New Insights into the Antibacterial Activity of Hydroxycoumarins against Ralstonia solanacearum
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antibacterial Activity of Screened Coumarins against R. solanacearum
2.2. MIC and MBC of Hydroxycoumarins against R. solanacearum
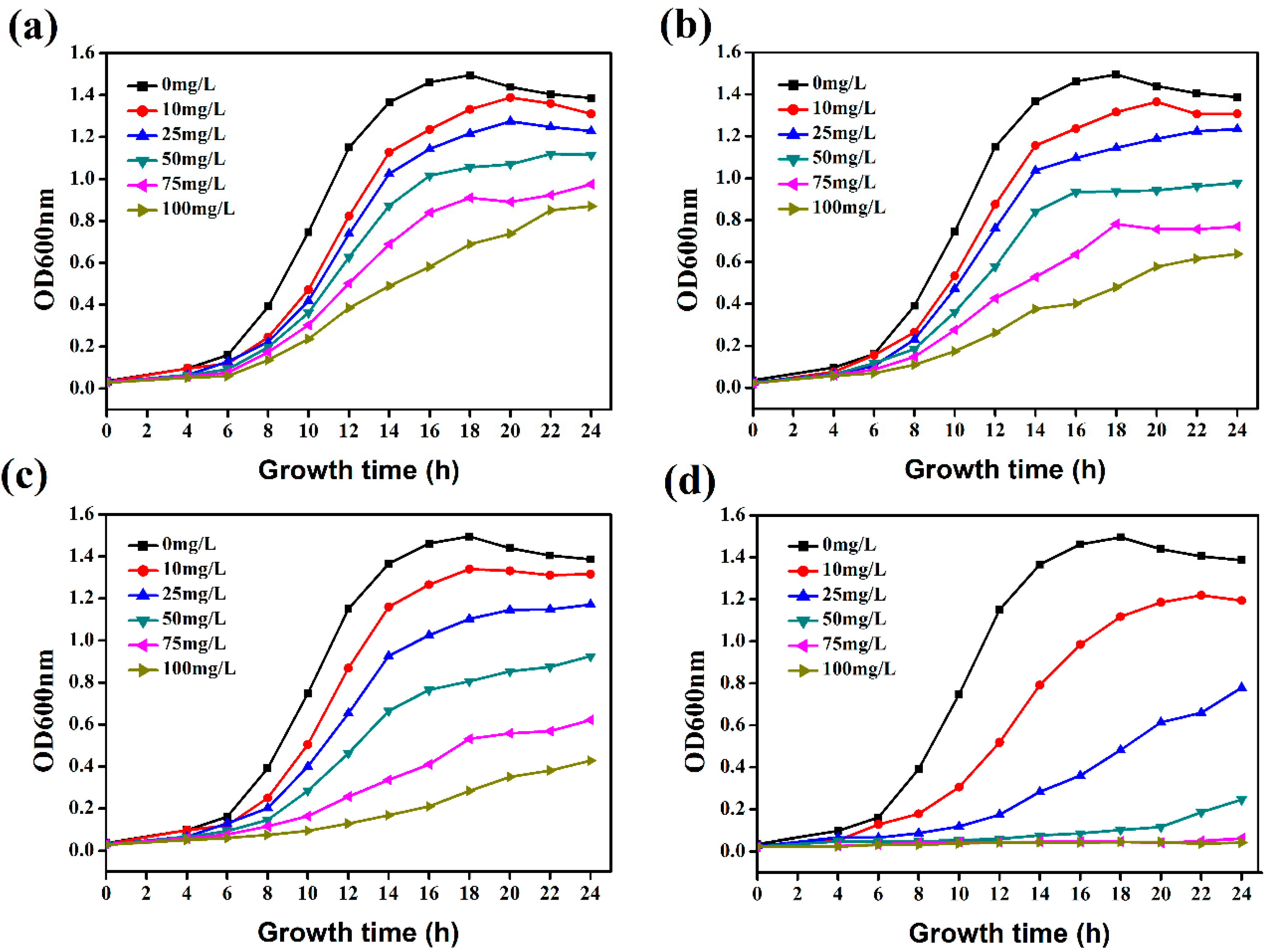
2.3. Hydroxycoumarins Inhibit the Growth of R. solanacearum
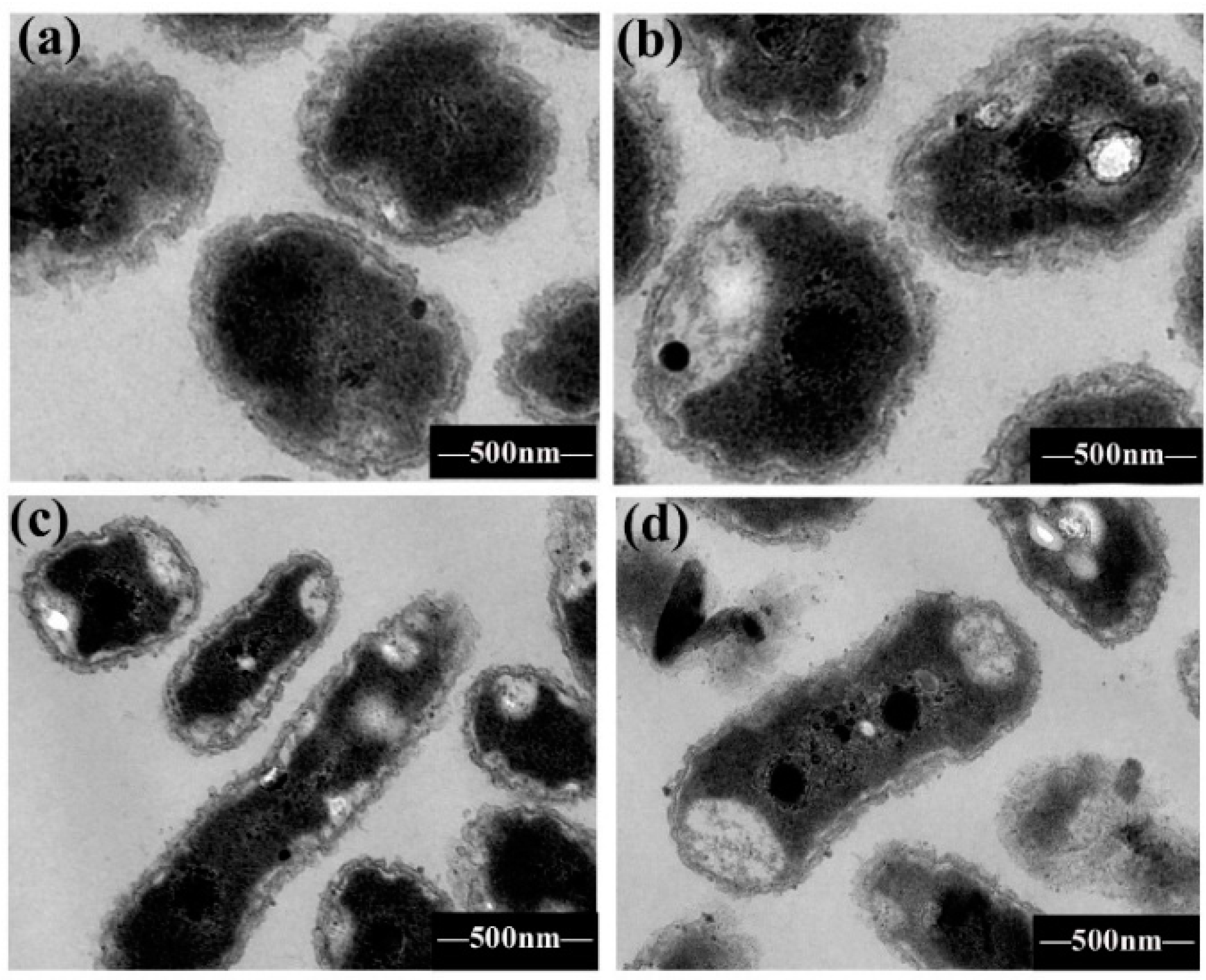
2.4. Bacterial Morphological Change by TEM
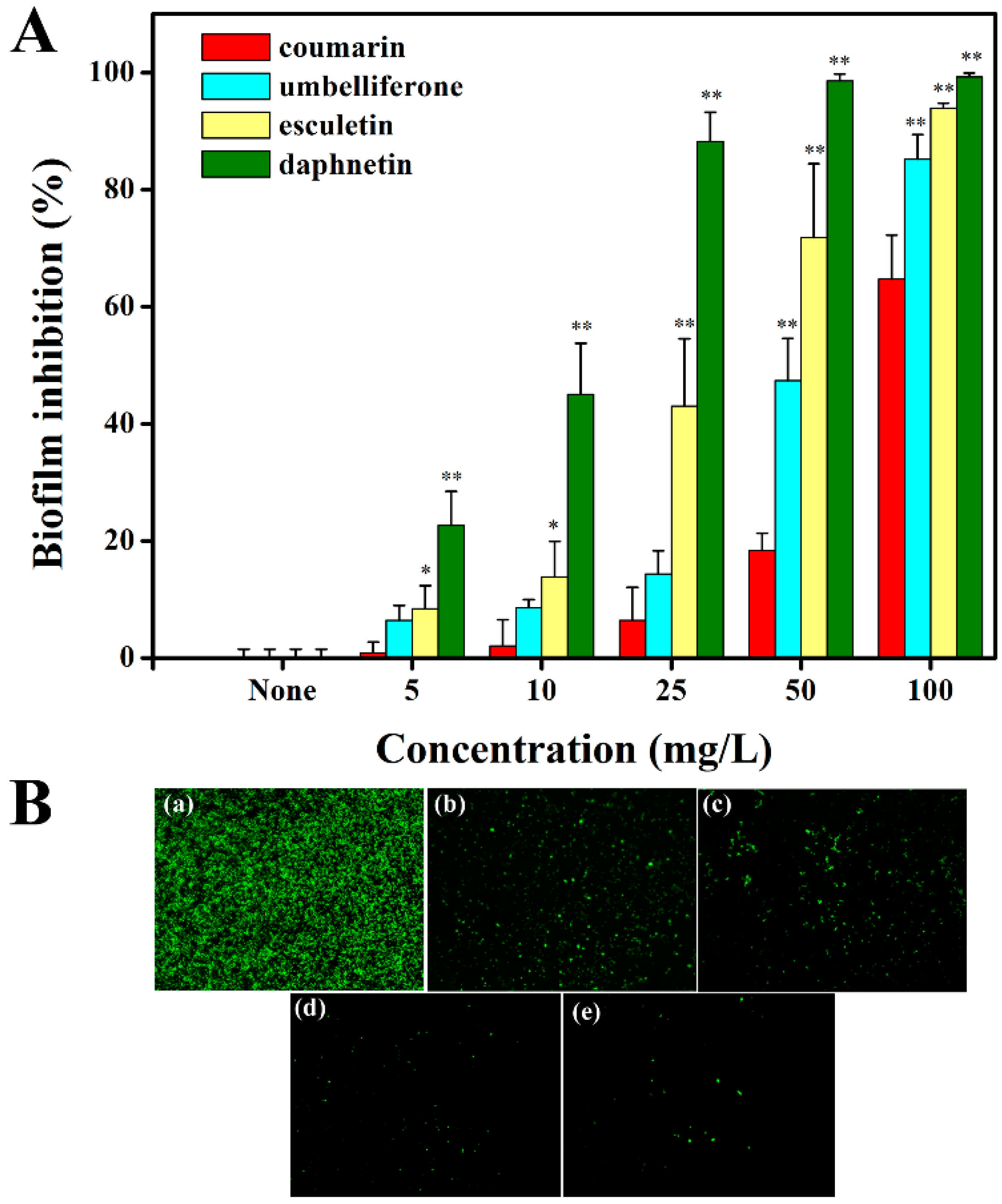
2.5. Hydroxycoumarins Reduce Biofilm Formation of R. solanacearum
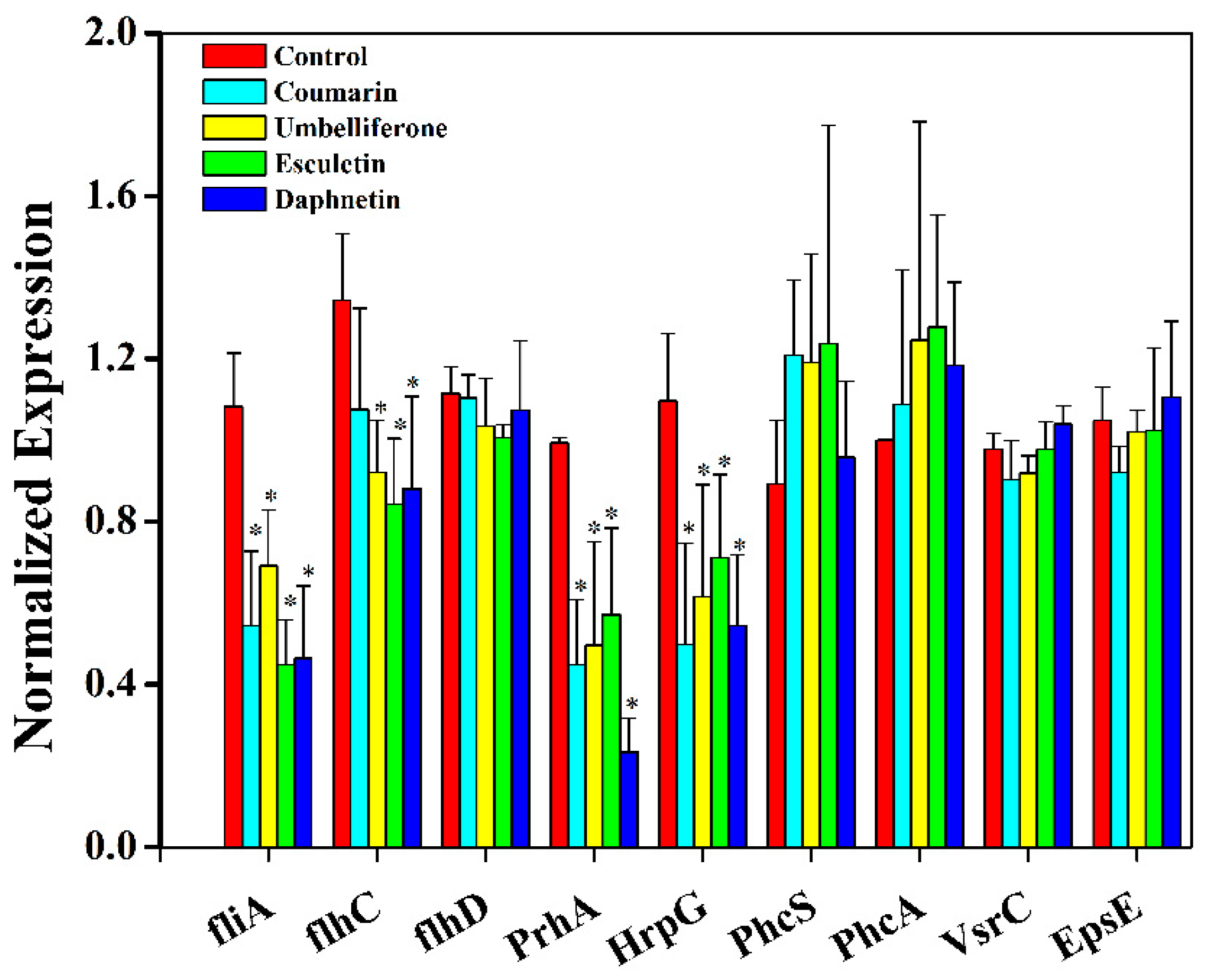
2.6. Hydroxycoumarins Repress Virulence-Associated Genes of R. solanacearum
3. Experimental Section
3.1. Bacterial Strains and Coumarins
3.2. Antibacterial Biological Assay to Screen Active Compounds
3.3. Determination of MIC and MBC
3.4. Bacterial Growth Curve
3.5. Biofilm Assay
3.6. Swimming Motility Assay
3.7. Fluorescence Microscopy Imaging
3.8. Cell Morphology Observation with TEM
3.9. RNA Isolation and Quantitative Real-Time RT-PCR
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Genin, S. Molecular traits controlling host range and adaptation to plants in Ralstonia solanacearum. New Phytologist. 2010, 187, 920–928. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Monteiro, F.M. Environmental Cues Controlling the Pathogenicity of “Ralstonia Solanacearum” on Plants: Señales Ambientales Que Determinan la Patogenicidad de “Ralstonia Solanacearum” en Plantas. Ph.D. Thesis, Universitat de Barcelona, Barcelona, Spain, 2013. [Google Scholar]
- Elphinstone, J.G.; Allen, C.; Prior, P.; Hayward, A.C. The current bacterial wilt situation: A global overview. In Bacterial Wilt Disease and the Ralstonia Solanacearum Species Complex; American Phytopathological Society: St Paul, MN, USA, 2005; pp. 9–28. [Google Scholar]
