Kinetic Analysis for the Catalytic Pyrolysis of Polypropylene over Low Cost Mineral Catalysts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Thermogravimetric Analysis
2.3. Kinetic Analysis
3. Results and Discussion
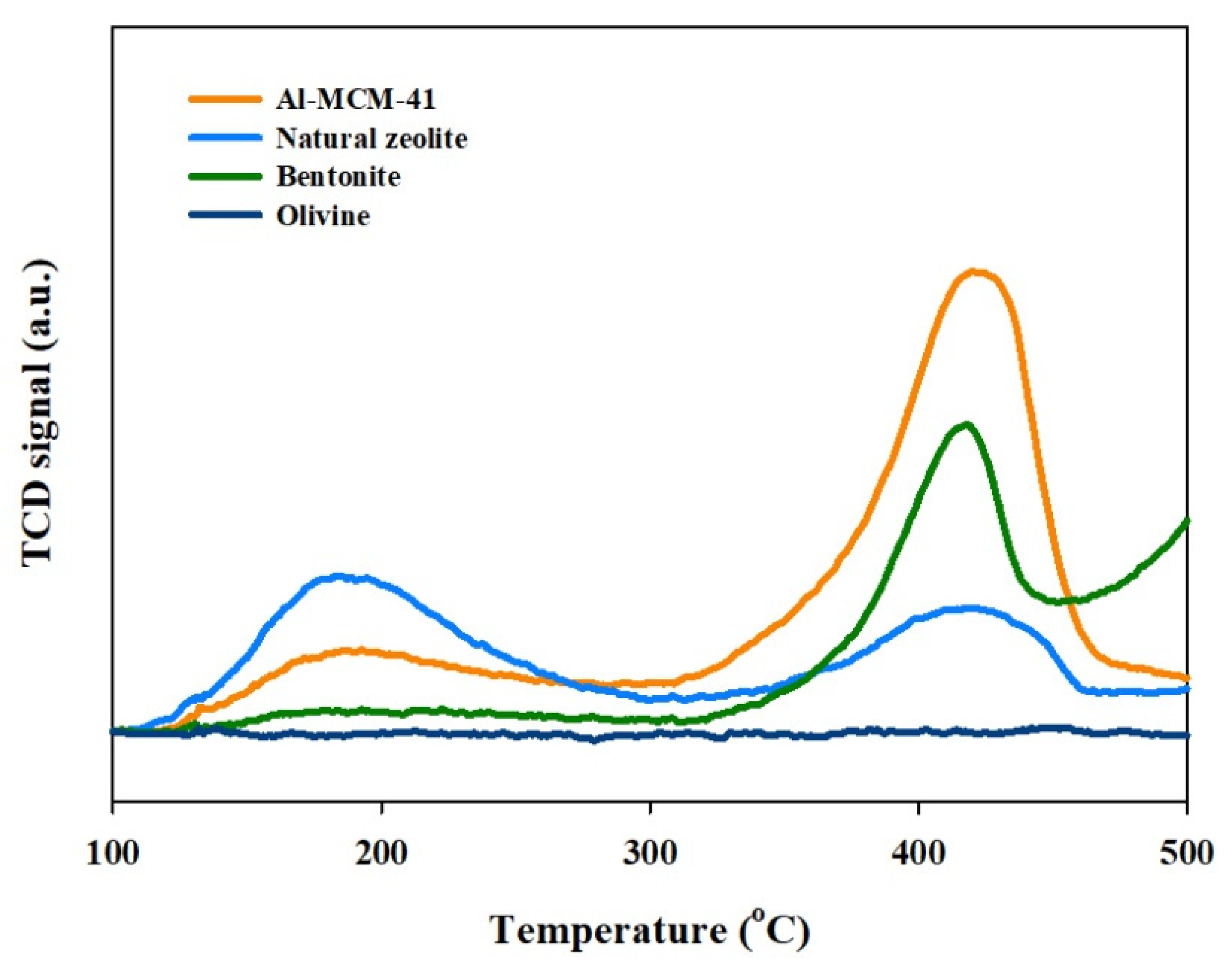
3.1. Characterization of Catalysts
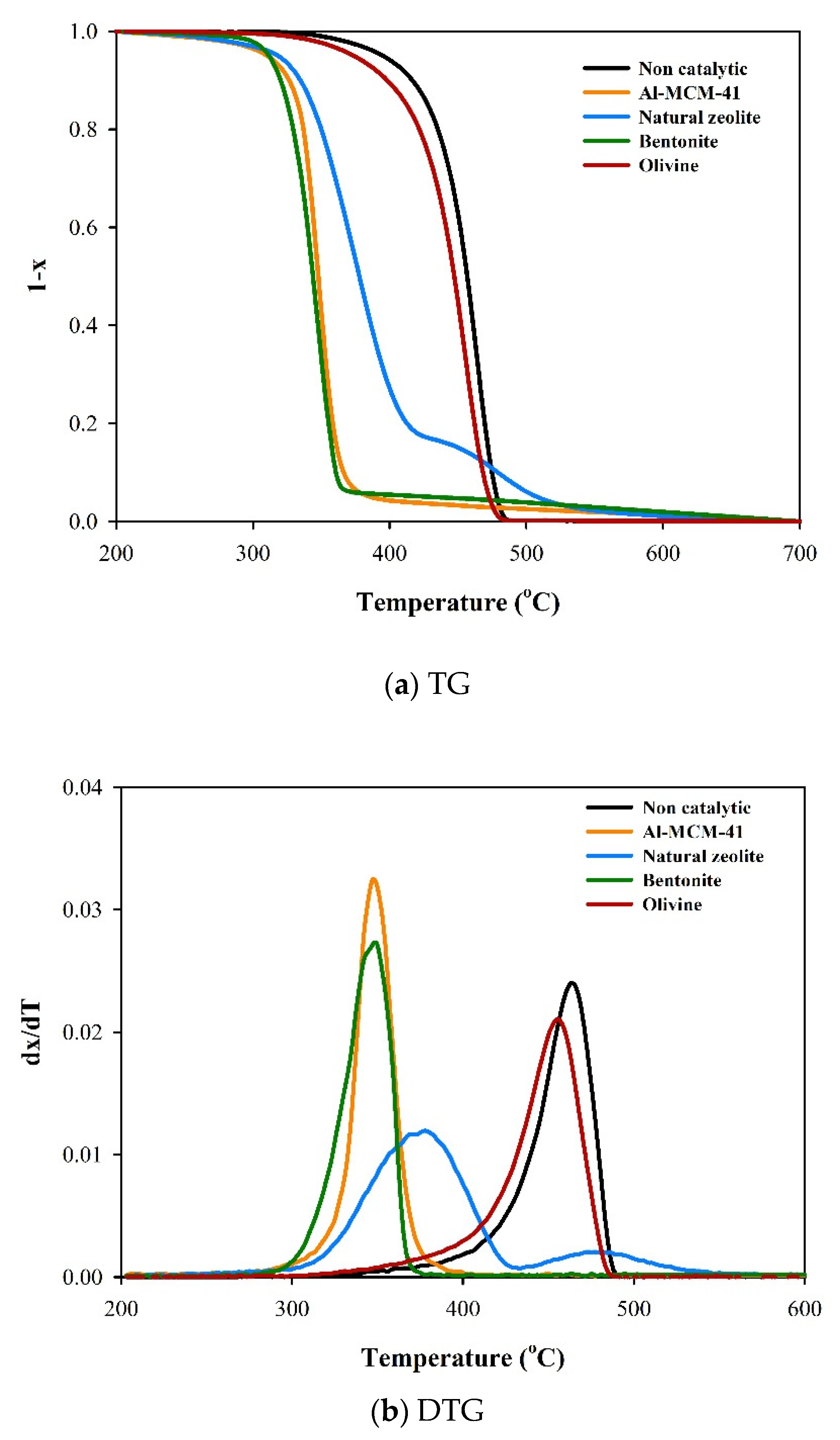
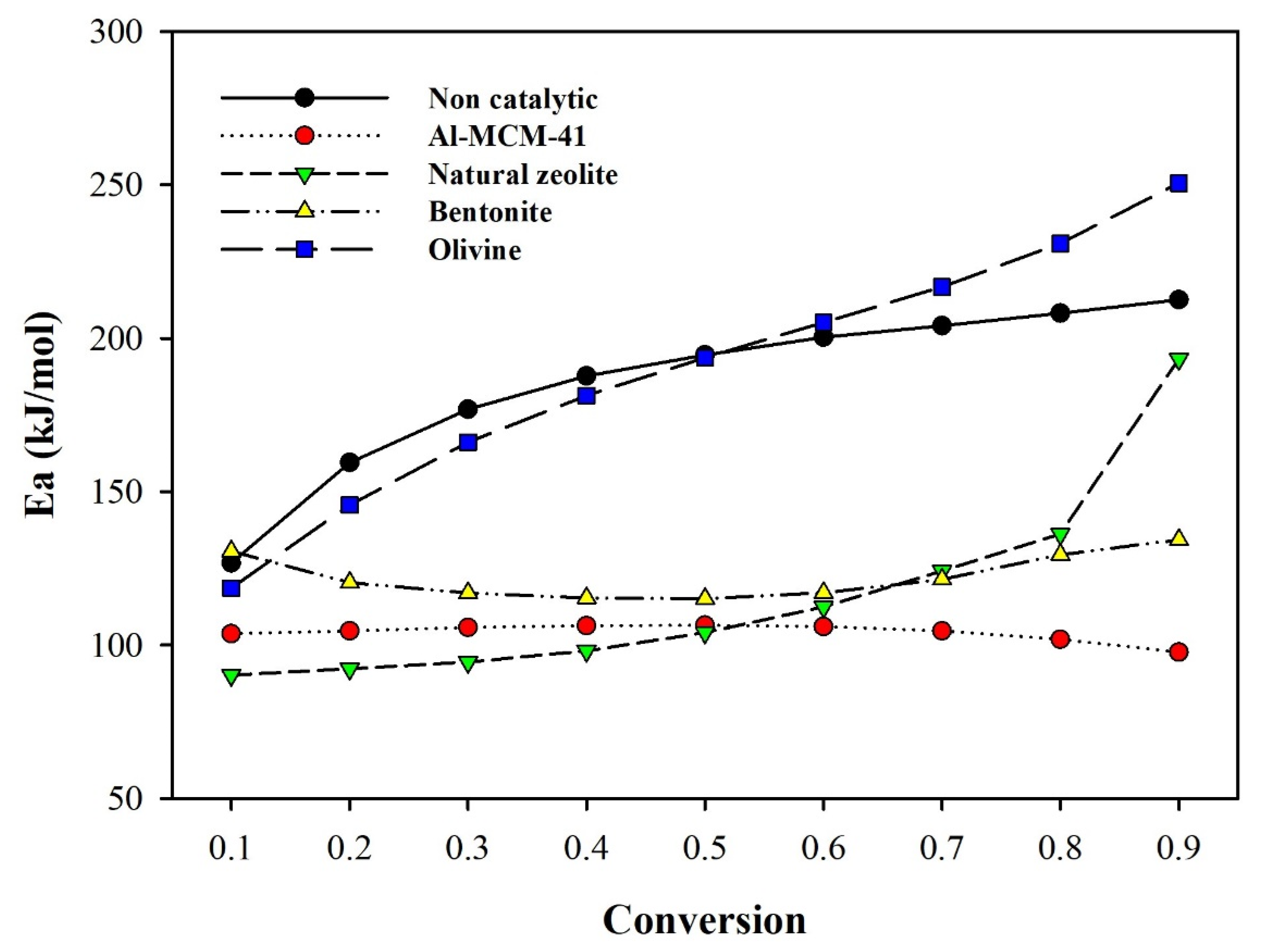
3.2. Thermogravimetric Analysis and Kinetic Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bora, R.R.; Wang, R.; You, F. Waste polypropylene plastic recycling toward climate change mitigation and circular economy: Energy, environmental, and technoeconomic perspectives. ACS Sustain. Chem. Eng. 2020, 8, 16350–16363. [Google Scholar] [CrossRef]
- Fadare, O.O.; Okoffo, E.D. Covid-19 face masks: A potential source of microplastic fibers in the environment. Sci. Total Environ. 2020, 737, 140279. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Park, Y.-K. Catalytic pyrolysis of polyethylene and polypropylene over desilicated beta and Al-MSU-F. Catalysts 2018, 8, 501. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Williams, P.T. Hydrogen production by steam gasification of polypropylene with various nickel catalysts. Appl. Catal. B Environ. 2009, 87, 152–161. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kim, S.; Kim, H.-J.; Yang, H.-S. Thermal properties of bio-flour-filled polyolefin composites with different compatibilizing agent type and content. Thermochim. Acta 2006, 451, 181–188. [Google Scholar] [CrossRef]
