Two-Photon Polymerization of Albumin Hydrogel Nanowires Strengthened with Graphene Oxide
Abstract
1. Introduction
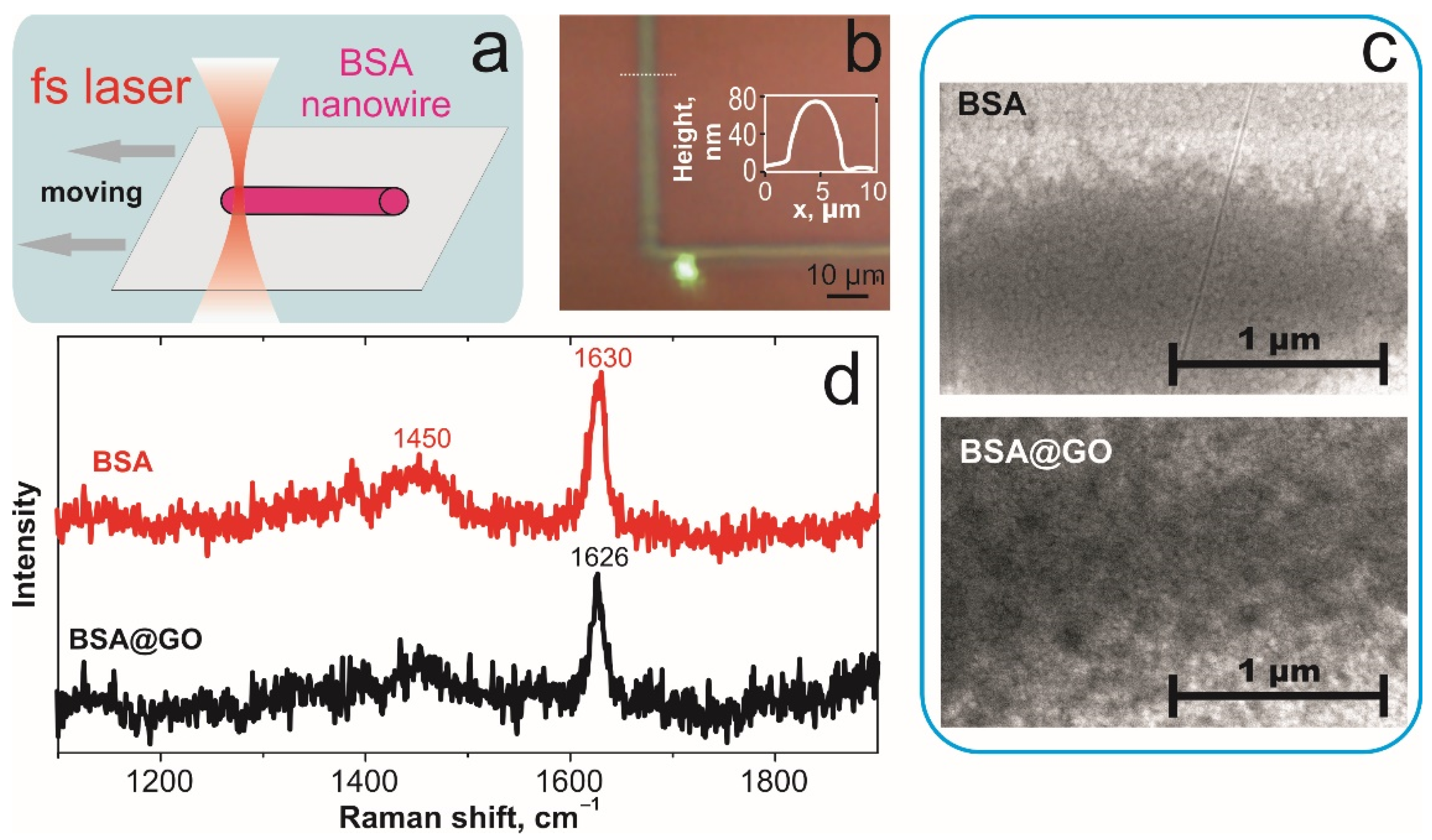
2. Materials and Methods
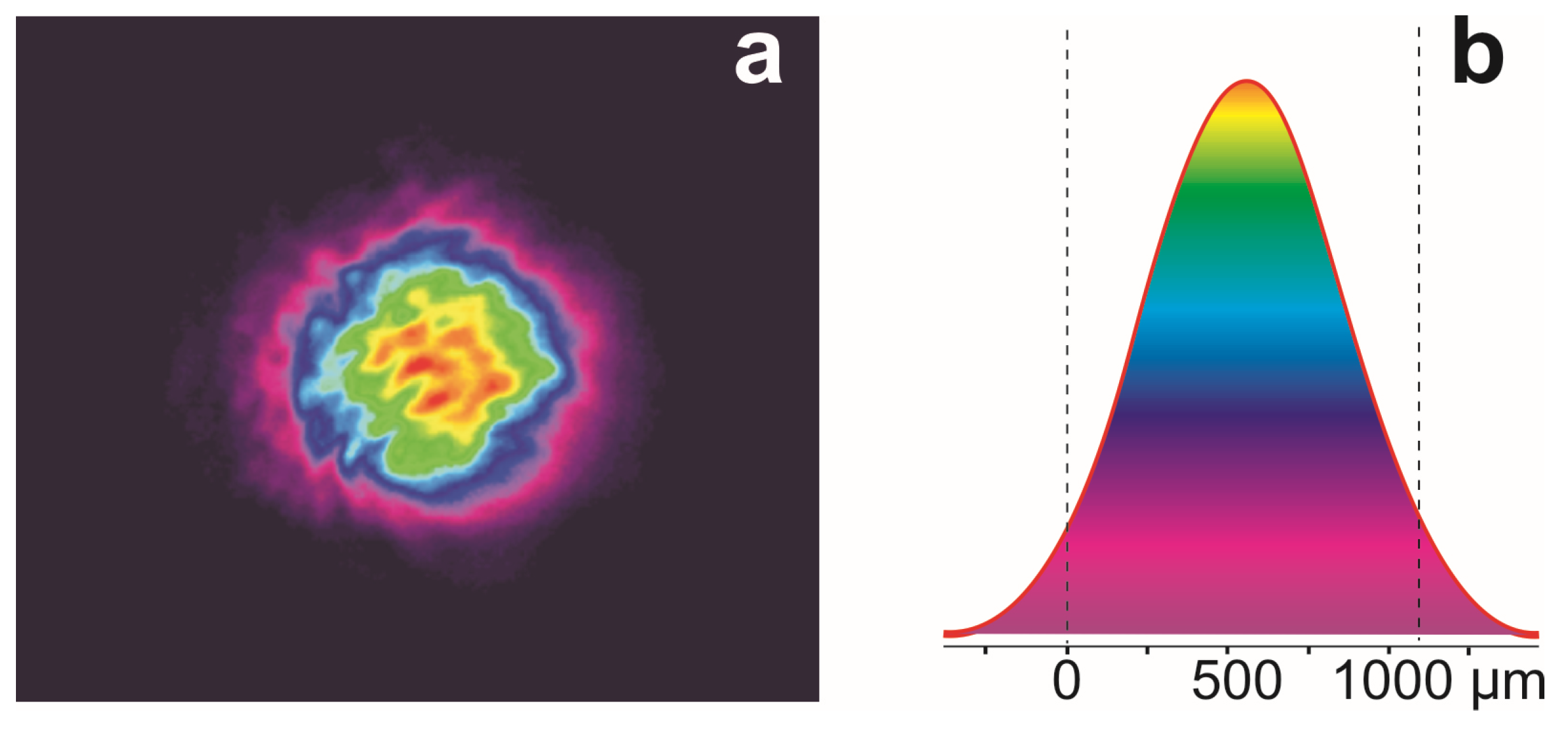
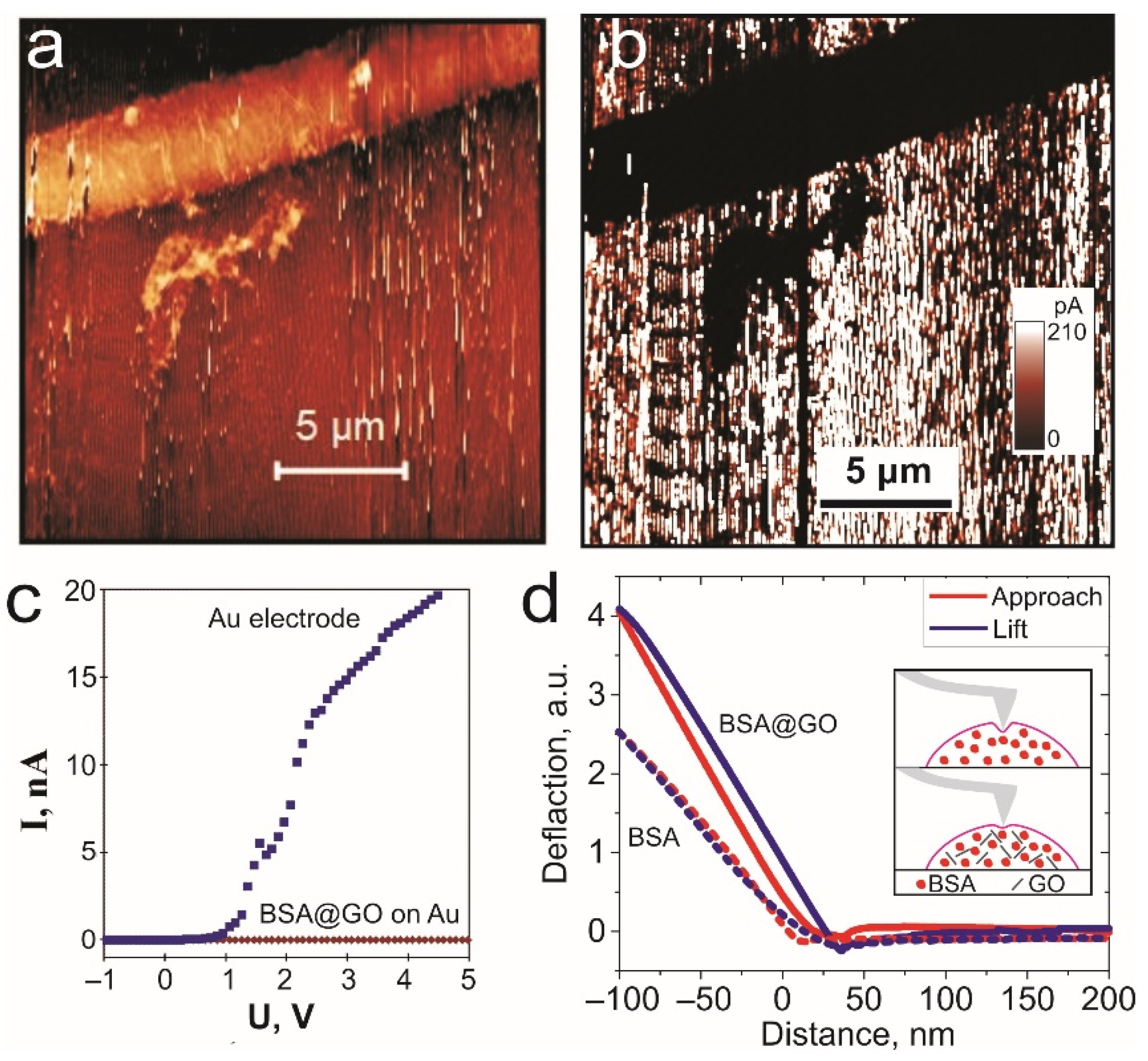
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Otuka, A.J.G.; Tomazio, N.B.; Paula, K.T.; Mendonça, C.R. Two-Photon Polymerization: Functionalized Microstructures, Micro-Resonators, and Bio-Scaffolds. Polymers 2021, 13, 1994. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, H.; Suhail, M.; Ren, H. Hydrogel Actuators and Sensors for Biomedical Soft Robots: Brief Overview with Impending Challenges. Biomimetics 2018, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Hu, Y.; Tang, P.; Wang, H.; Bin, Y. High stretchable, pH-sensitive and self-adhesive rGO/CMCNa/PAA composite conductive hydrogel with good strain-sensing performance. Compos. Commun. 2021, 24, 100669. [Google Scholar] [CrossRef]
- Cui, Z.; Zhou, M.; Greensmith, P.J.; Wang, W.; Hoyland, J.A.; Kinloch, I.A.; Freemont, T.; Saunders, B.R. A study of conductive hydrogel composites of pH-responsive microgels and carbon nanotubes. Soft Matter. 2016, 12, 4142–4153. [Google Scholar] [CrossRef]
- Bobrinetskii, I.I.; Morozov, R.A.; Podgaetskii, V.M.; Simunin, M.M.; Yaminskii, I.V. A study of bulky nanotube composites based on albumin by high-resolution microscopy. Biophysics 2011, 56, 194–199. [Google Scholar] [CrossRef]
- Simhon, D.; Gabay, I.; Shpolyansky, G.; Vasilyev, T.; Nur, I.; Meidler, R.; Hatoum, O.A.; Katzir, A.; Hashmonai, M.; Kopelman, D. Temperature-controlled laser-soldering system and its clinical application for bonding skin incisions. J. Biomed. Opt. 2015, 20, 128002. [Google Scholar] [CrossRef]
