Physico-Chemical Characteristics of Spodumene Concentrate and Its Thermal Transformations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Spodumene Concentrate Preparation
2.2. Particle Size Distribution
2.3. Thermal Treatment
2.4. XRD Analyses
2.5. SEM-EDS Analyses
2.6. Mineral Liberation Analysis
2.7. X-ray Fluorescence (XRF) Microscopy and Inductively Coupled Plasma—Optical Emission Spectrometry (ICP-OES)
3. Results and Discussion
3.1. Bulk Chemical Analysis
3.2. Mineralogy
3.3. Morphology, Texture of Particles and Mineralogy
3.4. Morphological Changes in Spodumene during Thermal Treatment
3.5. Conversion Extent of α-Spodumene
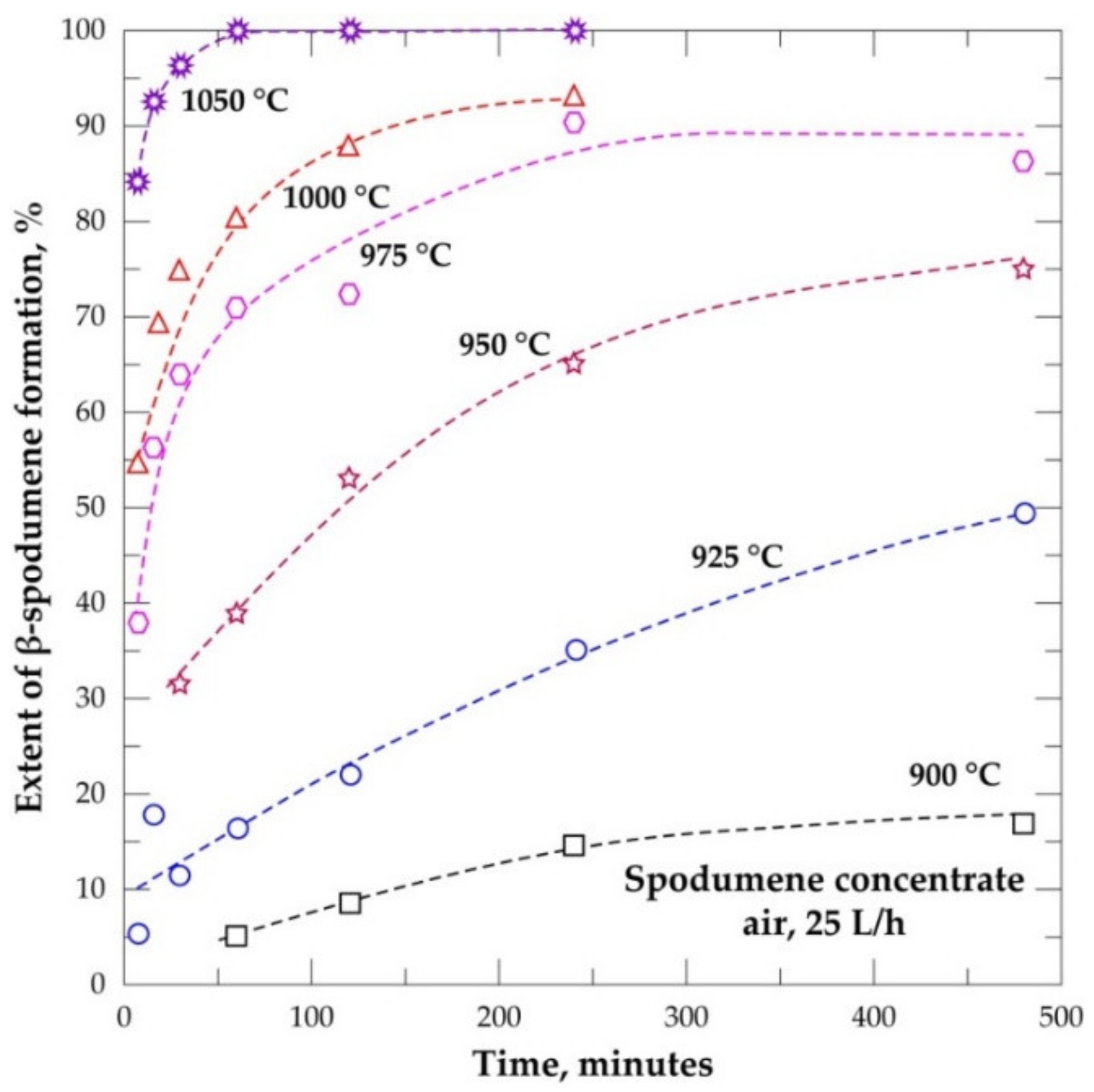
3.6. Evolution Extent of β-Spodumene
3.7. Evolution of the Relative γ-Spodumene
3.8. Phase Transformation and Kinetic Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bradley, D.C.; McCauley, A.D.; Stillings, L.M. Mineral-Deposit Model for Lithium-Cesium-Tantalum Pegmatites; U.S. Geological Survey: Reston, VA, USA, 2017. [Google Scholar]
- Pickles, C.A.; Marzoughi, O. Thermodynamic modelling of spodumene decrepitation. Miner. Process. Extr. Metall. Trans. Inst. Min. Metall. 2020, 1–15. [Google Scholar] [CrossRef]
- Moore, L.R.; Mann, J.P.; Montoya, A.; Haynes, B.S. In situ synchrotron XRD analysis of the kinetics of spodumene phase transitions. Phys. Chem. Chem. Phys. 2018, 20, 10753–10761. [Google Scholar] [CrossRef] [PubMed]
- Salakjani, N.K.; Singh, P.; Nikoloski, A.N. Production of Lithium–A Literature Review Part 1: Pretreatment of Spodumene. Miner. Process. Extr. Metall. Rev. 2020, 41, 335–348. [Google Scholar] [CrossRef]
- Yonghua, D.; Lishi, M.; Ping, L.; Yong, C. First-principles calculations of electronic structures and optical, phononic, and thermodynamic properties of monoclinic α-spodumene. Ceram. Int. 2017, 43, 6312–6321. [Google Scholar] [CrossRef]