- Kassargy, C.; Awad, S.; Burnens, G.; Kahine, K.; Tazerout, M. Gasoline and diesel-like fuel production by continuous catalytic pyrolysis of waste polyethylene and polypropylene mixtures over USY zeolite. Fuel 2018, 224, 764–773. [Google Scholar] [CrossRef]
- Santos, B.P.S.; Almeida, D.; Maria de Fatima, V.M.; Henriques, C.A. Petrochemical feedstock from pyrolysis of waste polyethylene and polypropylene using different catalysts. Fuel 2018, 215, 515–521. [Google Scholar] [CrossRef]
- Lee, H.W.; Choi, S.J.; Jeon, J.-K.; Park, S.H.; Jung, S.-C.; Park, Y.-K. Catalytic conversion of waste particle board and polypropylene over H-beta and HY zeolites. Renew. Energy 2015, 79, 9–13. [Google Scholar] [CrossRef]
- Ryu, S.; Lee, H.W.; Kim, Y.-M.; Jae, J.; Jung, S.-C.; Ha, J.-M.; Park, Y.-K. Catalytic fast co-pyrolysis of organosolv lignin and polypropylene over in-situ red mud and ex-situ HZSM-5 in two-step catalytic micro reactor. Appl. Surf. Sci. 2020, 511, 145521. [Google Scholar] [CrossRef]
- Ro, D.; Kim, Y.-M.; Lee, I.-G.; Jae, J.; Jung, S.-C.; Kim, S.C.; Park, Y.-K. Bench scale catalytic fast pyrolysis of empty fruit bunches over low cost catalysts and HZSM-5 using a fixed bed reactor. J. Clean. Prod. 2018, 176, 298–303. [Google Scholar] [CrossRef]
- Opfermann, J.R.; Kaisersberger, E.; Flammersheim, H.J. Model-free analysis of thermoanalytical data-advantages and limitations. Thermochim. Acta 2002, 391, 119–127. [Google Scholar] [CrossRef]
