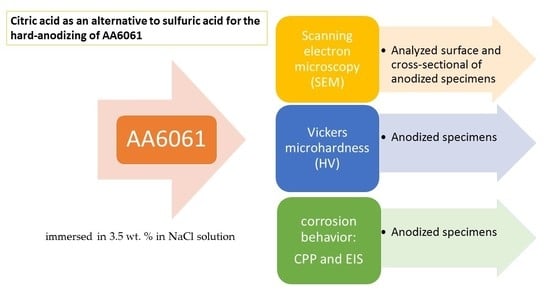
Citric Acid as an Alternative to Sulfuric Acid for the Hard-Anodizing of AA6061
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Anodizing Process
- ➢
- Degreased and pickling for 5 s in 50 wt. % HCl solution.
- ➢
- Three rinses in deionized water, each of 3 s duration.
- ➢
- Anodizing Bath:
- 2M sulfuric acid solution (196 g/L of H2SO4).
- 1M citric acid solution (192 g/L) with the addition of sulfuric acid of 5 and 10 mL/L.
- 2M citric acid solution (384 g/L) with the addition of sulfuric acid of 5 and 10 mL/L.
- The current generator used was a Model XLN30052-GL High Power Programmable DC Power Supply with a capacity of 300 Volts (V) and 5 Amperes (A). Current densities used, 3 and 4.5 A/dm2
- Time 60 min.
- Temperature at 5 °C ± 2 °C.
- ➢
- Rinse in deionized water for 5 s.
- ➢
- Sealing treatment, in H2O solution at 95 °C for 60 min.
2.3. Microstructural Characterization
2.4. Vickers Microhardness (HV)
2.5. Corrosion Test
3. Results
3.1. Chemical Composition
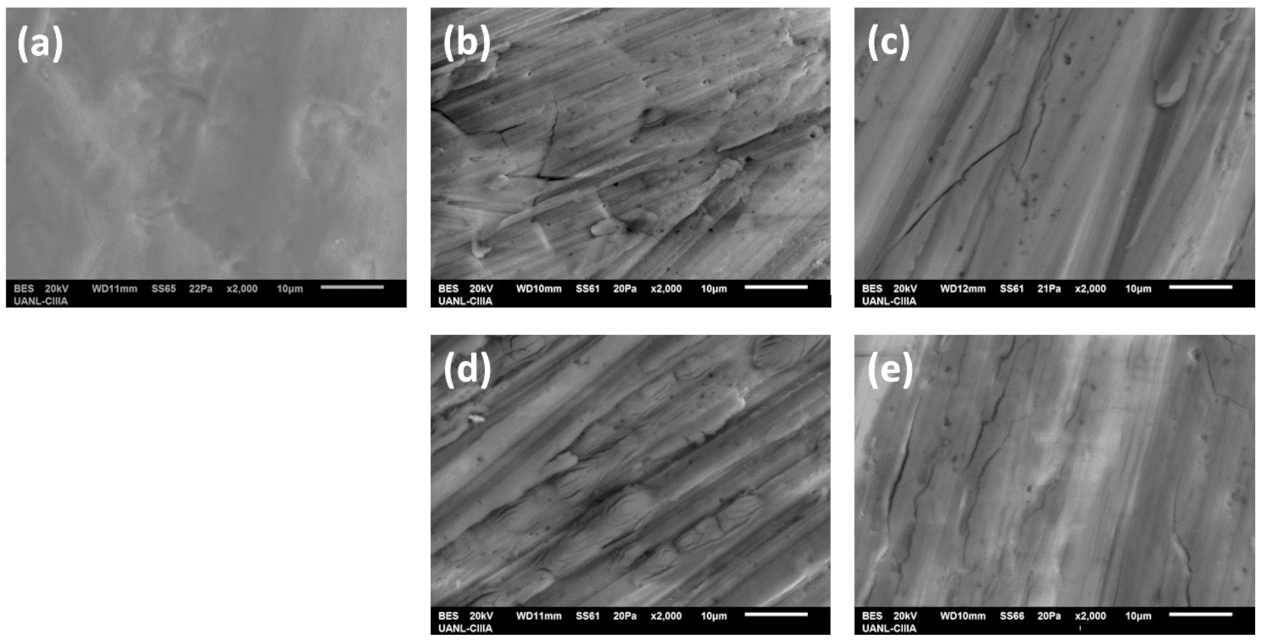
3.2. Microstructural Characterization (SEM)
3.2.1. Surface Morphology
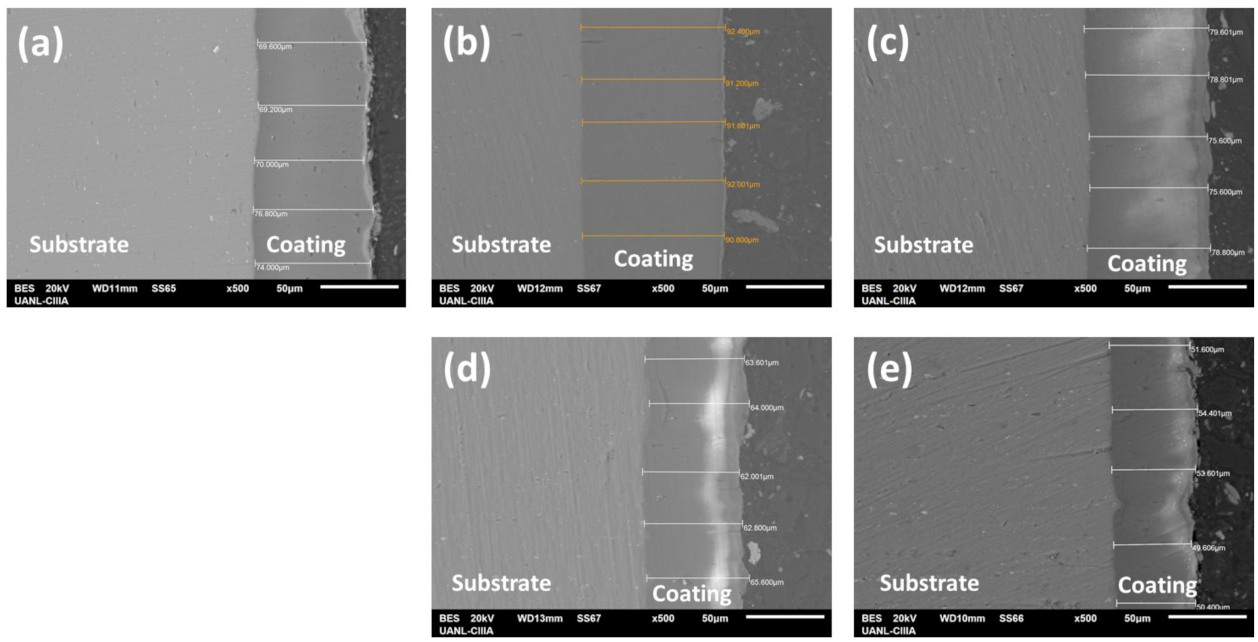
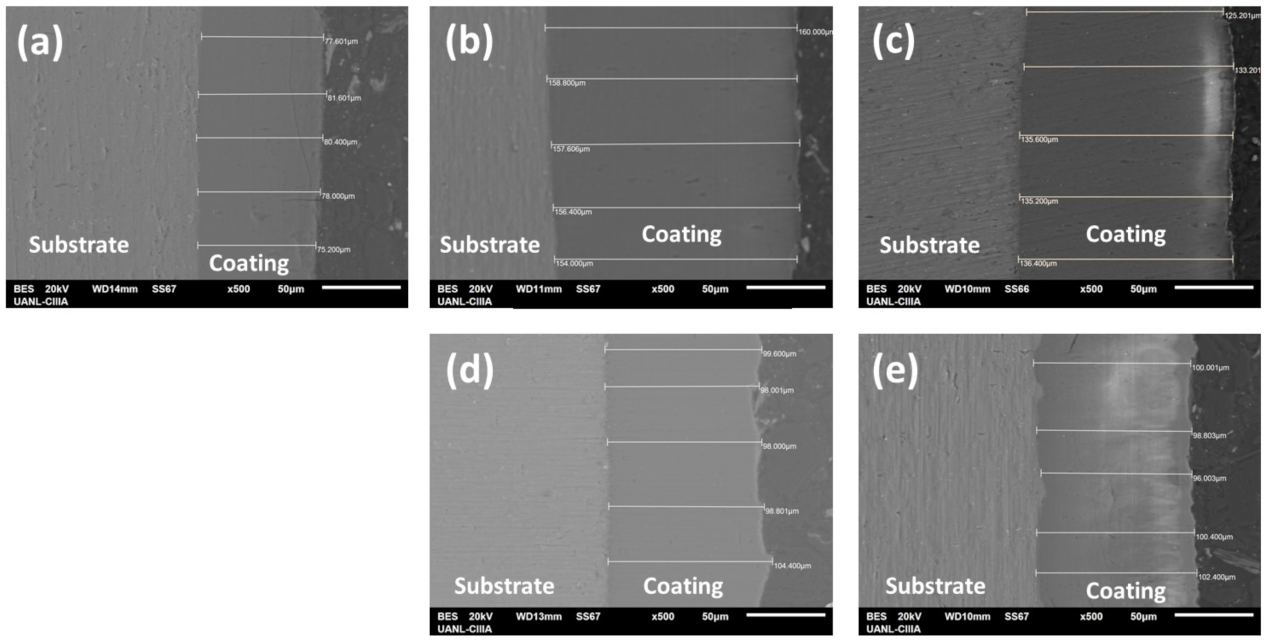
3.2.2. Cross-Sectional Morphology
3.2.3. Thickness of Anodized Materials
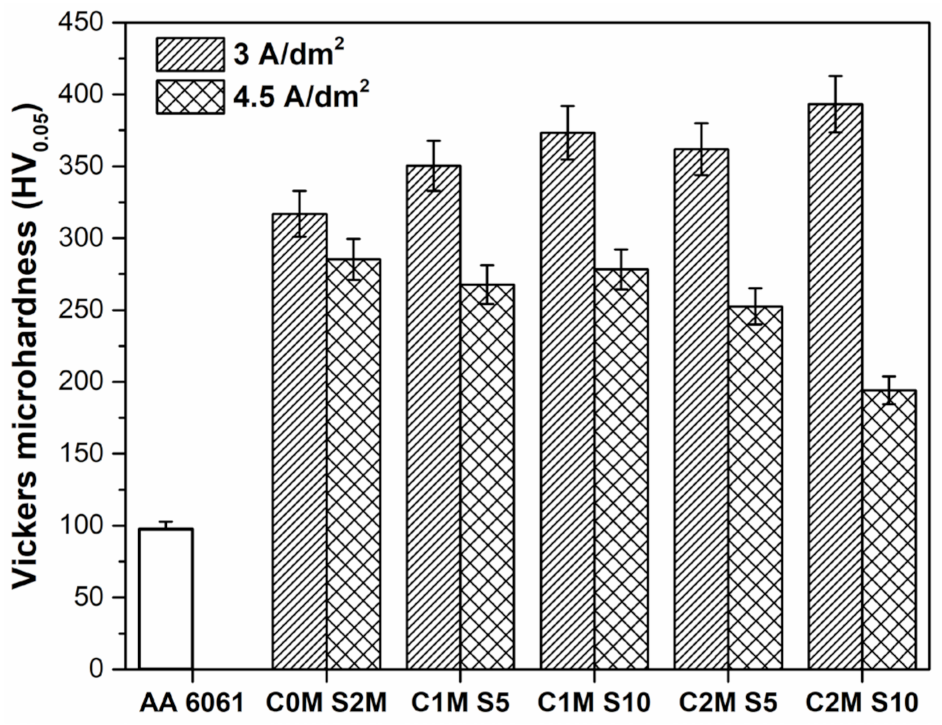
3.3. Vickers Microhardness (HV)
3.4. Corrosion Test
3.4.1. Cyclic Potentiodynamic Polarization (CPP)
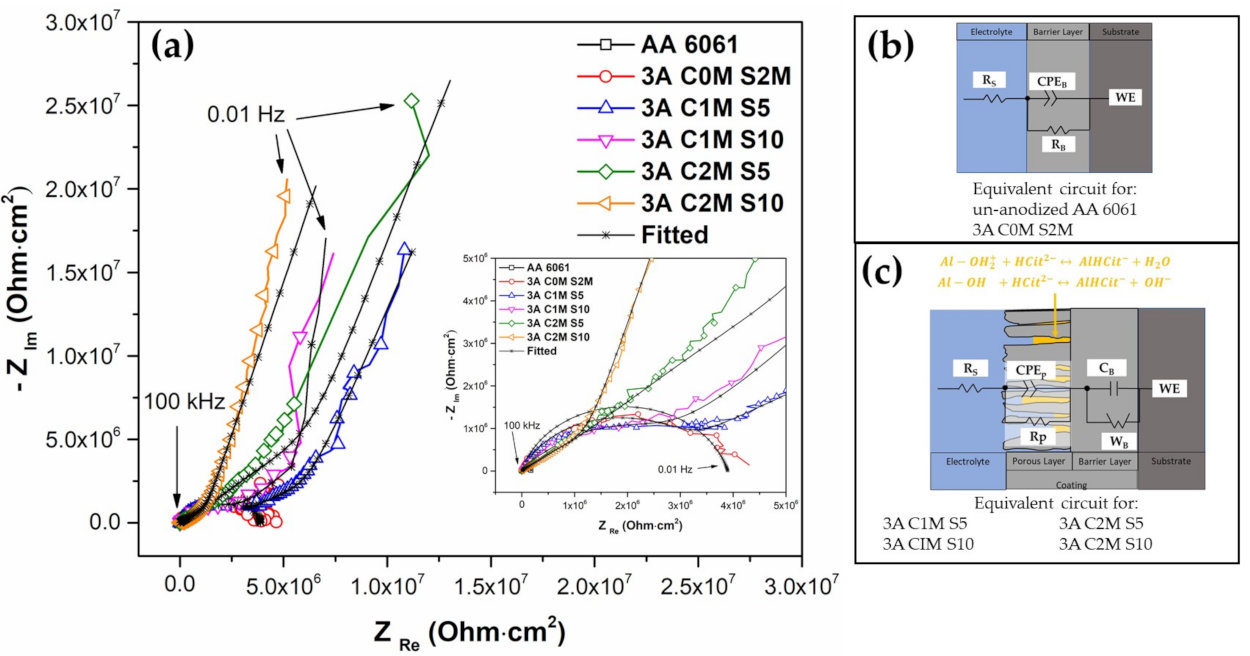
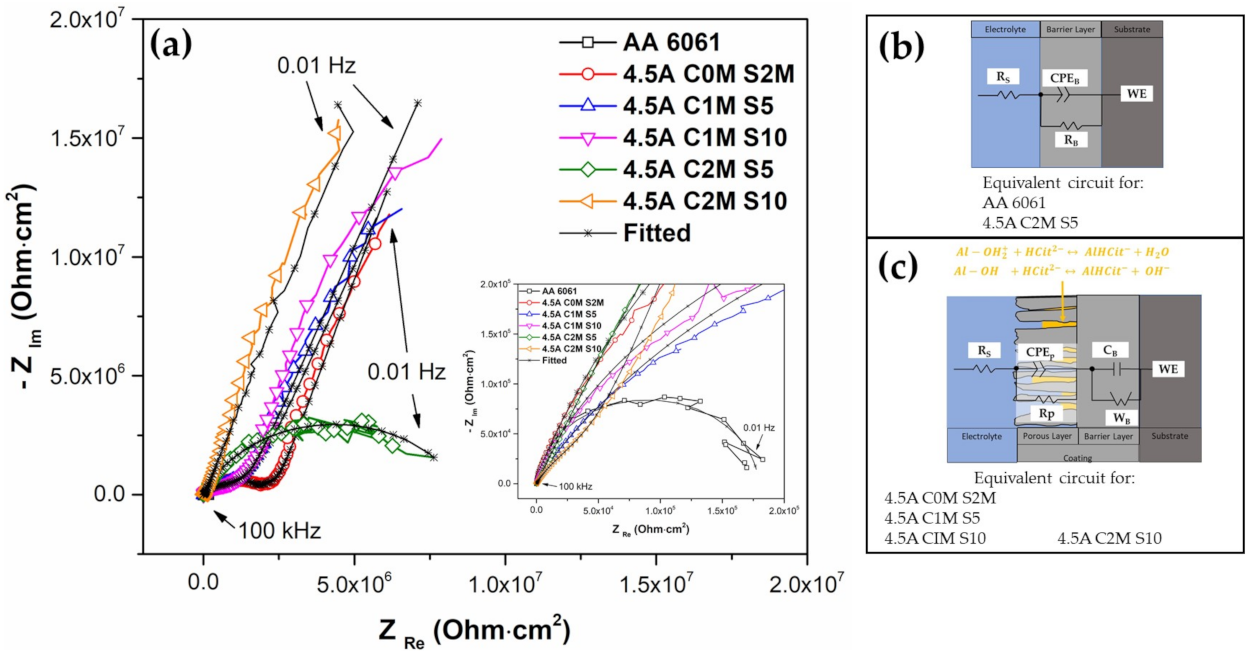
3.4.2. Electrochemical Impedance Spectroscopy (EIS)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evertsson, J.; Bertram, F.; Zhang, F.; Rullik, L.; Merte, L.R.; Shipilin, M.; Soldemo, M.; Ahmadi, S.; Vinogradov, N.; Carlà, F. The thickness of native oxides on aluminum alloys and single crystals. Appl. Surf. Sci. 2015, 349, 826–832. [Google Scholar] [CrossRef]
