A Review on Development of Bio-Inspired Implants Using 3D Printing
Abstract
:1. Introduction
- To replace or repair undermined organs with suitable implanted biomedical constructs.
- To build tissue/organ models for biological analysis and assessment, such as drug screening, toxicity analysis, cell-material interaction.
2. Ceramic Based AM with Added Functionalities and Application
2.1. Ceramic AM Using Biomimetic Designs
2.2. Recent Approaches Using Ceramic Biomimetic AM
2.3. Ceramic Processing for Biomimetic AM
2.4. Ceramic Modifiers for Biomimetic AM
3. Polymer-Based AM with Added Functionalities and Application
| Polymers | Biomimetic Functionality | Biomedical Application | AM Processing Technique | Advantages |
|---|---|---|---|---|
| Natural polymers (proteins and polysaccharides) | ||||
| Collagen [95] | Biomimicking native tissue | Skin replacement, hydrogel, bioink | Extrusion, Fusion | Mechanical stiffness, viscosity, biodegradability |
| Gelatin [96] | Biomimicking native tissue | Bioink, hydrogel | 3D Bioprinting, SLA, extrusion, inkjet printing, 4D printing, Laser printing | Biocompatible, biodegradable, flexible |
| Chitosan-HAp (hydroxyapatite) [97] | Biomimicking native tissue | Hydrogel, scaffolds, bone tissue engineering | 3D printing | Biocompatible, cell viability, cell-friendly environment, adequate mechanical properties |
| Hyaluronic Acid [98] | Biomimicking native tissue | Hydrogel in cartilage regeneration | 3D printing | Superabsorbent, cytocompatible |
| Alginate [99] | Biomimicking native tissue | Bioink, hydrogel | Bioprinting, extrusion printing | Cell-protective effect, cell viability |
| Silk Fibroin [99] | Biomimicking native tissue | Scaffold, bioink | Bioprinting, extrusion printing | Superior mechanical properties and tunable degradability |
| Fibrin ink | Biomimicking native tissue | Vascular constructs | Inkjet printing | [100] |
| Synthetic polymers | ||||
| Polycaprolactone (PCL) [101] | Biomimicking native tissue, multi-functionality | Tissue engineering wound dressing | FDM | Low melting point, Biocompatible |
| Polyurethane (PU) [91] | Biomimicking native tissue | Tissue engineering, prosthetic devices | Binder Jetting, FDM | Elasticity |
| Polyether ether ketone (PEEK) [102] | Biomimicking native tissue | Dental, orthopedic, trauma, and spinal implants | Laser printing, extrusion | Superior mechanical properties, inert, biocompatible |
| Polyethylene glycol [103] | Biomimicking native tissue | Porous scaffold, Implants, Drug delivery | Extrusion | Biocompatible |
| Polylactic acid (PLA) [104] | Biomimicking native tissue | Scaffolds, prosthetic devices | Extrusion based bioprinting | Mechanical strength |
| Acrylonitrile butadiene styrene (ABS) [105,106] | Functional models | Prototypes, cost-effective prosthetic devices | Extrusion | Low cost |
| Polymer composites | ||||
| Polyethylene glycol (PEG) derivatives mixed with fibroblasts [107] | Hollow tubular structures | Vascular constructs | Extrusion | Biocompatible |
| Polyglycolic acid/polylactic acid (PLA/PGA) scaffolds [108] | Native stiff bone-like constructs | Cartilage–Bone | 3D Printing | Biocompatible, stiffness |
| polyethylene glycol (PEG)/β-tricalcium phosphate (β-TCP) scaffold [109] | bio-inspired interface structures | Cartilage–Bone | Stereolithography | Biocompatible |
| PLA/HA screw-like scaffold [110] | Native bone | Bone | 3D Printing | Bio-active, mechanical properties |
| Chitosan-based polymers (N-succinyl chitosan grafted polyacrylamide [111] | Shape memory function (pH) | Drug delivery; Bone regenerative therapies | 4D Printing | Biocompatible, Better controlled release of drugs; Tunable mechanical properties |
| Gelatin-polycaprolactone (PCL) [112] | tubular structures | Bilayers, Cell-laden bioscaffolds for tissue engineering | 4D Printing | Compatible; Biodegradable |
| Poly-ethylene glycol (PEG) [113] | Shape memory function (Humidity) | Cell-laden bilayers | 4D Printing | Biocompatibility |
4. Metal-Based AM with Added Functionalities and Application
4.1. Importance of AM in Construction of Biomimicking Implants
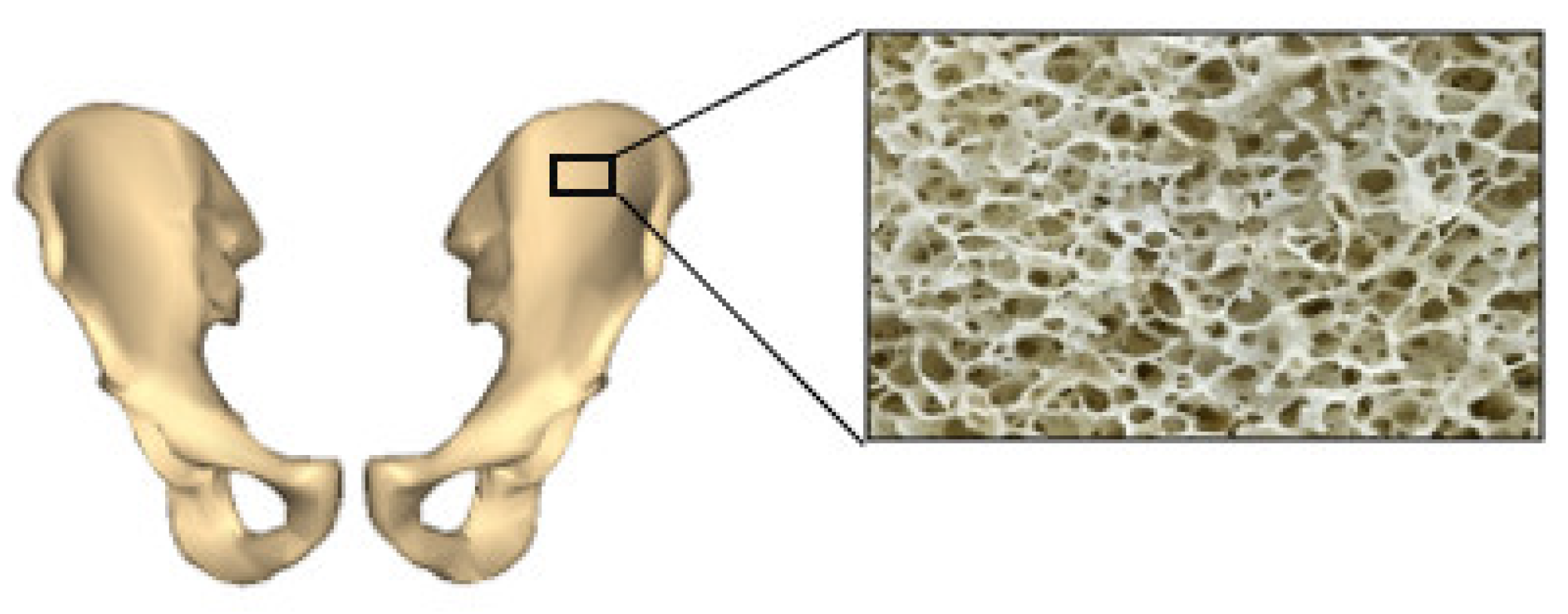
4.1.1. Mimicking Mechanical Properties of Natural Bone
4.1.2. Surgical Planning
4.2. Conventional Metal Processing and Its Biomimetics
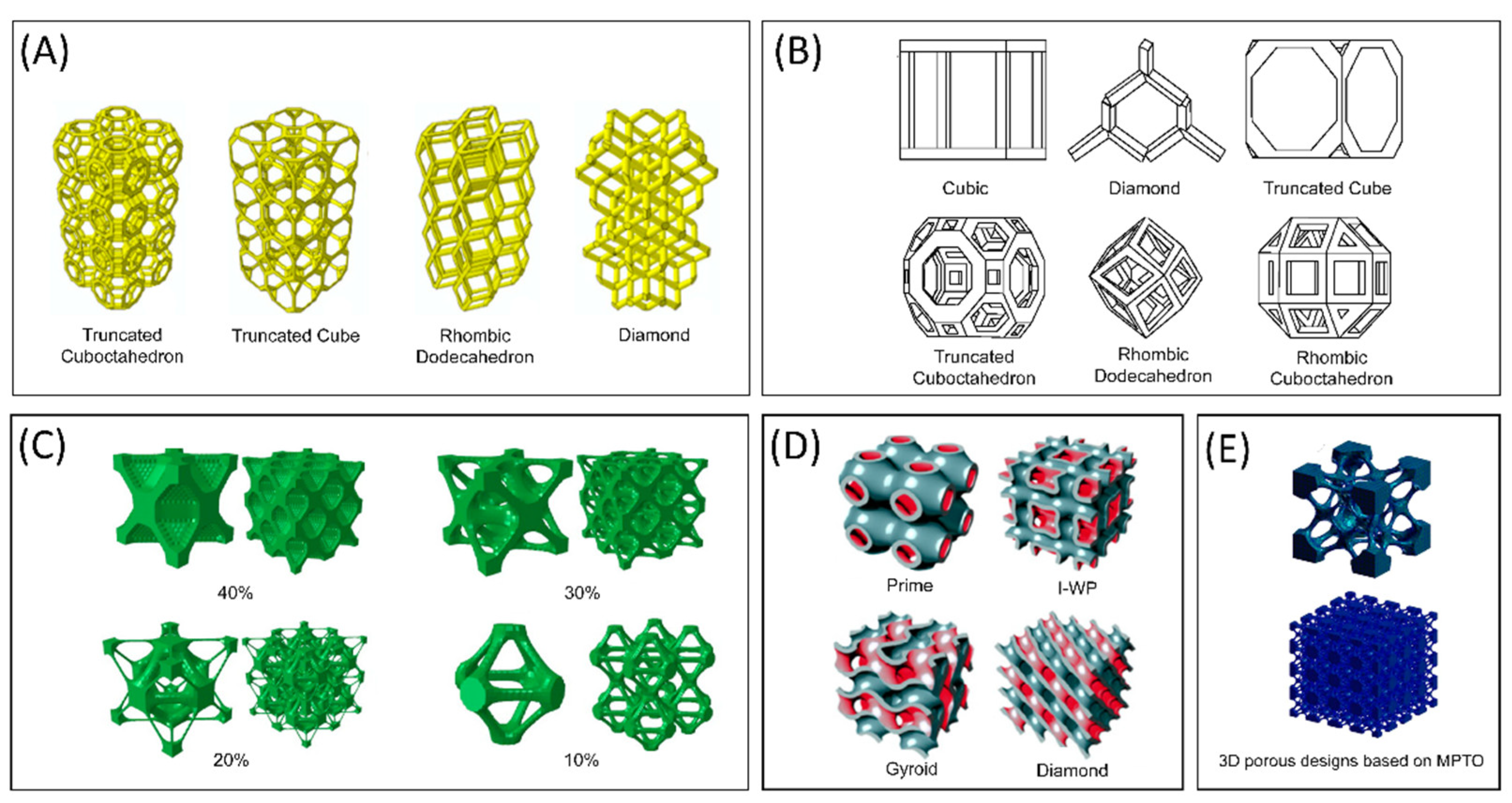
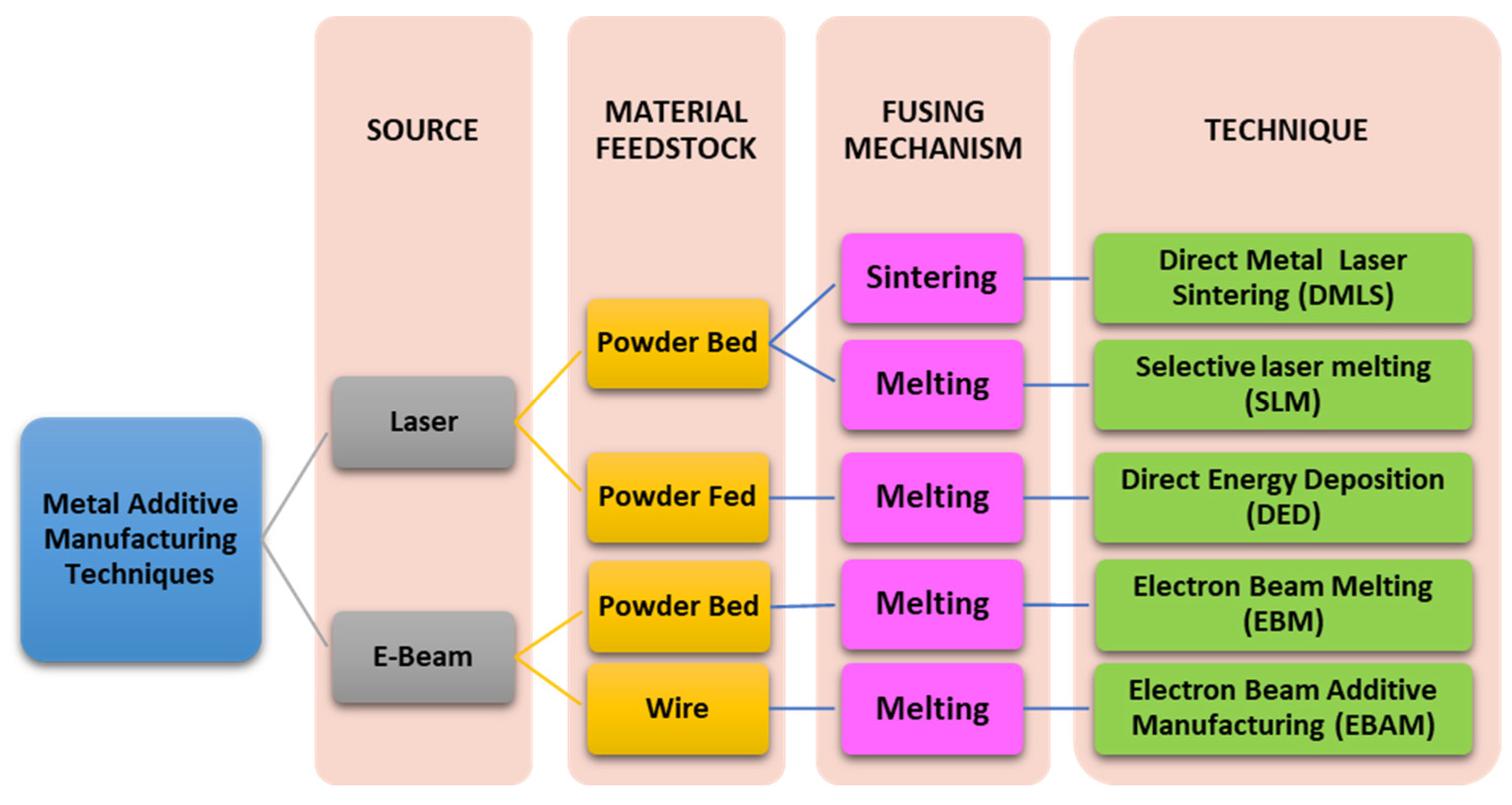
4.3. Techniques Used for Metal Powder Bed Fusion
| Technique | Description |
|---|---|
| EBAM | Among the other metal AM techniques, EBAM can be regarded as a faster and more cost-effective process, mainly due to its wire-feed system. The wire-feed system eliminates the wastage of powder and works faster by eliminating the powder’s recoating time. EBAM with a dual wire-feed nozzle has the added advantage of mixing two different metal alloys during the fabrication of a part, alternating between two different types of metals, and changing their ratio. At the same time, printing can be easily achieved in EBAM. This feature is not achievable in metal powder-bed and powder-fed fusion techniques due to the possibility of contaminating the part with unwanted metal powder. |
| LENS/DED | LENS systems use directed energy deposition (DED), where high-powered lasers build structures layer by layer directly from powdered metals, alloys, ceramics, or composites to produce fully-dense parts. DED has a coaxial laser and powder emission orifice, which often has inert gas, such as argon or nitrogen blown to sheath the melting region to prevent metal oxidation. This results in a high-speed, high-quality, affordable metal 3D printing process making complex metal parts easier, more precise with excellent mechanical and fatigue properties, and efficient and affordable to produce and repair. |
| DMLS | The wastage of powder in DMLS is fairly less than methods that involve widespread powder recoating using wipers. A benefit of using DMLS fabricated parts is that objects produced through DMLS do not possess any residual stresses or internal defects. However, the downside of such a high-end technique is its costly production and maintenance cost. |
| EBM | EBM is very similar to selective laser melting and produces dense and porous parts. The difference between the two techniques is that EBM uses an electron beam rather than a laser to melt the metal powder. Due to the use of an electron beam, the process takes place in a vacuum rather than an inert atmosphere, as in SLM. Another difference between SLM and EBM is that the powder bed in EBM can be pre-heated to up to 700 °C by the defocused electron beam. This plummets the temperature gradient by reducing the rapid heating and cooling. |
| SLM | SLM involves a high-powered laser that fully melts the metal powder particles and welds them together by melting, giving rise to more robust and denser objects than metal sintering techniques. The powder is heated to a temperature above the metal’s melting point for binding metal particles in a molten state. This rapid heating and cooling process gives rise to a broader temperature gradient, resulting in stress and dislocation in the final product, compromising the product’s mechanical properties. As powder flowability plays a vital role in SLM, only optimized metals are currently being fabricated, such as stainless steel, titanium alloys, chromium cobalt, and aluminum [46,147,148,149]. |
4.4. Post-Processing of AM Products
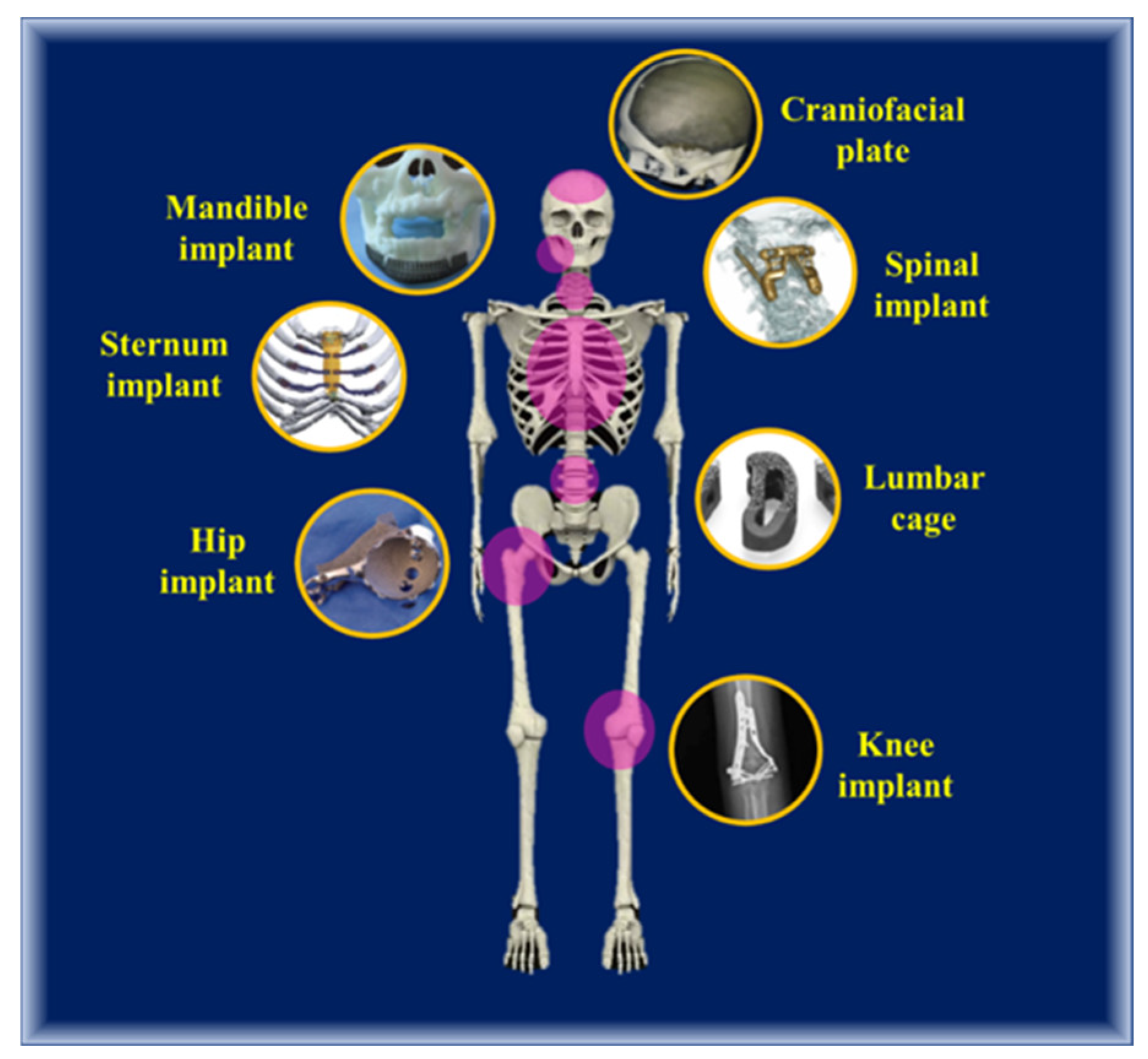
4.5. Various AM Biomimicking Metal Implants-with Clinical Case Studies
4.5.1. Cranioplasty Implants
4.5.2. Vascular Stent
4.5.3. Bone Fixtures
4.5.4. Hip and Knee Implant
4.5.5. Sternum Implant
4.5.6. Spinal Implant
4.5.7. Mandibular Implant
4.5.8. Dental Screws and Prostheses
4.5.9. Bone Scaffold
| S.No | Type of Implant | Purpose of Implant | Process | Material | Details of Case Study | Parameters/Specifications | Reference |
|---|---|---|---|---|---|---|---|