- Zergioti, I.; Karaiskou, A.; Papazoglou, D.G.; Fotakis, C.; Kapsetaki, M.; Kafetzopoulos, D. Femtosecond laser microprinting of biomaterials. Appl. Phys. Lett. 2005, 86, 163902. [Google Scholar] [CrossRef]
- Gerasimenko, A.Y.; Ten, G.N.; Ryabkin, D.I.; Shcherbakova, N.E.; Morozova, E.A.; Ichkitidze, L.P. The study of the interaction mechanism between bovine serum albumin and single-walled carbon nanotubes depending on their diameter and concentration in solid nanocomposites by vibrational spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 227, 117682. [Google Scholar] [CrossRef]
- Wang, X.; Wei, Z.; Baysah, C.Z.; Zheng, M.; Xing, J. Biomaterial-based microstructures fabricated by two-photon polymerization microfabrication technology. RSC Adv. 2019, 9, 34472–34480. [Google Scholar] [CrossRef]
- Ma, Z.-C.; Zhang, Y.-L.; Han, B.; Hu, X.-Y.; Li, C.-H.; Chen, Q.-D.; Sun, H.-B. Femtosecond laser programmed artificial musculoskeletal systems. Nat. Commun. 2020, 11, 4536. [Google Scholar] [CrossRef]
- Fouassier, J.-P.; Morlet-Savary, F.; Lalevée, J.; Allonas, X.; Ley, C. Dyes as Photoinitiators or Photosensitizers of Polymerization Reactions. Materials 2010, 3, 5130–5142. [Google Scholar] [CrossRef]
- Kaehr, B.; Shear, J.B. Multiphoton fabrication of chemically responsive protein hydrogels for microactuation. Proc. Natl. Acad. Sci. USA 2008, 105, 8850–8854. [Google Scholar] [CrossRef]
- Lay, C.L.; Lee, Y.H.; Lee, M.R.; Phang, I.Y.; Ling, X.Y. Formulating an Ideal Protein Photoresist for Fabricating Dynamic Microstructures with High Aspect Ratios and Uniform Responsiveness. ACS Appl. Mater. Interfaces 2016, 8, 8145–8153. [Google Scholar] [CrossRef]
