Properties of the PVA-VAVTD KOH Blend as a Gel Polymer Electrolyte for Zinc Batteries
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Characterization
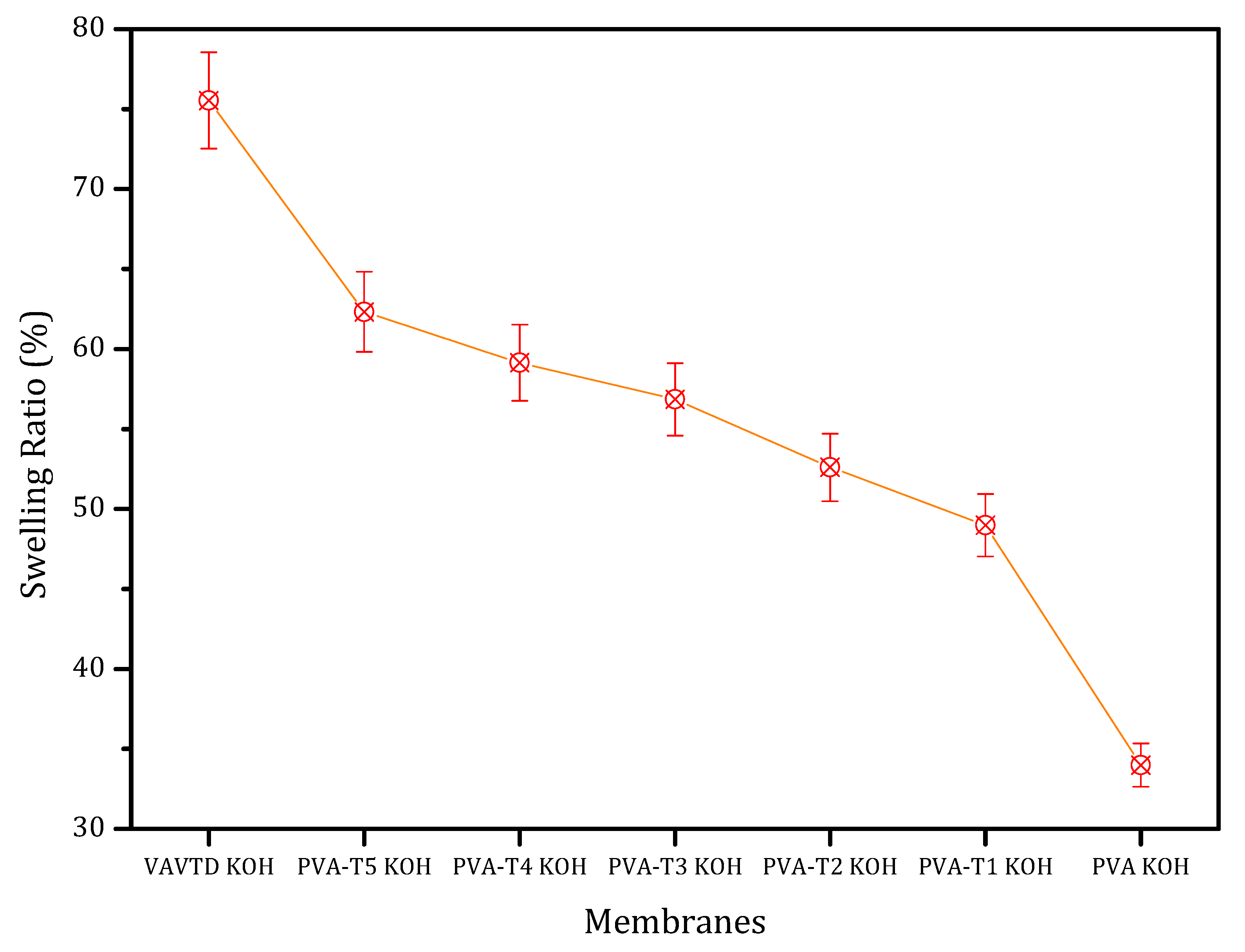
2.1.1. Swelling Ratio
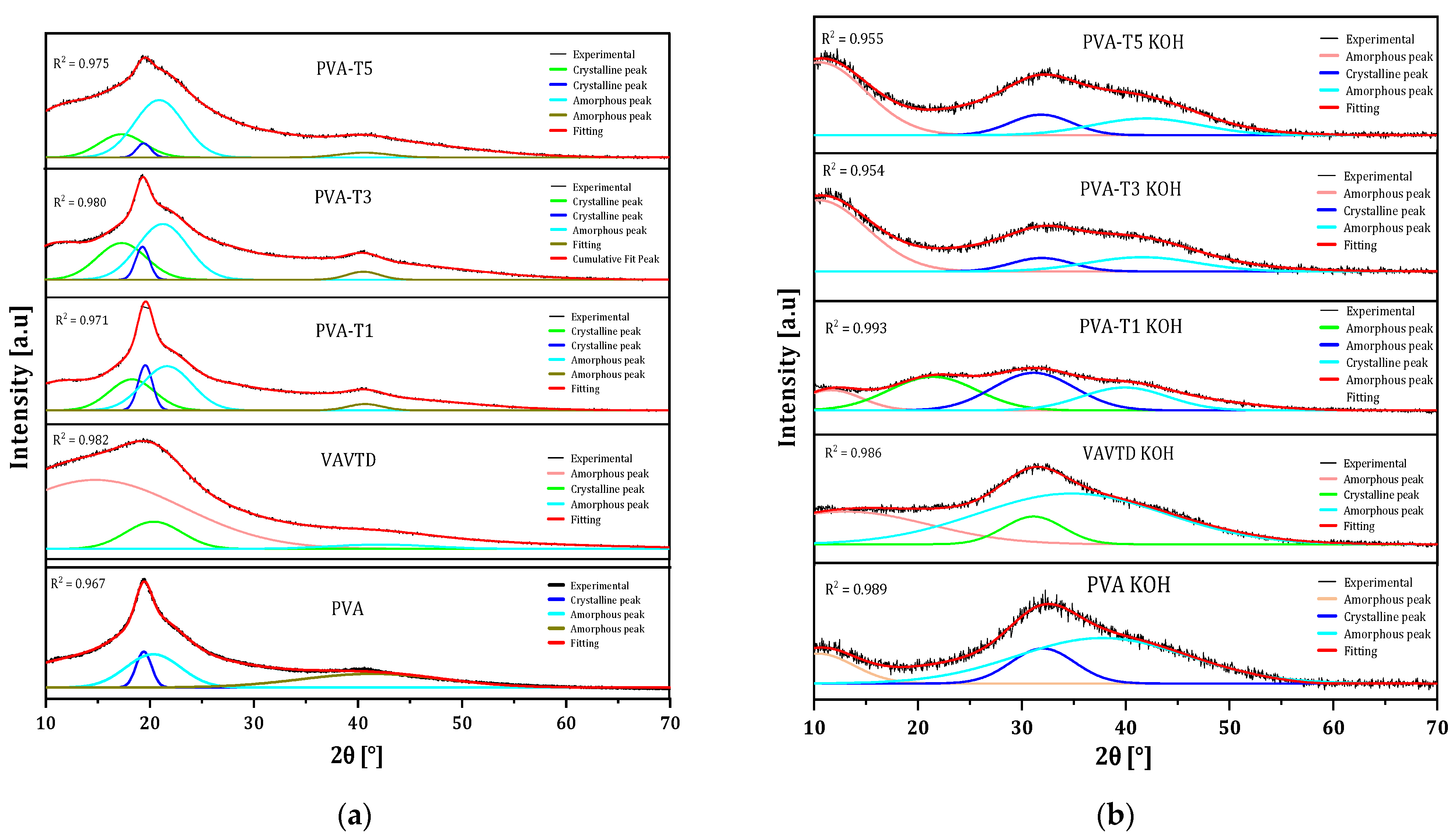
2.1.2. XRD Analysis
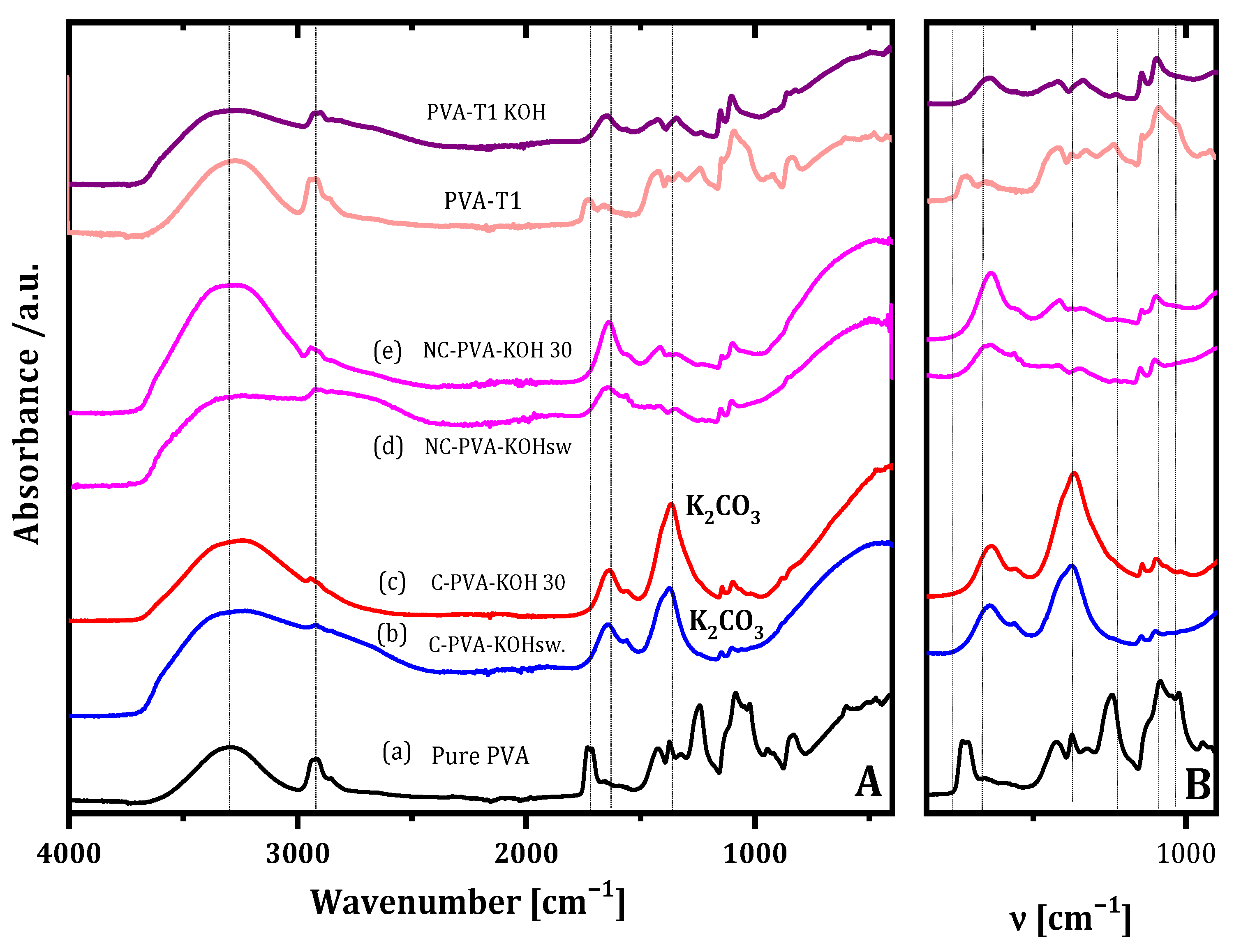
2.1.3. ATR-FTIR Analysis
2.1.4. Thermal Analysis
2.2. Electrochemical Characterization
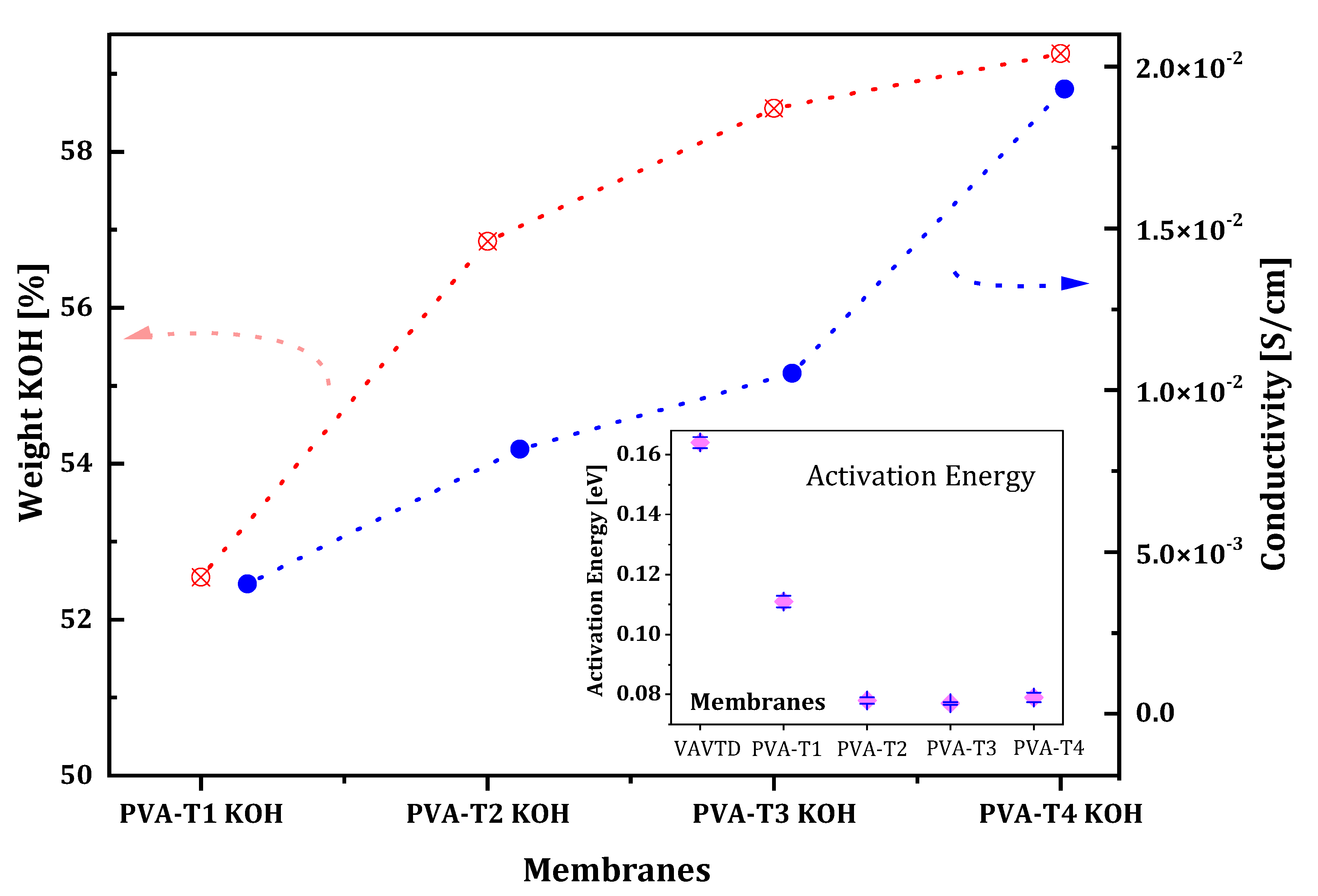
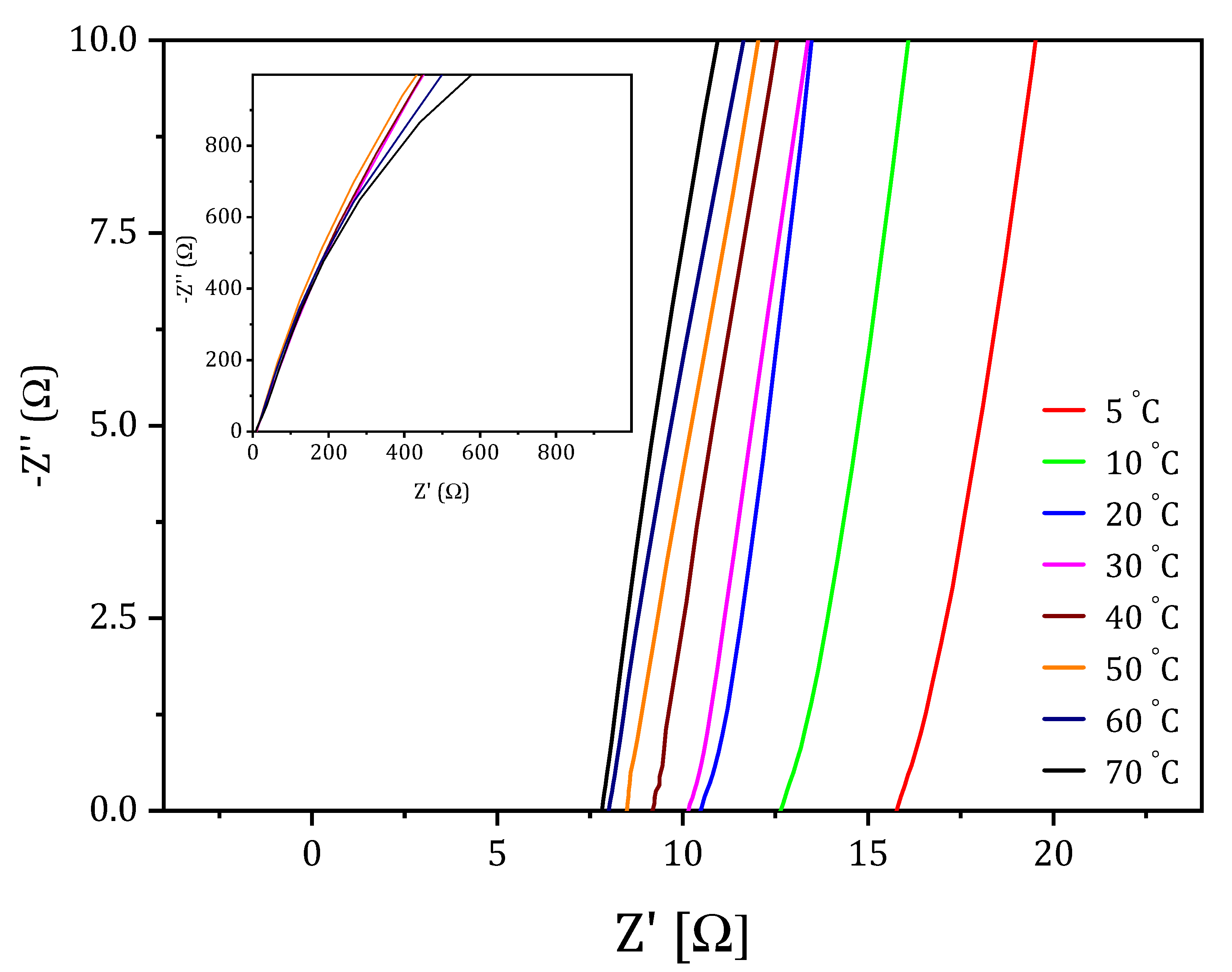
2.2.1. The Influence of VAVTD Content on Ionic Conductivity
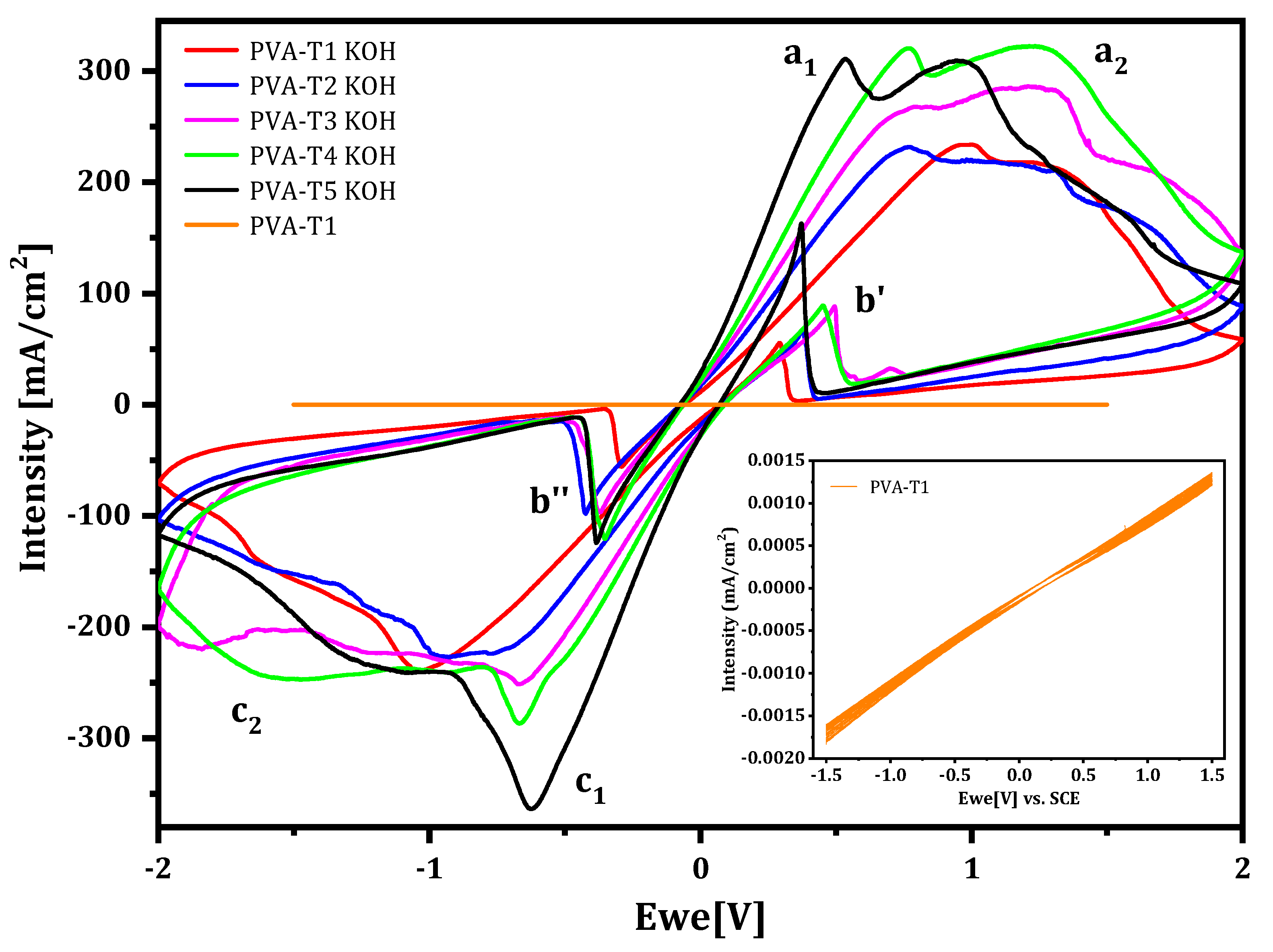
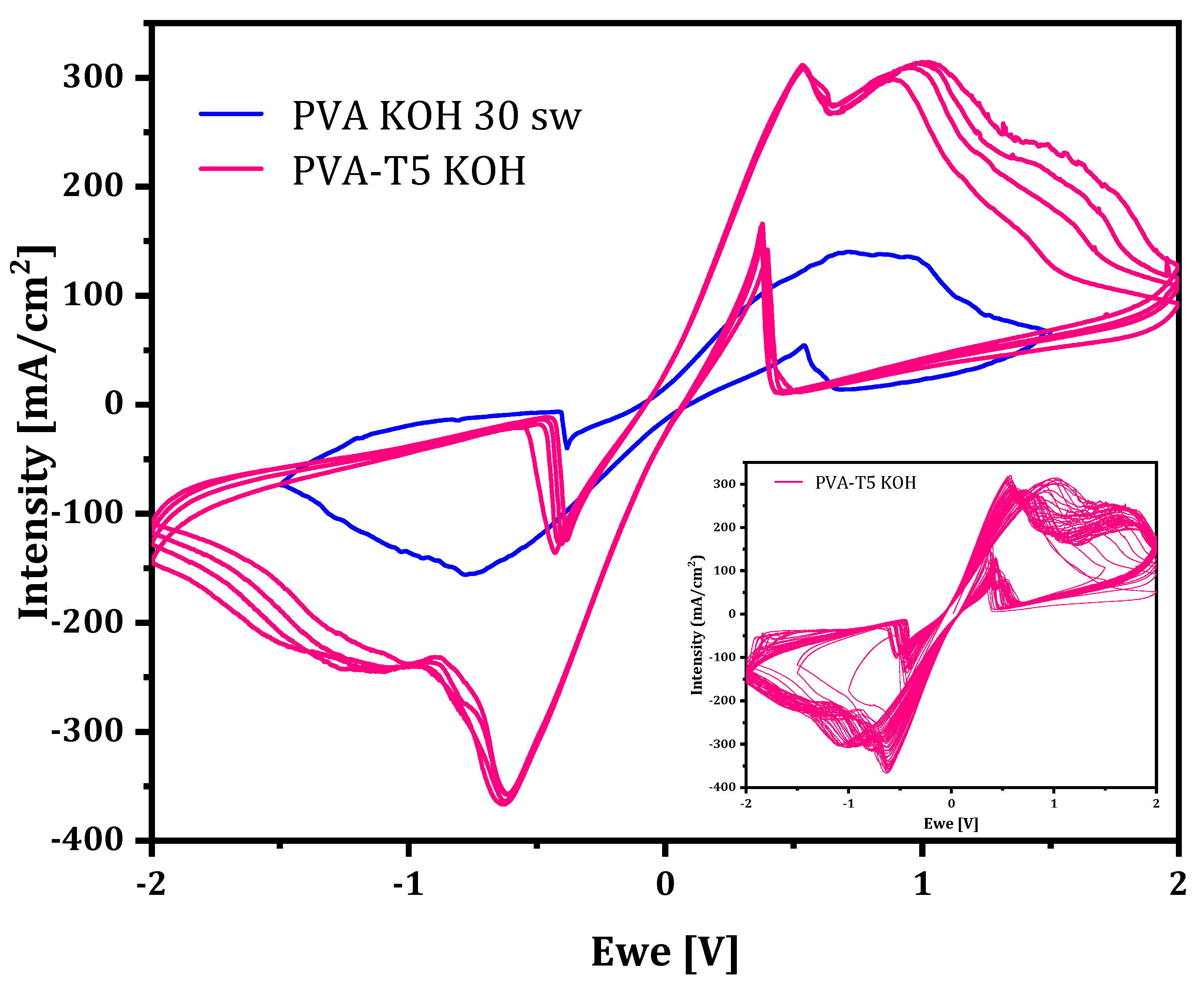
2.2.2. Cyclic Voltammetry
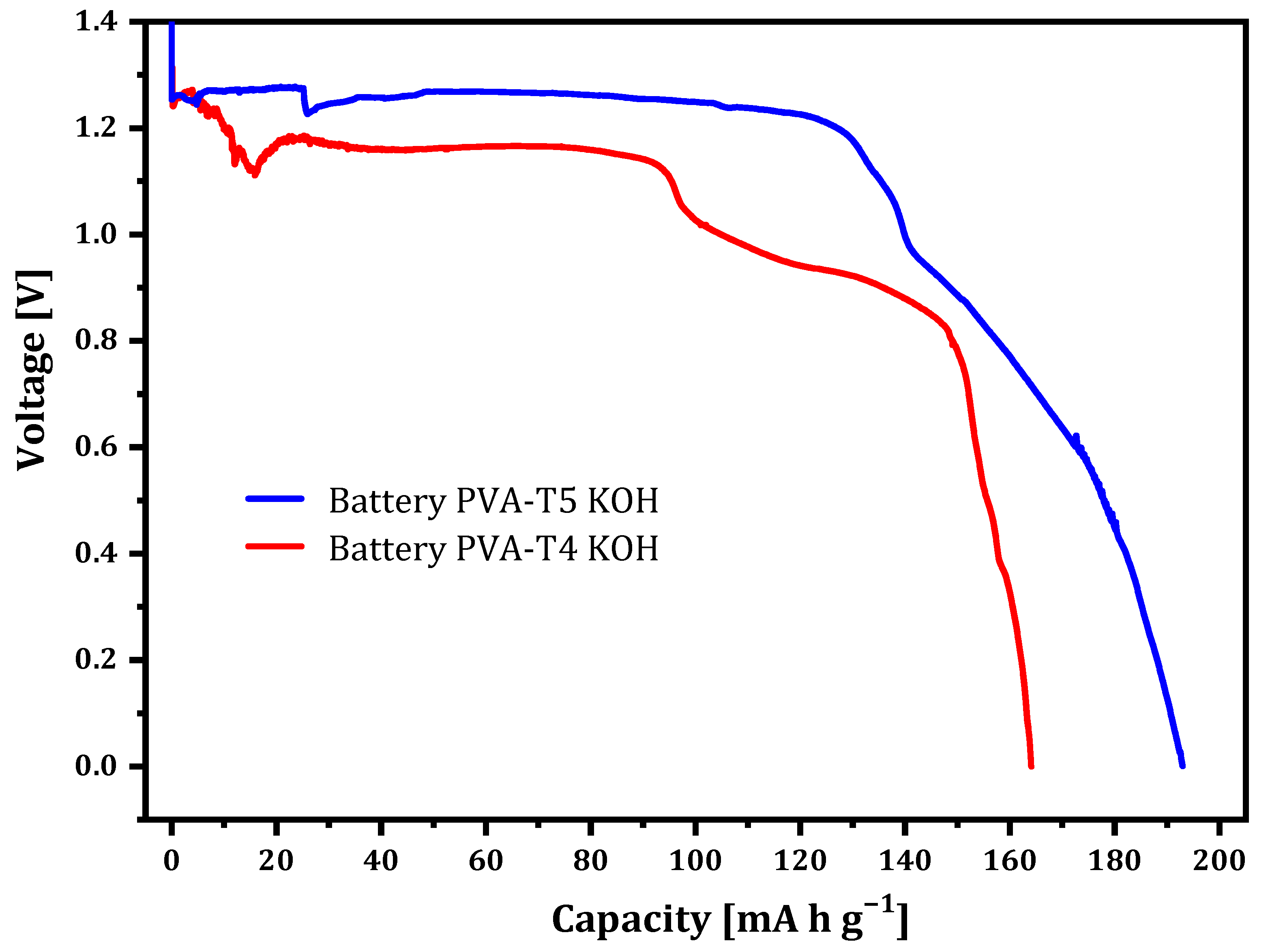
2.3. Zn/PVA-VAVTD KOH/Air Battery
3. Conclusions
4. Materials and Methods
4.1. VAVTD Synthesis
4.2. Preparation of PVA-VAVTD GPEs
4.3. Swelling Behavior of GPEs
4.4. Structural, Thermal, and Electrochemical Characterization Techniques
4.4.1. ATR-FTIR and XRD Methods
4.4.2. Thermal Analysis
4.4.3. Conductivity and Voltammetry Studies
4.5. Electrode Preparation and Battery Setup
4.5.1. Zn Electrode Preparation
4.5.2. Zn/PVA-VAVTD/Air Battery Fabrication
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fenton, D.E.; Parker, J.M.; Wright, P.V. Complexes of alkali metal ions with poly(ethylene oxide). Polymer 1973, 14, 589. [Google Scholar] [CrossRef]
- Michel, A. Polymers with Ionic Conductivity. Adv. Mater. 1990, 2, 278–286. [Google Scholar]
- Aziz, S.B.; Abidin, Z.H.Z. Ion-transport study in nanocomposite solid polymer electrolytes based on chitosan: Electrical and dielectric analysis. J. Appl. Polym. Sci. 2015, 132, 1–10. [Google Scholar] [CrossRef]
- Santos, F.; Tafur, J.P.; Abad, J.; Fernández Romero, A.J. Structural modifications and ionic transport of PVA-KOH hydrogels applied in Zn/Air batteries. J. Electroanal. Chem. 2019, 850, 113380. [Google Scholar] [CrossRef]
