The Current State of Knowledge of Shea Butter Tree (Vitellaria paradoxa C.F.Gaertner.) for Nutritional Value and Tree Improvement in West and Central Africa
Abstract
:1. Introduction
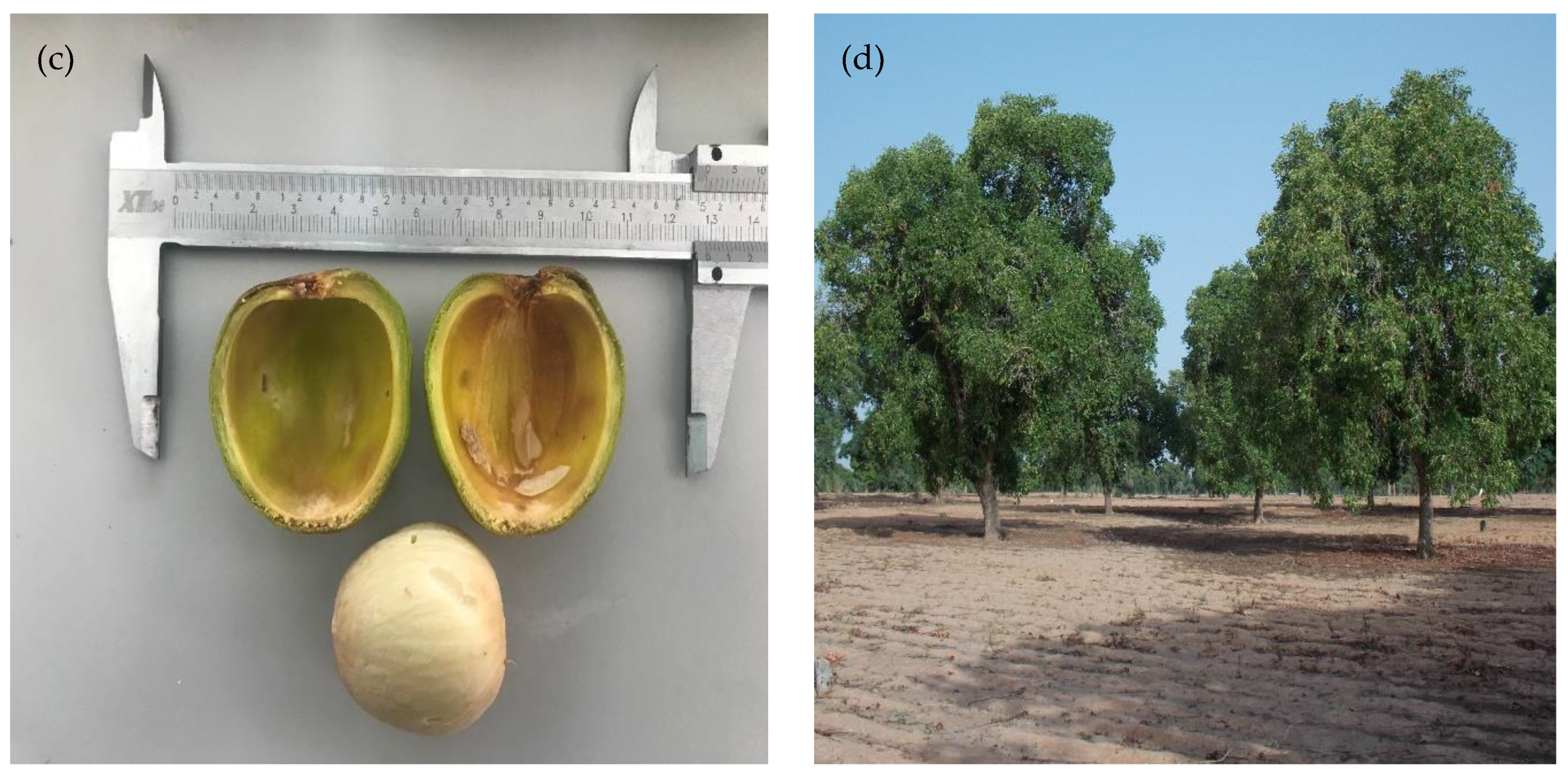
2. Taxonomy and Botanical Description
3. Ecological Requirements
3.1. Ecology
3.2. Phenology
3.3. Physiology and Reproductive Biology
4. Uses
4.1. Fruit Pulp and Kernels
4.2. Roots
4.3. Bark
4.4. Seed Husk
4.5. Timber and Environmental Services
5. Traditional Processing and Products Obtained from the Shea Fruit
6. Nutritional Composition
6.1. Nutritional Composition of Fruit Pulp
6.2. Biochemical and Phytochemical Composition of Shea Kernels and Butter
7. Importance of V. paradoxa in the Context of Agroforestry
8. Silvicultural Management of V. paradoxa
8.1. Tree Management
8.2. Propagation and Growth Performance
8.2.1. Generative Propagation
8.2.2. Vegetative Propagation
- Stem cuttings:
- Grafting:
- Air layering:
- In vitro propagation:
- Plan for rapid multiplication and how to shorten the long cycle:
8.3. Silvicultural Management of Trees
8.3.1. Parasitism
8.3.2. Diseases and Pests
8.4. Improvement Plan of V. paradoxa
- Strengthening forest management institutions through a local participatory approach for a suitable management plan about essential non-timber forests products (NTFPs) with high potential in the region (e.g., shea tree).
- Drawing a regional map of the model for sustainable management of NTFPs with a focus on livelihood improvement.
9. The Role of V. paradoxa in Food Security and Socio-Economic Development Diseases and Pests
10. Morphological and Genetic Diversity
11. Conclusions
Authors contribution
Funding
Acknowledgments
Conflicts of Interest
References
- Epanda, M.; Tsafack, R.; Ngo-Nonga, F.; Frynta, D.; Adi, N.N.; Willie, J.; Speelman, S. Contribution of Non-Timber Forest Product Valorisation to the Livelihood Assets of Local People in the Northern Periphery of the Dja Faunal Reserve, East Cameroon. Forests 2020, 11, 1019. [Google Scholar] [CrossRef]
- Leakey, R.R.B.; Tchoundjeu, Z.; Schreckenberg, K.; Shackleton, S.E.; Shackleton, C.M. Agroforestry Tree Products (AFTPs): Targeting Poverty Reduction and Enhanced Livelihoods. Int. J. Agric. Sustain. 2005, 3, 1–23. [Google Scholar] [CrossRef]
- Aleza, K.; Villamor, G.B.; Nyarko, B.K.; Wala, K.; Akpagana, K. Shea (Vitellaria paradoxa Gaertn C. F.) Fruit Yield Assessment and Management by Farm Households in the Atacora District of Benin. PLoS ONE 2018, 13, e0190234. [Google Scholar] [CrossRef]
- Tena, G. Sequencing Forgotten Crops. Nat. Plants 2019, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Hermans-Neumann, K.; Gerstner, K.; Geijzendorffer, I.R.; Herold, M.; Seppelt, R.; Wunder, S. Why Do Forest Products Become Less Available? A Pan-Tropical Comparison of Drivers of Forest-Resource Degradation. Environ. Res. Lett. 2016, 11, 125010. [Google Scholar] [CrossRef] [Green Version]
- Asprilla-Perea, J.; Díaz-Puente, J.M. Importance of Wild Foods to Household Food Security in Tropical Forest Areas. Food Secur. 2019, 11, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Billong, P.E.; Afiong Nana, N.; Betti, J.L.; Farick Njimbam, O.; Tientcheu Womeni, S.; Ávila Martin, E.; Ros Brull, G.; Okale, R.; Fa, J.E.; Funk, S.M. Ethnobotanical Survey of Wild Edible Plants Used by Baka People in Southeastern Cameroon. J. Ethnobiol. Ethnomed. 2020, 16, 64. [Google Scholar] [CrossRef] [PubMed]
- Dawson, I.K.; Leakey, R.; Clement, C.R.; Weber, J.C.; Cornelius, J.P.; Roshetko, J.M.; Vinceti, B.; Kalinganire, A.; Tchoundjeu, Z.; Masters, E.; et al. The Management of Tree Genetic Resources and the Livelihoods of Rural Communities in the Tropics: Non-Timber Forest Products, Smallholder Agroforestry Practices and Tree Commodity Crops. For. Ecol. Manag. 2014, 333, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Jamnadass, R.; Mumm, R.H.; Hale, I.; Hendre, P.; Muchugi, A.; Dawson, I.K.; Powell, W.; Graudal, L.; Yana-Shapiro, H.; Simons, A.J.; et al. Enhancing African Orphan Crops with Genomics. Nat. Genet. 2020, 52, 356–360. [Google Scholar] [CrossRef]
- Noordwijk, M.V.; Coe, R.; Sinclair, F. Agroforestry Paradigms. In Sustainable Development through Trees on Farms: Agroforestry in Its Fifth Decade; Van Noordwijk, M., Ed.; World Agroforestry (ICRAF): Bogor, Indonesia, 2019; pp. 1–14. [Google Scholar]
- Vinceti, B.; Termote, C.; Ickowitz, A.; Powell, B.; Kehlenbeck, K.; Hunter, D. The Contribution of Forests and Trees to Sustainable Diets. Sustainability 2013, 5, 4797–4824. [Google Scholar] [CrossRef] [Green Version]
- Dawson, I.K.; Lengkeek, A.; Weber, J.C.; Jamnadass, R. Managing Genetic Variation in Tropical Trees: Linking Knowledge with Action in Agroforestry Ecosystems for Improved Conservation and Enhanced Livelihoods. Biodivers. Conserv. 2009, 18, 969–986. [Google Scholar] [CrossRef]
- Asaah, E.K.; Tchoundjeu, Z.; Leakey, R.R.B.; Takousting, B.; Njong, J. Trees, Agroforestry and Multifunctional Agriculture in Cameroon. Int. J. Agric. Sustain. 2011, 9, 110–119. [Google Scholar] [CrossRef]
- Kindt, R.; Muchugi, A.; Hansen, O.; Kipruto, H.; Poole, J.; Dawson, I.; Jamnadass, R. Molecular Markers for Tropical Trees: Statistical Analysis of Dominant Data; ICRAF Technical Manual No. 13; World Agroforestry Centre: Nairobi, Kenya; Forest and Landscape Denmark: Frederiksberg, Denmark, 2009. [Google Scholar]