- Yuliar, Y.A.N.; Toyota, K. Recent trends in control methods for bacterial wilt diseases caused by Ralstonia solanacearum. Microbes Environ. 2015, 30, 1. [Google Scholar] [CrossRef] [PubMed]
- Denny, T. Plant pathogenic Ralstonia species. In Plant-Associated Bacteria; Springer: Dordrecht, The Netherlands, 2006; pp. 573–644. [Google Scholar]
- Lin, Y.; He, Z.; Rosskopf, E.N.; Conn, K.L.; Powell, C.A; Lazarovits, G. A nylon membrane bag assay for determination of the effect of chemicals on soilborne plant pathogens in soil. Plant Dis. 2010, 94, 201–206. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Han, H. Evaluation of antibacterial effects of carbon nanomaterials against copper-resistant Ralstonia solanacearum. Colloids Surf. B Biointerfaces 2013, 103, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, B.; Biosca, E.G.; López, M.M. On the life of Ralstonia solanacearum, a destructive bacterial plant pathogen. In Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology; Formatex Research Center: Badajoz, Spain, 2010; Volume 1. [Google Scholar]
- Hayward, A.C. Biology and epidemiology of bacterial wilt caused by Pseudomonas solanacearum. Annu. Rev. Phytopathol. 1991, 29, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Feng, X.; Tang, M.; Hao, W.; Han, Y.; Zhang, G.; Wan, S. Antibacterial activity of Lansiumamide B to tobacco bacterial wilt (Ralstonia solanacearum). Microbiol. Res. 2014, 169, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Mei, W.; Gong, M.; Zuo, W.; Bai, H.; Dai, H. Antibacterial activity of the flavonoids from Dalbergia odorifera on Ralstonia solanacearum. Molecules 2011, 16, 9775–9782. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.A.E.; Bereika, M.F.F.; Abo-Elnaga, H.I.G.; Sallam, M.A.A. Direct antimicrobial activity and induction of systemic resistance in potato plants against bacterial wilt disease by plant extracts. Plant Pathol. J. 2009, 25, 352–360. [Google Scholar] [CrossRef]
- Abo-Elyousr, K.A.; Seleim, M.A.; Abd-El-Moneem, K.M.; Saead, F.A. Integrated effect of Glomus mosseae and selected plant oils on the control of bacterial wilt disease of tomato. Crop Prot. 2014, 66, 67–71. [Google Scholar] [CrossRef]
- Paret, M.L.; Cabos, R.; Kratky, B.A.; Alvarez, A.M. Effect of plant essential oils on Ralstonia solanacearum race 4 and bacterial wilt of edible ginger. Plant Dis. 2010, 94, 521–527. [Google Scholar] [CrossRef]
- Fan, W.W.; Yuan, G.Q.; Li, Q.Q.; Lin, W. Antibacterial mechanisms of methyl gallate against Ralstonia solanacearum. Australas. Plant Pathol. 2014, 43, 1–7. [Google Scholar] [CrossRef]
- Barot, K.P.; Jain, S.V.; Kremer, L.; Singh, S.; Ghate, M.D. Recent advances and therapeutic journey of coumarins: Current status and perspectives. Med. Chem. Res. 2015, 24, 2771–2798. [Google Scholar] [CrossRef]
- Céspedes, C.L.; Avila, J.G.; Martínez, A.; Serrato, B.; Calderón-Mugica, J.C.; Salgado-Garciglia, R. Antifungal and antibacterial activities of Mexican tarragon (Tagetes lucida). J. Agric. Food Chem. 2006, 54, 3521–3527. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Nazir, M.; Ali, M.S.; Hussain, H.; Lee, Y.S.; Riaz, N.; Jabbar, A. Antimicrobial natural products: An update on future antibiotic drug candidates. Nat. Prod. Rep. 2010, 27, 238–254. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.M. The Antibacterial Effects and Mechanism of Several Botanical Compounds against Ralstonia solanacearum. Master’s Thesis, Southwest University, Chongqing, China, 2015. [Google Scholar]