- Nguyen, L.; Hashimoto, T.; Zakharov, D.N.; Stach, E.A.; Rooney, A.P.; Berkels, B.; Thompson, G.E.; Haigh, S.J.; Burnett, T.L. Atomic-scale insights into the oxidation of aluminum. ACS Appl. Mater. Interfaces 2018, 10, 2230–2235. [Google Scholar] [CrossRef] [PubMed]
- Dumas, P.; Dubarry-Barbe, J.P.; Riviere, D.; Levy, Y.; Corset, J. Growth of thin alumina film on aluminum at room temperature: A kinetic and spectroscopic study by surface plasmon excitation. J. Phys. Colloq. 1983, 44, C10-205–C10-208. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, W.; Wang, W.; Wang, L.; Xue, Q. Novel anodic oxide film with self-sealing layer showing excellent corrosion resistance. Sci. Rep. 2017, 7, 1–9. [Google Scholar]
- Hagelsieb, L.M. Anodic Aluminum Oxide Processing, Characterization and Application to DNA Hybridization Electrical Detection. Ph.D. Thesis, Universite Catholique de Louvain, Louvain-La-Neuve, Belgique, 2007. [Google Scholar]
- Sharma, A.K.; Bhojraj, H.; Kaila, V.K.; Narayanamurthy, H. Anodizing and inorganic black coloring of aluminum alloys for space applications. Met. Finish. 1997, 95, 14. [Google Scholar] [CrossRef]
- Dasquet, P.C.; Caillard, D.; Conforto, E.; Bonino, J.P.; Bes, R. Investigation of the anodic oxide layer on 1050 and 2024T3 aluminum alloys by electron microscopy and electrochemical impedance spectroscopy. Thin Solid Films 2000, 371, 183–190. [Google Scholar] [CrossRef]
- Thompson, G.E. Porous anodic alumina: Fabrication, characterization and applications. Thin Solid Films 1997, 297, 192–201. [Google Scholar] [CrossRef]
- Ofoegbu, S.U.; Fábio, A.; Fernandes, F.A.O.; Pereira, A.B. The sealing step in aluminum anodizing: A focus on sustainable strategies for enhancing both energy efficiency and corrosion resistance. Coatings 2020, 10, 226. [Google Scholar] [CrossRef] [Green Version]
- Naoi, K.; Takeda, M.; Kanno, H.; Sakakura, M.; Shimada, A. Simultaneous electrochemical formation of Al2O3/polypyrrole layers (I): Effect of electrolyte anion in formation process. Electrochim. Acta 2000, 45, 3413–3421. [Google Scholar] [CrossRef]