- Vimalathithan, P.K.; Barile, C.; Casavola, C.; Arunachalam, S.; Battisti, M.G.; Friesenbichler, W.; Vijayakumar, C.T. Thermal degradation kinetics of polypropylene/clay nanocomposites prepared by injection molding compounder. Polym. Compos. 2019, 40, 3634–3643. [Google Scholar] [CrossRef]
- Xu, F.; Wang, B.; Yang, D.; Hao, J.; Qiao, Y.; Tian, Y. Thermal degradation of typical plastics under high heating rate conditions by TG-FTIR: Pyrolysis behaviors and kinetic analysis. Energy Convers. Manag. 2018, 171, 1106–1115. [Google Scholar] [CrossRef]
- Chi, Y.; Xue, J.; Zhuo, J.; Zhang, D.; Liu, M.; Yao, Q. Catalytic co-pyrolysis of cellulose and polypropylene over all-silica mesoporous catalyst MCM-41 and Al-MCM-41. Sci. Total Environ. 2018, 633, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Aboulkas, A.; El harfi, K.; El Bouadili, A. Thermal degradation behaviors of polyethylene and polypropylene. Part I: Pyrolysis kinetics and mechanisms. Energy Convers. Manag. 2010, 51, 1363–1369. [Google Scholar] [CrossRef]
- Ali, G.; Nisar, J.; Iqbal, M.; Shah, A.; Abbas, M.; Shah, M.R.; Rashid, U.; Bhatti, I.A.; Khan, R.A.; Shah, F. Thermo-catalytic decomposition of polystyrene waste: Comparative analysis using different kinetic models. Waste Manag. Res. 2020, 38, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Pham, S.T.; Nguyen, M.B.; Le, G.H.; Nguyen, T.D.; Pham, C.D.; Le, T.S.; Vu, T.A. Influence of Brønsted and Lewis acidity of the modified Al-MCM-41 solid acid on cellulose conversion and 5-hydroxylmethylfurfuran selectivity. Chemosphere 2021, 265, 129062. [Google Scholar] [CrossRef]