- Leakey, R.R.B. Towards a Domestication Strategy for Indigenous Fruit Trees in the Tropics. In Multifunctional Agriculture; Elsevier: Amsterdam, The Netherlands, 2017; pp. 165–176. [Google Scholar] [CrossRef]
- Hendre, P.S.; Muthemba, S.; Kariba, R.; Muchugi, A.; Fu, Y.; Chang, Y.; Song, B.; Liu, H.; Liu, M.; Liao, X.; et al. African Orphan Crops Consortium (AOCC): Status of Developing Genomic Resources for African Orphan Crops. Planta 2019, 1, 989–1003. [Google Scholar] [CrossRef] [Green Version]
- Tsobeng, A.; Akem, M.; Avana, M.L.; Muchugi, A.; Degrande, A.; Tchoundjeu, Z.; Jamnadass, R.; Na’a, F. Tree-to-Tree Variation in Fruits of Three Populations of Trichoscypha acuminata (Engl.) in Cameroon. Sci. Afr. 2020, 7, e00235. [Google Scholar] [CrossRef]
- Sunil, K.; Liu, M.; Yssel, A.; Kariba, R.; Muthemba, S.; Jiang, S.; Song, B.; Hendre, P.S.; Muchugi, A.; Jamnadass, R.; et al. Draft Genomes of Two Artocarpus Plants, Jackfruit (A. heterophyllus) and Breadfruit (A. altilis). Genes 2020, 11, 27. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Liang, X.; Xuan, Y.; Geng, C.; Li, Y.; Lu, H.; Qu, S.; Mei, X.; Chen, H.; Yu, T.; et al. The Draft Genomes of Five Agriculturally Important African Orphan Crops. Gigascience 2017, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Mabhaudhi, T.; Chimonyo, V.G.P.; Hlahla, S.; Massawe, F.; Mayes, S.; Nhamo, L.; Modi, A.T. Prospects of Orphan Crops in Climate Change. Planta 2019, 250, 695–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okullo, J.B.L.; Hall, J.B.; Obua, J. Leafing, Flowering and Fruiting of Vitellaria paradoxa Subsp. Nilotica in Savanna Parklands in Uganda. Agrofor. Syst. 2004, 60, 77–91. [Google Scholar] [CrossRef]
- Byakagaba, P.; Eilu, G.; Okullo, J.B.L.; Tumwebaze, S.B.; Mwavu, E.N. Population Structure and Regeneration Status of Vitellaria paradoxa (C.F.Gaertn.) Under Different Land Management Regimes in Uganda. Agric. J. 2011, 6, 14–22. [Google Scholar] [CrossRef]
- Aremu, M.O.; Andrew, C.; Salau, R.B.; Atolaiye, B.O.; Yebpella, G.G.; Enemali, M.O. Comparative Studies on the Lipid Profile of Shea (Vitellaria paradoxa C.F. Gaertn.) Fruit Kernel and Pulp. J. Appl. Sci. 2019, 19, 480–486. [Google Scholar] [CrossRef]
- Moore, S. The Role of Vitellaria paradoxa in Poverty Reduction and Food Security in the Upper East Region of Ghana. Earth Environ. 2008, 3, 209–245. [Google Scholar]
- Boffa, J. Opportunities and Challenges in the Improvement of the Shea (Vitellaria paradoxa) Resource and Its Management; Report Submitted to the Global Shea Alliance; Occasional Paper 24; World Agroforestry: Nairobi, Kenya, 2015. [Google Scholar]
- Chen, T. Impact of the Shea Nut Industry on Women’s Empowerment in Burkina Faso; A Multi-Dimensional Study Focusing on the Central, Central-West and Hauts-Bassins regions; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2017. [Google Scholar]
- Kadu, C.A.C.; Imbuga, M.; Jamnadass, R.; Dawson, I.K. Genetic Management of Indigenous Fruit Trees in Southern Africa: A Case Study of Sclerocarya birrea Based on Nuclear and Chloroplast Variation. S. Afr. J. Bot. 2006, 72, 421–427. [Google Scholar] [CrossRef] [Green Version]
- Mkwezalamba, I.; Munthali, C.R.Y.; Missanjo, E. Phenotypic Variation in Fruit Morphology among Provenances of Sclerocarya birrea (A. Rich.) Hochst. Int. J. For. Res. 2015, 2015, 735418. [Google Scholar] [CrossRef] [Green Version]
- Sudrajat, D.J. Genetic Variation of Fruit, Seed, and Seedling Characteristics among 11 Populations of White Jabon in Indonesia. For. Sci. Technol. 2016, 12, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Oluoch, P.; Nyaboga, E.N.; Bargul, J.L. Analysis of Genetic Diversity of Passion Fruit (Passiflora edulis Sims) Genotypes Grown in Kenya by Sequence-Related Amplified Polymorphism (SRAP) Markers. Ann. Agrar. Sci. 2018, 16, 367–375. [Google Scholar] [CrossRef]
- Teklehaimanot, Z. Exploiting the Potential of Indigenous Agroforestry Trees: Parkia biglobosa and Vitellaria paradoxa in Sub-Saharan Africa. Agrofor. Syst. 2004, 61–62, 207–220. [Google Scholar] [CrossRef]
- Bremer, B.; Bremer, K.; Chase, M.W.; Fay, M.F.; Reveal, J.L.; Bailey, L.H.; Soltis, D.E.; Soltis, P.S.; Stevens, P.F. An Update of the Angiosperm Phylogeny Group Classification for the Orders and Families of Flowering Plants: APG III. Bot. J. Linn. Soc. 2009, 161, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Boffa, J.M.; Yameogo, G.; Nikiema, P.; Taoda, J.B. What Future for the Shea Tree? Agrofor. Today (ICRAF) 1996, 8, 5–9. [Google Scholar]
- Hall, J.B.; Aebischer, D.P.; Tomlinson, H.F.; Osei-Amaning, E.; Hindle, J.R. Vitellaria paradoxa: A Monograph; School of Agricultural and Forest Sciences; University of Wales: Bangor, UK, 1996; 105p. [Google Scholar]
- Zhang, J.; Kurita, M.; Shinozaki, T.; Ukiya, M.; Yasukawa, K.; Shimizu, N.; Tokuda, H.; Masters, E.T.; Akihisa, M.; Akihisa, T. Triterpene Glycosides and Other Polar Constituents of Shea (Vitellaria paradoxa) Kernels and Their Bioactivities. Phytochemistry 2014, 108, 157–170. [Google Scholar] [CrossRef]
- Baky, M.H.; Kamal, A.M.; Elgindi, M.R.; Haggag, E.G.; Mohamed Elgindi, C.R. A Review on Phenolic Compounds from Family Sapotaceae. J. Pharmacogn. Phytochem. 2016, 5, 280–287. [Google Scholar]
- Nikiema, A.; Umali, B.E. Vitellaria paradoxa C.F. Gaertn. Available online: https://www.prota4u.org/database/protav8.asp?g=pe&p=Vitellaria+paradoxa+C.F.Gaertn (accessed on 13 July 2020).