- Arlt, T.; Angel, R. Displacive phase transitions in C-centred clinopyroxenes: Spodumene, LiScSi2O6 and ZnSiO3. Phys. Chem. Miner. 2000, 27, 719–731. [Google Scholar] [CrossRef]
- Moore, R.L.; Haynes, B.S.; Montoya, A. Effect of the Local Atomic Ordering on the Stability of β-Spodumene. Inorg. Chem. 2016, 55, 6426–6434. [Google Scholar] [CrossRef]
- Li, C.-T.; Peacor, D.R. The crystal structure of LiAlSi2O6-II (“β spodumene″). Z. Krist. Mater. 1968, 126, 46–65. [Google Scholar] [CrossRef]
- Fosu, A.Y.; Kanari, N.; Vaughan, J.; Chagnes, A. Literature Review and Thermodynamic Modelling of Roasting Processes for Lithium Extraction from Spodumene. Metals 2020, 10, 1312. [Google Scholar] [CrossRef]
- Peltosaari, O.; Tanskanen, P.; Heikkinen, E.-P.; Fabritius, T. α→γ→β-phase transformation of spodumene with hybrid microwave and conventional furnaces. Miner. Eng. 2015, 82, 54–60. [Google Scholar] [CrossRef]
- Abdullah, A.A.; Oskierski, H.C.; Altarawneh, M.; Senanayake, G.; Lumpkin, G.; Dlugogorski, B.Z. Phase transformation mechanism of spodumene during its calcination. Miner. Eng. 2019, 140, 105883. [Google Scholar] [CrossRef]
- Salakjani, N.K.; Singh, P.; Nikoloski, A.N. Acid roasting of spodumene: Microwave vs. conventional heating. Miner. Eng. 2019, 138, 161–167. [Google Scholar] [CrossRef]
- Rosales, G.D.; Resentera, A.C.J.; Gonzalez, J.A.; Wuilloud, R.G.; Rodriguez, M.H. Efficient extraction of lithium from β-spodumene by direct roasting with NaF and leaching. Chem. Eng. Res. Des. 2019, 150, 320–326. [Google Scholar] [CrossRef]
- Rosales, G.D.; Ruiz, M.D.C.; Rodriguez, M.H. Novel process for the extraction of lithium from β-spodumene by leaching with HF. Hydrometallurgy 2014, 147, 1–6. [Google Scholar] [CrossRef]
- Barbosa, L.I.; Valente, G.; Orosco, R.P.; González, J.A. Lithium extraction from β-spodumene through chlorination with chlorine gas. Miner. Eng. 2014, 56, 29–34. [Google Scholar] [CrossRef]