- Shang, Y.; Wang, L.; Niu, D.; Liu, Z.; Wang, Y.; Liu, C. Effects of additive for anodizing electrolyte on anodic film of high silicon aluminum alloy. Int. J. Electrochem. Sci. 2016, 11, 1549–1557. [Google Scholar]
- Darwish, S. Anodization of aluminum in phosphate and carbonate solutions. Corrosion 1971, 27, 266–269. [Google Scholar] [CrossRef]
- Kikuchi, T.; Nakajima, D.; Nishinaga, O.; Natsui, S.; Suzuki, R.O. Porous aluminum oxide formed by anodizing in various electrolyte species. Curr. Nanosci. 2015, 11, 560–571. [Google Scholar] [CrossRef]
- Patra, N.; Salerno, M.; Losso, R.; Cingolani, R. Use of unconventional organic acids ass anodization electrolytes for fabrication of porous alumina. IEEE Nano 2009, 567–570. [Google Scholar]
- Kikuchi, T.; Nakajima, D.; Kawashima, J.; Natsui, S.; Suzuki, R.O. Fabrication of anodic porous alumina formed in malic acid solution. Appl. Suf. Sci. 2014, 313, 276–285. [Google Scholar] [CrossRef] [Green Version]
- MIL-A-8625F. Anodic Coatings for Aluminum and Aluminum Alloys; Departments and Agencies of the Department of Defense: The Pentagon Arlington, VA, USA, 1993; pp. 1–19. [Google Scholar]
- Edwards, J. Coating and Surface Treatment Systems for Metals; ASM International, Finishing Publications Ltd.: Naterials Park, OH, USA, 1994; Volume 38, pp. 35–40. [Google Scholar]
- Gabe, D.R. Hard anodizing—What do we mean by hard? Met. Finish. 2002, 100, 52–58. [Google Scholar] [CrossRef]
- Krishna, L.R.; Purnima, A.S.; Sundararajan, G. A comparative study of tribological behavior of microarc oxidation and hard-anodized coatings. Wear 2006, 261, 1095–1101. [Google Scholar] [CrossRef]
- Rajendra, A.; Biren, J.P.; Sharma, A.K.; Bhojraj, H.; Nayak, M.M.; Rajanna, K. Hard anodization of aluminium and its application to sensorics. Surf. Eng. 2005, 21, 193–197. [Google Scholar] [CrossRef]
- Jiang, C.X.; Tu, J.P.; Guo, S.Y.; Fu, M.F.; Zhao, X.B. Friction properties of oil-in filtrated porous AAO film on an aluminium substrate. Acta Metallurgica Sinica Engl. Lett. 2005, 18, 249–253. [Google Scholar]