- Guisao, J.P.T.; Romero, A.J.F. Interaction between Zn2+ cations and n-methyl-2-pyrrolidone in ionic liquid-based Gel Polymer Electrolytes for Zn batteries. Electrochim. Acta 2015, 176, 1447–1453. [Google Scholar] [CrossRef]
- Prakash, R.; Maiti, P. Functionalized thermoplastic polyurethane gel electrolytes for cosensitized TiO2/CdS/CdSe photoanode solar cells with high efficiency. Energy Fuels 2020, 34, 16847–16857. [Google Scholar] [CrossRef]
- Maria, M.B.; Sivasubramanian, G.; Kulasekaran, P.; Deivanayagam, P. Sulfonated polystyrene-block-poly(ethylene-ran-butylene)-block-polystyrene based membranes containing CuO@g-C3N4 embedded with 2,4,6-triphenylpyrylium tetrafluoroborate for fuel cell applications. Soft Matter. 2021, 17, 8387–8393. [Google Scholar] [CrossRef]
- Ni, J.; Zhou, J.; Bing, J.; Guan, X. Recycling the cathode materials of spent Li-ion batteries in a H-Shaped neutral water electrolysis cell. Sep. Purif. Technol. 2021, 278, 119485. [Google Scholar] [CrossRef]
- Asnawi, A.S.F.M.; Aziz, S.B.; Nofal, M.M.; Yusof, Y.M.; Brevik, I.; Hamsan, M.H.; Brza, M.A.; Abdulwahid, R.T.; Kadir, M.F.Z. Metal complex as a novel approach to enhance the amorphous phase and improve the EDLC performance of plasticized proton conducting chitosan-based polymer electrolyte. Membranes 2020, 10, 132. [Google Scholar] [CrossRef]
- Li, Q.; Itoh, T.; Imanishi, N.; Hirano, A.; Takeda, Y.; Yamamoto, O. All solid lithium polymer batteries with a novel composite polymer electrolyte. Solid State Ionics 2003, 159, 97–109. [Google Scholar] [CrossRef]
- Ngai, K.S.; Ramesh, S.; Ramesh, K.; Juan, J.C. A review of polymer electrolytes: Fundamental, approaches and applications. Ionics 2016, 22, 1259–1279. [Google Scholar] [CrossRef]
