Construction of Photoelectrochemical DNA Biosensors Based on TiO2@Carbon Dots@Black Phosphorous Quantum Dots
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. Apparatus
2.3. General Synthetic Method for Carbon Dots (CDs)
2.4. Synthesis of Black Phosphrous Quantum Dots (BPQDs)
2.5. Synthesis of TiO2@CDs Composites
2.6. Fabrication of TiO2@CDs@BPQDs Electrode
2.7. Detection of the Target DNA
3. Results and Discussion
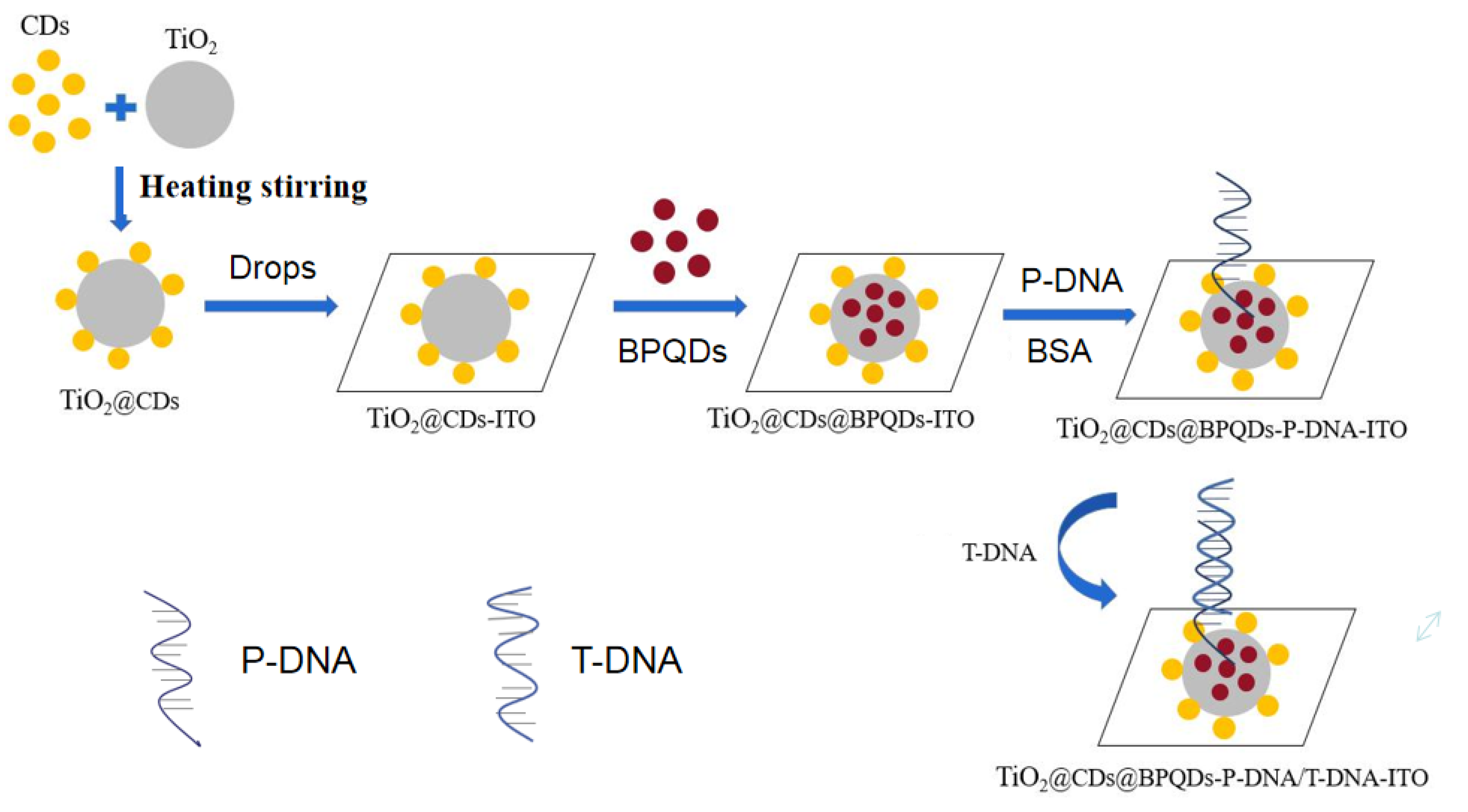
3.1. Construction of Biosensors Based on TiO2@CDs@BPQDs Nanocomposites
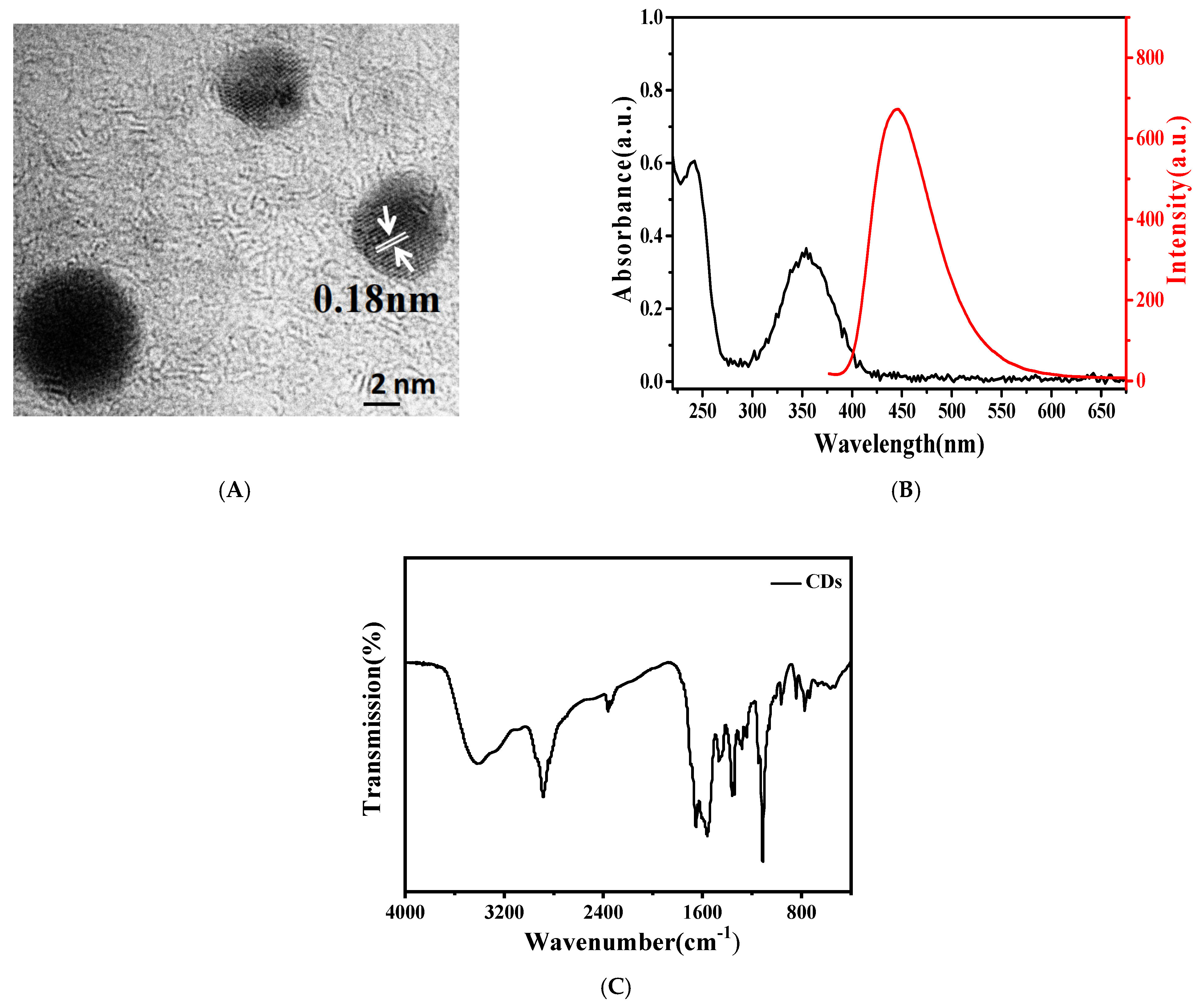
3.2. Characterization of the CDs
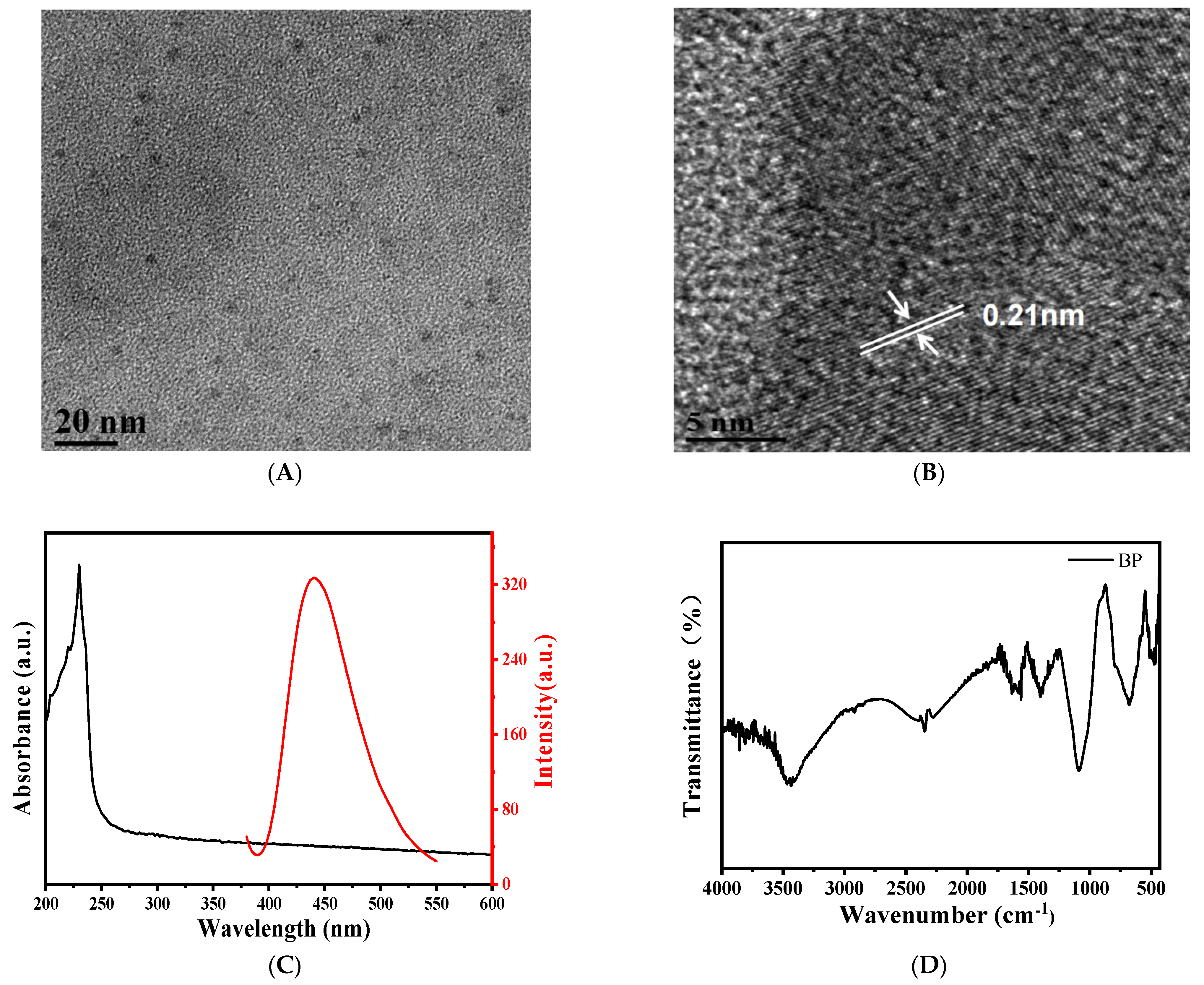
3.3. Characterization of BPQDs
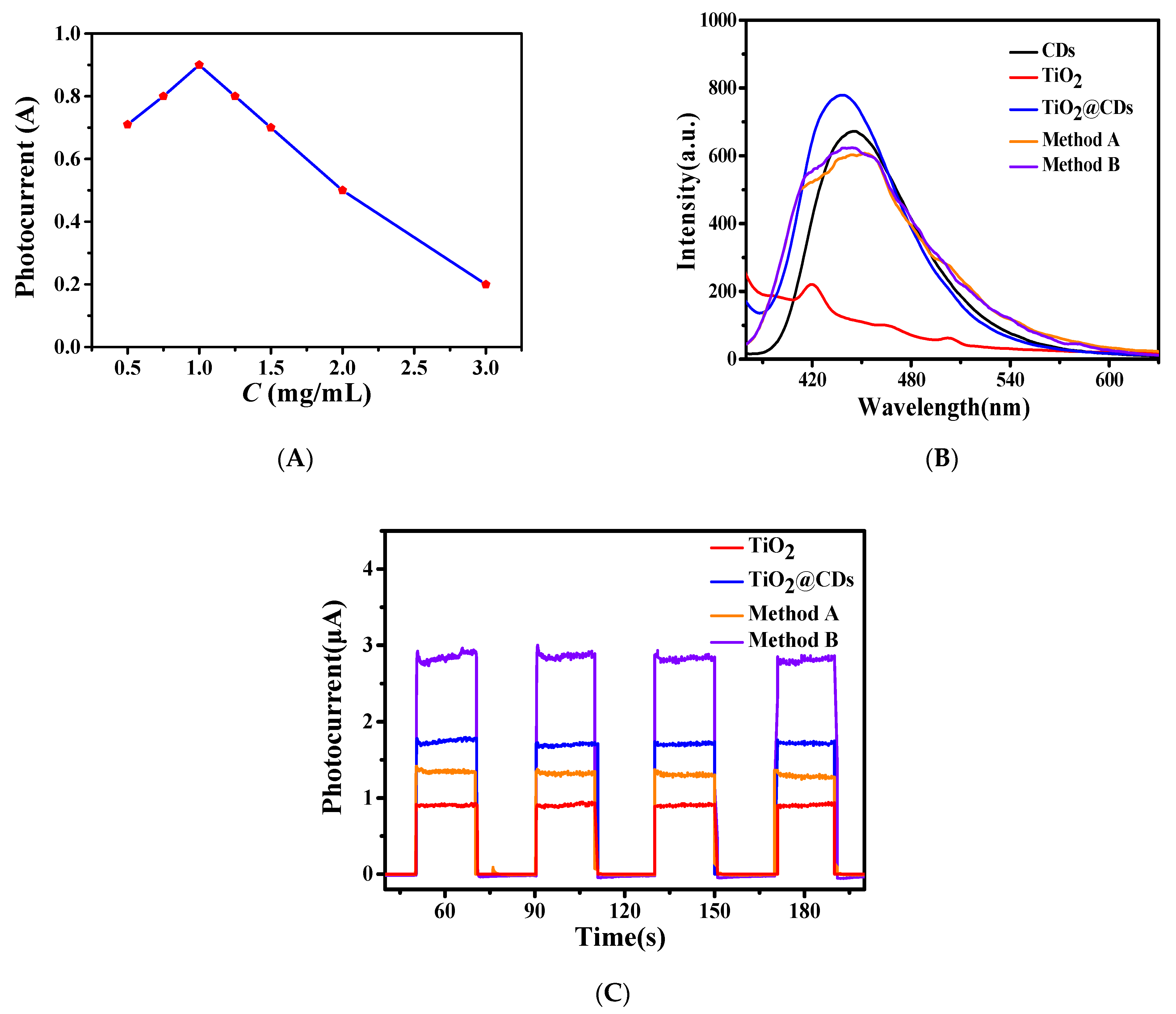
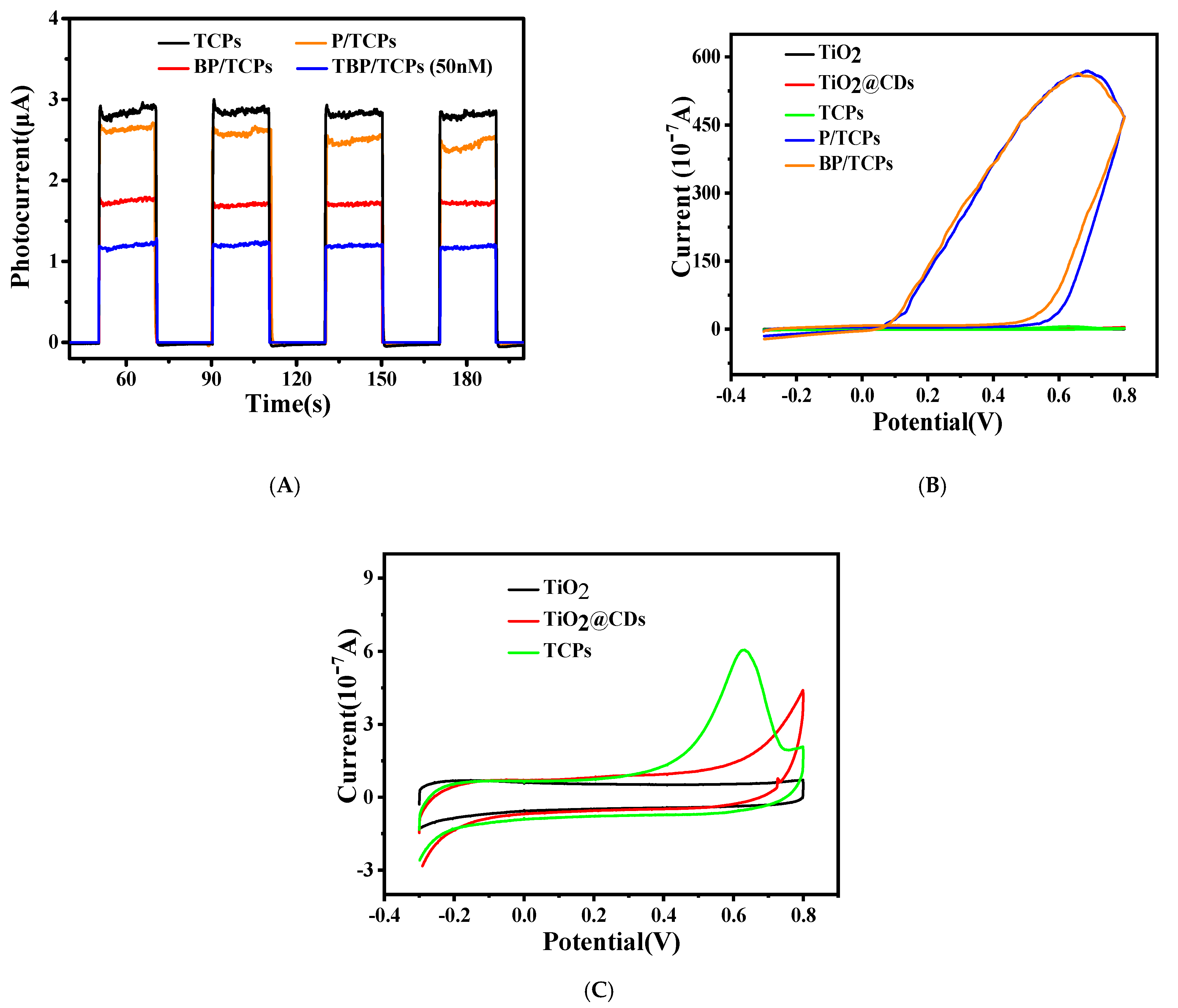
3.4. Preparation and Characterization of Photoanode
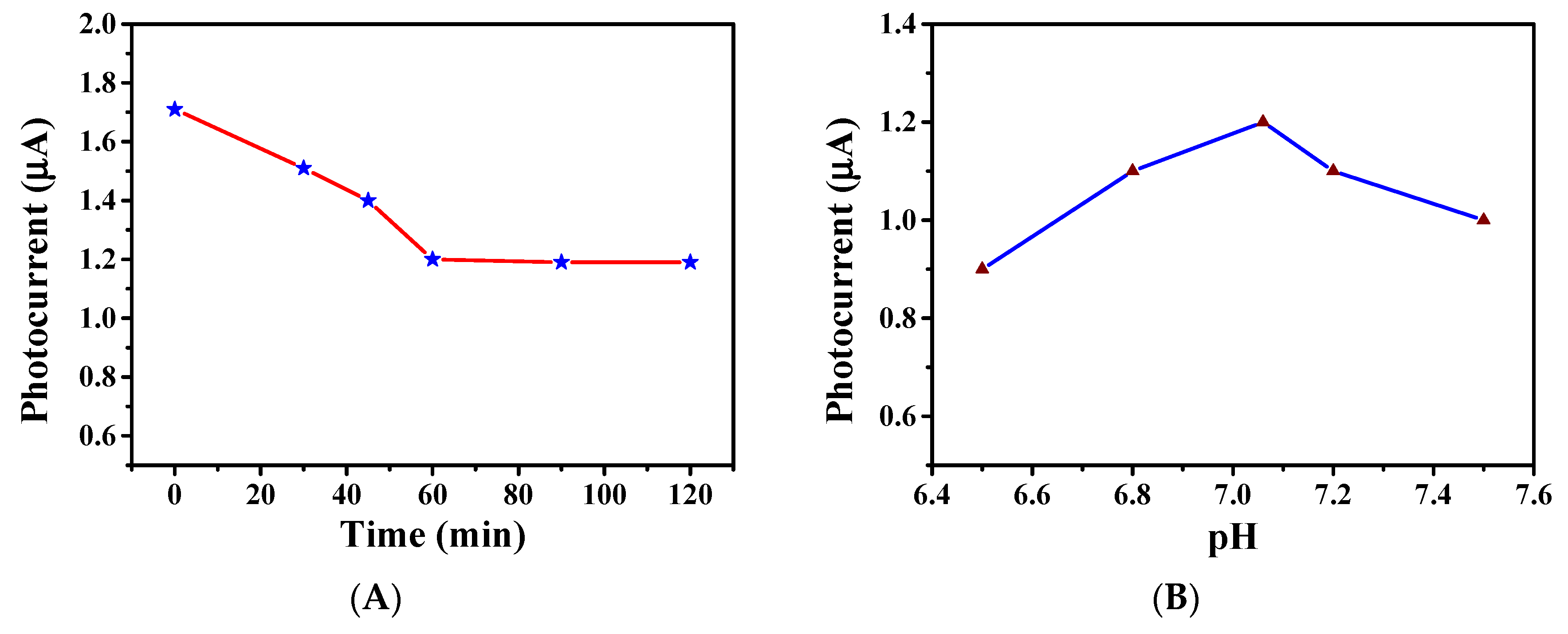
3.5. Construction and Condition Optimization of the Developed Biosensors
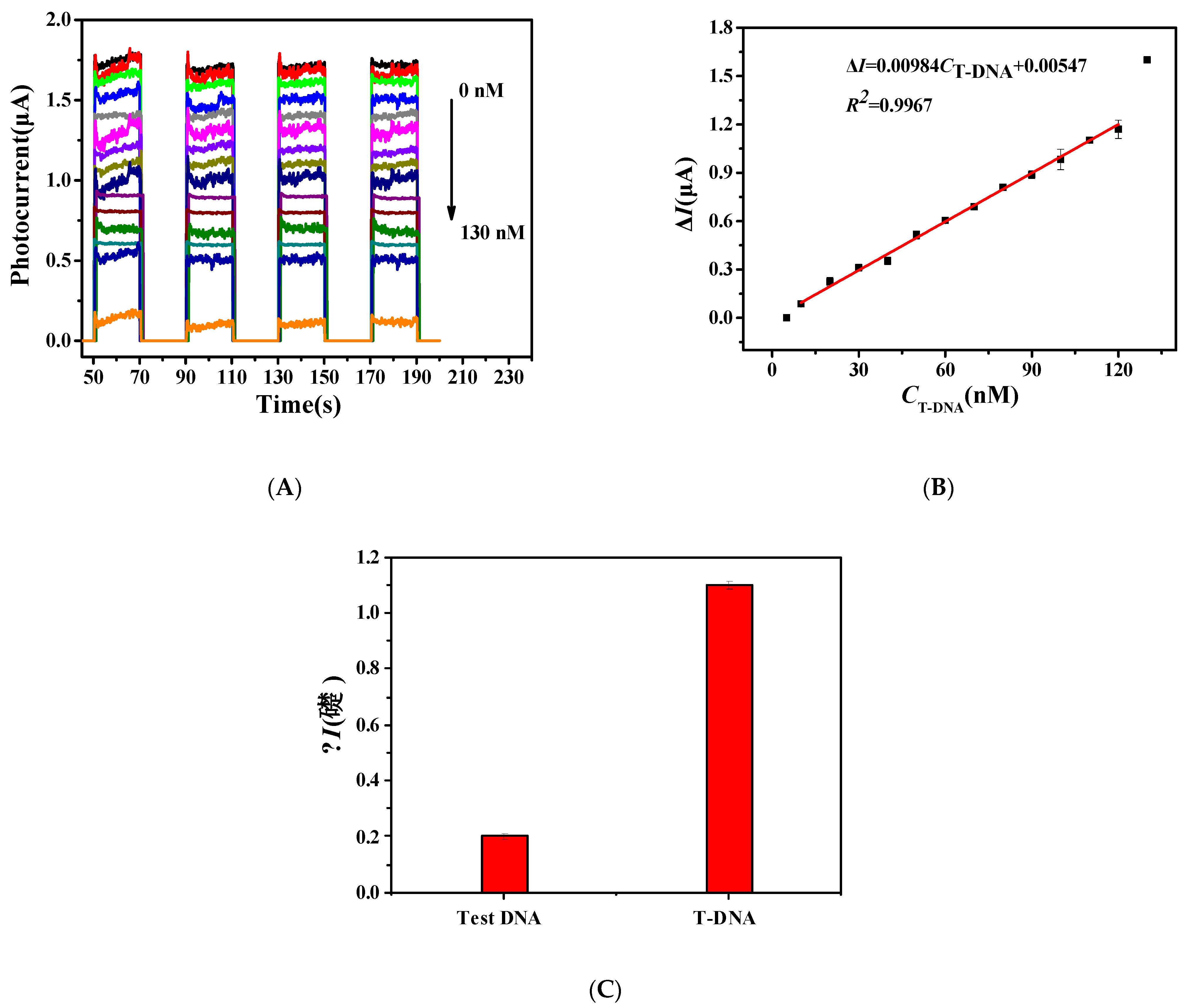