- Lee, W. The anodization of aluminium for nanotechnology applications. JOM 2010, 62, 57–63. [Google Scholar] [CrossRef]
- Choi, J. Fabrication of Monodomain Porous Alumina Using Nanoimprint Lithography and Its Applications. Ph.D. Thesis, Martin-Luther-Universitat Halle, Wittenberg, Germany, 2004. [Google Scholar]
- Khan, I.U.; John, P.; Sheikh, S.T.; Gulzar, N.; Rehman, A.U. Anodizing of aluminum with improved corrosion properties. J. Chem. Soc. Pak. 2010, 32, 46–51. [Google Scholar]
- Garcia-Vergara, S.J.; Skeldon, P.; Thompson, G.E.; Habazaki, H. A tracer investigation of chromic acid anodizing of aluminium. Surf. Interface Anal. 2007, 39, 860–864. [Google Scholar] [CrossRef]
- Stępniowski, W.J.; Michalska-Domańska, M.; Norek, M.; Czujko, T. Fast Fourier transform based arrangement analysis of poorly organized alumina nanopores formed via self-organized anodization in chromic acid. Mater. Lett. 2014, 117, 69–73. [Google Scholar] [CrossRef]
- Sulfuric Acid. Pubchem.ncbi.nlm.nih.gov. 2020. Retrieved 9 March 2020. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Sulfuric-acid#section=EPA-Safer-Chemical (accessed on 13 March 2020).
- Schaedel, F.C. Sulfuric/organic electrolytes and total quality improvement (TQI) for present/future anodizing requirements. NASF Surf. Technol. 2017, 81, 1–17. [Google Scholar]
- Norek, M.; Lazewski, M. Manufacturing of highly ordered porous anodic alumina with conical pore shape and tunable interpore distance in the range of 550 nm to 650 nm. Mater. Sci. Pol. 2017, 35, 511–518. [Google Scholar] [CrossRef] [Green Version]
- Koczera, A.E. The Effects of Carboxylic Acids in Aluminum Anodizing. Ph.D. Thesis, Honors Theses and Capstones, University of New Hampshire, Durham, NH, USA, 2017. [Google Scholar]
- Citric Acid. Pubchem.ncbi.nlm.nih.gov. (2020). Retrieved 9 March 2020. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Citric-acid#section=EPA-Safer-Chemical (accessed on 9 January 2020).