- Lin, M.H.; Chou, Y.S.; Tsai, Y.J.; Chou, D.S. Antioxidant properties of 5, 7-dihydroxycoumarin derivatives in in vitro cell-free and cell-containing systems. J. Exp. Clin. Med. 2011, 3, 126–131. [Google Scholar] [CrossRef]
- Jurd, L.; Corse, J.; King, A.D.; Bayne, H.; Mihara, K. Antimicrobial properties of 6,7-dihydroxy-, 7,8-dihydroxy-, 6-hydroxy-and 8-hydroxycoumarins. Phytochemistry 1971, 10, 2971–2974. [Google Scholar] [CrossRef]
- Nazari, Z.E.; Iranshahi, M. Biologically active sesquiterpene coumarins from Ferula species. Phytother. Res. 2011, 25, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Konc, J.T.; Hejchman, E.; Kruszewska, H.; Wolska, I.; Maciejewska, D. Synthesis and pharmacological activity of O-aminoalkyl derivatives of 7-hydroxycoumarin. Eur. J. Med. Chem. 2011, 46, 2252–2263. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Bhat, K.A.; Raja, A.F.; Shawl, A.S.; Alam, M.S. Isolation, characterisation and antibacterial activity studies of coumarins from Rhododendron lepidotum Wall. ex G. Don, Ericaceae. Rev. Bras. Farmacogn. 2010, 20, 886–890. [Google Scholar]
- Kabeya, L.M.; da Silva, C.H.; Kanashiro, A.; Campos, J.M.; Azzolini, A.E.C.; Polizello, A.C.M.; Pupo, M.T.; Lucisano-Valim, Y.M. Inhibition of immune complex-mediated neutrophil oxidative metabolism: A pharmacophore model for 3-phenylcoumarin derivatives using GRIND-based 3D-QSAR and 2D-QSAR procedures. Eur. J. Med. Chem. 2008, 43, 996–1007. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Shi, B.; Li, J.; Wu, W.; Zhang, J. Design and synthesis of new 2-aryl-4, 5-dihydro-thiazole analogues: In vitro antibacterial activities and preliminary mechanism of action. Molecules 2015, 20, 20118–20130. [Google Scholar] [CrossRef] [PubMed]