- Zhou, D.; Shanmukaraj, D.; Tkacheva, A.; Armand, M.; Wang, G. Polymer Electrolytes for Lithium-Based Batteries: Advances and Prospects. Chem 2019, 5, 2326–2352. [Google Scholar] [CrossRef]
- Aziz, S.B.; Woo, T.J.; Kadir, M.F.Z.; Ahmed, H.M. A conceptual review on polymer electrolytes and ion transport models. J. Sci. Adv. Mater. Devices. 2018, 3, 1–17. [Google Scholar] [CrossRef]
- Hamsan, M.H.; Nofal, M.M.; Aziz, S.B.; Brza, M.A.; Dannoun, E.M.A.; Murad, A.R.; Kadir, M.F.Z.; Muzakir, S.K. Plasticized Polymer Blend Electrolyte Based on Chitosan for Energy Storage Application: Structural, Circuit Modeling, Morphological and Electrochemical Properties. Polymers 2021, 13, 8. [Google Scholar] [CrossRef]
- Hosseini, S.; Lao-atiman, W.; Han, S.J.; Arpornwichanop, A.; Yonezawa, T.; Kheawhom, S. Discharge Performance of Zinc-Air Flow Batteries Under the Effects of Sodium Dodecyl Sulfate and Pluronic F-127. Sci. Rep. 2018, 8, 14909. [Google Scholar] [CrossRef] [Green Version]
- Santos, F.; Urbina, A.; Abad, J.; López, R.; Toledo, C.; Fernández Romero, A.J. Environmental and economical assessment for a sustainable Zn/air battery. Chemosphere 2020, 250, 126273. [Google Scholar] [CrossRef]
- Suren, S.; Kheawhom, S. Development of a High Energy Density Flexible Zinc-Air Battery. J. Electrochem. Soc. 2016, 163, A846–A850. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Li, X.; White, J.; Zhong, C.; Deng, Y.; Hu, W.; Ma, T. Metal–Air Batteries: From Static to Flow System. Adv. Energy Mater. 2018, 8, 1801396. [Google Scholar] [CrossRef]
- Santos, F.; Fernández Romero, A.J. Hydration as a solution to zinc batteries. Nat. Sustain. 2021, 2021, 1–2. [Google Scholar] [CrossRef]
- Fu, J.; Cano, Z.P.; Park, M.G.; Yu, A.; Fowler, M.; Chen, Z. Electrically Rechargeable Zinc–Air Batteries: Progress, Challenges, and Perspectives. Adv. Mater. 2017, 297, 1604685. [Google Scholar] [CrossRef]