- Karambiri, M.; Elias, M.; Vinceti, B.; Grosse, A. Exploring Local Knowledge and Preferences for Shea (Vitellaria paradoxa) Ethnovarieties in Southwest Burkina Faso through a Gender and Ethnic Lens. For. Trees Livelihoods 2017, 26, 13–28. [Google Scholar] [CrossRef] [Green Version]
- Orwa, C.; Mutua; Kindt, R.; Jamnadass, R.; Simons, A. A Tree Reference and Selection Guide Version 4.0. Agrofor. Database 2009, 4, 1–5. [Google Scholar]
- Jimoh, A.A.; Aina, S.T. Investigation of the Physical and Mechanical Properties of Shea Tree Timber (Vitellaria paradoxa) Used for Structural Applications in Kwara State, Nigeria. J. Appl. Sci. Environ. Manag. 2017, 21, 961. [Google Scholar] [CrossRef] [Green Version]
- Oyen, L.; Roeland, H. Ressources Végétales de l’Afrique Tropicale: Précurseur; Programme PROTA: Wageningen, The Netherlands, 2002. [Google Scholar]
- Delaney, A.; Dembele, A.; Nombré, I.; Gnane Lirasse, F.; Marshall, E.; Nana, A.; Vickery, J.; Tayleur, C.; Stout, J.C. Local-Scale Tree and Shrub Diversity Improves Pollination Services to Shea Trees in Tropical West African Parklands. J. Appl. Ecol. 2020, 57, 1504–1513. [Google Scholar] [CrossRef]
- Aubréville, A. Sapotacées. In Flore Du Cameroun; Museum National D’histoire Naturelle, Laboratoire de Phanerogamie: Paris, French, 1964. [Google Scholar]
- Sanogo, K.; Binam, J.; Bayala, J.; Villamor, G.B.; Kalinganire, A.; Dodiomon, S. Farmers’ Perceptions of Climate Change Impacts on Ecosystem Services Delivery of Parklands in Southern Mali. Agrofor. Syst. 2017, 91, 345–361. [Google Scholar] [CrossRef]
- Nasare, L.I.; Kwapong, P.K.; Doke, D.A. Insect Pollinator Dependence of Shea (Vitellaria paradoxa C.F. Gaertn.) in the Guinea Savanna Zone of Ghana. Ecol. Process. 2019, 8, 48. [Google Scholar] [CrossRef] [Green Version]
- Boffa, J.M. Agroforestry Parklands in Sub-Saharan Africa. FAO Conserv. Guid. 1999, 15, 694–700. [Google Scholar] [CrossRef]
- Chikamai, B.; Mbogga, M.; Eyog-Matig, O. Review and Appraisal on the Status of Indigenous Fruits in Eastern Africa; A Report Prepared for IPGRI-SAFORGEN in the Framework of AFREA/FORNESSA; Forestry Research Institute 2005: Nairobi, Kenya; 121p.
- Eshun, O. Purification, Physicochemical and Formulation Properties of Shea (Vitellaria paradoxa) Gum. Master’s Thesis, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana, 2012. [Google Scholar]
- Iddrisu, A. Anatomical, Germination and In Vitro Studies on Shea Tree (Vitellaria paradoxa C.F. Gaertn.) Seed. Ph.D. Thesis, University of Ghana, Accra, Ghana, 2013. [Google Scholar] [CrossRef]
- Iddrisu, A.; Elegba, W.; Klu, G.; Danso, K. Observations on Seed Embryo and Germination, Seedling Morphology and Development of Vitellaria paradoxa (C.F. Gaertn.). For. Trees Livelihoods 2019, 28, 1–15. [Google Scholar] [CrossRef]
- Maranz, S.; Wiesman, Z.; Bisgaard, J.; Bianchi, G. Germplasm Resources of Vitellaria paradoxa Based on Variations in Fat Composition across the Species Distribution Range. Agrofor. Syst. 2004, 60, 71–76. [Google Scholar] [CrossRef]
- International Plant Genetic Resources Institute. Descriptors for Shea Tree (Vitellaria paradoxa); International Plant Genetic Resources Institute: Rome, Italy, 2006; 54p. [Google Scholar]
- Sanou, H.; Picard, N.; Lovett, P.N.; Dembélé, M.; Korbo, A.; Diarisso, D.; Bouvet, J.M. Phenotypic Variation of Agromorphological Traits of the Shea Tree, Vitellaria paradoxa C.F. Gaertn., in Mali. Genet. Resour. Crop Evol. 2006, 53, 145–161. [Google Scholar] [CrossRef]
- Djossa, B.A.; Fahr, J.; Wiegand, T.; Ayihouénou, B.E.; Kalko, E.K.; Sinsin, B.A. Land Use Impact on Vitellaria paradoxa C.F. Gaerten. Stand Structure and Distribution Patterns: A Comparison of Biosphere Reserve of Pendjari in Atacora District in Benin. Agrofor. Syst. 2008, 72, 205–220. [Google Scholar] [CrossRef]
- Elias, M. Influence of Agroforestry Practices on the Structure and Spatiality of Shea Trees (Vitellaria paradoxa C.F. Gaertn.) in Central-West Burkina Faso. Agrofor. Syst. 2013, 87, 203–216. [Google Scholar] [CrossRef]
- Naughton, C.C.; Lovett, P.N.; Mihelcic, J.R. Land Suitability Modeling of Shea (Vitellaria paradoxa) Distribution across Sub-Saharan Africa. Appl. Geogr. 2015, 58, 217–227. [Google Scholar] [CrossRef]
- Okurut, I.T.; Bosco, J.; Okullo, L.; Waiswa, D.; Muyizzi, J. Modelling the Potential Distribution of Vitellaria paradoxa Subsp. Nilotica (C.F. Gaertn) across the Kidepo Landscape of Uganda in the Face of Climate Change. J. Geosci. Environ. Prot. 2020, 8, 14–24. [Google Scholar] [CrossRef]
- Abdulai, A.; Akwasi, A.; Iddrisu, A.M. Effect of Soil Variation on Quality of Shea Butter in Selected Areas of the Northern Region of Ghana. J. Agric. Biotechnol. Sustain. Dev. 2015, 7, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Bayala, J.; Ouédraogo, S.J.; Ong, C.K. Early Growth Performance and Water Use of Planted West African Provenances of Vitellaria paradoxa C.F. Gaertn (Karité) in Gonsé, Burkina Faso. Agrofor. Syst. 2009, 75, 117–127. [Google Scholar] [CrossRef]
- Ræbild, A.; Hansen, U.B.; Kambou, S. Regeneration of Vitellaria paradoxa and Parkia biglobosa in a Parkland in Southern Burkina Faso. Agrofor. Syst. 2012, 85, 443–453. [Google Scholar] [CrossRef]
- Nair, P.K.R. Classification of Agroforestry Systems. Agrofor. Syst. 1985, 3, 97–128. [Google Scholar] [CrossRef]
- Chevalier, A. L’arbre à Beurre d’Afrique et l’avenir de Sa Culture. Oléagineux 1946, 1, 7–11. [Google Scholar]
- Nkuutu, D.; Lovett, P.; Masters, E.; Ojok, P.; Obua, J. Tree Management and Plant Utilisation in the Agroforestry Parklands of Northern Uganda. In Proceedings of the Shea Tree (Vitellaria paradoxa subsp. nilotica): First Regional Conference for Eastern and Central Africa, Lira, Uganda, 26–30 June 2000; COVOL Uganda: Lira, Uganda, 2000. [Google Scholar]
- Agúndez, D.; Nouhoheflin, T.; Coulibaly, O.; Soliño, M.; Alía, R. Local Preferences for Shea Nut and Butter Production in Northern Benin: Preliminary Results. Forests 2020, 11, 13. [Google Scholar] [CrossRef] [Green Version]
- Bockel, L.; Veyrier, M.; Gopal, P.; Adu, A.; Ouedraogo, A. Shea Value Chain as a Key Pro-Poor Carbon Fixing Engine in West Africa; FAO and Global Shea Alliance: Accra, Ghana, 2020; 44p. [Google Scholar]
- Kelly, B.; Poudyal, M.; Bouvet, J. Variation of Vitellaria paradoxa Phenophases along the North-South Gradient in Mali. Res. Plant Biol. 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- Stout, J.; Nombre, I.; de Bruijn, B.; Delaney, A.; Doke, D.A.; Gyimah, T.; Kamano, F.; Kelly, R.; Lovett, P.; Marshall, E.; et al. Insect Pollination Improves Yield of Shea (Vitellaria paradoxa Subsp. Paradoxa) in the Agroforestry Parklands of West Africa. J. Pollinat. Ecol. 2018, 22, 11–20. [Google Scholar] [CrossRef]
- Lassen, K.M.; Nielsen, L.R.; Lompo, D.; Dupont, Y.L.; Kjær, E.D. Honey Bees Are Essential for Pollination of Vitellaria paradoxa Subsp. Paradoxa (Sapotaceae) in Burkina Faso. Agrofor. Syst. 2018, 92, 23–34. [Google Scholar] [CrossRef]
- Samaké, O.; Dakouo, J.M.; Kalinganire, A.; Bayala, J.; Bréhima, K. Techniques de déparasitage et gestion du karité. ICRAF Tech. Man. 2011, 15. Available online: http://apps.worldagroforestry.org/downloads/Publications/PDFS/MN11029.pdf (accessed on 1 December 2021).