| 1. | CRANIOFACIAL | To protect the brain and alleviate psychological affliction caused by the bone defect and restore the patient’s appearance and psychological stability. | EBM | Ti6Al4V ELI | A 38-year-old patient was referred to a craniofacial surgeon with a large cranial defect in the left parieto-temporal area. Cranial reconstruction surgery was performed. | Powder size—50–100 μm, Implant thickness—1.25 mm, Pore size diameter—700 μm Strut size—800 μm | [218] |
| DMLS | Ti6Al4V ELI | A 22-year-old male patient had a large post-trauma defect in the right frontal bone. Reconstruction of the cranial defect was required to restore the structural integrity of the skull and the patient’s facial aesthetics. | Cranial replacement area—12.5 × 8.4 cm2 | [219] | |||
| DMLS | Ti6Al4V ELI | A 28-year-old male patient had a large post-trauma defect in the right frontal bone. Reconstruction of the cranial defect was required to restore the structural integrity of the skull and the patient’s facial aesthetics. | Bone defect area—13.5 × 9.4 cm2, Total weight of prosthesis—82 g, Thickness—2–3 mm | [220] | |||
| EBM | Ti6Al4V ELI | A 7-year-old girl had a huge frontonasal bone defect with consequent hypertelorism. Reconstruction of the cranial defect was required to restore the structural integrity of the skull and the patient’s facial aesthetics. | Powder size—45–100 μm, Implant weight—12.20 g, Implant thickness—7 mm, Pore size diameter—2mm | [221] | |||
| EBM | Ti6Al4V | Three female patients (tumor—one patient, trauma—two patients) were chosen for the study. Reconstruction of the cranial defect was required to restore the structural integrity of the skull and the patient’s facial aesthetics. | Patient 1 defect size—12 × 14 cm2, Patient 2 defect size—14 × 11 cm2, Patient 3 defect size—15 × 15 cm2 | [222] | |||
| EBM | Ti6Al4V | A 27-year-old woman with a wide cranial vault lacuna in the upper part of the skull and slightly crossing the sagittal plane underwent reconstruction surgery to restore the shape and function of the cranium. | - | [173] | |||
| 2. | MAXILLOFACIAL | To achieve correct shape of orbital wall or jaw and reconstruction followed by resection of the tumor region. | DMLS | Ti6Al4V ELI | The patient was a 67-year-old male who had been in a severe accident. Reconstructive treatment was performed to achieve anatomically correct shape of the orbital wall and appearance of the eye symmetry. | Thickness—0.4 mm, Hole size—3 mm, Hole size (screw)—2 mm | [223] |
| EBM | Ti6Al4V ELI | Tumor treatment—The mandible section with the tumor on the patient’s left side was removed and replaced by mirroring the healthy right mandible. | Powder size—50–100 μm, Offset thickness—2 mm, Mesh size—0.4 mm, | [224] | |||
| EBM | Ti6Al4V ELI | A 40-year-old patient underwent a multilocular radiolucent lesion on the right posterior mandible. Reconstruction of the discontinuous mandible defect was performed. | - | [225] | |||
| SLM | Ti6Al4V-Grade 2 | The 50-year-old patient presented maxillary epidermoid carcinoma history with nasal affection addressed two years ago by a total maxillectomy and total nasal amputation. Nasal reconstruction was performed. | Thickness—0.4–0.7 mm, Pore dimension—860–1500 μm | [226] | |||
| SLM | cT4N1M0 | A 53-year-old male suffered osteoradionecrosis due to the radiation therapy after squamous cell carcinoma resection of attached gingiva in the left mandible. The reconstruction plate was fixed. | - | [205] | |||
| 3. | DENTAL IMPLANT | To restore the function of the tooth or jaw affected due to tumors or accidents. | SLS | Ti6Al4V | 16 patients with possible dental repair were voluntarily recruited for the clinical study. | Powder size—25–45 μm, Diameter—2.7 mm, Length—10 mm | [227] |
| DLMS | Ti6Al4V | 15 patients, 8 males and 7 females (age 39–55), were selected for the study based on the possibility of a dental repair. | Powder size—25–45 μm, Cylindrical implant: alveolar apex—3–5 mm | [228] | |||
| DMLS | Ti6Al4V | A 17-year-old male patient who sustained an injury to the anterior maxillary region leading to loss of upper front teeth along with bone was presented in this case study. | - | [229] | |||
| DMLS | Ti6Al4V | 44 males, 38 females, age range 26–67 years were voluntarily recruited for the study. | Laser wavelength—1054 nm, Laser power—200 W, Scanning rate—7 m/s, Laser spot size—0.1 mm, Powder size—25–45 μm | [211] | |||
| DMLS | Ti6Al4V | 39 males and 31 females, aged 62–79 years with dental repair were voluntarily enrolled for the study. | Laser wavelength—1070 nm, Laser power—50/W | [230] | |||
| 4. | SPINAL IMPLANT | Degenerative diseases, fractures, and other disorders can lead to the functional loss of the spine. Spinal fixation or spinal reconstruction can retain the function of the spine after the resection of the affected area | DMLS | Ti6Al4V Grade 5 | A 45-year-old man presented with neck and left arm pain combined with shoulder weakness. Imaging revealed significant destruction of the C3-C5 vertebrae, and chordoma diagnosis was confirmed by biopsy. | Laser power—200 W, Laser spot diameter—55 μm, Layer thickness—20–40 μm | [231] |
| DMLS | Ti6Al4V ELI | A 16-year-old boy had a severe kyphotic deformity of the thoracic spine resulting from neurofibromatosis type I. | Pore size—500–600 μm | [232] | |||
| A 63-year-old woman with progressive paralysis due to a severe cervicothoracic dissociation. | Implant width—10 mm, Depth—5 mm, Height—8 mm | ||||||
| EBM | Ti6Al4V | 9 patients (2 males and 7 females) were included in the study with a mean age of 31.4 years (12 to 59 years) for reconstruction following resection of the primary tumors of the upper cervical spine. | Powder size—45–100 μm | [233] | |||
| FDM | Ti6Al4V | A 12-year-old patient suffering from congenital scoliosis due to an L1 hemivertebra underwent a corpectomy and stabilization surgery from Th9 to L4. | - | [234] | |||
| 5. | FOOT/HAND IMPLANT | Foot or hand implants are used for reconstructing the defective/fracture/tumor-affected bone. | EBM | Ti6Al4V | A 40-year-old man presented with two-week-long paresthesia in his right hand and limited forearm rotation due to dislocation of the radial head attributed to a traumatic injury during childhood. | Length of the implant—15 cm, Weight—67 g, Pore size—700 and 1500 µm | [235] |
| EBM | Ti6Al4V ELI | A 23-year-old soldier was diagnosed with a calcaneal desmoplastic fibroma. Reconstruction surgery was performed for the bone tumor calcaneus. | Length of the implant—63.5 mm, Height—43.2 mm, Weight—104 g | [236] | |||
| EBM | Ti6Al4V | A 71-year-old man presented with a destructive and highly metabolic lesion in the right calcaneus. A total calcanectomy was performed, and the defect was reconstructed with 3D printed titanium calcaneal prosthesis. | Implant weight—280 g | [159] | |||
| EBM | Ti6Al4V ELI | 3 patients (one male and two females) had undergone surgery for oncological diagnosis, and reconstruction surgery was performed. | - | [237] | |||
| 6. | PELVIC IMPLANT | Pelvic implants provide support or replace the weaker bones due to arthritis, tumor, or fracture. | SLM | Ti6Al4V | A 65-year-old man presented with expansile osteolytic destruction at the anterior column of the left acetabulum. Pelvic tumor resection and prosthetic reconstruction of the bone defect were planned in the study. | - | [166] |
| EBM | Ti6Al4V ELI | 7 patients (3 males and 4 females) were chosen for the study based on existing pelvic/hip morbidity. Pelvic reconstruction was performed. | - | [237] | |||
| EBM | Ti6Al4V | 13 patients were chosen for the study, of which 3 patients had total hip replacement surgery, and 4 patients had pelvic resection surgery. | - | [238] | |||
| EBM | Ti6Al4V | A total of 35 patients (20 males and 15 females) underwent resection of pelvic tumor and reconstruction using 3D printed endoprostheses. | - | [239] | |||
| EBM | Ti6Al4V | 30 patients were involved in the study for trabecular bone reconstruction for early osteonecrosis of the femoral head. | Power of E-beam—3000 W, Diameter of electron beam—180 µm, Melting speed—55 to 80 cm3/h, Degree of vacuum work area <1 × 10−4 mbar | [185] | |||