3.6. The Detection of Target DNA(T-DNA)
3.7. Method Specificity Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hu, W.; Yang, S.; Yang, S. Surface modification of TiO2 for perovskite solar cells. Trends Chem. 2019, 2, 148–162. [Google Scholar] [CrossRef]
- Wang, C.; Sun, X.; Shan, H.; Zhang, H.; Xi, B. Degradation of landfill leachate using UV-TiO2 photocatalysis combination with aged waste reactors. Processes 2021, 9, 946. [Google Scholar] [CrossRef]
- Pang, Y.J.; Li, Y.W.; Xu, G.Q.; Hu, Y.T.; Kou, Z.K.; Feng, Q.; Lv, J.; Zhang, Y.; Wang, J.; Wu, Y.C. Z-scheme carbon-bridged Bi2O3/TiO2 nanotube arrays to boost photoelectrochemical detection performance. Appl. Catal. B Environ. 2019, 248, 255–263. [Google Scholar] [CrossRef]
- Jasim, K.E.; AldalIal, S.; Hassan, A.M. Natural dye-sensitized photovoltaic cell based on nanoporous TiO2. Int. J. Nanopart. 2011, 4, 359. [Google Scholar] [CrossRef]
- Mutalik, C.; Hsiao, Y.C.; Chang, Y.H.; Krisnawati, D.I.; Alimansur, M.; Jazidie, A.; Nuh, M.; Chang, C.C.; Wang, D.Y.; Kuo, T.R. High UV-Vis-NIR Light-Induced antibacterial activity by heterostructured TiO2-FeS2 nanocomposites. Int. J. Nanomed. 2020, 15, 8911–8920. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Wang, W.; Jaroniec, M. Nitrogen self-doped nanosized TiO2 sheets with exposed (001) facets for enhanced visible-light photocatalytic activity. Chem. Commun. 2011, 47, 6906–6908. [Google Scholar] [CrossRef]
- Liu, G.; Sun, C.; Smith, S.C.; Wang, L.; Gao, Q.L.; Cheng, H.M. Sulfur doped anatase TiO2 single crystals with a high percentage of (001) facets. J. Colloid Interf. Sci. 2010, 349, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Gu, X.; Sun, C.; Li, H.; Deng, Y.; Gao, F.; Dong, L. In situ loading of ultra-small Cu2O particles on TiO2 nanosheets to enhance the visible-light photoactivity. Nanoscale 2012, 4, 6351–6359. [Google Scholar] [CrossRef]