- Sanou, H.; Lamien, N. Vitellaria paradoxa, Karité. Conservation et Utilisation Durable Des Ressources Génétiques Des Espèces Ligneuses Alimentaires Prioritaires de l’Afrique Subsaharienne; INERA, Centre Régional de Recherches Environnementales et Agricoles: Ouagadougou, Burkina Faso, 2011. [Google Scholar]
- Bayala, J.; Sanou, J.; Teklehaimanot, Z.; Ouedraogo, S.J.; Kalinganire, A.; Coe, R.; van Noordwijk, M. Advances in Knowledge of Processes in Soil-Tree-Crop Interactions in Parkland Systems in the West African Sahel: A Review. Agric. Ecosyst. Environ. 2015, 205, 25–35. [Google Scholar] [CrossRef]
- Maranz, S.; Kpikpi, W.; Wiesman, Z.; De Saint Sauveur, A.; Chapagain, B. Nutritional Values and Indigenous Prefences for Shea Fruits (Vitellaria paradoxa C.V. Gaertn. F.) in African Agroforestry Parklands. Econ. Bot. 2004, 58, 588–600. [Google Scholar] [CrossRef]
- Alu, S.E.; Randa, E.A. Nutritional Value of Shea Butter (Vitellaria paradoxa) Seed Meal (SBSM) as Affected by Different Days of Natural Fermentation. Niger. Ann. PURE Appl. Sci. 2019, 1, 196–201. [Google Scholar] [CrossRef]
- Masters, E.T. The Phytochemical Diversity and Product Quality of Shea Butter Present Confounding Factors to Product Standardization in Research. Int. J. Basic Clin. Pharmacol. 2018, 7, 2070. [Google Scholar] [CrossRef]
- Honfo, F.G.; Akissoe, N.; Linnemann, A.R.; Soumanou, M.; Van Boekel, M.A.J.S. Nutritional Composition of Shea Products and Chemical Properties of Shea Butter: A Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 673–686. [Google Scholar] [CrossRef]
- Maranz, S.; Wiesman, Z. Influence of Climate on the Tocopherol Content of Shea Butter. J. Agric. Food Chem. 2004, 52, 2934–2937. [Google Scholar] [CrossRef]
- Muotono, P.; Maanikuu, I.; Peker, K. Medicinal and Nutritional Benefits from the Shea Tree-(Vitellaria paradoxa). J. Biol. Agric. Healthc. 2017, 7, 51–57. [Google Scholar]
- Poudyal, M. Chiefs and Trees: Tenures and Incentives in the Management and Use of Two Multipurpose Tree Species in Agroforestry Parklandsin Northern Ghana. Soc. Nat. Resour. 2011, 24, 1063–1077. [Google Scholar] [CrossRef]
- Tom-Dery, D.; Eller, F.; Reisdorff, C.; Jensen, K. Shea (Vitellaria paradoxa C.F. Gaertn.) at the Crossroads: Current Knowledge and Research Gaps. Agrofor. Syst. 2018, 92, 1353–1371. [Google Scholar] [CrossRef]
- Lamien, N.; Sidibe, A.; Bayala, J. The Joy of Cooking: Recipes for the Success of the Shea Tree. Agrofor. Today 1996, 8, 10–11. [Google Scholar]
- Dalziel, J. The Useful Plants of West Tropical Africa; Secretary of State for the Colonies by the Crown Agents for the Colonies: London, UK, 1937. [Google Scholar]
- Bonkoungou, E.G. Monographie du Karite, Butyrospermum paradoxum (Gaertn.f.) Hepper, Espece Agroforestiere a Usages Multiples; Institut de Recherche en Biologie et Ecologie Tropicale: Ouagadougou, Burkina Faso, 1987. [Google Scholar]
- Millee, J.K. Secondary products of species native to the Dinderesso Forest Reserve, Forestry Education and Development Project USAID, Ouagadougou. J. Dev. Agric. Econ. 1984, 3, 165–173. [Google Scholar]
- Vivien, J.; Faure, J.-J. Fruitiers Sauvages Du Cameroun. Fruits 1988, 43, 465–471. [Google Scholar]
- Masters, E.T. Traditional Food Plants of the Upper Aswa River Catchment of Northern Uganda—A Cultural Crossroads. J. Ethnobiol. Ethnomed. 2021, 17, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Bup, D.N.; Kapseu, C.; Tenin, D.; Kuitche, A.; Abi, C.F.; Tchiégang, C. Variation of the Physical Properties of Sheanut (Vitellaria paradoxa Gaertn.) Kernels during Convective Drying. Int. J. Food Eng. 2008, 4. [Google Scholar] [CrossRef]
- Baziari, F.; Henquinet, K.B.; Cavaleri, M.A. Understanding Farmers’ Perceptions and the Effects of Shea (Vitellaria paradoxa) Tree Distribution in Agroforestry Parklands of Upper West Region, Ghana. Agrofor. Syst. 2019, 93, 557–570. [Google Scholar] [CrossRef]
- Muoghalu, G.U.; Akah, P.A.; Okoye, T.C.; Ezenyi, I.C.; Ibeneme, S.; Okoli, C.O. Anti-Inflammatory Activity of Fatty Extract of Vitalleria Paradoxa Kernel (Shea Butter) and Pattern of Its Clinical Use in Arthritis in Enugu, South East Nigeria. Int. J. Basic Clin. Pharmacol. 2016, 5, 2345–2351. [Google Scholar] [CrossRef] [Green Version]
- Andersson, A.-C.; Alander, J. Shea Butter Extract for Bioactive Skin Care. Cosmet. Toilet. 2015, 130, 18–25. [Google Scholar]
- Tropical Plant Database. Vitellaria paradoxa—Useful Tropical Plants. Available online: http://tropical.theferns.info/viewtropical.php?id=Vitellaria+paradoxa (accessed on 22 July 2020).
- Adu-Ampomah, Y.; Amponsah, J.D.; Yidana, J.A. Collecting Germplasm of Sheanut (Vitellaria paradoxa) in Ghana. Plant Genet. Resour. Newsl. 1995, 1995, 37–38. [Google Scholar]
- Neya, T.; Neya, O.; Abunyewa, A.A. Agroforestry Parkland Profiles in Three Climatic Zones of Burkina Faso. Int. J. Biol. Chem. Sci. 2019, 12, 2119. [Google Scholar] [CrossRef]
- Gnonlonfoun, I.; Assogbadjo, A.E.; Gnanglè, C.P.; Glèlè Kakaï, R.L. New Indicators of Vulnerability and Resilience of Agroforestry Systems to Climate Change in West Africa: West African Agroforestry Systems and Climate Change. Agron. Sustain. Dev. 2019, 39, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Bup, D.N.; Mohagir, A.M.; Kapseu, C.; Mouloungui, Z. Production Zones and Systems, Markets, Benefits and Constraints of Shea (Vitellaria paradoxa Gaertn) Butter Processing. OCL Oilseeds Fats 2014, 21, D206. [Google Scholar] [CrossRef] [Green Version]
- Honfo, F.G. Quality of Traditionally Processed Shea (Vitellaria paradoxa) Kernels and Shea Butter; Wageningen University: Wageningen, The Netherland, 2015. [Google Scholar]
- Honfo, F.G.; Linnemann, A.R.; Akissoe, N.; Soumanou, M.M.; Van Boekel, M.A.J.S. Characteristics of Traditionally Processed Shea Kernels and Butter. Int. J. Food Sci. Technol. 2013, 48, 1714–1721. [Google Scholar] [CrossRef]
- Mumeen, A.; Didia, B.; Abdulai, A. Shea Butter Extraction Technologies: Current Status and Future Perspective. Afr. J. Biochem. Res. 2019, 13, 9–22. [Google Scholar] [CrossRef]
- Abdul-Mumeen, I.; Zakpaa, H.D.; Mills-Robertson, F.C. Biochemical and Microbiological Analysis of Shea Nut Cake: A Waste Product from Shea Butter Processing. J. Agric. Biotechnol. Sustain. Dev. 2013, 5, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Masters, E.T.; Yidana, J.A.; Lovett, P.N. Reinforcing Sound Management through Trade: Shea Tree Products in Africa. Unasylva 2004, 210, 46–52. [Google Scholar]
- Di Vincenzo, D.; Maranz, S.; Serraiocco, A.; Vito, R.; Wiesman, Z.; Bianchi, G. Regional Variation in Shea Butter Lipid and Triterpene Composition in Four African Countries. J. Agric. Food Chem. 2005, 53, 7473–7479. [Google Scholar] [CrossRef] [PubMed]
- Ugese, F.D.; Baiyeri, P.K.; Mbah, B.N. Nutritional Composition of Shea (Vitellaria paradoxa) Fruit Pulp across Its Major Distribution Zones in Nigeria. Fruits 2008, 63, 163–170. [Google Scholar] [CrossRef]