- Markov, A.; Wördenweber, R.; Ichkitidze, L.; Gerasimenko, A.; Kurilova, U.; Suetina, I.; Mezentseva, M.; Offenhäusser, A.; Telyshev, D. Biocompatible SWCNT Conductive Composites for Biomedical Applications. Nanomaterials 2020, 10, 2492. [Google Scholar] [CrossRef]
- Bobrinetskiy, I.I.; Seleznev, A.S.; Morozov, R.A.; Lopatina, O.A.; Podchernyaeva, R.Y.; Suetina, I.A. Investigation of the Effect of Local Electrical Stimulation on Cells Cultured on Conductive Single-Walled Carbon Nanotube/Albumin Films. J. Biomater. Nanobiotechnol. 2012, 3, 377–384. [Google Scholar] [CrossRef]
- Ding, Z.; Ma, H.; Chen, Y. Interaction of graphene oxide with human serum albumin and its mechanism. RSC Adv. 2014, 4, 55290–55295. [Google Scholar] [CrossRef]
- Nan, Z.; Hao, C.; Ye, X.; Feng, Y.; Sun, R. Interaction of graphene oxide with bovine serum albumin: A fluorescence quenching study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 210, 348–354. [Google Scholar] [CrossRef]
- Jokar, S.; Pourjavadi, A.; Adeli, M. Albumin–graphene oxide conjugates; carriers for anticancer drugs. RSC Adv. 2014, 4, 33001. [Google Scholar] [CrossRef]
- Joshi, S.; Singh, H.; Sharma, S.; Barman, P.; Saini, A.; Verma, G. Synthesis and characterization of graphene oxide-bovine serum albumin conjugate membrane for adsorptive removal of Cobalt(II) from water. Int. J. Environ. Sci. Technol. 2021, 18, 3915–3928. [Google Scholar] [CrossRef]
- Liu, X.; Yan, C.; Chen, K.L. Adsorption of Human Serum Albumin on Graphene Oxide: Implications for Protein Corona Formation and Conformation. Environ. Sci. Technol. 2019, 53, 8631–8639. [Google Scholar] [CrossRef]