| 7. | STERNUM IMPLANT | Sternum implants protect the heart, lungs, and chest blood vessels in people with a compromised sternum. The tumor-affected sternum can also be reconstructed using sternum implants. | EBM | Ti6Al4V | A 57-year-old man suffered from minor thoracic trauma because of prolonged chest pain and chest wall tumor in the chondrocostal junction. A segment of the sternum was replaced to restore the function. | Implant size—147.36 × 180.14 × 128.30 mm3 | [194] |
| DMLS | Ti6Al4V | A 70-year-old woman was affected by the sternal tumor and subtotal sternotomy. Resection of the sternal body with the adjacent sternocostal cartilage was performed. | Weight of the implant—53.5 g, Size of the implant—170 × 60 × 105 mm | [164] | |||
| EBM | Ti6Al4V | A 19-year-old woman presented with anterior chest wall instability and paradoxical movement with respiration. Reconstruction after anterior chest wall resection was performed. | - | [240] | |||
| SLM | Ti MG 1 | A 70-year-old male, with a right anterior pectoral mass approximately 10 by 9 cm was presented in the study for chest wall resection following wide local excision for bone tumor. | - | [241] | |||
| SLM | Ti6Al4V ELI | A 62-year-old female was presented with a mass located on the chest wall associated with foul smelly drainage. Reconstruction after chest wall resection was performed. | The thickness of the implant—2–3 mm, Weight—160 g | [242] |
5. Future Scope and Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aziz, M.S.; El Sherif, A.Y. Biomimicry as an approach for bio-inspired structure with the aid of computation. Alex. Eng. J. 2016, 55, 707–714. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.; Jeong, Y.; Park, J.M.; Lee, K.H.; Hong, J.W.; Choi, J. Biomimetics: Forecasting the future of science, engineering, and medicine. Int. J. Nanomed. 2015, 10, 5701–5713. [Google Scholar] [CrossRef] [Green Version]
- Peng, T.; Kellens, K.; Tang, R.; Chen, C.; Chen, G. Sustainability of additive manufacturing: An overview on its energy demand and environmental impact. Addit. Manuf. 2018, 21, 694–704. [Google Scholar] [CrossRef]
- Zhakeyev, A.; Wang, P.; Zhang, L.; Shu, W.; Wang, H.; Xuan, J. Additive Manufacturing: Unlocking the Evolution of Energy Materials. Adv. Sci. 2017, 4, 1700187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, R.B.; Mcadams, D.A. Translating terms of the functional basis into biologically meaningful keywords. In Proceedings of the ASME 2008 International Design Engineering Technical Conference & Computers and Information in Engineering Conference, New York, NY, USA, 3–6 August 2008; pp. 1–12. [Google Scholar]
- Li, Y.-C.E. Sustainable Biomass Materials for Biomedical Applications. ACS Biomater. Sci. Eng. 2019, 5, 2079–2092. [Google Scholar] [CrossRef]
- Ruban, W.; Vijayakumar, V.; Dhanabal, P.; Pridhar, T. Effective process parameters in selective laser sintering. Int. J. Rapid Manuf. 2014, 4, 148. [Google Scholar] [CrossRef]
- Prashanth, K.G.; Löber, L.; Klauss, H.-J.; Kühn, U.; Eckert, J. Characterization of 316L Steel Cellular Dodecahedron Structures Produced by Selective Laser Melting. Technologies 2016, 4, 34. [Google Scholar] [CrossRef] [Green Version]
- Olakanmi, E.O.; Cochrane, R.; Dalgarno, K. A review on selective laser sintering/melting (SLS/SLM) of aluminium alloy powders: Processing, microstructure, and properties. Prog. Mater. Sci. 2015, 74, 401–477. [Google Scholar] [CrossRef]
- Srinivasan, R.; Ruban, W.; Deepanraj, A.; Bhuvanesh, R.; Bhuvanesh, T. Effect on infill density on mechanical properties of PETG part fabricated by fused deposition modelling. Mater. Today Proc. 2020, 27, 1838–1842. [Google Scholar] [CrossRef]
- Prashanth, K.G.; Scudino, S.; Chaubey, A.K.; Löber, L.; Wang, P.; Attar, H.; Schimansky, F.P.; Pyczak, F.; Eckert, J. Processing of Al–12Si–TNM composites by selective laser melting and evaluation of compressive and wear properties. J. Mater. Res. 2016, 31, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Prashanth, K.; Scudino, S.; Eckert, J. Defining the tensile properties of Al-12Si parts produced by selective laser melting. Acta Mater. 2017, 126, 25–35. [Google Scholar] [CrossRef]
- Prashanth, K.; Scudino, S.; Klauss, H.; Surreddi, K.; Löber, L.; Wang, Z.; Chaubey, A.; Kühn, U.; Eckert, J. Microstructure and mechanical properties of Al–12Si produced by selective laser melting: Effect of heat treatment. Mater. Sci. Eng. A 2014, 590, 153–160. [Google Scholar] [CrossRef]
- Kellens, K.; Baumers, M.; Gutowski, T.G.; Flanagan, W.; Lifset, R.; Duflou, J. Environmental Dimensions of Additive Manufacturing: Mapping Application Domains and Their Environmental Implications. J. Ind. Ecol. 2017, 21, S49–S68. [Google Scholar] [CrossRef] [Green Version]
- Gokuldoss, P.K.; Kolla, S.; Eckert, J. Additive Manufacturing Processes: Selective Laser Melting, Electron Beam Melting and Binder Jetting—Selection Guidelines. Materials 2017, 10, 672. [Google Scholar] [CrossRef] [Green Version]
- Scudino, S.; Unterdörfer, C.; Prashanth, K.; Attar, H.; Ellendt, N.; Uhlenwinkel, V.; Eckert, J. Additive manufacturing of Cu–10Sn bronze. Mater. Lett. 2015, 156, 202–204. [Google Scholar] [CrossRef]
- Prashanth, K.; Damodaram, R.; Maity, T.; Wang, P.; Eckert, J. Friction welding of selective laser melted Ti6Al4V parts. Mater. Sci. Eng. A 2017, 704, 66–71. [Google Scholar] [CrossRef]
- Gokuldoss Prashanth, K.; Scudino, S.; Eckert, J. Tensile Properties of Al-12Si Fabricated via Selective Laser Melting (SLM) at Different Temperatures. Technologies 2016, 4, 38. [Google Scholar] [CrossRef] [Green Version]
- Prashanth, K.G.; Scudino, S.; Chatterjee, R.P.; Salman, O.O.; Eckert, J. Additive Manufacturing: Reproducibility of Metallic Parts. Technologies 2017, 5, 8. [Google Scholar] [CrossRef] [Green Version]
- Salman, O.; Brenne, F.; Niendorf, T.; Eckert, J.; Prashanth, K.; He, T.; Scudino, S. Impact of the scanning strategy on the mechanical behavior of 316L steel synthesized by selective laser melting. J. Manuf. Process. 2019, 45, 255–261. [Google Scholar] [CrossRef]
- Xi, L.X.; Zhang, H.; Wang, P.; Li, H.C.; Prashanth, K.G.; Lin, K.J.; Kaban, I.; Gu, D.D. Comparative investigation of microstructure, mechanical properties and strengthening mechanisms of Al-12Si/TiB2 fabricated by selective laser melting and hot pressing. Ceram. Int. 2018, 44, 17635–17642. [Google Scholar] [CrossRef]
- Maity, T.; Chawake, N.; Kim, J.; Eckert, J.; Prashanth, K. Anisotropy in local microstructure—Does it affect the tensile properties of the SLM samples? Manuf. Lett. 2018, 15, 33–37. [Google Scholar] [CrossRef]
- Whenish, R.; Antony, M.; Balaji, T.; Selvam, A. Design and performance of additively manufactured lightweight bionic hand Design and Performance of Additively Manufactured Lightweight Bionic Hand. AIP Conf. Proc. 2021, 2317, 020028. [Google Scholar]
- Wang, Z.; Xie, M.; Li, Y.; Zhang, W.; Yang, C.; Kollo, L.; Eckert, J.; Prashanth, K.G. Premature failure of an additively manufactured material. NPG Asia Mater. 2020, 12, 1–10. [Google Scholar] [CrossRef]
- Babu, S.; Goodridge, R. Additive manufacturing. Mater. Sci. Technol. 2014, 31, 881–883. [Google Scholar] [CrossRef]
- Attar, H.; Prashanth, K.G.; Zhang, L.-C.; Calin, M.; Okulov, I.V.; Scudino, S.; Yang, C.; Eckert, J. Effect of Powder Particle Shape on the Properties of In Situ Ti–TiB Composite Materials Produced by Selective Laser Melting. J. Mater. Sci. Technol. 2015, 31, 1001–1005. [Google Scholar] [CrossRef]
- Attar, H.; Prashanth, K.; Chaubey, A.; Calin, M.; Zhang, L.; Scudino, S.; Eckert, J. Comparison of wear properties of commercially pure titanium prepared by selective laser melting and casting processes. Mater. Lett. 2015, 142, 38–41. [Google Scholar] [CrossRef]
- Additive Manufacturing Market Analysis by Material Type, by Metal Type, by Polymer Type, by Ceramics Type, by Process, by End-Use, and Segment Forecasts to 2027. Research and Markets. 2020. Available online: https://www.researchandmarkets.com/reports/5206602/additive-manufacturing-market-analysis-by (accessed on 3 July 2021).