- Kadji, B.; Kone, F.; Sika, A.; Dabonne, S. Physico-Chemical Properties of Safou (Dacryodes edulis) Fruits Grown in Côte d’Ivoire. J. Appl. Biosci. 2016, 105, 10103. [Google Scholar] [CrossRef] [Green Version]
- Okullo, J.; Omujal, F.; Agea, J.; Vuzi, P.; Namutebi, A.; Okello, J.; Nyanzi, S. Proximate and Mineral Composition of Shea (Vitellaria paradoxa C.F. Gaertn.) Fruit Pulp in Uganda. Afr. J. Food Agric. Nutr. Dev. 2010, 10, 4430–4443. [Google Scholar] [CrossRef] [Green Version]
- Kalinganire, A.; Weber, J.C.; Uwamariya, A.; Kone, B. Improving Rural Livelihoods through Domestication of Indigenous Fruit Trees in the Parklands of the Sahel. In Indigenous Fruit Trees in the Tropics: Domestication, Utillization and Commercialization; CABI Publishing: Wallingford, Oxfordshire, UK, 2007; pp. 186–203. [Google Scholar] [CrossRef] [Green Version]
- Raimi, M.; Adegoke, B.M.; Afolabi, O. Nutritional Composition of Seed and Physicochemical Properties of Seed Oil of Vitellaria paradoxa. Sci. Res. J. 2014, 2, 35–39. [Google Scholar]
- Aguzue, O.C.; Akanji, F.T.; Tafida, M.A.; Kamal, M. Nutritional and Some Elemental Composition of Shea (Vitellaria paradoxa) Fruit Pulp. Arch. Appl. Sci. Res. 2013, 5, 63–65. [Google Scholar]
- Ugese, F.; Baiyeri, K.; Mbah, B. Mineral Content of the Pulp of Shea Butter Fruit (Vitellaria paradoxa CF Gaertn.) Sourced from Seven Locations in the Savanna Ecology of Nigeria. Tree Sci. Biotechnol. 2008, 2, 40–42. [Google Scholar]
- Busson, F. Plantes Alimentaires de l’Ouest Africain: Étude Botanique, Biologique et Chimique; Leconte, M., Ed.; L’Imprimerie Leconte: Marseille, France, 1965. [Google Scholar]
- Tallantire, A.; Goode, P. A Preliminary Study of the Food Plants of the West Nile and Madi Districts of Uganda. East Afr. Agric. For. J. 1975, 40, 233–255. [Google Scholar] [CrossRef]
- Mbaiguinam, M.; Mbayhoudel, K.; Djekota, C. Physical and Chemical Characteristics of Fruits, Pulps, Kernels and Butter of Shea Butyrospermum parkii (Sapotaceae) from Mandoul, Southern Chad. Asian J. Biochem. 2007, 2, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Greenwood, M. Shea Nuts and Shea Butter; Bulletin of the Agricultural Department: Lagos, Nigeria, 1929; pp. 59–100. [Google Scholar]
- Megnanou, R.-M.; Niamke, S.; Diopoh, J. Physicochemical and Microbiological Characteristics of Optimized and Traditional Shea Butters from Côte d’Ivoire. Afr. J. Biochem. Res. 2007, 1, 041–047. [Google Scholar]
- Chukwu, O.; Adgidzi, P.P. Evaluation of Some Physico-Chemical Properties of Shea-Butter (Butyrospermum paradoxum) Related to Its Value for Food and Industrial Utilisation. Int. J. Postharvest Technol. Innov. 2008, 1, 320–326. [Google Scholar] [CrossRef]
- Honfo, F.G.; Hell, K.; Akissoé, N.; Coulibaly, O.; Fandohan, P.; Hounhouigan, J. Effect of Storage Conditions on Microbiological and Physicochemical Quality of Shea Butter. J. Food Sci. Technol. 2011, 48, 274–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duke, J.A.; Atchley, A.A. Handbook of Proximate Analysis Tables of Higher Plants; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar] [CrossRef]
- Maranz, S.; Wiesman, Z.; Garti, N. Phenolic Constituents of Shea (Vitellaria paradoxa) Kernels. J. Agric. Food Chem. 2003, 51, 6268–6273. [Google Scholar] [CrossRef] [PubMed]
- Akihisa, T.; Kojima, N.; Katoh, N.; Ichimura, Y.; Suzuki, H.; Fukatsu, M.; Maranz, S.; Masters, E.T. Triterpene Alcohol and Fatty Acid Composition of Shea Nuts from Seven African Countries. J. Oleo Sci. 2010, 59, 351–360. [Google Scholar] [CrossRef] [Green Version]
- Tano-Debrah, K.; Ohta, Y. Enzyme-Assisted Aqueous Extraction of Fat from Kernels of the Shea Tree, Butyrospermum parkii. J. Am. Oil Chem. Soc. 1994, 71, 979–983. [Google Scholar] [CrossRef]
- Alhassan, E.; Agbemava, S.E.; Adoo, N.A.; Agbodemegbe, V.Y.; Bansah, C.Y.; Della, R.; Appiah, G.I.; Kombat, E.O.; Nyarko, B.J.B. Determination of Trace Elements in Ghanaian Shea Butter and Shea Nut by Neutron Activation Analysis (NAA). Res. J. Appl. Sci. Eng. Technol. 2011, 3, 22–25. [Google Scholar]
- Akihisa, T.; Kojima, N.; Kikuchi, T.; Yasukawa, K.; Tokuda, H.; Masters, E.T.; Manosroi, A.; Manosroi, J. Anti-Inflammatory and Chemopreventive Effects of Triterpene Cinnamates and Acetates from Shea Fat. J. Oleo Sci. 2010, 59, 273–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dijkstra, A. Palm Oil. In Encyclopedia of Food and Health; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 199–204. [Google Scholar] [CrossRef]
- Megnanou, R.; Niamke, S. Improving the Optimized Shea Butter Quality: A Great Potential of Utilization for Common Consumers and Industrials. SpringerPlus 2015, 4, 667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Njoku, O.U.; Eneh, F.U.; Ononogbu, I.C.; Adikwu, M.U. Compositional and Toxicological Studies on Shea Butter. J. Nutraceuticals Funct. Med. Foods. 2000, 2, 33–39. [Google Scholar] [CrossRef]
- Nkouam, G.B.; Kapseu, C.; Barth, D.; Dirand, M.; Tchatchueng, J.B. Oil Extraction from Sheanut Kernel (Vitellaria paradoxa Gaertn) and Canarium Pulp (Canarium schweinfurthii Engl.) Using Supercritical Co 2. Res. J. Appl. Sci. 2007, 2, 646–652. [Google Scholar]
- Badifu, G.I.O. Lipid Composition of Nigerian Butyrospermum paradoxum Kernel. J. Food Compos. Anal. 1989, 2, 238–244. [Google Scholar] [CrossRef]
- Ugese, F.D. Proximate traits of the seed and seed cake of shea buttertree (Vitellaria paradoxa C.F. Gaertn.) in Nigeria’s savanna ecozone. J. Appl. Biosci. 2010, 31, 1935–1941. [Google Scholar]
- Kapseu, C.; Nono, Y.; Parmentier, M.; Dirand, M.; Dellacherie, D. Fatty Acids and Triglycerides of Cameroon Shea Butter. Riv. Ital. Sostanze Grasse 2001, 78, 31–34. [Google Scholar]
- Okoye, N.N.; Ajaghaku, D.L.; Okeke, H.N.; Ilodigwe, E.E.; Nworu, C.S.; Okoye, F.B.C. Beta-Amyrin and Alpha-Amyrin Acetate Isolated from the Stem Bark of Alstonia Boonei Display Profound Anti-Inflammatory Activity. Pharm. Biol. 2014, 52, 1478–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salminen, A.; Lehtonen, M.; Suuronen, T.; Kaarniranta, K.; Huuskonen, J. Terpenoids: Natural Inhibitors of NF-ΚB Signaling with Anti-Inflammatory and Anticancer Potential. Cell. Mol. Life Sci. 2008, 65, 2979–2999. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xiao, J.; Liu, W.; Zhang, C.; Akihisa, T.; Abe, M.; Masters, E.; Zhai, W.; Feng, F.; Zhang, J. Vitellaria paradoxa Nutshells from Seven Sub-Saharan Countries as Potential Herbal Medicines for Treating Diabetes Based on Chemical Compositions, HPLC Fingerprints and Bioactivity Evaluation. Chin. J. Nat. Med. 2019, 17, 446–460. [Google Scholar] [CrossRef]
- Zhang, J.; Kurita, M.; Ebina, K.; Ukiya, M.; Tokuda, H.; Yasukawa, K.; Masters, E.T.; Shimizu, N.; Akihisa, M.; Feng, F.; et al. Melanogenesis-Inhibitory Activity and Cancer Chemopreventive Effect of Glucosylcucurbic Acid from Shea (Vitellaria paradoxa) Kernels. Chem. Biodivers. 2015, 12, 547–558. [Google Scholar] [CrossRef]