- Witaicenis, A.; Seito, L.N.; da Silveira Chagas, A.; de Almeida, L.D.; Luchini, A.C.; Rodrigues-Orsi, P.; Cestari, S.H.; Di Stasi, L.C. Antioxidant and intestinal anti-inflammatory effects of plant-derived coumarin derivatives. Phytomedicine 2014, 21, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Turkmen, N.; Velioglu, Y.S.; Sari, F.; Polat, G. Effect of extraction conditions on measured total polyphenol contents and antioxidant and antibacterial activities of black tea. Molecules 2007, 12, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Maddox, C.E.; Laur, L.M.; Tian, L. Antibacterial activity of phenolic compounds against the phytopathogen Xylella fastidiosa. Curr. Microbiol. 2010, 60, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhou, F.; Ji, B.P.; Pei, R.S.; Xu, N. The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Lett. Appl. Microbiol. 2008, 47, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Mathesius, U. The role of flavonoids in root-rhizosphere signalling: Opportunities and challenges for improving plant-microbe interactions. J. Exp. Bot. 2012, 63, 3429–3444. [Google Scholar] [CrossRef] [PubMed]
- Kawase, M.; Sakagami, H.; Hashimoto, K.; Tani, S.; Hauer, H.; Chatterjee, S.S. Structure-cytotoxic activity relationships of simple hydroxylated coumarins. Anticancer Res. 2002, 23, 3243–3246. [Google Scholar]
- Yao, J.; Allen, C. The plant pathogen Ralstonia solanacearum needs aerotaxis for normal biofilm formation and interactions with its tomato host. J. Bacteriol. 2007, 189, 6415–6424. [Google Scholar] [CrossRef] [PubMed]