- Singh, N.; Hameed, P.; Ummethala, R.; Manivasagam, G.; Prashanth, K.; Eckert, J. Selective laser manufacturing of Ti-based alloys and composites: Impact of process parameters, application trends, and future prospects. Mater. Today Adv. 2020, 8, 100097. [Google Scholar] [CrossRef]
- Wang, Z.; Ummethala, R.; Singh, N.; Tang, S.; Suryanarayana, C.; Eckert, J.; Prashanth, K.G. Selective Laser Melting of Aluminum and Its Alloys. Materials 2020, 13, 4564. [Google Scholar] [CrossRef]
- Wang, P.; Eckert, J.; Prashanth, K.-G.; Wu, M.-W.; Kaban, I.; Xi, L.-X.; Scudino, S. A review of particulate-reinforced aluminum matrix composites fabricated by selective laser melting. Trans. Nonferrous Met. Soc. China 2020, 30, 2001–2034. [Google Scholar] [CrossRef]
- Salman, O.O.O.; Gammer, C.; Eckert, J.; Salih, M.Z.Z.; Abdulsalam, E.H.H.; Prashanth, K.G.G.; Scudino, S. Selective laser melting of 316L stainless steel: Influence of TiB2 addition on microstructure and mechanical properties. Mater. Today Commun. 2019, 21, 100615. [Google Scholar] [CrossRef]
- Hameed, P.; Liu, C.-F.; Ummethala, R.; Singh, N.; Huang, H.-H.; Manivasagam, G.; Prashanth, K.G. Biomorphic porous Ti6Al4V gyroid scaffolds for bone implant applications fabricated by selective laser melting. Prog. Addit. Manuf. 2021, 6, 455–469. [Google Scholar] [CrossRef]
- Velasco-Hogan, A.; Xu, J.; Meyers, M.A. Additive Manufacturing as a Method to Design and Optimize Bioinspired Structures. Adv. Mater. 2018, 30, 1800940. [Google Scholar] [CrossRef]
- Selvam, A.; Mayilswamy, S.; Whenish, R. Strength Improvement of Additive Manufacturing Components by Reinforcing Carbon Fiber and by Employing Bioinspired Interlock Sutures. J. Vinyl Addit. Technol. 2020, 26, 511–523. [Google Scholar] [CrossRef]
- Sharma, N.; Ostas, D.; Rotar, H.; Brantner, P.; Thieringer, F.M. Design and Additive Manufacturing of a Biomimetic Customized Cranial Implant Based on Voronoi Diagram. Front. Physiol. 2021, 12, 647923. [Google Scholar] [CrossRef]
- Mahmoud, D.; Elbestawi, M.A. Lattice Structures and Functionally Graded Materials Applications in Additive Manufacturing of Orthopedic Implants: A Review. J. Manuf. Mater. Process. 2017, 1, 13. [Google Scholar] [CrossRef]
- Kladovasilakis, N.; Tsongas, K.; Tzetzis, D. Finite Element Analysis of Orthopedic Hip Implant with Functionally Graded Bioinspired Lattice Structures. Biomimetics 2020, 5, 44. [Google Scholar] [CrossRef] [PubMed]
- Robles-Linares, J.A.; Ramírez-Cedillo, E.; Siller, H.R.; Rodríguez, C.A.; Martínez-López, J.I. Parametric Modeling of Biomimetic Cortical Bone Microstructure for Additive Manufacturing. Materials 2019, 12, 913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maguire, A.; Pottackal, N.; Saadi, M.A.S.R.; Rahman, M.M.; Ajayan, P.M. Additive manufacturing of polymer-based structures by extrusion technologies. Oxf. Open Mater. Sci. 2020, 1, itaa004. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, C.; Goldberg, P.; Cohen, D.; Pan, Y.; Arpin, T.; Bar-Yosef, O. Early Pottery at 20,000 Years Ago in Xianrendong Cave, China. Science 2012, 336, 1696–1700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darsell, J.; Bose, S.; Hosick, H.L.; Bandyopadhyay, A. From CT Scan to Ceramic Bone Graft. J. Am. Ceram. Soc. 2003, 86, 1076–1080. [Google Scholar] [CrossRef]
- Kamboj, N.; Rodríguez, M.A.; Rahmani, R.; Prashanth, K.G.; Hussainova, I. Bioceramic scaffolds by additive manufacturing for controlled delivery of the antibiotic vancomycin. Proc. Est. Acad. Sci. 2019, 68, 185–190. [Google Scholar] [CrossRef]
- Holovenko, Y.; Kollo, L.; Saarna, M.; Rahmani, R.; Soloviova, T.; Antonov, M.; Prashanth, K.G.; Cygan, S.; Veinthal, R. Effect of lattice surface treatment on performance of hardmetal—Titanium interpenetrating phase composites. Int. J. Refract. Met. Hard Mater. 2019, 86, 105087. [Google Scholar] [CrossRef]
- Rahmani, R.; Antonov, M.; Kollo, L.; Holovenko, Y.; Prashanth, K.G. Mechanical Behavior of Ti6Al4V Scaffolds Filled with CaSiO3 for Implant Applications. Appl. Sci. 2019, 9, 3844. [Google Scholar] [CrossRef] [Green Version]
- Rahmani, R.; Brojan, M.; Antonov, M.; Prashanth, K.G. Perspectives of metal-diamond composites additive manufacturing using SLM-SPS and other techniques for increased wear-impact resistance. Int. J. Refract. Met. Hard Mater. 2020, 88, 105192. [Google Scholar] [CrossRef]
- Aramian, A.; Sadeghian, Z.; Prashanth, K.G.; Berto, F. In situ fabrication of TiC-NiCr cermets by selective laser melting. Int. J. Refract. Met. Hard Mater. 2019, 87, 105171. [Google Scholar] [CrossRef]
- Aramian, A.; Sadeghian, Z.; Razavi, S.M.J.; Prashanth, K.G.; Berto, F. Effect of selective laser melting process parameters on microstructural and mechanical properties of TiC–NiCr cermet. Ceram. Int. 2020, 46, 28749–28757. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, S.; Scudino, S.; Ivanov, Y.; Qu, R.; Wang, D.; Yang, C.; Zhang, W.; Greer, A.; Eckert, J.; et al. Additive manufacturing of a martensitic Co–Cr–Mo alloy: Towards circumventing the strength–ductility trade-off. Addit. Manuf. 2020, 37, 101725. [Google Scholar] [CrossRef]
- Miranda, P.; Saiz, E.; Gryn, K.; Tomsia, A.P. Sintering and robocasting of β-tricalcium phosphate scaffolds for orthopaedic applications. Acta Biomater. 2006, 2, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Myers, K.; Cortes, P.; Conner, B.; Wagner, T.; Hetzel, B.; Peters, K. Structure property relationship of metal matrix syntactic foams manufactured by a binder jet printing process. Addit. Manuf. 2014, 5, 54–59. [Google Scholar] [CrossRef]
- Vaezi, M.; Yang, S. Freeform fabrication of nanobiomaterials using 3D printing. Rapid Prototyp. Biomater. 2014, 41–92. [Google Scholar] [CrossRef]
- Harrer, W.; Schwentenwein, M.; Lube, T.; Danzer, R. Fractography of zirconia-specimens made using additive manufacturing (LCM) technology. J. Eur. Ceram. Soc. 2017, 37, 4331–4338. [Google Scholar] [CrossRef]
- Bhaskar, S.; Lim, S. Engineering protein nanocages as carriers for biomedical applications. NPG Asia Mater. 2017, 9, e371. [Google Scholar] [CrossRef]
- Holzwarth, J.M.; Ma, P.X. Biomimetic nanofibrous scaffolds for bone tissue engineering. Biomaterials 2011, 32, 9622–9629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barabaschi, G.D.G.; Manoharan, V.; Li, Q.; Bertassoni, L.E. Engineering Pre-vascularized Scaffolds for Bone Regeneration. In Engineering Mineralized and Load Bearing Tissues; Springer: Berlin/Heidelberg, Germany, 2015; Volume 881, pp. 79–94. [Google Scholar] [CrossRef]
- Launey, M.E.; Munch, E.; Alsem, D.H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. A novel biomimetic approach to the design of high-performance ceramic–metal composites. J. R. Soc. Interface 2009, 7, 741–753. [Google Scholar] [CrossRef] [Green Version]
- Moon, Y.-W.; Choi, I.-J.; Koh, Y.-H.; Kim, H.-E. Porous alumina ceramic scaffolds with biomimetic macro/micro-porous structure using three-dimensional (3-D) ceramic/camphene-based extrusion. Ceram. Int. 2015, 41, 12371–12377. [Google Scholar] [CrossRef]
- Vijayavenkataraman, S.; Kuan, L.Y.; Lu, W.F. 3D-printed ceramic triply periodic minimal surface structures for design of functionally graded bone implants. Mater. Des. 2020, 191, 108602. [Google Scholar] [CrossRef]
- Stanciuc, A.-M.; Sprecher, C.M.; Adrien, J.; Roiban, L.I.; Alini, M.; Gremillard, L.; Peroglio, M. Robocast zirconia-toughened alumina scaffolds: Processing, structural characterisation and interaction with human primary osteoblasts. J. Eur. Ceram. Soc. 2018, 38, 845–853. [Google Scholar] [CrossRef]
- Bose, S.; Darsell, J.; Kintner, M.; Hosick, H.; Bandyopadhyay, A. Pore size and pore volume effects on alumina and TCP ceramic scaffolds. Mater. Sci. Eng. C 2003, 23, 479–486. [Google Scholar] [CrossRef]
- Dawson, J.; Wahl, D.A.; Lanham, S.A.; Kanczler, J.; Czernuszka, J.T.; Oreffo, R. Development of specific collagen scaffolds to support the osteogenic and chondrogenic differentiation of human bone marrow stromal cells. Biomaterials 2008, 29, 3105–3116. [Google Scholar] [CrossRef]
- Ge, X.; Zhao, J.; Esmeryan, K.D.; Lu, X.; Li, Z.; Wang, K.; Ren, F.; Wang, Q.; Wang, M.; Qian, B. Cicada-inspired fluoridated hydroxyapatite nanostructured surfaces synthesized by electrochemical additive manufacturing. Mater. Des. 2020, 193, 108790. [Google Scholar] [CrossRef]
- Wu, S.; Zuber, F.; Maniura-Weber, K.; Brugger, J.; Ren, Q. Nanostructured surface topographies have an effect on bactericidal activity. J. Nanobiotechnology 2018, 16, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kavasi, R.-M.; Coelho, C.; Platania, V.; Quadros, P.; Chatzinikolaidou, M. In Vitro Biocompatibility Assessment of Nano-Hydroxyapatite. Nanomaterials 2021, 11, 1152. [Google Scholar] [CrossRef]
- Montalbano, G.; Molino, G.; Fiorilli, S.; Vitale-Brovarone, C. Synthesis and incorporation of rod-like nano-hydroxyapatite into type I collagen matrix: A hybrid formulation for 3D printing of bone scaffolds. J. Eur. Ceram. Soc. 2020, 40, 3689–3697. [Google Scholar] [CrossRef]
- Luo, Y.; Zhai, D.; Huan, Z.; Zhu, H.; Xia, L.; Chang, J.; Wu, C. Three-Dimensional Printing of Hollow-Struts-Packed Bioceramic Scaffolds for Bone Regeneration. ACS Appl. Mater. Interfaces 2015, 7, 24377–24383. [Google Scholar] [CrossRef]
- Stevanovic, S.; Chavanne, P.; Braissant, O.; Pieles, U.; Gruner, P.; Schumacher, R. Improvement of Mechanical Properties of 3d Printed Hydroxyapatite Scaffolds by Polymeric Infiltration. Bioceram. Dev. Appl. 2013, 3, 10–12. [Google Scholar] [CrossRef]
- Li, X.; Yuan, Y.; Liu, L.; Leung, Y.-S.; Chen, Y.; Guo, Y.; Chai, Y.; Chen, Y. 3D printing of hydroxyapatite/tricalcium phosphate scaffold with hierarchical porous structure for bone regeneration. Bio-Design Manuf. 2019, 3, 15–29. [Google Scholar] [CrossRef]
- Chen, G.; Sun, Y.; Lu, F.; Jiang, A.; Subedi, D.; Kong, P.; Wang, X.; Yu, T.; Chi, H.; Song, C.; et al. A three-dimensional (3D) printed biomimetic hierarchical scaffold with a covalent modular release system for osteogenesis. Mater. Sci. Eng. C 2019, 104, 109842. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Lin, C.-Y.; Liu, F.-H.; Chen, M.H.-C.; Lin, C.-P.; Ho, H.-N.; Liao, Y.-S. 3D Printing Bioceramic Porous Scaffolds with Good Mechanical Property and Cell Affinity. PLoS ONE 2015, 10, e0143713. [Google Scholar] [CrossRef]
- Maazouz, Y.; Montufar, E.B.; Guillem-Marti, J.; Fleps, I.; Öhman, C.; Persson, C.; Ginebra, M.P. Robocasting of biomimetic hydroxyapatite scaffolds using self-setting inks. J. Mater. Chem. B 2014, 2, 5378–5386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Shi, Y.; Zhang, X.; Ma, J. Biomimetic synthesis of Mg-substituted hydroxyapatite nanocomposites and three-dimensional printing of composite scaffolds for bone regeneration. J. Biomed. Mater. Res. Part A 2019, 107, 2512–2521. [Google Scholar] [CrossRef]
- Thammarakcharoen, F.; Suwanprateeb, J. Biomimetically Co-Deposition of Bovine Serum Albumin and Calcium Phosphate on 3D Printed Hydroxyapatite: Influence of Time, Temperature and Concentration. Key Eng. Mater. 2018, 766, 83–87. [Google Scholar] [CrossRef]