- Zhang, J.; Li, D.; Lv, Q.; Ye, F.; Jing, X.; Masters, E.T.; Shimizu, N.; Abe, M.; Akihisa, T.; Feng, F. Compositions and Melanogenesis-Inhibitory Activities of the Extracts of Defatted Shea (Vitellaria paradoxa) Kernels from Seven African Countries. J. Food Compos. Anal. 2018, 70, 89–97. [Google Scholar] [CrossRef]
- Talla, E.; Nyemb, J.; Tiabou, A.; Djou, S.; Biyanzi, P.; Sophie, L.; Elst, L.; Tanyi, J. Antioxidant Activity and a New Ursane-Type Triterpene from Vitellaria paradoxa (Sapotaceae) Stem Barks. Eur. J. Med. Plants 2016, 16, 1–20. [Google Scholar] [CrossRef]
- Falana, M.B.; Bankole, M.O.; Ojo, D.A.; Omemu, A.M. Comparative Phytochemical Investigation of the Various Parts of Vitellaria paradoxa. J. Nat. Sci. Res. 2016, 6, 74–80. [Google Scholar]
- Boyejo, I.; Azeez, S.; Owolabi, A.; Issah, O. Antifungal and Phytochemical Screening of Extract from Vitellaria paradoxa (Shea Butter Tree) Leaves, Barks and Roots on Dermatophytes. Int. J. Sci. Res. Publ. 2019, 9, p90129. [Google Scholar] [CrossRef]
- Estella, U.; Victoria, O. Pharmacognostic Profiling and Antidiabetic Effects of Leaves of Vitellaria paradoxa C.F. Gaertn (Sapotaceae). J. Glob. Biosci. 2020, 9, 7280–7306. [Google Scholar]
- Aleza, K.; Wala, K.; Bayala, J.; Villamor, G.B.; Dourma, M.; Atakpama, W.; Akpagana, K. Population Structure and Regeneration Status of Vitellaria paradoxa (C.F. Gaertner) under Different Land Management Regimes in Atacora Department, Benin. Agrofor. Syst. 2015, 89, 511–523. [Google Scholar] [CrossRef]
- Luedeling, E.; Neufeldt, H. Carbon Sequestration Potential of Parkland Agroforestry in the Sahel. Clim. Chang. 2012, 115, 443–461. [Google Scholar] [CrossRef] [Green Version]
- Sanogo, K.; Gebrekirstos, A.; Bayala, J.; Villamor, G.B.; Kalinganire, A.; Dodiomon, S. Potential of Dendrochronology in Assessing Carbon Sequestration Rates of Vitellaria paradoxa in Southern Mali, West Africa. Dendrochronologia 2016, 40, 26–35. [Google Scholar] [CrossRef]
- Dimobe, K.; Kuyah, S.; Dabré, Z.; Ouédraogo, A.; Thiombiano, A. Diversity-Carbon Stock Relationship across Vegetation Types in W National Park in Burkina Faso. For. Ecol. Manag. 2019, 438, 243–254. [Google Scholar] [CrossRef]
- Kelly, B.A.; Gourlet-Fleury, S.; Bouvet, J.M. Impact of Agroforestry Practices on the Flowering Phenology of Vitellaria paradoxa in Parklands in Southern Mali. Agrofor. Syst. 2007, 71, 67–75. [Google Scholar] [CrossRef]
- Kelly, B.A.; Poudyal, M.; Bouvet, J.-M. Impact of Land Use and Land Use History on Fruits Production of Vitellaria paradoxa (Shea Tree) According to Agroclimatic Zones in Mali (West Africa). Curr. Bot. 2019, 10, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Nyamekye, C.; Thiel, M.; Schönbrodt-Stitt, S.; Zoungrana, B.; Amekudzi, L. Soil and Water Conservation in Burkina Faso, West Africa. Sustainability 2018, 10, 3182. [Google Scholar] [CrossRef] [Green Version]
- Ogwok, G.; Alele, P.O.; Kizza, S. Influence of Shea Tree (Vitellaria paradoxa) on Maize and Soybean Production. PLoS ONE 2019, 14, e0201329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gwali, S.; Okullo, J.B.L.; Eilu, G.; Nakabonge, G.; Nyeko, P.; Vuzi, P. Traditional Management and Conservation of Shea Trees (Vitellaria paradoxa Subspecies Nilotica) in Uganda. Environ. Dev. Sustain. 2012, 14, 347–363. [Google Scholar] [CrossRef]
- Bazié, H.R.; Bayala, J.; Zombré, G.; Sanou, J.; Ilstedt, U. Separating Competition-Related Factors Limiting Crop Performance in an Agroforestry Parkland System in Burkina Faso. Agrofor. Syst. 2012, 84, 377–388. [Google Scholar] [CrossRef]
- Lovett, P.N.; Haq, N. Evidence for Anthropic Selection of the Sheanut Tree (Vitellaria paradoxa). Agrofor. Syst. 2000, 48, 273–288. [Google Scholar] [CrossRef]
- Lamien, N.; Ouédraogo, S.J.; Diallo, O.B.; Guinko, S. Productivité Fruitiére Du Karité (Vitellaria paradoxa Gaertn. C. F., Sapotaceae) Dans Les Parcs Agroforestiers Traditionnels Au Burkina Faso. Fruits 2004, 59, 423–429. [Google Scholar] [CrossRef] [Green Version]
- Bokary, K.; Bouvet, J.M.; Picard, N. Size Class Distribution and Spatial Pattern of Vitellaria paradoxa in Relation to Farmers’ Practices in Mali. Agrofor. Syst. 2004, 60, 3–11. [Google Scholar] [CrossRef]
- Diarrassouba, N.; Didier, S.; Yao, M.; Alui, K.A. Comment Restaurer Les Parcs à Karité Dégradés Par La Technique de Régénération Naturelle Assistée (RNA)? Afr. Sci. 2020, 16, 307–314. [Google Scholar]
- Tchinmegni, F.; Tsobeng, A.; Ngonkeu, E. Valorisation of Non-Timber Forest Product (NTFP): Case of Allanblackia Floribunda Oliver. Int. J. Res. Pharm. Biosci. 2016, 3, 9–20. [Google Scholar]
- Alonge, A.; Olaniyan, A. Problems of Shea Butter Processing in Africa. In Proceedings of the American Society of Agricultural and Biological Engineers—International Conference on Crop Harvesting and Processing, Louisville, KY, USA, 11–14 February 2007; pp. 69–91. [Google Scholar] [CrossRef]
- Bayala, J.; Sanon, Z.; Bazié, P.; Sanou, J.; Roupsard, O.; Jourdan, C.; Ræbild, A.; Kelly, B.; Okullo, J.B.L.; Thiam, M.; et al. Relationships between Climate at Origin and Seedling Traits in Eight Panafrican Provenances of Vitellaria paradoxa C.F. Gaertn. under Imposed Drought Stress. Agrofor. Syst. 2018, 92, 1455–1467. [Google Scholar] [CrossRef]
- Dianda, M.; Bayala, J.; Diop, T.; Ouédraogo, S.J. Improving Growth of Shea Butter Tree (Vitellaria paradoxa C.F. Gaertn.) Seedlings Using Mineral N, P and Arbuscular Mycorrhizal (AM) Fungi. Biotechnol. Agron. Soc. Environ. 2009, 13, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Alphonse, A.K.; Nafan, D.; Didier Martial, Y.S. Germination Test of Shea Seeds (Vitellaria paradoxa C.F. Gaertn) in Nursery on Substrates of Northern Côte d’Ivoire. Asian Plant Res. J. 2020, 3, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Adu-Gyamfi, P.; Barnor, M.; Dadzie, A.; Lowor, S.; Opoku, S.; Opoku-Ameyaw, K.; Bissah, M.; Padi, F. Preliminary Investigation on Somatic Embryogenesis from Immature Cotyledon Explants of Shea (Vitellaria paradoxa G). J. Agric. Sci. Technol. B 2012, 2, 1171–1176. [Google Scholar]
- Mjunjuga, M.; Ofori, D.; Sawe, C.; Asaah, E.; Anegbeh, P.; Peprah, T.; Mpanda, M.; Mwaura, L.; Mtui, E.; Sirito, C.; et al. Allanblackia Propagation Protocol; World Agroforestry Centre: Nairobi, Kenya, 2008; 44p. [Google Scholar]
- Tchoundjeu, Z.; Degrande, A.; Leakey, R.R.; Nimino, G.; Kemajou, E.; Asaah, E.; Facheux, C.; Mbile, P.; Mbosso, C.; Sado, T.; et al. Impacts of Participatory Tree Domestication on Farmer Livelihoods in West and Central Africa. For. Trees Livelihoods 2010, 19, 217–234. [Google Scholar] [CrossRef]
- Mbile, P.; Tchoundjeu, Z.; Degrande, A.; Avana, M.; Tsobeng, A.C. Non-Mist Vegetative Propagation by Resource-Poor, Rural Farmers of the Forest Zone of Cameroon: Some Technology Adaptations to Enhance Practice. For. Trees Livelihoods 2004, 14, 43–52. [Google Scholar] [CrossRef]
- Atangana, A.R.; Tchoundjeu, Z.; Asaah, E.K.; Simons, A.J.; Khasa, D.P. Domestication of Allanblackia Floribunda: Amenability to Vegetative Propagation. For. Ecol. Manag. 2006, 237, 246–251. [Google Scholar] [CrossRef]