- Amunátegui, B.; Ibáñez, A.; Sierra, M.; Pérez, M. Electrochemical energy storage for renewable energy integration: Zinc-air flow batteries. J. Appl. Electrochem. 2018, 48, 627–637. [Google Scholar] [CrossRef]
- Abdullah, O.G.; Aziz, S.B.; Rasheed, M.A. Incorporation of NH4NO3 into MC-PVA blend-based polymer to prepare proton-conducting polymer electrolyte films. Ionics 2018, 24, 777–785. [Google Scholar] [CrossRef]
- Saeed, M.A.M.; Abdullah, O.G. Effect of high ammonium salt concentration and temperature on the structure, morphology, and ionic conductivity of proton-conductor solid polymer electrolytes based pva. Membranes 2020, 10, 262. [Google Scholar] [CrossRef] [PubMed]
- Tiong, T.S.; Buraidah, M.H.; Teo, L.P.; Arof, A.K. Conductivity studies of poly(ethylene oxide)(PEO)/poly(vinyl alcohol) (PVA) blend gel polymer electrolytes for dye-sensitized solar cells. Ionics 2016, 22, 2133–2142. [Google Scholar] [CrossRef]
- Merle, G.; Wessling, M.; Nijmeijer, K. Anion exchange membranes for alkaline fuel cells: A review. J. Memb. Sci. 2011, 377, 1–35. [Google Scholar] [CrossRef]
- Asua, J.M. Polymer Reaction Engineering; Blackwell Publishing Ltd.: Oxford, UK, 2008; ISBN 1405144424. [Google Scholar]
- Lewandowski, A.; Skorupska, K.; Malinska, J. Novel poly(vinyl alcohol)-KOH-H O alkaline polymer electrolyte. Solid State Ion. 2000, 133, 265–271. [Google Scholar] [CrossRef]
- Gupta, S.; Pramanik, A.K.; Kailath, A.; Mishra, T.; Guha, A.; Nayar, S.; Sinha, A. Composition dependent structural modulations in transparent poly(vinyl alcohol) hydrogels. Colloids Surf. B Biointerfaces. 2009, 74, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Barrios, A.; González, G.; Reinoso, C.; Santiana, J.; Quiroz, F.; Chango, J.I.; Vera, C.C.; Caniglia, L.; Salazar, V.; Fernández-Delgado, M. In situ synthesis and long-term stabilization of nanosilver in poly(vinyl acetate-co-butyl acrylate-co-neodecanoate) matrix for antibacterial applications. Mater. Chem. Phys. 2020, 255, 123476. [Google Scholar] [CrossRef]