- Thammarakcharoen, F.; Suwanprateeb, J. Effect of Process Parameters on Biomimetic Deposition of Calcium Phosphate on 3D Printed Hydroxyapatite. Key Eng. Mater. 2017, 751, 599–604. [Google Scholar] [CrossRef]
- Jalota, S.; Bhaduri, S.B.; Tas, A.C. In vitro testing of calcium phosphate (HA, TCP, and biphasic HA-TCP) whiskers. J. Biomed. Mater. Res. Part A 2006, 78, 481–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mygind, T.; Stiehler, M.; Baatrup, A.; Li, H.; Zou, X.; Flyvbjerg, A.; Kassem, M.; Bünger, C. Mesenchymal stem cell ingrowth and differentiation on coralline hydroxyapatite scaffolds. Biomaterials 2007, 28, 1036–1047. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-F.; Long, L.; Wang, R.; Chen, D.; Duan, S.; Xu, F.-J. Surface-Modified Hydroxyapatite Nanoparticle-Reinforced Polylactides for Three-Dimensional Printed Bone Tissue Engineering Scaffolds. J. Biomed. Nanotechnol. 2018, 14, 294–303. [Google Scholar] [CrossRef]
- Tarafder, S.; Bose, S. Polycaprolactone-Coated 3D Printed Tricalcium Phosphate Scaffolds for Bone Tissue Engineering: In Vitro Alendronate Release Behavior and Local Delivery Effect on In Vivo Osteogenesis. ACS Appl. Mater. Interfaces 2014, 6, 9955–9965. [Google Scholar] [CrossRef]
- Canillas, M.; Pena, P.; de Aza, A.H.; Rodríguez, M.A. Calcium phosphates for biomedical applications. Boletín Soc. Española Cerámica Vidr. 2017, 56, 91–112. [Google Scholar] [CrossRef]
- Fielding, G.; Bose, S. SiO2 and ZnO dopants in three-dimensionally printed tricalcium phosphate bone tissue engineering scaffolds enhance osteogenesis and angiogenesis in vivo. Acta Biomater. 2013, 9, 9137–9148. [Google Scholar] [CrossRef] [Green Version]
- Bose, S.; Tarafder, S.; Bandyopadhyay, A. Effect of Chemistry on Osteogenesis and Angiogenesis Towards Bone Tissue Engineering Using 3D Printed Scaffolds. Ann. Biomed. Eng. 2016, 45, 261–272. [Google Scholar] [CrossRef] [Green Version]
- Bidgoli, M.R.; Alemzadeh, I.; Tamjid, E.; Khafaji, M.; Vossoughi, M. Fabrication of hierarchically porous silk fibroin-bioactive glass composite scaffold via indirect 3D printing: Effect of particle size on physico-mechanical properties and in vitro cellular behavior. Mater. Sci. Eng. C 2019, 103, 109688. [Google Scholar] [CrossRef]
- Guo, X.; Zheng, Q.; Yang, S.; Shao, Z.; Yuan, Q.; Pan, Z.; Tang, S.; Liu, K.; Quan, D. Repair of full-thickness articular cartilage defects by cultured mesenchymal stem cells transfected with the transforming growth factor β1gene. Biomed. Mater. 2006, 1, 206–215. [Google Scholar] [CrossRef]
- Mastrogiacomo, M.; Muraglia, A.; Komlev, V.; Peyrin, F.; Rustichelli, F.; Crovace, A.; Cancedda, R. Tissue engineering of bone: Search for a better scaffold. Orthod. Craniofacial Res. 2005, 8, 277–284. [Google Scholar] [CrossRef]
- Singh, S.; Ramakrishna, S.; Singh, R. Material issues in additive manufacturing: A review. J. Manuf. Process. 2017, 25, 185–200. [Google Scholar] [CrossRef]
- Ha, N.S.; Lu, G. A review of recent research on bio-inspired structures and materials for energy absorption applications. Compos. Part B Eng. 2019, 181, 107496. [Google Scholar] [CrossRef]
- Nguyen, N.A.; Barnes, S.H.; Bowland, C.C.; Meek, K.M.; Littrell, K.C.; Keum, J.K.; Naskar, A.K. A path for lignin valorization via additive manufacturing of high-performance sustainable composites with enhanced 3D printability. Sci. Adv. 2018, 4, eaat4967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benmeridja, L.; De Moor, L.; De Maere, E.; Vanlauwe, F.; Ryx, M.; Tytgat, L.; Vercruysse, C.; Dubruel, P.; Van Vlierberghe, S.; Blondeel, P.; et al. High-throughput fabrication of vascularized adipose microtissues for 3D bioprinting. J. Tissue Eng. Regen. Med. 2020, 14, 840–854. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.Z.; Chou, D.-T.; Hong, D.; Roy, A.; Kumta, P.N. Biomimetic Rotated Lamellar Plywood Motifs by Additive Manufacturing of Metal Alloy Scaffolds for Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2017, 3, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Whatley, B.R.; Kuo, J.; Shuai, C.; Damon, B.J.; Wen, X. Fabrication of a biomimetic elastic intervertebral disk scaffold using additive manufacturing. Biofabrication 2011, 3, 015004. [Google Scholar] [CrossRef]
- Limmahakhun, S.; Oloyede, A.; Sitthiseripratip, K.; Xiao, Y.; Yan, C. 3D-printed cellular structures for bone biomimetic implants. Addit. Manuf. 2017, 15, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.; Felismina, R.; Mateus, A.; Parreira, P.; Malça, C. Application of a Hybrid Additive Manufacturing Methodology to Produce a Metal/Polymer Customized Dental Implant. Procedia Manuf. 2017, 12, 150–155. [Google Scholar] [CrossRef]
- Sabaté Rovira, D.; Nielsen, H.M.; Taboryski, R.; Bunea, A.-I. Additive manufacturing of polymeric scaffolds for biomimetic cell membrane engineering. Mater. Des. 2021, 201, 109486. [Google Scholar] [CrossRef]
- Chan, W.W.; Yeo, D.C.L.; Tan, V.; Singh, S.; Choudhury, D.; Naing, M.W. Additive Biomanufacturing with Collagen Inks. Bioengineering 2020, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, C.; Chu, P.K.; Gelinsky, M. 3D printing of hydrogels: Rational design strategies and emerging biomedical applications. Mater. Sci. Eng. R Rep. 2020, 140, 100543. [Google Scholar] [CrossRef]
- Zafeiris, K.; Brasinika, D.; Karatza, A.; Koumoulos, E.; Karoussis, I.; Kyriakidou, K.; Charitidis, C. Additive manufacturing of hydroxyapatite–chitosan–genipin composite scaffolds for bone tissue engineering applications. Mater. Sci. Eng. C 2020, 119, 111639. [Google Scholar] [CrossRef]
- Del Bakhshayesh, A.R.; Asadi, N.; Alihemmati, A.; Nasrabadi, H.T.; Montaseri, A.; Davaran, S.; Saghati, S.; Akbarzadeh, A.; Abedelahi, A. An overview of advanced biocompatible and biomimetic materials for creation of replacement structures in the musculoskeletal systems: Focusing on cartilage tissue engineering. J. Biol. Eng. 2019, 9, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashammakhi, N.; Ahadian, S.; Xu, C.; Montazerian, H.; Ko, H.; Nasiri, R.; Barros, N.; Khademhosseini, A. Bioinks and bioprinting technologies to make heterogeneous and biomimetic tissue constructs. Mater. Today Bio 2019, 1, 100008. [Google Scholar] [CrossRef]
- Cui, X.; Boland, T. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials 2009, 30, 6221–6227. [Google Scholar] [CrossRef] [Green Version]
- Hajiali, F.; Tajbakhsh, S.; Shojaei, A. Fabrication and Properties of Polycaprolactone Composites Containing Calcium Phosphate-Based Ceramics and Bioactive Glasses in Bone Tissue Engineering: A Review. Polym. Rev. 2017, 58, 164–207. [Google Scholar] [CrossRef]
- Wang, L.; He, S.; Wu, X.; Liang, S.; Mu, Z.; Wei, J.; Deng, F.; Deng, Y.; Wei, S. Polyetheretherketone/nano-fluorohydroxyapatite composite with antimicrobial activity and osseointegration properties. Biomaterials 2014, 35, 6758–6775. [Google Scholar] [CrossRef]
- Yoo, D.-J. Recent trends and challenges in computer-aided design of additive manufacturing-based biomimetic scaffolds and bioartificial organs. Int. J. Precis. Eng. Manuf. 2014, 15, 2205–2217. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, Z.Y.; Wenger, A.C.; Tam, K.C.; Tang, X. 3D bioprinting of liver-mimetic construct with alginate/cellulose nanocrystal hybrid bioink. Bioprinting 2018, 9, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, R.; Pridhar, T.; Sree Charan, N.; Ramprasath, L.; Charan, N.S.; Ruban, W. Prediction of tensile strength in FDM printed ABS parts using response surface methodology (RSM). Mater. Today Proc. 2020, 27, 1827–1832. [Google Scholar] [CrossRef]
- Singh, M.; Jonnalagadda, S. Advances in bioprinting using additive manufacturing. Eur. J. Pharm. Sci. 2019, 143, 105167. [Google Scholar] [CrossRef]
- Christensen, K.; Xu, C.; Chai, W.; Zhang, Z.; Fu, J.; Huang, Y. Freeform inkjet printing of cellular structures with bifurcations. Biotechnol. Bioeng. 2014, 112, 1047–1055. [Google Scholar] [CrossRef]
- Ding, C.; Qiao, Z.; Jiang, W.; Li, H.; Wei, J.; Zhou, G.; Dai, K. Regeneration of a goat femoral head using a tissue-specific, biphasic scaffold fabricated with CAD/CAM technology. Biomaterials 2013, 34, 6706–6716. [Google Scholar] [CrossRef]
- Zhang, W.; Lian, Q.; Li, D.; Wang, K.; Hao, D.; Bian, W.; Jin, Z. The effect of interface microstructure on interfacial shear strength for osteochondral scaffolds based on biomimetic design and 3D printing. Mater. Sci. Eng. C 2015, 46, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Xue, G.-H.; Sun, M.; Shao, H.-F.; Ma, C.-Y.; Gao, Q.; Gou, Z.-R.; Yan, S.-G.; Liu, Y.-M.; He, Y. 3D Printing Surgical Implants at the clinic: A Experimental Study on Anterior Cruciate Ligament Reconstruction. Sci. Rep. 2016, 6, 21704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukhopadhyay, P.; Sarkar, K.; Bhattacharya, S.; Bhattacharyya, A.; Mishra, R.; Kundu, P. pH sensitive N-succinyl chitosan grafted polyacrylamide hydrogel for oral insulin delivery. Carbohydr. Polym. 2014, 112, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Stroganov, V. 4D Biofabrication Using Self-Folding Polymers. Ph.D. Thesis, University of Bayreuth, Bayreuth, Germany, 2018. [Google Scholar]
- Wan, Z.; Zhang, P.; Liu, Y.; Lv, L.; Zhou, Y. Four-dimensional bioprinting: Current developments and applications in bone tissue engineering. Acta Biomater. 2019, 101, 26–42. [Google Scholar] [CrossRef]
- Abraham, C.M. A Brief Historical Perspective on Dental Implants, Their Surface Coatings and Treatments. Open Dent. J. 2014, 8, 50–55. [Google Scholar] [CrossRef]
- Saini, M. Implant biomaterials: A comprehensive review. World J. Clin. Cases 2015, 3, 52–57. [Google Scholar] [CrossRef]
- Katti, K.; Verma, D. Materials for joint replacement. In Joint Replacement Technology; Woodhead Publishing: Cambridge, UK, 2008; pp. 81–104. [Google Scholar] [CrossRef]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Ummethala, R.; Karamched, P.S.; Rathinavelu, S.; Singh, N.; Aggarwal, A.; Sun, K.; Ivanov, E.; Kollo, L.; Okulov, I.; Eckert, J.; et al. Selective laser melting of high-strength, low-modulus Ti–35Nb–7Zr–5Ta alloy. Materialia 2020, 14, 100941. [Google Scholar] [CrossRef]
- Schwab, H.; Prashanth, K.G.; Löber, L.; Kühn, U.; Eckert, J. Selective Laser Melting of Ti-45Nb Alloy. Metals 2015, 5, 686–694. [Google Scholar] [CrossRef] [Green Version]
- Zhuravleva, K.; Bönisch, M.; Prashanth, K.G.; Hempel, U.; Helth, A.; Gemming, T.; Calin, M.; Scudino, S.; Schultz, L.; Eckert, J.; et al. Production of Porous β-Type Ti–40Nb Alloy for Biomedical Applications: Comparison of Selective Laser Melting and Hot Pressing. Materials 2013, 6, 5700–5712. [Google Scholar] [CrossRef] [PubMed]
- Ridzwan, M.I.Z.; Shuib, S.; Hassan, A.Y.; Shokri, A.A.; Mohammad Ibrahim, M.N. Problem of Stress Shielding and Improvement to the Hip Implant Designs: A Review. J. Med Sci. 2007, 7, 460–467. [Google Scholar] [CrossRef] [Green Version]
- Gong, Q.; Hu, X. Photonic crystals: Principles and applications. MRS Bull. 2014, 39, 824–825. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, J.W. Titanium Alloys for Dental Implants: A Review. Prosthesis 2020, 2, 100–116. [Google Scholar] [CrossRef]
- Bone Healing—Physiopedia. Available online: https://www.physio-pedia.com/Bone_Healing (accessed on 3 June 2021).