- Kai, K.; Shimizu, B.I.; Mizutani, M.; Watanabe, K.; Sakata, K. Accumulation of coumarins in Arabidopsis thaliana. Phytochemistry 2006, 67, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Gnonlonfin, G.B.; Sanni, A.; Brimer, L. Review scopoletin—A coumarin phytoalexin with medicinal properties. Crit. Rev. Plant Sci. 2012, 31, 47–56. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [PubMed]
- Strathmann, M.; Wingender, J.; Flemming, H.C. Application of fluorescently labelled lectins for the visualization and biochemical characterization of polysaccharides in biofilms of Pseudomonas aeruginosa. J. Microbiol. Methods 2002, 50, 237–248. [Google Scholar] [CrossRef]
- Genin, S.; Denny, T.P. Pathogenomics of the Ralstonia solanacearum species complex. Annu. Rev. Phytopathol. 2012, 50, 67–89. [Google Scholar] [CrossRef] [PubMed]
- Peeters, N.; Guidot, A.; Vailleau, F.; Valls, M. Ralstonia solanacearum, a widespread bacterial plant pathogen in the post-genomic era. Mol. Plant Pathol. 2013, 14, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, J.; Xu, J.; Zhang, H.; He, L.; Feng, J. TssB is essential for virulence and required for type VI secretion system in Ralstonia solanacearum. Microb. Pathog. 2014, 74, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Ding, W.; Zhang, Y.; Liu, X.; Yang, L. Oleanolic acid induces the type III secretion system of Ralstonia solanacearum. Front. Microbiol. 2015, 6, 1466. [Google Scholar] [CrossRef] [PubMed]
- Yoshimochi, T.; Zhang, Y.; Kiba, A.; Hikichi, Y.; Ohnishi, K. Expression of hrpG and activation of response regulator HrpG are controlled by distinct signal cascades in Ralstonia solanacearum. J. Gen. Plant Pathol. 2009, 75, 196–204. [Google Scholar] [CrossRef]