- Ismail, A.S.; Darwish, M.S.A.; Ismail, E.A. Synthesis and characterization of hydrophilic chitosan-polyvinyl acetate blends and their sorption performance in binary methanol–water mixture. Egypt. J. Pet. 2017, 26, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Aziz, S.B.; Marf, A.S.; Dannoun, E.M.A.; Brza, M.A.; Abdullah, R.M. The study of the degree of crystallinity, electrical equivalent circuit, and dielectric properties of polyvinyl alcohol (PVA)-based biopolymer electrolytes. Polymers 2020, 12, 2184. [Google Scholar] [CrossRef] [PubMed]
- Brza, M.A.; Aziz, S.B.; Anuar, H.; Dannoun, E.M.A.; Ali, F.; Abdulwahid, R.T.; Al-Zangana, S.; Kadir, M.F.Z. The study of EDLC device with high electrochemical performance fabricated from proton ion conducting PVA-based polymer composite electrolytes plasticized with glycerol. Polymers 2020, 12, 1896. [Google Scholar] [CrossRef]
- Tretinnikov, O.N.; Zagorskaya, S.A. Determination of the degree of crystallinity of poly(Vinyl alcohol) by ftir spectroscopy. J. Appl. Spectrosc. 2012, 79, 521–526. [Google Scholar] [CrossRef]
- Qiao, J.; Fu, J.; Lin, R.; Ma, J.; Liu, J. Alkaline solid polymer electrolyte membranes based on structurally modified PVA/PVP with improved alkali stability. Polymer 2010, 51, 4850–4859. [Google Scholar] [CrossRef]
- Seki, T.; Chiang, K.Y.; Yu, C.C.; Yu, X.; Okuno, M.; Hunger, J.; Nagata, Y.; Bonn, M. The bending mode of water: A powerful probe for hydrogen bond structure of aqueous systems. J. Phys. Chem. Lett. 2020, 11, 8459–8469. [Google Scholar] [CrossRef] [PubMed]