- Biria, S.; Hosein, I.D. Control of Morphology in Polymer Blends through Light Self-Trapping: An in Situ Study of Structure Evolution, Reaction Kinetics, and Phase Separation. Macromolecules 2017, 50, 3617–3626. [Google Scholar] [CrossRef]
- Jacobsen, A.J.; Barvosa-Carter, W.; Nutt, S. Micro-scale Truss Structures formed from Self-Propagating Photopolymer Waveguides. Adv. Mater. 2007, 19, 3892–3896. [Google Scholar] [CrossRef]
- Lykina, A.; Artemyev, D.; Bratchenko, I. Analysis of albumin Raman scattering registration efficiency from different volume and shape cuvette. J. Biomed. Photonics Eng. 2017, 3, 020309. [Google Scholar] [CrossRef][Green Version]
- Murayama, K.; Tomida, M. Heat-Induced Secondary Structure and Conformation Change of Bovine Serum Albumin Investigated by Fourier Transform Infrared Spectroscopy. Biochemistry 2004, 43, 11526–11532. [Google Scholar] [CrossRef]
- Yu, H.; Ding, H.; Zhang, Q.; Gu, Z.; Gu, M. Three-Dimensional Direct Laser Writing of PEGda Hydrogel Microstructures with Low Threshold Power using a Green Laser Beam. Light Adv. Manuf. 2021, 2, 1–8. [Google Scholar] [CrossRef]
- Biscar, J.P.; Dhall, P.; Pennison, J. Raman behavior of bovine serum albumin. Chem. Phys. Lett. 1972, 14, 569–572. [Google Scholar] [CrossRef]
- Taneva, S.G.; Krumova, S.; Bogár, F.; Kincses, A.; Stoichev, S.; Todinova, S.; Danailova, A.; Horváth, J.; Násztor, Z.; Kelemen, L.; et al. Insights into graphene oxide interaction with human serum albumin in isolated state and in blood plasma. Int. J. Biol. Macromol. 2021, 175, 19–29. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q.; Chen, X.; Gu, M. Giant refractive-index modulation by two-photon reduction of fluorescent graphene oxides for multimode optical recording. Sci. Rep. 2013, 3, 2819. [Google Scholar] [CrossRef]