- Barbosa, L.I.; González, J.A.; del Carmen Ruiz, M. Extraction of lithium from β-spodumene using chlorination roasting with calcium chloride. Thermochim. Acta 2015, 605, 63–67. [Google Scholar] [CrossRef]
- Aylmore, M.G.; Merigot, K.; Rickard, W.D.A.; Evans, N.J.; Mcdonald, B.J.; Spitalny, P. Assessment of a spodumene ore by advanced analytical and mass spectrometry techniques to determine its amenability to processing for the extraction of lithium. Miner. Eng. 2018, 119, 137–148. [Google Scholar] [CrossRef] [Green Version]
- Fandrich, R.; Gu, Y.; Burrows, D.; Moeller, K. Modern SEM-based mineral liberation analysis. Int. J. Miner. Process. 2007, 84, 310–320. [Google Scholar] [CrossRef]
- Gu, Y. Automated Scanning Electron Microscope Based Mineral Liberation Analysis. An Introduction to JKMRC/FEI Mineral Liberation Analyser. J. Miner. Mater. Charact. Eng. 2003, 02, 33–41. [Google Scholar] [CrossRef]
- Tian, Q.; Chen, B.; Chen, Y.; Ma, L.; Shi, X. Roasting and leaching behavior of spodumene in sulphuric acid process. Xiyou Jinshu Chin. J. Rare Met. 2011, 35, 118–123. [Google Scholar] [CrossRef]
- White, G.D.; McVay, T.N. Some Aspects Of The Recovery Of Lithium From Spodumene; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1958. [Google Scholar]
- Salakjani, N.K.; Singh, P.; Nikoloski, A.N. Mineralogical transformations of spodumene concentrate from Greenbushes, Western Australia. Part 1: Conventional heating. Miner. Eng. 2016, 98, 71–79. [Google Scholar] [CrossRef]
- Gasalla, H.J.; Aglietti, E.F.; Lopez, J.M.P.; Pereira, E. Changes in physicochemical properties of α-spodumene by mechanochemical treatment. Mater. Chem. Phys. 1987, 17, 379–389. [Google Scholar] [CrossRef]
- Dessemond, C.; Soucy, G.; Harvey, J.P.; Ouzilleau, P. Phase transitions in the α-γ-β spodumene thermodynamic system and impact of γ-spodumene on the efficiency of lithium extraction by acid Leaching. Minerals 2020, 10, 519. [Google Scholar] [CrossRef]