- Pak, J.; Han, S.J.; Wee, J.H. Precipitation of potassium-based carbonates for carbon dioxide fixation via the carbonation and re-carbonation of KOH dissolved aqueous ethanol solutions. Chem. Eng. J. 2022, 427, 131669. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, K.; Tang, D.; Liu, W.; Meng, F.; Huang, Q.; Liu, J. Recent Progress in Electrolytes for Zn–Air Batteries. Front. Chem. 2020, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Fan, X.; Ding, J.; Hu, W.; Zhong, C.; Lu, J. Challenges in Zinc Electrodes for Alkaline Zinc-Air Batteries: Obstacles to Commercialization. ACS Energy Lett. 2019, 4, 2259–2270. [Google Scholar] [CrossRef]
- Jackson, P.; Robinson, K.; Puxty, G.; Attalla, M. In situ Fourier Transform-Infrared (FT-IR) analysis of carbon dioxide absorption and desorption in amine solutions. Energy Procedia 2009, 1, 985–994. [Google Scholar] [CrossRef] [Green Version]
- Gilman, J.W.; VanderHart, D.L.; Kashiwagi, T. Thermal Decomposition Chemistry of Poly(Vinyl Alcohol); Gordon, L.N., Ed.; Florida Institute of Technology: Melbourne, VIC, Australia, 1995; Volume 599, pp. 161–185. [Google Scholar]
- Restrepo, I.; Medina, C.; Meruane, V.; Akbari-Fakhrabadi, A.; Flores, P.; Rodríguez-Llamazares, S. The effect of molecular weight and hydrolysis degree of poly(vinyl alcohol)(PVA) on the thermal and mechanical properties of poly(lactic acid)/PVA blends. Polímeros 2018, 28, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Duquesne, S.; Lefebvre, J.; Delobel, R.; Camino, G.; LeBras, M.; Seeley, G. Vinyl acetate/butyl acrylate copolymers—Part 1: Mechanism of degradation. Polym. Degrad. Stab. 2004, 83, 19–28. [Google Scholar] [CrossRef]
- Ludwig, B.; Harald, C.; Manfred. F.; Willi, K.; Arnold, S. Comprehensive Polymer Science: The Synthesis, Characteri-Zation, Reactions and Applications of Polymers, 7th ed.; Pergamon Press: Oxford, UK, 1989; ISBN 0-08-03251 6-5. [Google Scholar]