- Bobrinetskiy, I.I.; Emelianov, A.V.; Smagulova, S.A.; Komarov, I.A.; Otero, N.; Romero, P.M. Laser direct 3D patterning and reduction of graphene oxide film on polymer substrate. Mater. Lett. 2017, 187, 20–23. [Google Scholar] [CrossRef]
- Zhou, J.; Cai, Q.; Xu, F. Nanoscale Mechanical Properties and Indentation Recovery of PI@GO Composites Measured Using AFM. Polymers 2018, 10, 1020. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]

| Mechanical Parameters | BSA | BSA@GO |
|---|---|---|
| Increase of hardness | 1 | 1.6 |
| Indentation depth | 35 nm | 20 nm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nekrasov, N.; Yakunina, N.; Nevolin, V.; Bobrinetskiy, I.; Vasilevsky, P.; Gerasimenko, A.Y. Two-Photon Polymerization of Albumin Hydrogel Nanowires Strengthened with Graphene Oxide. Biomimetics 2021, 6, 66. https://doi.org/10.3390/biomimetics6040066
Nekrasov N, Yakunina N, Nevolin V, Bobrinetskiy I, Vasilevsky P, Gerasimenko AY. Two-Photon Polymerization of Albumin Hydrogel Nanowires Strengthened with Graphene Oxide. Biomimetics. 2021; 6(4):66. https://doi.org/10.3390/biomimetics6040066
Chicago/Turabian StyleNekrasov, Nikita, Natalya Yakunina, Vladimir Nevolin, Ivan Bobrinetskiy, Pavel Vasilevsky, and Alexander Yu. Gerasimenko. 2021. "Two-Photon Polymerization of Albumin Hydrogel Nanowires Strengthened with Graphene Oxide" Biomimetics 6, no. 4: 66. https://doi.org/10.3390/biomimetics6040066
APA StyleNekrasov, N., Yakunina, N., Nevolin, V., Bobrinetskiy, I., Vasilevsky, P., & Gerasimenko, A. Y. (2021). Two-Photon Polymerization of Albumin Hydrogel Nanowires Strengthened with Graphene Oxide. Biomimetics, 6(4), 66. https://doi.org/10.3390/biomimetics6040066







