | νCO (secondary OH group) | |||
| 1038 | 1018 | 1004 | 1012 | 1018 | 1023 | - | νCO (primary OH group) | |||
| - | 992 | - | Geopolymer | |||||||
| 969 | 969 | 964 | Palygorskite | |||||||
| - | - | 947 | Geopolymer | |||||||
| 899 | 899 | - | 893 | 899 | 896 | - | pyranose ring | |||
| 783 | 786 | 780 | Palygorskite | |||||||
| - | - | 774 | Quartz | |||||||
| 661 | δNH out of plane | |||||||||
| 602 | δOH out of plane | |||||||||
| β-Chitosan | Mont-β Chitosan | Pal-β Chitosan | GCM-β Chitosan | Assignment | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Montmorillonite Content (wt.%) | Palygorskite Content (wt.%) | GCMContent (wt.%) | ||||||||
| 3 | 5 | 25 | 3 | 5 | 25 | 3 | 5 | 25 | ||
| 1621 | 1635 | 1635 | 1635 | 1635 | 1635 | 1635 | 1635 | 1635 | 1635 | νCO (amide I) |
| 1537 | 1537 | 1537 | 1537 | 1537 | 1537 | 1549 | 1539 | 1537 | δ-NH3+ | |
| 1431 | 1404 | 1404 | 1404 | 1395 | 1395 | 1395 | 1406 | 1400 | 1400 | δ CH2 (CH2OH) |
| 1388 | δ CH3 (NHCOCH3) | |||||||||
| 1323 | 1331 | 1331 | 1331 | δ CH (pyranose ring) | ||||||
| 1261 | 1256 | 1256 | 1256 | 1256 | 1256 | 1256 | 1257 | 1257 | 1257 | NHCO group (amide III) |
| 1111 | 1152 | 1152 | 1152 | 1149 | 1149 | 1149 | 1152 | 1152 | 1152 | νCOC (glycosidiclinkage) |
| 1067 | 1067 | - | 1061 | 1061 | 1061 | 1063 | 1063 | 1063 | νCO (secondary OH group) | |
| 1038 | 1017 | 1017 | 993 | 1013 | 1013 | 1013 | 1007 | 1007 | 1007 | νCO (primary OH group) |
| - | - | 973 | Palygorskite | |||||||
| 888 | 897 | 897 | 897 | 900 | 900 | - | 894 | 894 | 894 | pyranose ring |
| - | - | 784 | Quartz | |||||||
| 661 | 699 | 699 | 707 | δNH out of plane | ||||||
| 610 | δOH out of plane | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mourak, A.; Hajjaji, M.; Alagui, A.; Martin, P.; Joly, N. Effects of Geomaterial-Originated Fillers on Microstructure and Mechanical/Physical Properties of α- and β-Chitosan-Based Films. Molecules 2021, 26, 7514. https://doi.org/10.3390/molecules26247514
Mourak A, Hajjaji M, Alagui A, Martin P, Joly N. Effects of Geomaterial-Originated Fillers on Microstructure and Mechanical/Physical Properties of α- and β-Chitosan-Based Films. Molecules. 2021; 26(24):7514. https://doi.org/10.3390/molecules26247514
Chicago/Turabian StyleMourak, Abdellah, Mohamed Hajjaji, Abdelhakim Alagui, Patrick Martin, and Nicolas Joly. 2021. "Effects of Geomaterial-Originated Fillers on Microstructure and Mechanical/Physical Properties of α- and β-Chitosan-Based Films" Molecules 26, no. 24: 7514. https://doi.org/10.3390/molecules26247514
APA StyleMourak, A., Hajjaji, M., Alagui, A., Martin, P., & Joly, N. (2021). Effects of Geomaterial-Originated Fillers on Microstructure and Mechanical/Physical Properties of α- and β-Chitosan-Based Films. Molecules, 26(24), 7514. https://doi.org/10.3390/molecules26247514