- Zelkó, R.; Szakonyi, G. The effect of water on the solid state characteristics of pharmaceutical excipients: Molecular mechanisms, measurement techniques, and quality aspects of final dosage form. Int. J. Pharm. Investig. 2012, 2, 18. [Google Scholar] [CrossRef] [Green Version]
- Strydom, C.A.; Collins, A.C.; Bunt, J.R. The influence of various potassium compound additions on the plasticity of a high-swelling South African coal under pyrolyzing conditions. J. Anal. Appl. Pyrolysis 2015, 112, 221–229. [Google Scholar] [CrossRef]
- Hatta, F.F.; Yahya, M.Z.A.; Ali, A.M.M.; Subban, R.H.Y.; Harun, M.K.; Mohamad, A.A. Electrical Conductivity Studies on PVA/PVP-KOH Alkaline Solid Polymer Blend Electrolyte. Ionics 2005, 11, 418–422. [Google Scholar] [CrossRef]
- Fan, L.; Wang, M.; Zhang, Z.; Qin, G.; Hu, X.; Chen, Q. Preparation and characterization of PVA alkaline solid polymer electrolyte with addition of bamboo charcoal. Materials 2018, 11, 679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Han, H.; Wu, S.; Xu, S.; Yang, Y.; Zhou, C.; Zhao, X. Conductive carbon nanoparticles hybrid PEO/P(VDF-HFP)/SiO2 nanocomposite polymer electrolyte type dye sensitized solar cells. Solid State Ionics 2007, 178, 1595–1601. [Google Scholar] [CrossRef]
- Cai, M.; Park, S. Spectroelectrochemical Studies on Dissolution and Passivation of Zinc Electrodes in Alkaline Solutions. J. Electrochem. Soc. 1996, 143, 2125–2131. [Google Scholar] [CrossRef]
- Santiana, A.; Jessica, M. Desarrollo de una Emulsión Polimérica Vinil-Acrílica Con Características Hidrofóbicas. Bachelor’s Thesis, Escuela Politecnica Nacional, Quito, Ecuador, 2017. Available online: http://bibdigital.epn.edu.ec/handle/15000/17319 (accessed on 17 May 2017).
- Ramesh, S.; Upender, G.; Raju, K.C.J.; Padmaja, G.; Reddy, S.M.; Reddy, C.V. Effect of Ca on the Properties of Gd-Doped Ceria for IT-SOFC. J. Mod. Phys. 2013, 04, 859–863. [Google Scholar] [CrossRef] [Green Version]