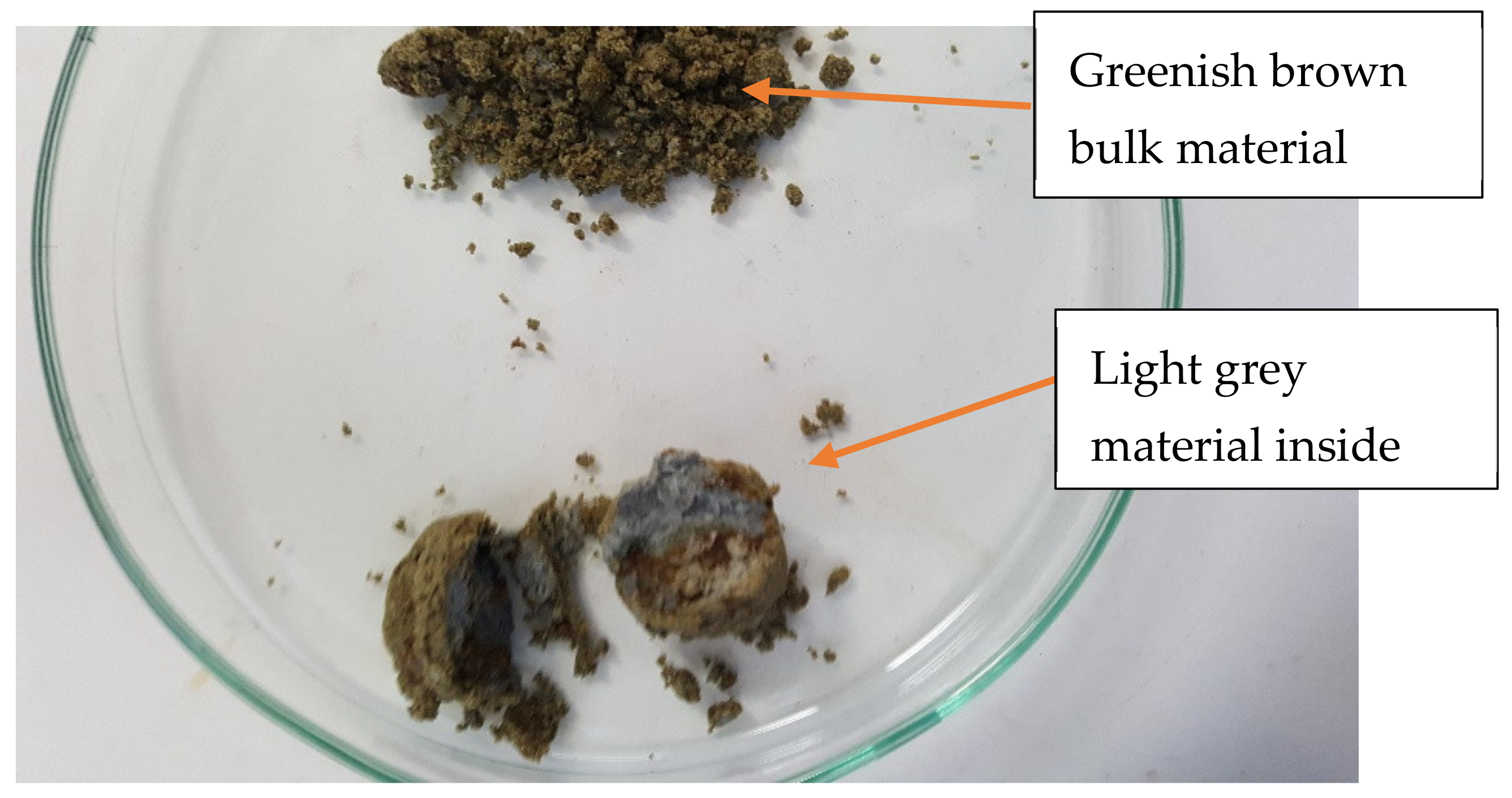












| Major | Al2O3 | CaO | Cr2O3 | Fe2O3 | K2O | MgO | MnO | Na2O | P2O5 | SO3 | SiO2 | TiO2 | Li | Cs | Rb | Ta | Nb | Sn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (wt.%) | 20.79 | 1.72 | 0.03 | 4.29 | 1.26 | 1.30 | 0.32 | 1.14 | 0.33 | 0.15 | 61.31 | 0.10 | 2.14 | |||||
| (ppm) | 116 | 1033 | 202 | 180 | 140 |
| Mineral | Formula | wt.% |
|---|---|---|
| Spodumene | LiAl(Si2O6) | 60.21 |
| Pyrite | FeS2 | 0.13 |
| Quartz | SiO2 | 7.58 |
| Orthoclase | KAlSi3O8 | 4 |
| Albite | NaAlSi3O8 | 9.1 |
| Anorthite | CaAl2Si2O8 | 1 |
| Biotite | KMg2.5Fe2+0.5AlSi3O10(OH)1.75F0.25 | 3.32 |
| Muscovite | KAl3Si3O10(OH)1.9F0.1 | 4.9 |
| Chlorite | (Mg,Fe)3(Si,Al)4O10(OH)2·(Mg,Fe)3(OH)6 | 0.33 |
| Amphibole | CaFeSi2O6 | 7.3 |
| Spessartine | Mn2+3Al2(SiO4)3 | 0.61 |
| Tantalite-(Mn) | MnTa2O6 | 0.12 |
| Calcite | Ca(CO3) | 0.47 |
| Apatite | Ca5(PO4)(F,Cl,OH) | 0.93 |
| Li | Al | Si | C | S | Fe | K | Na | Ta | P | Mn | Mg | Ca | Cl | F | H | O |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.96 | 11.58 | 29.47 | 0.06 | 0.07 | 2.0 | 1.34 | 0.76 | 0.09 | 0.17 | 0.22 | 0.5 | 1.97 | 0.02 | 0.09 | 0.04 | 49.39 |
| Elements | Spot “1” | Spot “2” | Spot ”3” | Spot “4” | Spot “5” | Spot “6” | Spot “7” | Spot “8” | Spot “9” | Spot “10” |
|---|---|---|---|---|---|---|---|---|---|---|
| O | 64.7 | 60.9 | 54.4 | 66.7 | 61.9 | 61.6 | 60.1 | 67.3 | 56.6 | 67.8 |
| Al | 11.5 | 5.2 | 4.1 | 7.7 | 7.5 | 0.4 | ||||
| Si | 23.6 | 16.8 | 16.1 | 33.3 | 23.3 | 23.2 | 0.6 | |||
| Fe | 0.2 | 6.2 | 2.2 | 38.9 | 1.9 | |||||
| Mg | 4.3 | 10.6 | 0.1 | |||||||
| Ca | 4.8 | 0.2 | 20.2 | |||||||
| Mn | 0.2 | 8.5 | ||||||||
| Na | 1.1 | 6.9 | 2.4 | |||||||
| K | 0.5 | 3.9 | 5.2 | |||||||
| F | 8.6 | 8.6 | ||||||||