- Dalgaard, P.; Ross, T.; Kamperman, L.; Neumeyer, K.; McMeekin, T.A. Estimation of bacterial growth rates from turbidimetric and viable count data. Int. J. Food Microbiol. 1994, 23, 391–404. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48 (Suppl. 1), 5–16. [Google Scholar] [CrossRef] [PubMed]
- Smyth, T.; Ramachandran, V.N.; Smyth, W.F. A study of the antimicrobial activity of selected naturally occurring and synthetic coumarins. Int. J. Antimicrob. Agents 2009, 33, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Galal, A.A.; Kehil, Y.E.I.; El-Daoudi, Y.H.; Shihata, Z.A.; Ouf, M.F. A Comparative Study on the Identification of Races and Biovars of some Egyptian Isolates of Ralsionia solanaceamm. Egypt J. Phytopathol. 2003, 31, 103–117. [Google Scholar]
- Tans-Kersten, J.; Brown, D.; Allen, C. Swimming motility, a virulence trait of Ralstonia solanacearum, is regulated by FlhDC and the plant host environment. Mol. Plant Microbe Interact. 2004, 17, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Peng, H.; Wang, X.; Shao, F.; Yuan, Z.; Han, H. Graphene oxide exhibits broad-spectrum antimicrobial activity against bacterial phytopathogens and fungal conidia by intertwining and membrane perturbation. Nanoscale 2014, 6, 1879–1889. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, F.; Genin, S.; van Dijk, I.; Valls, M. A luminescent reporter evidences active expression of Ralstonia solanacearum type III secretion system genes throughout plant infection. Microbiology 2012, 158, 2107–2116. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 1–18 are available from the authors.