- Salman, M.; Nisar, S.; Hussain, Z.; Salman, H.; Kazimi, M.R. TGA-DSC: A screening tool for the evaluation of hydrocracking catalyst performance. Am. J. Anal. Chem. 2015, 6, 364–375. [Google Scholar] [CrossRef] [Green Version]
- Güleç, F.; Meredith, W.; Snape, C.E. Progress in the CO2 Capture Technologies for Fluid Catalytic Cracking (FCC) Units—A Review. Front. Energy Res. 2020, 8, 62. [Google Scholar] [CrossRef]
- Locus, R.; Verboekend, D.; Zhong, R.; Houthoofd, K.; Jaumann, T.; Oswald, S.; Giebeler, L.; Baron, G.; Sels, B.F. Enhanced acidity and accessibility in Al-MCM-41 through aluminum activation. Chem. Mater. 2016, 28, 7731–7743. [Google Scholar] [CrossRef]
- Elfadly, A.; Zeid, I.; Yehia, F.; Abouelela, M.; Rabie, A. Production of aromatic hydrocarbons from catalytic pyrolysis of lignin over acid-activated bentonite clay. Fuel Process. Technol. 2017, 163, 1–7. [Google Scholar] [CrossRef]
- Durmuş, A.; Koç, S.N.; Pozan, G.S.; Kaşgöz, A. Thermal-catalytic degradation kinetics of polypropylene over BEA, ZSM-5 and MOR zeolites. Appl. Catal. B Environ. 2005, 61, 316–322. [Google Scholar] [CrossRef]
- Khan, M.A.; Nisar, J.; Iqbal, M.; Shah, A.; Khan, R.A.; Bhatti, I.A.; Amin, R. Pyrolysis of polypropylene over a LZ-Y52 molecular sieve: Kinetics and the product distribution. Iran. Polym. J. 2019, 28, 839–847. [Google Scholar] [CrossRef]