- Wojciech, J.S.; Moneta, M.; Norek, M.; Michalska-Domańska, M.; Scarpellini, A.; Salerno, M. The influence of electrolyte composition on the growth of nanoporous anodic alumina. Electroch. Acta 2016, 211, 453–460. [Google Scholar]
- Franco, M.; Krishna, T.H.; Pillai, A.M.; Rajendra, A.; Sharma, A.K. A comparative study on the corrosion behavior of hard anodic coatings on aa 6061 obtained using dc and pulsed dc power sources. Acta Metall. Sin. Engl. Lett. 2013, 26, 647–656. [Google Scholar] [CrossRef] [Green Version]
- ASTM E3-95. Standard Practice for Preparation of Metallographic Specimens; ASTM International: West Conshohocken, PA, USA, 1995. [Google Scholar]
- ASTM E407-07. Standard Practice for Microetching Metals and Alloys; ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar]
- Huang, Y.L.; Shih, H.; Huang, H.C.; Daugherty, J.; Wu, S.; Ramanathan, S.; Chang, C.; Mansfeld, F. Evaluation of the corrosion resistance of anodized aluminum 6061 using electrochemical impedance spectroscopy (EIS). Corros. Sci. 2008, 50, 3569–3575. [Google Scholar] [CrossRef]
- Zuo, Y.; Zhao, P.H.; Zhao, J.M. The influences of sealing methods on corrosion behavior of anodized aluminum alloys in NaCl solutions. Surf. Coat. Technol. 2003, 166, 237–242. [Google Scholar] [CrossRef]
- Suay, J.J.; Gimenez, E.; Rodriguez, T.; Habbib, K.; Saura, J.J. Characterization of anodized and sealed aluminium by EIS. Corros. Sci. 2003, 45, 611–624. [Google Scholar] [CrossRef]
- Cabral-Miramontes, J.A.; Barceinas-Sánchez, J.D.O.; Poblano-Salas, C.A.; Pedraza-Basulto, G.K.; Nieves-Mendoza, D.; Zambrano-Robledo, P.C.; Almeraya-Calderón, F.; Chacón-Nava, J.G. Corrosion Behavior of AISI 409Nb Stainless Steel Manufactured by Powder Metallurgy Exposed in H2SO4 and NaCl Solutions. Int. J. Electrochem. Sci. 2013, 8, 564–577. [Google Scholar]
- Cabral Miramontes, J.A.; Barceinas Sánchez, J.D.O.; Almeraya Calderón, F.; Martínez Villafañe, A.; Chacón Nava, J.G. Effect of Boron Additions on Sintering and Densification of a Ferritic Stainless Steel. J. Mater. Eng. Perform. 2010, 19, 880–884. [Google Scholar] [CrossRef]
- ASTM G5-11. Standard Reference Test Method for Making Potentiostatic and Potentiodynamic Anodic Polarization Measurements; ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar]
- ASTM G106-15. Standard Practice for Verification of Algorithm and Equipment for Electrochemical Impedance Measurements; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Setyarini, P.H.; Soenoko, R.; Suprapto, A.; Irawan, Y.S. Effect of low potential anodizing of AA 6061. J. Eng. Appl. Sci. 2017, 12, 6419–6422. [Google Scholar]
- Gilling, F.G. WADC Technical Report, Study of Hard Coating for Aluminum Alloys; Cornell Aeronautical Laboratory, Wright Air Development Center, Wright-Patterson Air Force Base: Dayton, OH, USA, 1987; pp. 53–151. [Google Scholar]
- Military Handbook, MIL_HDBK-132A (ORD), Department of the Army Technical Bulletin TB-700-801D-1, Chapter 5: Elec-trochemical conversion coatings (anodic coatings). In Protective Finishes for Metal and Wood Surfaces; Federal and Military Specification and Standars are Government Publications; The Naval Publication and Dorms Center: Philadelphia, PA, USA, 1984; pp. 83–100.
- López, V.; Otero, E.; Bautista, A.; González, J.A. Sealing of anodic films obtained in oxalic acid baths. Surf. Coat. Technol. 2000, 124, 76–84. [Google Scholar] [CrossRef]
- Van Der Linden, B.; Terryn, H.; Vereecken, J. Investigation of anodic aluminium oxide layers by electrochemical impedance spectroscopy. J. Appl. Electrochem. 1990, 20, 798–803. [Google Scholar] [CrossRef]
- Moutarlier, V.; Gigandet, M.P.; Ricq, L.; Pagetti, J. Electrochemical characterization of anodic oxidation films formed in presence of corrosion inhibitors. Appl. Surf. Sci. 2001, 183, 1–9. [Google Scholar] [CrossRef]
- Schneider, M.; Liebmann, T.; Langklotz, U.; Michaelis, A. Microelectrochemical investigation of anodic oxide formation on the aluminum alloy AA2024. Electrochim. Acta 2017, 249, 198–205. [Google Scholar] [CrossRef]
- Peng, L.; Li, M. Improved technology for hard anodizing dissolution of aluminum alloy part. The Open Mat. Sci. J. 2015, 9, 82–85. [Google Scholar] [CrossRef] [Green Version]
- Chu, S.Z.; Wada, K.; Inoue, S.; Isogai, M.; Yasumori, A. Fabrication of ideally ordered nanoporous alumina films and integrated alumina nanotubule arrays by high-field anodization. Adv. Mater. 2005, 17, 2115–2119. [Google Scholar] [CrossRef]
- Schwirn, K.; Lee, W.; Hillebrand, R.; Steinhart, M.; Nielsch, K.; Gösele, U. Self-ordered anodic aluminum oxide formed by H2SO4 hard anodization. ACS Nano 2008, 2, 302–310. [Google Scholar] [CrossRef]
- Noh, K.; Brammer, K.; Kim, H.; Jung, S.; Seong, T.; Jin, S. Highly self-assembled nanotubular aluminum oxide by hard anodization. J. Mater. Res. 2011, 26, 186–193. [Google Scholar] [CrossRef]
- Lerner, L.M. Hard anodizing of aerospace aluminium alloys. Trans. Inst. Met. Finish. 2010, 88, 21–24. [Google Scholar] [CrossRef]