- Arabnejad, S.; Johnston, B.; Tanzer, M.; Pasini, D. Fully porous 3D printed titanium femoral stem to reduce stress-shielding following total hip arthroplasty. J. Orthop. Res. 2016, 35, 1774–1783. [Google Scholar] [CrossRef]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic Biomaterials: Current Challenges and Opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef]
- Rotta, G.; Seramak, T.; Zasińska, K. Estimation of Young’s Modulus of the Porous Titanium Alloy with the Use of Fem Package. Adv. Mater. Sci. 2015, 15, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Mehboob, H.; Tarlochan, F.; Mehboob, A.; Chang, S.-H.; Ramesh, S.; Harun, W.S.W.; Kadirgama, K. A novel design, analysis and 3D printing of Ti-6Al-4V alloy bio-inspired porous femoral stem. J. Mater. Sci. Mater. Med. 2020, 31, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kim, M.-Y.; Knowles, J.C.; Choi, S.; Kang, H.; Park, S.-H.; Park, S.-M.; Kim, H.-W.; Park, J.-T.; Lee, J.-H.; et al. Mechanophysical and biological properties of a 3D-printed titanium alloy for dental applications. Dent. Mater. 2020, 36, 945–958. [Google Scholar] [CrossRef]
- El-Hajje, A.; Kolos, E.C.; Wang, J.K.; Maleksaeedi, S.; He, Z.; Wiria, F.E.; Choong, C.; Ruys, A.J. Physical and mechanical characterisation of 3D-printed porous titanium for biomedical applications. J. Mater. Sci. Mater. Med. 2014, 25, 2471–2480. [Google Scholar] [CrossRef]
- Miura, H.; Okawachi, K.; Kang, H.G.; Tsumori, F.; Kurata, K.; Arimoto, N. Laser Forming of Ti-6Al-7Nb Alloy Porous Parts for Medical Devices; Trans Tech Publications, Ltd.: Bäch, Switzerland, 2010; Available online: https://www.pm-review.com/articles/laser-forming-of-ti-6al-7nb-alloy-porous-parts-for-medical-devices/ (accessed on 20 August 2021).
- Szymczyk, P.; Ziółkowski, G.; Junka, A.; Chlebus, E. Application of Ti6Al7Nb Alloy for the Manufacture of Biomechanical Functional Structures (BFS) for Custom-Made Bone Implants. Materials 2018, 11, 971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verlee, B.; Dormal, T.; Lecomte-Beckers, J. Density and porosity control of sintered 316L stainless steel parts produced by additive manufacturing. Powder Met. 2012, 55, 260–267. [Google Scholar] [CrossRef]
- Čapek, J.; Machová, M.; Fousová, M.; Kubásek, J.; Vojtěch, D.; Fojt, J.; Jablonská, E.; Lipov, J.; Ruml, T. Highly porous, low elastic modulus 316L stainless steel scaffold prepared by selective laser melting. Mater. Sci. Eng. C 2016, 69, 631–639. [Google Scholar] [CrossRef]
- Bobbert, F.; Lietaert, K.; Eftekhari, A.A.; Pouran, B.; Ahmadi, S.; Weinans, H.; Zadpoor, A.A. Additively manufactured metallic porous biomaterials based on minimal surfaces: A unique combination of topological, mechanical, and mass transport properties. Acta Biomater. 2017, 53, 572–584. [Google Scholar] [CrossRef] [Green Version]
- Bai, L.; Gong, C.; Chen, X.; Sun, Y.; Zhang, J.; Cai, L.; Zhu, S.; Xie, S.Q. Additive Manufacturing of Customized Metallic Orthopedic Implants: Materials, Structures, and Surface Modifications. Metals 2019, 9, 1004. [Google Scholar] [CrossRef] [Green Version]
- Van Bael, S.; Chai, Y.C.; Truscello, S.; Moesen, M.; Kerckhofs, G.; Van Oosterwyck, H.; Kruth, J.-P.; Schrooten, J. The effect of pore geometry on the in vitro biological behavior of human periosteum-derived cells seeded on selective laser-melted Ti6Al4V bone scaffolds. Acta Biomater. 2012, 8, 2824–2834. [Google Scholar] [CrossRef]
- Hollander, D.A.; Wirtz, T.; von Walter, M.; Linker, R.; Schultheis, A.; Paar, O. Development of Individual Three-Dimensional Bone Substitutes Using “Selective Laser Melting”. Eur. J. Trauma Emerg. Surg. 2003, 29, 228–234. [Google Scholar] [CrossRef]
- Anatomography. Wikipedia, via Wikimedia Commons. 2021. Available online: https://en.wikipedia.org/wiki/Anatomography (accessed on 6 July 2021).
- Siemer, P. Wikipedia, via Wikimedia Commons. Available online: https://creativecommons.org/licenses/by/2.0%3E (accessed on 6 July 2021).
- Chung, K.J.; Hong, D.Y.; Kim, Y.T.; Yang, I.; Park, Y.W.; Kim, H.N. Preshaping Plates for Minimally Invasive Fixation of Calcaneal Fractures Using a Real-Size 3D-Printed Model as a Preoperative and Intraoperative Tool. Foot Ankle Int. 2014, 35, 1231–1236. [Google Scholar] [CrossRef] [PubMed]
- Starosolski, Z.A.; Kan, J.H.; Rosenfeld, S.D.; Krishnamurthy, R.; Annapragada, A. Application of 3-D printing (rapid prototyping) for creating physical models of pediatric orthopedic disorders. Pediatr. Radiol. 2013, 44, 216–221. [Google Scholar] [CrossRef]
- Victor, J.; Dujardin, J.; Vandenneucker, H.; Arnout, N.; Bellemans, J. Patient-specific Guides Do Not Improve Accuracy in Total Knee Arthroplasty: A Prospective Randomized Controlled Trial. Clin. Orthop. Relat. Res. 2014, 472, 263–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guarino, J.; Tennyson, S.; McCain, G.; Bond, L.; Shea, K.; King, H. Rapid Prototyping Technology for Surgeries of the Pediatric Spine and Pelvis. J. Pediatr. Orthop. 2007, 27, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Victor, J.; Premanathan, A. Virtual 3D planning and patient specific surgical guides for osteotomies around the knee: A feasibility and proof-of-concept study. Bone Jt. J. 2013, 95, 153–158. [Google Scholar] [CrossRef]
- Additive Manufacturing Market Report | Global Analysis & Forecast. Reports and Data. Available online: https://www.reportsanddata.com/report-detail/additive-manufacturing-market (accessed on 17 November 2021).
- Wang, P.; Li, H.; Prashanth, K.; Eckert, J.; Scudino, S. Selective laser melting of Al-Zn-Mg-Cu: Heat treatment, microstructure and mechanical properties. J. Alloy. Compd. 2017, 707, 287–290. [Google Scholar] [CrossRef]
- Wang, P.; Gammer, C.; Brenne, F.; Prashanth, K.G.; Mendes, R.G.; Rümmeli, M.H.; Gemming, T.; Eckert, J.; Scudino, S. Microstructure and mechanical properties of a heat-treatable Al-3.5Cu-1.5Mg-1Si alloy produced by selective laser melting. Mater. Sci. Eng. A 2018, 711, 562–570. [Google Scholar] [CrossRef]
- Jia, Y.D.; Ma, P.; Prashanth, K.G.; Wang, G.; Yi, J.; Scudino, S.; Cao, F.Y.; Sun, J.F.; Eckert, J. Microstructure and thermal expansion behavior of Al-50Si synthesized by selective laser melting. J. Alloys Compd. 2017, 699, 548–553. [Google Scholar] [CrossRef]
- Mfusi, B.J.; Mathe, N.R.; Tshabalala, L.C.; Popoola, P.A. The Effect of Stress Relief on the Mechanical and Fatigue Properties of Additively Manufactured AlSi10Mg Parts. Metals 2019, 9, 1216. [Google Scholar] [CrossRef] [Green Version]
- Roehling, J.D.; Smith, W.L.; Roehling, T.T.; Vrancken, B.; Guss, G.M.; McKeown, J.T.; Hill, M.R.; Matthews, M.J. Reducing residual stress by selective large-area diode surface heating during laser powder bed fusion additive manufacturing. Addit. Manuf. 2019, 28, 228–235. [Google Scholar] [CrossRef]
- Li, C.; Liu, Z.; Fang, X.; Guo, Y. Residual Stress in Metal Additive Manufacturing. Procedia CIRP 2018, 71, 348–353. [Google Scholar] [CrossRef]
- Tong, Z.; Ren, X.; Jiao, J.; Zhou, W.; Ren, Y.; Ye, Y.; Larson, E.A.; Gu, J. Laser additive manufacturing of FeCrCoMnNi high-entropy alloy: Effect of heat treatment on microstructure, residual stress and mechanical property. J. Alloy. Compd. 2019, 785, 1144–1159. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Cheng, X.; Wang, H.; Huang, Z. Effect of heat treatment on microstructure, mechanical and corrosion properties of austenitic stainless steel 316L using arc additive manufacturing. Mater. Sci. Eng. A 2018, 715, 307–314. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, H.; Zhu, J.; Nie, X.; Hu, Z.; Zhu, H.; Zeng, X. Mechanical behavior and microstructure evolution of Al-Cu-Mg alloy produced by laser powder bed fusion: Effect of heat treatment. Mater. Charact. 2020, 165, 110364. [Google Scholar] [CrossRef]
- Kumbhar, N.N.; Mulay, A.V. Post Processing Methods used to Improve Surface Finish of Products which are Manufactured by Additive Manufacturing Technologies: A Review. J. Inst. Eng. (India) Ser. C 2016, 99, 481–487. [Google Scholar] [CrossRef]
- Tan, K.; Yeo, S.H. Surface modification of additive manufactured components by ultrasonic cavitation abrasive finishing. Wear 2017, 378-379, 90–95. [Google Scholar] [CrossRef]
- Witkin, D.B.; Patel, D.N.; Helvajian, H.; Steffeney, L.; Diaz, A. Surface Treatment of Powder-Bed Fusion Additive Manufactured Metals for Improved Fatigue Life. J. Mater. Eng. Perform. 2018, 28, 681–692. [Google Scholar] [CrossRef]
- Imanishi, J.; Choong, P.F. Three-dimensional printed calcaneal prosthesis following total calcanectomy. Int. J. Surg. Case Rep. 2015, 10, 83–87. [Google Scholar] [CrossRef] [Green Version]
- Ni, J.; Ling, H.; Zhang, S.; Wang, Z.; Peng, Z.; Benyshek, C.; Zan, R.; Miri, A.K.; Li, Z.; Zhang, X.; et al. Three-dimensional printing of metals for biomedical applications. Mater. Today Bio 2019, 3, 100024. [Google Scholar] [CrossRef]
- Jardini, A.L.; Larosa, M.A.; Macedo, M.F.; Bernardes, L.F.; Lambert, C.S.; Zavaglia, C.A.C.; Filho, R.M.; Calderoni, D.R.; Ghizoni, E.; Kharmandayan, P. Improvement in Cranioplasty: Advanced Prosthesis Biomanufacturing. Procedia CIRP 2016, 49, 203–208. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Possel, J.K.; Wacongne, C.; van Ham, A.F.; Klink, C.; Roelfsema, P. 3D printing and modelling of customized implants and surgical guides for non-human primates. J. Neurosci. Methods 2017, 286, 38–55. [Google Scholar] [CrossRef] [PubMed]
- Phan, K.; Sgro, A.; Maharaj, M.M.; D’Urso, P.; Mobbs, R.J. Application of a 3D custom printed patient specific spinal implant for C1/2 arthrodesis. J. Spine Surg. 2016, 2, 314–318. [Google Scholar] [CrossRef] [Green Version]
- Dzian, A.; Zivcak, J.; Penciak, R.; Hudak, R. Implantation of a 3D-printed titanium sternum in a patient with a sternal tumor. World J. Surg. Oncol. 2018, 16, 7. [Google Scholar] [CrossRef] [Green Version]
- Captiva Spine’s TirboLOX-L Titanium Lumbar Cages Receives FDA Clearance. Available online: https://spinalnewsinternational.com/captiva-spine-tirbolox/ (accessed on 17 November 2021).