- Nisar, J.; Khan, M.A.; Ali, G.; Iqbal, M.; Shah, A.; Shah, M.R.; Sherazi, S.T.H.; Shah, L.A.; Rehman, N.U. Pyrolysis of polypropylene over zeolite mordenite ammonium: Kinetics and products distribution. J. Polym. Eng. 2019, 39, 785–793. [Google Scholar] [CrossRef]
- Kim, B.-S.; Kim, Y.-M.; Lee, H.W.; Jae, J.; Kim, D.H.; Jung, S.-C.; Watanabe, C.; Park, Y.-K. Catalytic Copyrolysis of Cellulose and Thermoplastics over HZSM-4 and HY. ACS Sustain. Chem. Eng. 2016, 4, 1354–1363. [Google Scholar] [CrossRef]

| Proximate Analysis (wt.%) | Ultimate Analysis (wt.%) | ||
|---|---|---|---|
| Volatile | 99.8 | C | 85.7 |
| Fixed Carbon | 0.2 | H | 14.3 |
| Sum | 100 | Sum | 100 |
| Catalyst | SBET (m2/g) | Average Pore Size (nm) |
|---|---|---|
| Al-MCM-41 | 512 | 2.7 |
| Natural zeolite | 157 | 0.5 |
| Bentonite | 23 | 29.8 |
| Olivine | 0.2 | 7.9 |
| Catalyst | Tmax (°C) |
|---|---|
| Non catalytic | 464 |
| Al-MCM-41 | 347 |
| Natural zeolite | 1st: 376 |
| 2nd: 474 | |
| Bentonite | 348 |
| Olivine | 456 |
| Catalyst | Tmax | Ea | Applied Method | Ref. |
|---|---|---|---|---|
| Non catalytic | 411 | 114 | [22] | |
| BEA zeolite | 224 | 59 | ||
| ZSM-5 (SiO2/Al2O3 = 12.5) | 228 | 98 | ||
| ZSM-5 (SiO2/Al2O3 = 25) | 264 | 62 | ||
| MOR zeolite | 280 | 51 | ||
| Non catalytic | 397 | - | - | [23] |
| LZ-Y52 molecular sieve | 373 | 64.6 | Ozawa–Flynn–Wall | |
| 57.5 | modified Coats–Redfern | |||