- Kwolek, P.; Krupa, K.; Obłój, A.; Kocurek, P.; Wierzbin´ska, M.; Sieniawski, J. Tribological Properties of the Oxide Coatings Produced onto 6061-T6 Aluminum Alloy in the Hard Anodizing Process. J. Mater. Eng. Perform. 2018, 27, 3268–3275. [Google Scholar] [CrossRef] [Green Version]
- Kwolek, P. Hard anodic coatings on aluminum alloys. Adv. Manuf. Sci. Tech. 2017, 41, 35–46. [Google Scholar]
- Thomas, R.W. Hard Anodizing of Aluminum; Brace, A.W., Ed.; Technicopy Ltd.: Stonehouse, UK, 1987; pp. 1–7. [Google Scholar]
- Wang, L.W.; Tian, H.Y.; Gao, H.; Xie, F.Z.; Zhao, K.; Cui, Z.Y. Electrochemical and XPS analytical investigation of the accelerative effect of bicarbonate/carbonate ions on AISI 304 in alkaline environment. Appl. Surf. Sci. 2019, 492, 792–807. [Google Scholar] [CrossRef]
- Yang, Y.J.; Zhang, Z.P.; Jin, Z.S.; Sun, W.C.; Xia, C.Q.; Ma, M.Z.; Zhang, X.Y.; Li, G.; Liu, R.P. A study on the corrosion behavior of the in-situ Ti-based bulk metallic glass matrix composites in acid solutions. J. Alloys Compd. 2019, 782, 927–935. [Google Scholar] [CrossRef]
- Khamaj, J.A. Cyclic polarization analysis of corrosion behavior of ceramic coating on 6061 Al/SiCp composite for marine applications. Prot. Met. Phys. Chem. Surf. 2016, 52, 886–893. [Google Scholar] [CrossRef]
- Loto, R.T.; Adeleke, A.J. Corrosion of Aluminum Alloy Metal Matrix Composites in Neutral Chloride Solutions. Failure Anal. Prev. 2016, 16, 874–885. [Google Scholar] [CrossRef]
- Silverman, D.C. Practical Corrosion Prediction Using Electrochemical Techniques, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 1129–1166. [Google Scholar] [CrossRef]
- McCafferty, E. Introduction to Corrosion Science; Springer: New York, NY, USA, 2010. [Google Scholar]
- El-Hameed, A.M.A.; Abdel-Aziz, Y.A.; El-Tokhy, F.S. Anodic Coating Characteristics of Different Aluminum Alloys for Spacecraft Materials Applications. J. Mater. Sci. Appl. 2017, 8, 197–208. [Google Scholar] [CrossRef] [Green Version]
- Arredondo-Rea1, P.S.; Corral-Higuera, R.; Neri-Flores, M.A.; Gómez-Soberón, J.; Almeraya-Calderón, F.; Castorena-González, K.H.; Almaral-Sánchez, J.L. Electrochemical Corrosion and Electrical Resistivity of Reinforced Recycled Aggregate Concrete. Int. J. Electrochem. Sci. 2011, 6, 475–483. [Google Scholar]
- Jalal, H.; Hussein, K.; Karabet, F.; Saound, Y. Study of the effect mechanism of organic additives on the aluminum alloy 6066 anodization. J. Miner. Met. Mater. Eng. 2019, 5, 73–81. [Google Scholar]
- Sposito, G. The Environmental Chemistry of Aluminum, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1996; pp. 173–174, 303–305. [Google Scholar]
- Hidber, P.C.; Graule, T.J.; Gauckler, L.J. Citric Acid—A Dispersant for Aqueous Alumina Suspensions. J. Am. Ceram. Soc. 1996, 79, 1857–1867. [Google Scholar] [CrossRef]
- Wieland, E.; Stumm, W. Dissolution kinetics of kaolinite in acidic aqueous solutions at 25 °C. Geochim. Acta 1992, 56, 3339–3355. [Google Scholar] [CrossRef]
- Šeruga, M.; Hasenay, D. Electrochemical and surface properties of aluminium in citric acid solutions. J. Appl. Electrochem. 2001, 31, 961–967. [Google Scholar] [CrossRef]
- Huang, Y.; Esra, K.; Mansfeld, F. Evaluation of the corrosion resistance of anodizing aluminum samples using electrochemical impedance spectroscopy. In Proceedings of the Annual Graduate Student Research Symposium, Los Angeles, CA, USA; 2007. [Google Scholar]
- Corral, H.R.; Arredondo, R.S.P.; Neri, F.M.; Gómez, S.J.M.; Almeraya, C.F.; Castorena, G.J.H.; Almaral, S.J. Sulfate attack and reinforcement corrosion in concrete with recycled concrete aggregates and supplementary cementing materials. Int. J. Electrochem. Sci. 2011, 6, 613–621. [Google Scholar]
- Cabral-Miramontes, J.A.; Gaona-Tiburcio, C.; Almeraya-Calderón, F.; Estupiñan-Lopez, H.F.; Pedraza-Basulto, G.; Poblano-Salas, C. Parameter Studies on High-Velocity Oxy-Fuel Spraying of CoNiCrAlY Coatings Used in the Aeronautical Industry. Int. J. Corros. 2014, 703806, 1–8. [Google Scholar] [CrossRef]
- Dzhurinskiy, D.V.; Dautov, S.S.; Shornikov, P.G.; Akhatov, I.S. Surface Modification of Aluminum 6061-O Alloy by Plasma Electrolytic Oxidation to Improve Corrosion Resistance Properties. Coatings 2021, 11, 4. [Google Scholar] [CrossRef]
- Ryu, H.S.; Mun, S.-J.; Lim, T.S.; Kim, H.-C.; Shin, K.-S.; Hong, S.-H.; Tanasini, P.; Diethelm, S.; Van Herle, J.; Favrat, D. Microstructure evolution during plasma electrolytic oxidation and its effects on the electrochemical properties of AZ91D Mg alloy. J. Electrochem. Soc. 2011, 158, 266–273. [Google Scholar] [CrossRef]
- Domingues, L.; Fernandes, J.C.S.; Belo, M.D.C.; Ferreira, M.G.S.; Guerra-Rosa, L. Anodizing of Al 2024-T3 in a modified sulphuric acid/boric acid bath for aeronautical applications. Corros. Sci. 2003, 45, 149–160. [Google Scholar] [CrossRef]
- Analysis of Alternatives for Chromium Sealing in the Aerospace Industry—GCCA_AoA_Potassium Dichromate—2007. Available online: https://echa.europa.eu/documents/10162/d6c88cd1-ef18-460b-8493-e1d59211a888 (accessed on 5 November 2019).