- Kouakou, K.; Dao, J.P.; Kouassi, K.I.; Beugré, M.M.; Koné, M.; Baudoin, J.P.; Bi, I.A.Z. Propagation of Garcinia kola (Heckel) by Stem and Root Cuttings. Silva Fenn. 2016, 50, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Takoutsing, B.; Tsobeng, A.; Tchoundjeu, Z.; Degrande, A.; Asaah, E. Vegetative Propagation of Garcinia lucida Vesque (Clusiaceae) Using Leafy Stem Cuttings and Grafting. Afr. Focus 2017, 27. [Google Scholar] [CrossRef] [Green Version]
- Amissah, N.; Akakpo, B.; Yeboah, J.; Blay, E. Asexual Propagation of Sheanut Tree (Vitellaria paradoxa CF Gaertn.) Using a Container Layering Technique. Am. J. Plant Sci. 2013, 4, 1758–1765. [Google Scholar] [CrossRef]
- Akakpo, D.B.; Amissah, N.; Yeboah, J.; Blay, E. Effect of Indole 3-Butyric Acid and Media Type on Adventitious Root Formation in Sheanut Tree (Vitellaria paradoxa CF Gaertn.) Stem Cuttings. Am. J. Plant Sci. 2014, 5, 313–318. [Google Scholar] [CrossRef] [Green Version]
- Yeboah, J.; Lowor, S.T.; Amoah, F.M. The Rooting Performance of Shea (Vitellaria paradoxa C.F. Gaertn) Cuttings Leached in Water and Application of Rooting Hormone in Different Media. J. Plant Sci. 2009, 4, 10–14. [Google Scholar] [CrossRef]
- Sawadogo, B. Greffage In Situ de Vitellaria paradoxa C.F. Gaertn Dans La Province du Sanguie. Master’s Thesis, Universite Nazi Boni (UNB), Dindéresso, Burkina Faso, 2018. [Google Scholar]
- Sanou, H.; Kambou, S.; Teklehaimanot, Z.; Dembélé, M.; Yossi, H.; Sina, S.; Djingdia, L.; Bouvet, J.-M. Vegetative Propagation of Vitellaria paradoxa by Grafting. Agrofor. Syst. 2004, 60, 93–99. [Google Scholar] [CrossRef]
- Yeboah, J.; Akrofi, A.; Owusu-Ansah, F. Influence of Selected Fungicides and Hormone on the Rooting Success of Shea (Vitellaria paradoxa Gaernt) Stem Cuttings. Agric. Biol. J. N. Am. 2010, 1, 313–320. [Google Scholar] [CrossRef]
- Lovett, P.N.; Haq, N. Progress in Developing in Vitro Systems for Shea Tree (Vitellaria paradoxa C.F. Gaertn.) Propagation. For. Trees Livelihoods 2013, 22, 60–69. [Google Scholar] [CrossRef]
- Boussim, J.; Sallé, G.; Guinko, S. Tapinanthus Parasite Du Karité Au Burkina Faso. 1ère Partie Identification et Distribution. Bois For. Trop. 1993, 238, 45–52. [Google Scholar] [CrossRef]
- Odebiyi, J.; Bada, S.; Awodoyin, R.; Oni, P.; Omoloye, A. Population Structure of Vitelaria paradoxa Gaernt. F. and Parkia biglobosa (Jacq.) Benth. in the Agroforestry Parklands of Nigerian Humid Savanna. West Afr. J. Appl. Ecol. 2004, 5, 31–39. [Google Scholar]
- Yao, M.; Diarrassouba, N.; Konan, A.; Koua, G.; Zadjehi, K.; Fofana, J. Loranthaceae Species Infesting Shea Trees (Vitellaria paradoxa Gaertn. C.F.) and Factors Involving Attacks in Northern Côte D’Ivoire. Ecologia 2020, 10, 101–109. [Google Scholar] [CrossRef]
- Sallé, G.; Boussim, J.I.; Raynal-Roques, A.; Brunck, F. Le Karité: Une Richesse Potentielle. Perspectives de Recherche Pour Améliorer Sa Production. Bois For. Trop. 1991, 228, 11–23. [Google Scholar]
- Lamien, N.; Tigabu, M.; Dabiré, R.; Guinko, S.; Oden, P.C. Insect (Salebria Sp.) Infestation and Impact on Vitellaria paradoxa C.F. Gaertn. Fruit Production in Agroforestry Parklands. Agrofor. Syst. 2008, 72, 15–22. [Google Scholar] [CrossRef]
- Bondé, L.; Camara Assis, J.; Benavides-Gordillo, S.; Canales-Gomez, E.; Fajardo, J.; Marrón-Becerra, A.; Noguera-Urbano, E.A.; Weidlich, A.E.W.; Ament, J.M. Scenario-Modelling for the Sustainable Management of Non-Timber Forest Products in Tropical Ecosystems Scenario-Modelling for the Sustainable Management of Non-Timber Forest Products in Tropical Ecosystems. Biota Neotrop. 2020, 20. [Google Scholar] [CrossRef]
- FAO; IFAD; UNICEFWFP; WHO. The State of Food Security and Nutrition in the World 2020; FAO, IFAD, UNICEF, WFP and WHO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Pouliot, M.; Ouedraogo, B.; Simonsen, H.L.; Smith-Hall, C. Household-Level Studies of Forests and Poverty in Burkina Faso: Contextual Information, Methods and Preliminary Results; Forest & Landscape Working Papers: Copenhagen, Denmark, 2010. [Google Scholar]
- Pouliot, M. Contribution of “Women’s Gold” to West African Livelihoods: The Case of Shea (Vitellaria paradoxa) in Burkina Faso. Econ. Bot. 2012, 66, 237–248. [Google Scholar] [CrossRef]
- Laube, W. Global Shea Nut Commodity Chains and Poverty Eradication in Northern Ghana: Myth or Reality? Int. J. Dev. 2015, 2, 1–20. [Google Scholar]
- Seghieri, J. Shea Tree (Vitellaria paradoxa Gaertn. f.): From Local Constraints to Multi-Scale Improvement of Economic, Agronomic and Environmental Performance in an Endemic Sudanian Multipurpose Agroforestry Species. Agrofor. Syst. 2019, 93, 2313–2330. [Google Scholar] [CrossRef]
- Bondé, L.; Ouédraogo, O.; Ouédraogo, I.; Thiombiano, A.; Boussim, J.I. Variability and Estimating in Fruiting of Shea Tree (Vitellaria paradoxa C.F. Gaertn) Associated to Climatic Conditions in West Africa: Implications for Sustainable Management and Development. Plant Prod. Sci. 2019, 22, 143–158. [Google Scholar] [CrossRef] [Green Version]
- Bondé, L.; Ouédraogo, O.; Traoré, S.; Thiombiano, A.; Boussim, J.I. Impact of Environmental Conditions on Fruit Production Patterns of Shea Tree (Vitellaria paradoxa C.F.Gaertn) in West Africa. Afr. J. Ecol. 2019, 57, 353–362. [Google Scholar] [CrossRef]
- Diarassouba, N.; Koffi, K.E.; N’Guessan, K.A.; Van Damme, P.; Sangare, A. Connaissances Locales et Leur Utilisation Dans La Gestion Des Parcs à Karité En Côte d’Ivoire. Afr. Focus 2008, 21, 77–96. [Google Scholar] [CrossRef]
- Okiror, P.; Agea, J.G.; Okia, C.A.; Okullo, J.B.L. On-Farm Management of Vitellaria paradoxa C.F. Gaertn. in Amuria District, Eastern Uganda. Int. J. For. Res. 2012, 2012, 768946. [Google Scholar] [CrossRef]
- Porth, I.; El-Kassaby, Y. Assessment of the Genetic Diversity in Forest Tree Populations Using Molecular Markers. Diversity 2014, 6, 283–295. [Google Scholar] [CrossRef] [Green Version]
- Diarrassouba, N.; Bup Nde, D.; Kapseu, C.; Kouame, C.; Sangare, A. Phenotypic Diversity of Shea (Vitellaria paradoxa C. F. Gaertn.) Populations across Four Agro-Ecological Zones of Cameroon. J. Crop. Sci. Biotechnol. 2007, 10, 211–218. [Google Scholar]
- Diarrassouba, N.; Fofana, I.; Issali, A.; Bup-Nde, D.; Sangare, A. Typology of Shea Trees (Vitellaria paradoxa) Using Qualitative Morphological Traits in Cote d’Ivoire. Gene Conserv. 2009, 8, 1–21. [Google Scholar]
- Abdulai, I.; Krutovsky, K.V.; Finkeldey, R. Morphological and Genetic Diversity of Shea Tree (Vitellaria paradoxa) in the Savannah Regions of Ghana. Genet. Resour. Crop Evol. 2017, 64, 1253–1268. [Google Scholar] [CrossRef]
- Sanou, H.; Lovett, P.N.; Bouvet, J.M. Comparison of Quantitative and Molecular Variation in Agroforestry Populations of the Shea Tree (Vitellaria paradoxa C.F. Gaertn) in Mali. Mol. Ecol. 2005, 14, 2601–2610. [Google Scholar] [CrossRef] [PubMed]
- Akpona, T.J.D.; Akpona, H.A.; Djossa, B.A.; Savi, M.K.; Daïnou, K.; Ayihouenou, B.; Glèlè Kakaï, R. Impact of Land Use Practices on Traits and Production of Shea Butter Tree (Vitellaria paradoxa C.F. Gaertn.) in Pendjari Biosphere Reserve in Benin. Agrofor. Syst. 2016, 90, 607–615. [Google Scholar] [CrossRef]