- Qi, L.; Yu, J.; Jaroniec, M. Preparation and enhanced visible-light photocatalytic H2-production activity of CdS-sensitized Pt/TiO2 nanosheets with exposed (001) facets. Phys. Chem. Chem. Phys. 2011, 13, 8915–8923. [Google Scholar] [CrossRef]
- Zhu, S.Y.; Liang, S.J.; Gu, Q.; Xie, L.; Wang, J.; Ding, Z.X.; Liu, P. Effect of Au supported TiO2 with dominant exposed {001) facets on the visible-light photocatalytic activity. Appl. Catal. B Environ. 2012, 119, 146–155. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, S.J.; Qin, H.Y.; Zhou, J.H.; Peng, X.G. Oxygen stabilizes photoluminescence of CdSe/CdS core/shell quantum dots via deionization. J. Am. Chem. Soc. 2020, 142, 4254–4264. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, M.; Guo, Z.; Miao, L.; Han, S.T.; Wang, Z.; Zhang, X.; Zhang, H.; Peng, Z. Recent advances in black phosphorus-based photonics, electronics, sensors and energy devices. Mater. Horiz. 2017, 4, 997–1019. [Google Scholar] [CrossRef]
- Xia, F.N.; Wang, H.; Jia, Y.C. Rediscovering black phosphorus as an anisotropic layered material for optoelectronics and electronics. Nat. Commun. 2014, 5, 4458. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Huynh, T.; Xiu, P.; Zhou, B.; Ye, C.; Luan, B.; Zhou, R. Revealing the importance of surface morphology of nanomaterials to biological responses: Adsorption of the villin headpiece onto graphene and phosphorene. Carbon 2015, 94, 895–902. [Google Scholar] [CrossRef]
- Zaini, M.S.; Liew, J.; Ahmad, S.; Mohmad, A.R.; Kamarudin, M.A. Quantum confinement effect and photoenhancement of photoluminescence of PbS and PbS/MnS quantum dots. Appl. Sci. 2020, 10, 6282. [Google Scholar] [CrossRef]
- Xu, Y.H.; Wang, Z.T.; Guo, Z.N.; Huang, H.; Xiao, Q.L.; Zhang, H.; Yu, X.F. Solvothermal synthesis and ultrafast photonics of black phosphorus quantum dots. Adv. Opt. Mater. 2016, 4, 1223–1229. [Google Scholar] [CrossRef]
- Sahu, S.; Behera, B.; Maiti, T.K.; Mohapatra, S. Simple one-step synthesis of highly luminescent carbon dots from orange juice: Application as excellent bio-imaging agents. Chem. Commun. 