- Mansfeld, F.; Shih, H.; Greene, H.; Tsai, C.H. Analysis of EIS-data for common corrosion processes. In Electrochemical Impedance: Analysis and Interpretation, ASTM STP 1188; Scully, J.R., Silverman, D.D., Kendig, M.W., Eds.; ASTM: San Diego, CA, USA, 1991. [Google Scholar] [CrossRef]
- MacDonald, J.R.; Johnson, W.B. Impedance Spectroscopy; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1987; pp. 154–156. [Google Scholar]
- Fonseca, C.; Barbosa, M.A. Corrosion behaviour of titanium in biofluids containing H2O2 studied by electrochemical impedance spectroscopy. Corros. Sci. 2001, 43, 547–559. [Google Scholar] [CrossRef]
- Macdonald, D. Reflections on the history of electrochemical impedance spectroscopy. Electrochim. Acta 2006, 51, 1376–1388. [Google Scholar] [CrossRef]
- Liu, J.C.; Park, S.W.; Nagao, S.J.; Nogi, M.; Koga, H.; Ma, J.S. The role of Zn precipitates and Cl anions in pitting corrosion of Sn–Zn solder alloys. Corros. Sci. 2015, 92, 263–271. [Google Scholar] [CrossRef]
- Cabral-Miramontes, J.; Gaona-Tiburcio, C.; Estupinán-López, F.; Lara-Banda, M.; Zambrano-Robledo, P.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Chacón-Nava, J.; Almeraya-Calderón, F. Corrosion Resistance of Hard Coat Anodized AA 6061 in Citric–Sulfuric Solutions. Coatings 2020, 10, 601. [Google Scholar] [CrossRef]
- Khadiri, M.; Elyaagoubi, M.; Idouhli, R.; Koumya, Y.; Zakir, O.; Benzakour, J.; Benyaich, A.; Abouelfida, A.; Outzourhit, A. Electrochemical Study of Anodized Titanium in Phosphoric. Acid. Adv. Mater. Sci. Eng. 2020, 2020, 1–11. [Google Scholar] [CrossRef]
- Macdonald, D.D. Review of mechanistic analysis by electrochemical impedance spectroscopy. Electrochim. Acta 1990, 35, 1509–1525. [Google Scholar] [CrossRef]
- Cesiulis, H.; Tsyntsaru, N.; Ramanavicius, A.; Ragoisha, G. The Study of Thin Films by Electrochemical Impedance Spectroscopy. Chapter, I. In Nanostructures and Thin Films for Multifunctional Applications; Tiginyanu, I., Topala, P., Ursaki, V., Eds.; NanoScience and Technology; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
- Kramer, G.R.; Mendez, C.M.; Ares, A.E. Evaluation of corrosion resistance of aluminum-based alloys in bioethanol produced in Misiones. Proc. Mat. Sci. 2015, 9, 341–349. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, R.; Seshadri, S.K. Phosphoric acid anodization of Ti–6Al–4V– Structural and corrosion aspects. Corros. Sci. 2007, 49, 542–558. [Google Scholar] [CrossRef]
- Shao, L.; Li, H.; Jiang, B.; Liu, C.; Gu, X.; Chen, D. A Comparative Study of Corrosion Behavior of Hard Anodized and Micro-Arc Oxidation Coatings on 7050 Aluminum Alloy. Metals 2018, 8, 165. [Google Scholar] [CrossRef] [Green Version]
- Ban, C.L.; He, Y.D.; Xin, S. Effect of citric acid on microstructure and electrochemical characteristics of high voltage anodized alumina film formed on etched Al Foils. Trans. Nonferrous Met. Soc. China 2011, 21, 133–138. [Google Scholar] [CrossRef]
- Xiangfeng, M.; Guoying, W.; Hongliang, G.; Yundan, Y.; Ying, C.; Dettinger, H. Anodization for 2024 Al Alloy from Sulfuric-Citric Acid and Anticorrosion Performance of Anodization Films. Int. J. Electrochem. Sci. 2013, 8, 10660–10671. [Google Scholar]
- Tang, C.W. The Study of Anodic Treatment of Aluminum in Tertiary Mixed Acid after High Temperature Pre-Immersing. Ph.D. Thesis, Tatung University, Taipei City, Taiwan, 2005. [Google Scholar]
- Martinez-Villafañe, A.; Chacon-Nava, J.G.; Gaona-Tiburcio, C.; Almeraya-Calderon, F.; Domínguez-Patiño, G.; Gonzalez-Rodríguez, G. Oxidation performance of a Fe–13Cr alloy with additions of rare earth elements. Mater. Sci. Eng. A 2003, 363, 15–19. [Google Scholar] [CrossRef]
- Gaona-Tiburcio, C.; Montoya, R.M.; Cabral, M.J.A.; Estupiñan, L.F.; Zambrano, R.P.; Orozco, C.R.; Chacon-Nava, J.G.; Baltazar, Z.M.A.; Almeraya-Calderon, F. Corrosion resistance of multilayer coatings deposited by PVD on inconel 718 using electrochemical impedance spectroscopy technique. Coatings 2020, 10, 521. [Google Scholar] [CrossRef]




| Alloy | Anodizing | Sealing | Nomenclature | |||
|---|---|---|---|---|---|---|
| Bath | Current Density (A/dm2) | Time (min) | ||||
| Citric Acid | Sulfuric Acid | |||||
| AA6061 | --- | 2M | 3 | 60 | Deionized water at 95 °C for 60 min | 3A C0M S2M |
| 1M | 5 mL/L | 3A C1M S5 | ||||