- Wong, K.C.; Kumta, S.M.; Geel, N.V.; Demol, J. One-step reconstruction with a 3D-printed, biomechanically evaluated custom implant after complex pelvic tumor resection. Comput. Aided Surg. 2015, 20, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Shuang, F.; Hu, W.; Shao, Y.; Li, H.; Zou, H. Treatment of Intercondylar Humeral Fractures With 3D-Printed Osteosynthesis Plates. Medicine 2016, 95, e2461. [Google Scholar] [CrossRef]
- Cranioplasty | Johns Hopkins Medicine. Available online: https://www.hopkinsmedicine.org/health/treatment-tests-and-therapies/cranioplasty (accessed on 15 June 2021).
- Wind, J.; Cooke, R.S.; Gray, J.; Fannin, T.; Fegan, T. Medical rapid prototyping and 3D CT in the manufacture of custom made cranial titanium plates. J. Med. Eng. Technol. 1999, 23, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Mazzoli, A. Selective laser sintering in biomedical engineering. Med. Biol. Eng. Comput. 2012, 51, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Mazzoli, A.; Germani, M.; Raffaeli, R. Direct fabrication through electron beam melting technology of custom cranial implants designed in a PHANToM-based haptic environment. Mater. Des. 2009, 30, 3186–3192. [Google Scholar] [CrossRef]
- Ran, Q.; Yang, W.; Hu, Y.; Shen, X.; Yu, Y.; Xiang, Y.; Cai, K. Osteogenesis of 3D printed porous Ti6Al4V implants with different pore sizes. J. Mech. Behav. Biomed. Mater. 2018, 84, 1–11. [Google Scholar] [CrossRef]
- Marzola, A.; Buonamici, F.; Furferi, R.; Governi, L.; Genitori, L.; Mussa, F. Additive Manufacturing and Reverse Engineering in Cranioplasty: A Personalized Approach to Minimize Skin Flap Complications. Appl. Sci. 2021, 11, 4926. [Google Scholar] [CrossRef]
- Cascino, T.; Shea, M.J. Percutaneous Coronary Interventions (PCI)—Cardiovascular Disorders—MSD Manual Professional Edition. Available online: https://www.msdmanuals.com/en-in/professional/cardiovascular-disorders/cardiovascular-tests-and-procedures/percutaneous-coronary-interventions-pci (accessed on 15 June 2021).
- Guerra, A.J.; Ciurana, J. Stent’s Manufacturing Field: Past, Present, and Future Prospects. Angiography 2019. [Google Scholar] [CrossRef] [Green Version]
- Demir, A.G.; Previtali, B. Additive manufacturing of cardiovascular CoCr stents by selective laser melting. Mater. Des. 2017, 119, 338–350. [Google Scholar] [CrossRef] [Green Version]
- World’s First 3D Printed Nitinol Stent—CSIRO. Available online: https://www.csiro.au/en/research/production/materials/3D-printed-Nitinol-stent (accessed on 15 June 2021).
- Orla, A.; Mcgee, M.; Geraghty, S.; Hughes, C.; Jamshidi, P.; Kenny, D.P.; Attallah, M.M.; Lally, C. An Investigation into Patient-Specific 3D Printed Titanium Stents and the use of Etching to Overcome Selective Laser Melting Design Constraints. bioRxiv 2021. [Google Scholar] [CrossRef]
- Tilton, M.; Lewis, G.; Bok Wee, H.; Armstrong, A.; Hast, M.W.; Manogharan, G. Additive manufacturing of fracture fixation implants: Design, material characterization, biomechanical modeling and experimentation. Addit. Manuf. 2020, 33, 101137. [Google Scholar] [CrossRef]
- Jason, A.; Lowe, M. AAOS. Available online: https://orthoinfo.aaos.org/en/treatment/internal-fixation-for-fractures/ (accessed on 16 June 2021).
- Smith, K.E.; Dupont, K.M.; Safranski, D.L.; Blair, J.W.; Buratti, D.R.; Zeetser, V.; Callahan, R.; Lin, J.S.; Gall, K. Use of 3D Printed Bone Plate in Novel Technique to Surgically Correct Hallux Valgus Deformities. Tech. Orthop. 2016, 31, 181–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, J.N.; McQueen, P.; Kim, W.; Hutchinson, M.R. Bioabsorbable interference screw failure in anterior cruciate ligament reconstruction: A case series and review of the literature. Knee 2015, 22, 256–261. [Google Scholar] [CrossRef]
- Harvey, A.; Thomas, N.P.; Amis, A.A. Fixation of the graft in reconstruction of the anterior cruciate ligament. J. Bone Jt. Surg. Ser. B 2005, 87, 593–603. [Google Scholar] [CrossRef]
- Huang, Y.-M.; Huang, C.-C.; Tsai, P.-I.; Yang, K.-Y.; Huang, S.-I.; Shen, H.-H.; Lai, H.-J.; Huang, S.-W.; Chen, S.-Y.; Lin, F.-H.; et al. Three-Dimensional Printed Porous Titanium Screw with Bioactive Surface Modification for Bone–Tendon Healing: A Rabbit Animal Model. Int. J. Mol. Sci. 2020, 21, 3628. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Sun, R.; Jia, Y.; Chen, X.; Liu, Y.; Oyang, H.; Feng, L. A new 3D printed titanium metal trabecular bone reconstruction system for early osteonecrosis of the femoral head. Medicine 2018, 97, e11088. [Google Scholar] [CrossRef] [PubMed]
- Cronskär, M. The Use of Additive Manufacturing in the Custom Design of Orthopedic Implants. Ph.D. Thesis, Mid Sweden University, Östersund, Sweden, 2011. Available online: http://miun.diva-portal.org/smash/record.jsf?pid=diva2:436633 (accessed on 17 June 2021).
- Vundelinckx, B.J.; Bruckers, L.; De Mulder, K.; De Schepper, J.; Van Esbroeck, G. Functional and Radiographic Short-Term Outcome Evaluation of the Visionaire System, a Patient-Matched Instrumentation System for Total Knee Arthroplasty. J. Arthroplast. 2013, 28, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Stronach, B.M.; Pelt, C.E.; Erickson, J.; Peters, C.L. Patient-specific Total Knee Arthroplasty Required Frequent Surgeon-directed Changes knee. Clin. Orthop. Relat. Res. 2013, 471, 169–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maniar, R.N.; Singhi, T. Patient specific implants: Scope for the future. Curr. Rev. Musculoskelet. Med. 2014, 7, 125–130. [Google Scholar] [CrossRef] [Green Version]
- Murr, L.E.; Gaytan, S.M.; Martinez, E.; Medina, F.; Wicker, R.B. Next Generation Orthopaedic Implants by Additive Manufacturing Using Electron Beam Melting. Int. J. Biomater. 2012, 2012, 1–14. [Google Scholar] [CrossRef]
- Narra, S.P.; Mittwede, P.N.; DeVincent Wolf, S.; Urish, K.L. Additive Manufacturing in Total Joint Arthroplasty. Orthop. Clin. N. Am. 2019, 50, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Rib and Sternum Repair | UTMB Health | UTMB Home. Available online: https://www.utmbhealth.com/services/cardiovascular-thoracic-surgery/procedures-conditions/chest-wall-repair/Rib-Sternum-Repair (accessed on 16 June 2021).
- Rupprecht, L.; Schmid, C. Deep Sternal Wound Complications: An Overview of Old and new Therapeutic Options. Open J. Cardiovasc. Surg. 2013, 6, OJCS-S11199. [Google Scholar] [CrossRef] [Green Version]
- Aragón, J.; Méndez, I.P. Dynamic 3D printed titanium copy prosthesis: A novel design for large chest wall resection and reconstruction. J. Thorac. Dis. 2016, 8, E385–E389. [Google Scholar] [CrossRef] [Green Version]
- The Additive Manufacturing Trend in Spine Today. Available online: https://orthostreams.com/2018/05/the-additive-manufacturing-trend-in-spine-today/ (accessed on 17 June 2021).
- Additively Manufactured Spinal Implants Mimic the Mechanical Properties of Bone. Available online: https://www.eurekamagazine.co.uk/design-engineering-features/technology/3d-printed-spinal-implants-1/214814/ (accessed on 17 June 2021).
- Sheha, E.D.; Gandhi, S.D.; Colman, M.W. 3D printing in spine surgery. Ann. Transl. Med. 2019, 7, S164. [Google Scholar] [CrossRef]
- Burnard, J.L.; Parr, W.C.H.; Choy, W.J.; Walsh, W.R.; Mobbs, R.J. 3D-printed spine surgery implants: A systematic review of the efficacy and clinical safety profile of patient-specific and off-the-shelf devices. Eur. Spine J. 2019, 29, 1248–1260. [Google Scholar] [CrossRef]
- Xu, N.; Wei, F.; Liu, X.; Jiang, L.; Cai, H.; Li, Z.; Yu, M.; Wu, F.; Liu, Z. Reconstruction of the Upper Cervical Spine Using a Personalized 3D-Printed Vertebral Body in an Adolescent With Ewing Sarcoma. Spine 2016, 41, E50–E54. [Google Scholar] [CrossRef]
- Mobbs, R.J.; Parr, W.C.; Choy, W.J.; McEvoy, A.; Walsh, W.R.; Phan, K. Anterior Lumbar Interbody Fusion Using a Personalized Approach: Is Custom the Future of Implants for Anterior Lumbar Interbody Fusion Surgery? World Neurosurg. 2019, 124, 452–458.e1. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Guo, W.; Yang, R.; Tang, X.; Yang, Y.; Ji, T.; Liang, H. Reconstruction of the pelvic ring after total en bloc sacrectomy using a 3D-printed sacral endoprosthesis with re-establishment of spinopelvic stability: A retrospective comparative study. Bone Jt. J. 2019, 101B, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Breeland, G.; Patel, B.C. Anatomy, Head and Neck, Mandible; StatPearls Publishing: Treasure Island, FL, USA, 2019. Available online: http://www.ncbi.nlm.nih.gov/pubmed/30335325 (accessed on 17 June 2021).
- Kumar, B.P.; Venkatesh, V.; Kumar, K.A.J.; Yadav, B.Y.; Mohan, S.R. Mandibular Reconstruction: Overview. J. Maxillofac. Oral Surg. 2015, 15, 425–441. [Google Scholar] [CrossRef]
- Xia, Y.; Feng, Z.C.; Li, C.; Wu, H.; Tang, C.; Wang, L.; Li, H. Application of additive manufacturing in customized titanium mandibular implants for patients with oral tumors. Oncol. Lett. 2020, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Odkhuu, M.; Cho, S.; Li, J.; Park, B.-Y.; Kim, J.-W. 3D-printed titanium implant with pre-mounted dental implants for mandible reconstruction: A case report. Maxillofac. Plast. Reconstr. Surg. 2020, 42, 1–4. [Google Scholar] [CrossRef]
- Gaviria, L.; Salcido, J.P.; Guda, T.; Ong, J.L. Current trends in dental implants. J. Korean Assoc. Oral Maxillofac. Surg. 2014, 40, 50–60. [Google Scholar] [CrossRef]
- Pillai, S.; Upadhyay, A.; Khayambashi, P.; Farooq, I.; Sabri, H.; Tarar, M.; Lee, K.T.; Harb, I.; Zhou, S.; Wang, Y.; et al. Dental 3D-Printing: Transferring Art from the Laboratories to the Clinics. Polymers 2021, 13, 157. [Google Scholar] [CrossRef] [PubMed]
- Barazanchi, A.; Li, K.C.; Al-Amleh, B.; Lyons, K.; Waddell, J. Additive Technology: Update on Current Materials and Applications in Dentistry. J. Prosthodont. 2016, 26, 156–163. [Google Scholar] [CrossRef]
- Padrós, R.; Punset, M.; Molmeneu, M.; Velasco, A.B.; Herrero-Climent, M.; Rupérez, E.; Gil, F.J. Mechanical Properties of CoCr Dental-Prosthesis Restorations Made by Three Manufacturing Processes. Influence of the Microstructure and Topography. Metals 2020, 10, 788. [Google Scholar] [CrossRef]
- Clinical Trial Finds 3D Printed Dentures are Preferred to Conventional Cast Metal Dentures—3D Printing Industry. Available online: https://3dprintingindustry.com/news/clinical-trial-finds-3d-printed-dentures-preferred-conventional-cast-metal-dentures-118320/ (accessed on 17 June 2021).