2012, 48, 8835–8837. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Shen, W.; Gao, Z.Q. Carbon quantum dots and their applications. Chem. Soc. Rev. 2014, 44, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.J.; Bai, X.L.; Liu, Y.M.; Liao, X. Functionalized carbon quantum dots with dopamine for tyrosinase activity analysis. Anal. Chim. Acta 2017, 995, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Mayorga-Martinez, C.C.; Sofer, Z.; Pumera, M. Layered black phosphorus as a selective vapor sensor. Angew. Chem. Int. Ed. 2015, 54, 14317–14320. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.N.; Zhang, H.; Lu, S.B.; Wang, Z.T.; Tang, S.Y.; Shao, J.D.; Sun, Z.B.; Xie, H.H.; Wang, H.Y.; Yu, X.F.; et al. From black phosphorus to phosphorene: Basic solvent exfoliation, evolution of Raman scattering, and applications to ultrafast photonics. Adv. Funct. Mater. 2015, 25, 6996–7002. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, H.M.; Liu, Z.D.; Tan, C.L.; Luo, Z.M.; Li, H.; Lin, J.D.; Sun, L.Q.; Chen, W.; Xu, Z.C.; et al. Black phosphorus quantum dots. Angew. Chem. Int. Ed. 2015, 54, 3653–3657. [Google Scholar] [CrossRef] [PubMed]
- Song, M.Y.; Wang, L.J.; Li, J.X.; Guan, R.Q.; Zhai, C.J.; Gao, X.C.; Li, X.H.; Zhao, Z.; Sun, Z.C. Defect density modulation of La2TiO5: An effective method to suppress electron-hole recombination and improve photocatalytic nitrogen fixation. J. Colloid Interf. Sci. 2021, 602, 748–755. [Google Scholar] [CrossRef]
- Lee, A.Y.; Ra, H.S.; Kwak, D.; Jeong, M.H.; Park, J.H.; Kang, Y.S.; Chae, W.S.; Lee, J.S. Hybrid black phosphorus-0D quantum dots phototransistors: Tunable photodoping and enhanced photoresponsivity. ACS Appl. Mater. Inter. 2018, 10, 16033–16040. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Z.; Han, S.T.; Zhou, Y. Recent advances in flexible field-effect transistors towards wearable sensors. Adv. Intell. Syst. 2020, 2, 2000113. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, K.; Lin, J.; Zhuang, Y.; Han, Z.; Chen, J. Construction of Photoelectrochemical DNA Biosensors Based on TiO2@Carbon Dots@Black Phosphorous Quantum Dots. Micromachines 2021, 12, 1523. https://doi.org/10.3390/mi12121523
Song K, Lin J, Zhuang Y, Han Z, Chen J. Construction of Photoelectrochemical DNA Biosensors Based on TiO2@Carbon Dots@Black Phosphorous Quantum Dots. Micromachines. 2021; 12(12):1523. https://doi.org/10.3390/mi12121523
Chicago/Turabian StyleSong, Kai, Jianwei Lin, Yafeng Zhuang, Zhizhong Han, and Jinghua Chen. 2021. "Construction of Photoelectrochemical DNA Biosensors Based on TiO2@Carbon Dots@Black Phosphorous Quantum Dots" Micromachines 12, no. 12: 1523. https://doi.org/10.3390/mi12121523
APA StyleSong, K., Lin, J., Zhuang, Y., Han, Z., & Chen, J. (2021). Construction of Photoelectrochemical DNA Biosensors Based on TiO2@Carbon Dots@Black Phosphorous Quantum Dots. Micromachines, 12(12), 1523. https://doi.org/10.3390/mi12121523