| 1M | 10 mL/L | 3A C1M S10 | ||||
| 2M | 5 mL/L | 3A C2M S5 | ||||
| 2M | 10 mL/L | 3A C2M S10 | ||||
| --- | 2M | 4.5 | 60 | Deionized water at 95 °C for 60 min | 4.5A C0M S2M | |
| 1M | 5 mL/L | 4.5A C1M S5 | ||||
| 1M | 10 mL/L | 4.5A C1M S10 | ||||
| 2M | 5 mL/L | 4.5A C2M S5 | ||||
| 2M | 10 mL/L | 4.5A C2M S10 | ||||
| Elements | Al | Cu | Mg | Mn | Fe | Si | Zn | Cr | Ti |
|---|---|---|---|---|---|---|---|---|---|
| 6061 | Bal. | 0.08 ± 0.004 | 2.14 ± 0.107 | 0.03 ± 0.0015 | 0.11 ± 0.0055 | 2.14 ± 0.107 | 0.15 ± 0.0075 | 0.12 ± 0.006 | 0.08 ± 0.004 |
| Samples | Ecorr (V vs. SCE) | Epit (V vs. SCE) | E(Anodic to Cathodic Transition) (V vs. SCE) | ipass (A/cm2) | icorr (A/cm2) | Hysteresis |
|---|---|---|---|---|---|---|
| AA6061 | −1.02 | −0.689 | −0.858 | 2.22 × 10−5 | 9.47 × 10−6 | + |
| 3A C0M S2M | −0.767 | −0.124 | −0.793 | 7.41 × 10−9 | 4.48 × 10−9 | − |
| 3A C1M S5 | −0.230 | --- | 0.112 | 4.32 × 10−9 | 3.14 × 10−10 | − |
| 3A C1M S10 | −0.551 | −0.161 | --- | 2.06 × 10−9 | 1.02 × 10−9 | + |
| 3A C2M S5 | −0.734 | −0.387 | −0.844 | 3.55 × 10−9 | 1.57 × 10−9 | + |
| 3A C2M S10 | −0.857 | −0.540 | −0.820 | 1.30 × 10−9 | 6.47 × 10−9 | + |
| 4.5A C0M S2M | −0.293 | --- | 0.131 | 5.91 × 10−9 | 1.27 × 10−9 | − |
| 4.5A C1M S5 | −0.590 | --- | 0.065 | 4.09 × 10−9 | 4.79 × 10−10 | − |
| 4.5A C1M S10 | −0.230 | --- | 0.150 | 5.39 × 10−9 | 1.15 × 10−9 | − |
| 4.5A C2M S5 | −0.926 | --- | --- | 3.16 × 10−9 | 8.35 × 10−10 | − |
| Sample | Rs (Ω·cm2) | CPEp (Ω−1 sαp cm−2) | Rp (Ω·cm2) | αp | CB (F/cm2) | RB (Ω·cm2) | αB | WB (Ω·cm2/s0.5) | Error (%) | χ2 |
|---|---|---|---|---|---|---|---|---|---|---|
| AA 6061 | 21.61 | --- | --- | --- | 6.84 × 10−6 | 0.17 × 106 | 0.94 | --- | <2.58 | 3 × 10−2 |
| 3A C0M S2M | 27.28 | --- | --- | --- | 1.82 × 10−9 | 3.90 × 106 | 0.83 | --- | <1.33 | 1 × 10−2 |
| 3A C1M S5 | 14.17 | 0.46 × 10−8 | 2.67 × 106 | 0.77 | 1.04 × 10−9 | --- | --- | 8.63 × 106 | <2.35 | 8 × 10−3 |
| 3A C1M S10 | 26.34 | 0.94 × 10−8 | 2.32 × 106 | 0.84 | 5.49 × 10−9 | --- | --- | 20.60 × 106 | <2.01 | 3 × 10−2 |
| 3A C2M S5 | 14.99 | 7.40 × 10−8 | 1.05 × 106 | 0.62 | 31.10 × 10−9 | --- | --- | 23.90 × 106 | <5.39 | 1 × 10−2 |
| 3A C2M S10 | 18.85 | 85.30 × 10−8 | 0.02 × 106 | 0.67 | 1.53 × 10−9 | --- | --- | 3.20 × 106 | <2.50 | 3 × 10−3 |
| 4.5A C0M S2M | 15.20 | 0.44 × 10−8 | 1.54 × 106 | 0.79 | 2.74 × 10−9 | --- | --- | 2.40 × 106 | <2.83 | 1 × 10−3 |
| 4.5A C1M S5 | 24.73 | 7.18 × 10−8 | 0.30 × 106 | 0.68 | 15.20 × 10−9 | --- | --- | 3.32 × 106 | <3.61 | 2 × 10−3 |
| 4.5A C1M S10 | 35.38 | 5.03 × 10−8 | 0.40 × 106 | 0.72 | 6.71 × 10−9 | --- | --- | 3.00 × 106 | <2.56 | 2 × 10−3 |
| 4.5A C2M S5 | 42.74 | --- | --- | --- | 228.00 × 10−9 | 8.65 × 106 | 0.76 | --- | <1.73 | 1 × 10−2 |
| 4.5A C2M S10 | 21.67 | 62.3 × 10−8 | 0.04 × 106 | 0.70 | 551.00 × 10−9 | --- | --- | 128.00 × 106 | <3.55 | 5 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabral-Miramontes, J.; Almeraya-Calderón, F.; López, F.E.; Lara Banda, M.; Olguín-Coca, J.; López-León, L.D.; Castañeda-Robles, I.; Alcalá, M.Á.E.; Zambrano-Robledo, P.; Gaona-Tiburcio, C. Citric Acid as an Alternative to Sulfuric Acid for the Hard-Anodizing of AA6061. Metals 2021, 11, 1838. https://doi.org/10.3390/met11111838
Cabral-Miramontes J, Almeraya-Calderón F, López FE, Lara Banda M, Olguín-Coca J, López-León LD, Castañeda-Robles I, Alcalá MÁE, Zambrano-Robledo P, Gaona-Tiburcio C. Citric Acid as an Alternative to Sulfuric Acid for the Hard-Anodizing of AA6061. Metals. 2021; 11(11):1838. https://doi.org/10.3390/met11111838
Chicago/Turabian StyleCabral-Miramontes, José, Facundo Almeraya-Calderón, Francisco Estupinán López, María Lara Banda, Javier Olguín-Coca, Luis Daimir López-León, Ivan Castañeda-Robles, Miguel Ángel Esneider Alcalá, Patricia Zambrano-Robledo, and Citlalli Gaona-Tiburcio. 2021. "Citric Acid as an Alternative to Sulfuric Acid for the Hard-Anodizing of AA6061" Metals 11, no. 11: 1838. https://doi.org/10.3390/met11111838
APA StyleCabral-Miramontes, J., Almeraya-Calderón, F., López, F. E., Lara Banda, M., Olguín-Coca, J., López-León, L. D., Castañeda-Robles, I., Alcalá, M. Á. E., Zambrano-Robledo, P., & Gaona-Tiburcio, C. (2021). Citric Acid as an Alternative to Sulfuric Acid for the Hard-Anodizing of AA6061. Metals, 11(11), 1838. https://doi.org/10.3390/met11111838