| P | 14.6 | |||||||||
| Nb | 12.1 | |||||||||
| Ta | 10.3 | 0.4 | ||||||||
| N | 6.2 | |||||||||
| Sn | 25.6 |
| Elements | Spot “1” | Spot “2” | Spot “3, 5 and 6” | Spot “4” | Spot “7” | Spot “8” | Spot “9” |
|---|---|---|---|---|---|---|---|
| O | 64.3 | 62.8 | 66.7 | 60.1 | 67.3 | 56.9 | |
| Al | 11.4 | 3.4 | 6.6 | 0.4 | |||
| Si | 23.1 | 19.5 | 24.3 | 33.3 | 0.6 | ||
| Fe | 0.2 | 14.4 | 0.8 | 38.9 | 1.9 | ||
| Mg | 0.2 | 3.9 | 0.3 | 0.1 | |||
| Ca | 0.1 | 1.7 | 0.5 | 2.4 | |||
| Mn | 0.1 | 0.2 | 8.5 | 0.3 | |||
| Na | 0.6 | 0.9 | 2.0 | ||||
| K | 0.2 | 0.8 | 2.7 | ||||
| Ti | 0.3 | ||||||
| F | 7.9 | ||||||
| P | 14.5 | ||||||
| Nb | 12.1 | ||||||
| Ta | 10.3 |
| T °C | 900 | 925 | 975 | 1000 | 1025 | 1050 |
|---|---|---|---|---|---|---|
| (min−1) | 0.0004 | 0.0019 | 0.0224 | 0.0054 | 0.0224 | 0.085 |
| (min−1) | 0.0048 | 0.0192 | 0.065 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fosu, A.Y.; Kanari, N.; Bartier, D.; Hodge, H.; Vaughan, J.; Chagnes, A. Physico-Chemical Characteristics of Spodumene Concentrate and Its Thermal Transformations. Materials 2021, 14, 7423. https://doi.org/10.3390/ma14237423
Fosu AY, Kanari N, Bartier D, Hodge H, Vaughan J, Chagnes A. Physico-Chemical Characteristics of Spodumene Concentrate and Its Thermal Transformations. Materials. 2021; 14(23):7423. https://doi.org/10.3390/ma14237423
Chicago/Turabian StyleFosu, Allen Yushark, Ndue Kanari, Danièle Bartier, Harrison Hodge, James Vaughan, and Alexandre Chagnes. 2021. "Physico-Chemical Characteristics of Spodumene Concentrate and Its Thermal Transformations" Materials 14, no. 23: 7423. https://doi.org/10.3390/ma14237423
APA StyleFosu, A. Y., Kanari, N., Bartier, D., Hodge, H., Vaughan, J., & Chagnes, A. (2021). Physico-Chemical Characteristics of Spodumene Concentrate and Its Thermal Transformations. Materials, 14(23), 7423. https://doi.org/10.3390/ma14237423