- Cardie, C.; Vaillant, A.; Sanou, H.; Kelly, B.; Bouvet, J. Characterization of Microsatellite Markers in the Shea Tree (Vitellaria paradoxa C. F Gaertn) in Mali. Mol. Ecol. Notes 2005, 5, 524–526. [Google Scholar] [CrossRef]
- Allal, F.; Vaillant, A.; Sanou, H.; Kelly, B.; Bouvet, J.-M. Isolation and Characterization of New Microsatellite Markers in Shea Tree (Vitellaria paradoxa C.F. Gaertn). Mol. Ecol. Resour. 2008, 8, 822–824. [Google Scholar] [CrossRef] [PubMed]
- Gwali, S.; Vaillant, A.; Nakabonge, G.; Okullo, J.B.L.; Eilu, G.; Muchugi, A.; Bouvet, J.M. Genetic Diversity in Shea Tree (Vitellaria paradoxa Subspecies Nilotica) Ethno-Varieties in Uganda Assessed with Microsatellite Markers. For. Trees Livelihoods 2015, 24, 163–175. [Google Scholar] [CrossRef]
- Logossa, Z.; Camus-Kulandaivelu, L.; Allal, F.; Vaillant, A.; Sanou, H.; Kokou, K.; Bouvet, J.-M. Molecular Data Reveal Isolation by Distance and Past Population Expansion for the Shea Tree (Vitellaria paradoxa C.F. Gaertn) in West Africa. Mol. Ecol. 2011, 20, 4009–4027. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Ji, B.; Siewers, V.; Xu, D.; Halkier, B.A.; Nielsen, J. Identification of Genes Involved in Shea Butter Biosynthesis from Vitellaria paradoxa Fruits through Transcriptomics and Functional Heterologous Expression. Appl. Microbiol. Biotechnol. 2019, 103, 3727–3736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontaine, C.; Lovett, P.N.; Sanou, H.; Maley, J.; Bouvet, J.M. Genetic Diversity of the Shea Tree (Vitellaria paradoxa C.F. Gaertn), Detected by RAPD and Chloroplast Microsatellite Markers. Heredity 2004, 93, 639–648. [Google Scholar] [CrossRef] [Green Version]
- Allal, F.; Sanou, H.; Millet, L.; Vaillant, A.; Camus-Kulandaivelu, L.; Logossa, Z.A.; Lefèvre, F.; Bouvet, J.M. Past Climate Changes Explain the Phylogeography of Vitellaria paradoxa over Africa. Heredity 2011, 107, 174–186. [Google Scholar] [CrossRef] [Green Version]



| Phenology | Period |
|---|---|
| Falling of leaves | November–March |
| The emergence of young flower buds | November–April |
| Flowering | December–April |
| The emergence of young leaves | December–April |
| Fruit development | December–April |
| Fruit ripening | March–August |
| Fruit harvest | April–September |
| Type of Use | Uses | Parts Used |
|---|---|---|
| Food | Raw or in the form of drinks and jam | Fruit pulp |
| Cooking oil [80] | Kernel | |
| Nectar processed into honey by bees | Flowers | |
| Industry | Soap making [46] | Butter |
| Cream for hair and skin, shampoo [81] | Butter | |
| Replacement of cocoa butter in chocolate | Butter | |
| Feed | Flowers | |
| Pharmacopoeia | Stomach pain, headaches, eye problems [82] | Leaves, bark |
| Throat pain, treatment of wounds, rheumatism [82] | Butter | |
| Diarrhoea, stomach problems, teeth cleaning | Root | |
| Malaria | Kernels | |
| Gynaecological problems [24] | Latex | |
| Facilitation of delivery lactation [24] | Bark | |
| Firewood | Trunk and branches | |
| Lighting | Butter | |
| Cosmetics | Cream, perfume, shampoo [81] | Butter |
| Handcrafts | Musical instruments [34] | Bark, sap (used as glue/adhesive) |
| Hunting | The stem latex used to capture small animals [83] | Latex and glue |
| Pesticides | [81] | Seed residue after extraction of butter |
| Soil conservation | [84] | Leaves |
| Composition/Minerals | (Alu and Randa 2019) a | Muotono et al. (2017) b | Raimi et al. (2014) c | Aguzue et al. (2013) d | Okullo et al. (2010) e | Ugese et al. (2008) f |
|---|---|---|---|---|---|---|
| Moisture (%) | NE | 67 | 2.84 ± 0.13 | 4.58 | 72.4 ± 0.1 | 8.8 |
| Energy (kJ/100 g dw) | NE | NE | 2348± 0.41 | NE | NE | 198.8 |
| Carbohydrates (mg/100 g dw) | NE | 8.1 | 21.8 ±0.27 | 72.0 | 19.4 ± 0.6 | 42.5 |
| Crude protein (mg/100 g dw) | 15.2 ± 0.63 | 4.2 | 9.3 ± 0.05 | 3.5 | 3.1 ± 0.1 | 3.5 |
| Ash (mg/100 g dw) | 3.75 ± 1.10 | 5.1 | 4.18 ± 0.10 | 8.95 | 3.6 ± 0.2 | 4.6 |
| Crude fibre (mg/100 g dw) | 6.38 ± 0.10 | 42.2 | 12.68 ±0.13 | 9.6 | 14.5 ± 1.7 | 39 |
| Crude fat (mg/100 g dw) | NE | 1.3 | NE | NE | 1.5 ± 0.7 | NE |
| Ca | 0.18 ± 0.01 | 117.3 | 30.24 ±0.04 | 2.3 | 69.4 ± 0.1 | NE |
| Cu | NE | 0.1 | 0.80 ± 0.00 | NE | NE | NE |
| Fe | NE | 8.5 | 52.00 ±0.11 | 0.02 | 3.6 ± 0.1 | NE |
| K | 0.36 ± 0.02 | 830.3 | 61.70 ±0.30 | NE | 47.9 ± 0.2 | NE |
| Mg | 0.27 ± 0.02 | 57.2 | 6.24 ± 0.01 | 0.5 | 18.1 ± 0.3 | NE |
| Mn | NE | 0.6 | 0.30 ± 0.02 | 0.2 | NE | NE |
| P | 0.35 ± 0.02 | 39.8 | NE | NE | NE | NE |
| Na | 0.09 ± 0.01 | 19.3 | 5.10 ± 0.01 | NE | 8.9 ± 0.1 | NE |
| Zn | NE | 2.1 | 0.72 ± 0.00 | NE | NE | NE |
| Kernel | References | Butter | References | |
|---|---|---|---|---|
| Macronutrients | ||||
| Moisture (%) | 6.8 | [108,109,110] | 1.4 | [75,111,112,113,114] |
| Carbohydrates (g/100 g dw) | 30.9 | [109,111,115] | 22.3 | [113] |
| Crude protein (g/100 g dw) | 8.1 | [109,111,115] | ||
| Crude lipids (g/100 g dw) | 45.2 | [100,110,116,117] | 75 | [113] |
| Crude fibre (g/100 g dw) | 9.1 | [111,115] | ||
| Ash (g/100 g dw) | 2.5 | [111,115,118] | 2.3 | [113] |
| Minerals (mg/100 g dw) | ||||
| Ca | 71.8 | [109,112,115,119] | 9.6 | [112] |
| Cu | 0.3 | [112] | 0.8 | [112] |
| Fe | 1.6 | [112,115] | 3.6 | [112] |
| K | 0.1 | [119] | 2.2 | [112] |
| Mg | 142.6 | [112] | 4.5 | [112] |
| Mn | 0.4 | [119] | 0.006 | [119] |
| P | 0.04 | [109,115] | ||
| Zn | 0.9 | [112] | 2.7 | [112] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choungo Nguekeng, P.B.; Hendre, P.; Tchoundjeu, Z.; Kalousová, M.; Tchanou Tchapda, A.V.; Kyereh, D.; Masters, E.; Lojka, B. The Current State of Knowledge of Shea Butter Tree (Vitellaria paradoxa C.F.Gaertner.) for Nutritional Value and Tree Improvement in West and Central Africa. Forests 2021, 12, 1740. https://doi.org/10.3390/f12121740
Choungo Nguekeng PB, Hendre P, Tchoundjeu Z, Kalousová M, Tchanou Tchapda AV, Kyereh D, Masters E, Lojka B. The Current State of Knowledge of Shea Butter Tree (Vitellaria paradoxa C.F.Gaertner.) for Nutritional Value and Tree Improvement in West and Central Africa. Forests. 2021; 12(12):1740. https://doi.org/10.3390/f12121740
Chicago/Turabian StyleChoungo Nguekeng, Patrick Bustrel, Prasad Hendre, Zacharie Tchoundjeu, Marie Kalousová, Armelle Verdiane Tchanou Tchapda, Dennis Kyereh, Eliot Masters, and Bohdan Lojka. 2021. "The Current State of Knowledge of Shea Butter Tree (Vitellaria paradoxa C.F.Gaertner.) for Nutritional Value and Tree Improvement in West and Central Africa" Forests 12, no. 12: 1740. https://doi.org/10.3390/f12121740
APA StyleChoungo Nguekeng, P. B., Hendre, P., Tchoundjeu, Z., Kalousová, M., Tchanou Tchapda, A. V., Kyereh, D., Masters, E., & Lojka, B. (2021). The Current State of Knowledge of Shea Butter Tree (Vitellaria paradoxa C.F.Gaertner.) for Nutritional Value and Tree Improvement in West and Central Africa. Forests, 12(12), 1740. https://doi.org/10.3390/f12121740