| 57.97 | Tang–Wanjun method | |||
| Non catalytic | 376.2 | - | - | [24] |
| 10% zeolite mordenite ammonium | 359.7 | 73.3 | Ozawa–Flynn–Wall | |
| 66.1 | modified Coats–Redfern | |||
| 65.8 | Tang–Wanjun method | |||
| Non catalytic | 464 | 185.6 | Ozawa–Flynn–Wall | This study |
| Olivine | 456 | 189.8 | ||
| Bentonite | 348 | 122.2 | ||
| Al-MCM-41 | 347 | 104.1 | ||
| NZ | 1st peak: 376 2nd peak: 474 | 116.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-M.; Pyo, S.; Hakimian, H.; Yoo, K.-S.; Rhee, G.-H.; Park, Y.-K. Kinetic Analysis for the Catalytic Pyrolysis of Polypropylene over Low Cost Mineral Catalysts. Sustainability 2021, 13, 13386. https://doi.org/10.3390/su132313386
Kim Y-M, Pyo S, Hakimian H, Yoo K-S, Rhee G-H, Park Y-K. Kinetic Analysis for the Catalytic Pyrolysis of Polypropylene over Low Cost Mineral Catalysts. Sustainability. 2021; 13(23):13386. https://doi.org/10.3390/su132313386
Chicago/Turabian StyleKim, Young-Min, Sumin Pyo, Hanie Hakimian, Kyung-Seun Yoo, Gwang-Hoon Rhee, and Young-Kwon Park. 2021. "Kinetic Analysis for the Catalytic Pyrolysis of Polypropylene over Low Cost Mineral Catalysts" Sustainability 13, no. 23: 13386. https://doi.org/10.3390/su132313386
APA StyleKim, Y.-M., Pyo, S., Hakimian, H., Yoo, K.-S., Rhee, G.-H., & Park, Y.-K. (2021). Kinetic Analysis for the Catalytic Pyrolysis of Polypropylene over Low Cost Mineral Catalysts. Sustainability, 13(23), 13386. https://doi.org/10.3390/su132313386