| Electrolyte | Xc (%) | |
|---|---|---|
| Dried | Soaked in KOH | |
| PVA | 41.97 | 17.81 |
| VAVTD | 9.773 | 1.612 |
| PVA-T1 | 25.48 | 11.90 |
| PVA-T3 | 19.01 | 7.16 |
| PVA-T5 | 15.95 | 5.06 |
| Electrolyte | SR (%) | Ea (eV) | σ (S/cm) |
|---|---|---|---|
| VAVTD KOH | 75.2 | 0.164 | 0.001 |
| PVA-T1 KOH | 52.5 | 0.111 | 0.004 |
| PVA-T2 KOH | 56.8 | 0.078 | 0.008 |
| PVA-T3 KOH | 58.6 | 0.077 | 0.011 |
| PVA-T4 KOH | 59.3 | 0.079 | 0.019 |
| PVA-T5 KOH | 62.7 | - | 0.019 |
| Electrolyte | Sample Code |
|---|---|
| Poly (vinyl alcohol) | PVA |
| PVA + VAVTD (1:1) | PVA-T1 |
| PVA + VAVTD (1:2) | PVA-T2 |
| PVA + VAVTD (1:3) | PVA-T3 |
| PVA + VAVTD (1:4) | PVA-T4 |
| PVA + VAVTD (1:5) | PVA-T5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velez, A.A.I.; Reyes, E.; Diaz-Barrios, A.; Santos, F.; Fernández Romero, A.J.; Tafur, J.P. Properties of the PVA-VAVTD KOH Blend as a Gel Polymer Electrolyte for Zinc Batteries. Gels 2021, 7, 256. https://doi.org/10.3390/gels7040256
Velez AAI, Reyes E, Diaz-Barrios A, Santos F, Fernández Romero AJ, Tafur JP. Properties of the PVA-VAVTD KOH Blend as a Gel Polymer Electrolyte for Zinc Batteries. Gels. 2021; 7(4):256. https://doi.org/10.3390/gels7040256
Chicago/Turabian StyleVelez, Alisson A. Iles, Edwin Reyes, Antonio Diaz-Barrios, Florencio Santos, Antonio J. Fernández Romero, and Juan P. Tafur. 2021. "Properties of the PVA-VAVTD KOH Blend as a Gel Polymer Electrolyte for Zinc Batteries" Gels 7, no. 4: 256. https://doi.org/10.3390/gels7040256
APA StyleVelez, A. A. I., Reyes, E., Diaz-Barrios, A., Santos, F., Fernández Romero, A. J., & Tafur, J. P. (2021). Properties of the PVA-VAVTD KOH Blend as a Gel Polymer Electrolyte for Zinc Batteries. Gels, 7(4), 256. https://doi.org/10.3390/gels7040256