- Tunchel, S.; Blay, A.; Kolerman, R.; Mijiritsky, E.; Shibli, J.A. 3D Printing/Additive Manufacturing Single Titanium Dental Implants: A Prospective Multicenter Study with 3 Years of Follow-Up. Int. J. Dent. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hesse, H.; Özcan, M. A review on current additive manufacturing technologies and materials used for fabrication of metal-ceramic fixed dental prosthesis. J. Adhes. Sci. Technol. 2021, 1–18. [Google Scholar] [CrossRef]
- Ghassemi, T.; Shahroodi, A.; Ebrahimzadeh, M.H.; Mousavian, A.; Movaffagh, J.; Moradi, A. Current Concepts in Scaffolding for Bone Tissue Engineering. Arch. Bone Jt. Surg. 2018, 6, 90–99. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; O’Neal, C.; Bhuiyan, A.; Egan, P.F. Design and Mechanical Testing of 3D Printed Hierarchical Lattices Using Biocompatible Stereolithography. Designs 2020, 4, 22. [Google Scholar] [CrossRef]
- De Wild, M.; Ghayor, C.; Zimmermann, S.; Rüegg, J.; Nicholls, F.; Schuler, F.; Chen, T.-H.; Weber, F.E. Osteoconductive Lattice Microarchitecture for Optimized Bone Regeneration. 3D Print. Addit. Manuf. 2019, 6, 40–49. [Google Scholar] [CrossRef]
- Crovace, A.M.; Lacitignola, L.; Forleo, D.M.; Staffieri, F.; Francioso, E.; Di Meo, A.; Becerra, J.; Crovace, A.; Santos-Ruiz, L. 3D Biomimetic Porous Titanium (Ti6Al4V ELI) Scaffolds for Large Bone Critical Defect Reconstruction: An Experimental Study in Sheep. Animals 2020, 10, 1389. [Google Scholar] [CrossRef] [PubMed]
- Moiduddin, K.; Darwish, S.; Al-Ahmari, A.; ElWatidy, S.; Mohammad, A.; Ameen, W. Structural and mechanical characterization of custom design cranial implant created using additive manufacturing. Electron. J. Biotechnol. 2017, 29, 22–31. [Google Scholar] [CrossRef]
- Jardini, A.L.; Larosa, M.A.; Filho, R.M.; Zavaglia, C.A.D.C.; Bernardes, L.F.; Lambert, C.S.; Calderoni, D.R.; Kharmandayan, P. Cranial reconstruction: 3D biomodel and custom-built implant created using additive manufacturing. J. Cranio-Maxillofac. Surg. 2014, 42, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Jardini, A.L.; Larosa, M.A.; de Carvalho Zavaglia, C.A.; Bernardes, L.F.; Lambert, C.S.; Kharmandayan, P.; Calderoni, D.; Maciel Filho, R. Customised titanium implant fabricated in additive manufacturing for craniomaxillofacial surgery: This paper discusses the design and fabrication of a metallic implant for the reconstruction of a large cranial defect. Virtual Phys. Prototyp. 2014, 9, 115–125. [Google Scholar] [CrossRef]
- Volpe, Y.; Furferi, R.; Governi, L.; Uccheddu, F.; Carfagni, M.; Mussa, F.; Scagnet, M.; Genitori, L. Surgery of complex craniofacial defects: A single-step AM-based methodology. Comput. Methods Programs Biomed. 2018, 165, 225–233. [Google Scholar] [CrossRef]
- Cho, H.R.; Roh, T.S.; Shim, K.W.; Kim, Y.O.; Lew, D.H.; Yun, I.S. Skull Reconstruction with Custom Made Three-Dimensional Titanium Implant. Arch. Craniofacial Surg. 2015, 16, 11–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salmi, M.; Tuomi, J.; Paloheimo, K.; Björkstrand, R.; Paloheimo, M.; Salo, J.; Kontio, R.; Mesimäki, K.; Mäkitie, A.A. Patient-specific reconstruction with 3D modeling and DMLS additive manufacturing. Rapid Prototyp. J. 2012, 18, 209–214. [Google Scholar] [CrossRef]
- Moiduddin, K. Implementation of Computer-Assisted Design, Analysis, and Additive Manufactured Customized Mandibular Implants. J. Med. Biol. Eng. 2018, 38, 744–756. [Google Scholar] [CrossRef]
- Jo, Y.-Y.; Kim, S.-G.; Kim, M.-K.; Shin, S.-H.; Ahn, J.; Seok, H. Mandibular Reconstruction Using a Customized Three-Dimensional Titanium Implant Applied on the Lingual Surface of the Mandible. J. Craniofacial Surg. 2018, 29, 415–419. [Google Scholar] [CrossRef]
- Qassemyar, Q.; Assouly, N.; Madar, Y.; Temam, S.; Kolb, F. Total nasal reconstruction with 3D custom made porous titanium prosthesis and free thoracodorsal artery perforator flap: A case report. Microsurgery 2018, 38, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Mangano, F.; Pozzi-Taubert, S.; Zecca, P.A.; Luongo, G.; Sammons, R.; Mangano, C. Immediate Restoration of Fixed Partial Prostheses Supported by One-Piece Narrow-Diameter Selective Laser Sintering Implants: A 2-year prospective study in the posterior jaws of 16 patients. Implant. Dent. 2013, 22, 388–393. [Google Scholar] [CrossRef]
- Mangano, F.; De Franco, M.; Caprioglio, A.; Macchi, A.; Piattelli, A.; Mangano, C. Immediate, non-submerged, root-analogue direct laser metal sintering (DLMS) implants: A 1-year prospective study on 15 patients. Lasers Med Sci. 2013, 29, 1321–1328. [Google Scholar] [CrossRef]
- Kumar Malyala, S.; Kumar, R.Y.; Alwala, A.M. A 3D-printed osseointegrated combined jaw and dental implant prosthesis—A case study. Rapid Prototyp. J. 2017, 23, 1164–1169. [Google Scholar] [CrossRef]
- Cerea, M.; Dolcini, G.A. Custom-Made Direct Metal Laser Sintering Titanium Subperiosteal Implants: A Retrospective Clinical Study on 70 Patients. BioMed Res. Int. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Parr, W.C.; Burnard, J.L.; Singh, T.; McEvoy, A.; Walsh, W.R.; Mobbs, R.J. C3-C5 Chordoma Resection and Reconstruction with a Three-Dimensional Printed Titanium Patient-Specific Implant. World Neurosurg. 2019, 136, 226–233. [Google Scholar] [CrossRef]
- Willemsen, K.; Nizak, R.; Noordmans, H.J.; Castelein, R.M.; Weinans, H.; Kruyt, M.C. Challenges in the design and regulatory approval of 3D-printed surgical implants: A two-case series. Lancet Digit. Health 2019, 1, e163–e171. [Google Scholar] [CrossRef] [Green Version]
- Wei, F.; Li, Z.; Liu, Z.; Liu, X.; Jiang, L.; Yu, M.; Xu, N.; Wu, F.; Dang, L.; Zhou, H.; et al. Upper cervical spine reconstruction using customized 3D-printed vertebral body in 9 patients with primary tumors involving C2. Ann. Transl. Med. 2020, 8, 332. [Google Scholar] [CrossRef]
- Eltes, P.E.; Kiss, L.; Bartos, M.; Gyorgy, Z.M.; Csakany, T.; Bereczki, F.; Lesko, V.; Puhl, M.; Varga, P.P.; Lazary, A. Geometrical accuracy evaluation of an affordable 3D printing technology for spine physical models. J. Clin. Neurosci. 2020, 72, 438–446. [Google Scholar] [CrossRef] [Green Version]
- Park, J.W.; Song, C.A.; Kang, H.G.; Kim, J.H.; Lim, K.M.; Kim, H.-S. Integration of a Three-Dimensional-Printed Titanium Implant in Human Tissues: Case Study. Appl. Sci. 2020, 10, 553. [Google Scholar] [CrossRef] [Green Version]
- Park, J.W.; Kang, H.G.; Lim, K.M.; Kim, J.H.; Kim, H.S. Three-Dimensionally Printed Personalized Implant Design and Reconstructive Surgery for a Bone Tumor of the Calcaneus. JBJS Case Connect. 2018, 8, e25. [Google Scholar] [CrossRef]
- Park, J.W.; Kang, H.G.; Kim, J.H.; Kim, H.-S. New 3-dimensional implant application as an alternative to allograft in limb salvage surgery: A technical note on 10 cases. Acta Orthop. 2020, 91, 489–496. [Google Scholar] [CrossRef]
- Angelini, A.; Trovarelli, G.; Berizzi, A.; Pala, E.; Breda, A.; Ruggieri, P. Three-dimension-printed custom-made prosthetic reconstructions: From revision surgery to oncologic reconstructions. Int. Orthop. 2018, 43, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Liang, H.; Ji, T.; Zhang, Y.; Wang, Y.; Guo, W. Reconstruction with 3D-printed pelvic endoprostheses after resection of a pelvic tumour. Bone Jt. J. 2017, 99B, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Kamel, M.K.; Cheng, A.; Vaughan, B.; Stiles, B.; Altorki, N.; Spector, J.A.; Port, J.L. Sternal Reconstruction Using Customized 3D-Printed Titanium Implants. Ann. Thorac. Surg. 2019, 109, e411–e414. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, I.; Evans, P.L.; Goodrum, H.; Warbrick-Smith, J.; Bragg, T. Chest wall reconstruction with an anatomically designed 3-D printed titanium ribs and hemi-sternum implant. 3D Print. Med. 2020, 6, 1–7. [Google Scholar] [CrossRef]
- Turna, A.; Kavakli, K.; Sapmaz, E.; Arslan, H.; Caylak, H.; Gokce, H.S.; Demirkaya, A. Reconstruction with a patient-specific titanium implant after a wide anterior chest wall resection. Interact. Cardiovasc. Thorac. Surg. 2013, 18, 234–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mertz, L. Dream It, Design It, Print It in 3-D: What Can 3-D Printing Do for You? IEEE Pulse 2013, 4, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Abdulhameed, O.; Al-Ahmari, A.; Ameen, W.; Mian, S.H. Additive manufacturing: Challenges, trends, and applications. Adv. Mech. Eng. 2019, 11, 1687814018822880. [Google Scholar] [CrossRef] [Green Version]


| Materials | Tensile Strength (MPa) | Elastic Modulus (GPa) |
|---|---|---|
| Natural Bone | ||
| a. Tibia | 140 | 18.1 |
| b. Femur | 121 | 17.2 |
| c. Radius | 149 | 18.6 |
| d. Humerus | 130 | 17.2 |
| e. Cervical | 3.1 | 0.23 |
| f. Lumbar | 3.7 | 0.16 |
| Conventional Metals/Alloys | ||
| a. CP Ti | 785 | 105 |
| b. Ti-6Al-4V | 970 | 110 |
| c. Ti-6Al-7Nb | 1024 | 105 |
| d. Stainless steel 316L | 460–950 | 200 |
| e. Co-Cr alloys | 655–1896 | 210–250 |
| AM Porous Metals/Alloys | ||
| a. CP Ti | 78–245.5 | 5.5–8.5 |
| b. Ti-6Al-4V | 64–409 | 3.8–7.8 |
| c. Ti-6Al-7Nb | 105 | 1.2–4-5 |
| d. Stainless steel 316L | 300 | 0.15–0.12 |
| e. Co-Cr alloys | 60–150 | 20–25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raheem, A.A.; Hameed, P.; Whenish, R.; Elsen, R.S.; G, A.; Jaiswal, A.K.; Prashanth, K.G.; Manivasagam, G. A Review on Development of Bio-Inspired Implants Using 3D Printing. Biomimetics 2021, 6, 65. https://doi.org/10.3390/biomimetics6040065
Raheem AA, Hameed P, Whenish R, Elsen RS, G A, Jaiswal AK, Prashanth KG, Manivasagam G. A Review on Development of Bio-Inspired Implants Using 3D Printing. Biomimetics. 2021; 6(4):65. https://doi.org/10.3390/biomimetics6040065
Chicago/Turabian StyleRaheem, Ansheed A., Pearlin Hameed, Ruban Whenish, Renold S. Elsen, Aswin G, Amit Kumar Jaiswal, Konda Gokuldoss Prashanth, and Geetha Manivasagam. 2021. "A Review on Development of Bio-Inspired Implants Using 3D Printing" Biomimetics 6, no. 4: 65. https://doi.org/10.3390/biomimetics6040065
APA StyleRaheem, A. A., Hameed, P., Whenish, R., Elsen, R. S., G, A., Jaiswal, A. K., Prashanth, K. G., & Manivasagam, G. (2021). A Review on Development of Bio-Inspired Implants Using 3D Printing. Biomimetics, 6(4), 65. https://doi.org/10.3390/biomimetics6040065