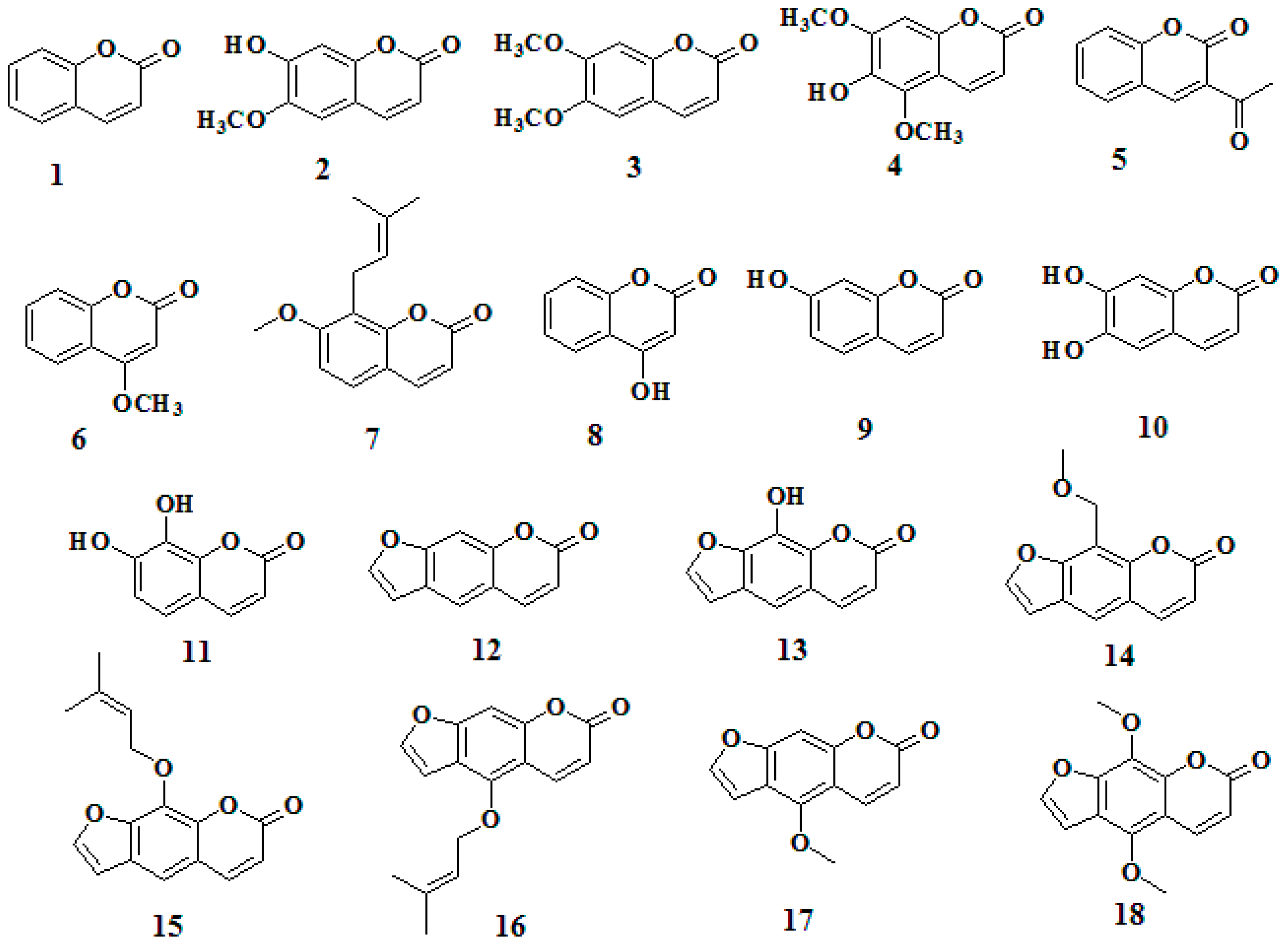
| Number | Compound | Antibacterial Rate (%) (Mean ± SD) a | |
|---|---|---|---|
| 10 mg/L | 100 mg/L | ||
| 1 | Coumarin | 3.9 ± 1.1 * | 50.3 ± 3.3 * |
| 2 | Scopoletin | 1.5 ± 7.6 | 32.6 ± 4.9 * |
| 3 | Scoparone | 1.0 ± 7.0 | 17.3 ± 2.9 * |
| 4 | Isofraxidin | 2.5 ± 3.2 * | 0.7 ± 1.4 * |
| 5 | 3-Acetyl-2H-chromen-2-one | 6.3 ± 4.7 | 10.7 ± 2.7 * |
| 6 | 4-Methoxycoumarin | 7.3 ± 1.9 * | 54.1 ± 4.2 * |
| 7 | Osthole | 0.2 ± 1.9 * | 3.1 ± 3.3 * |
| 8 | 4-Hydroxycoumarin | 2.1 ± 5.7 * | 4.9 ± 6.6 * |
| 9 | Umbelliferone | 7.3 ± 3.0 | 59.7 ± 3.8 |
| 10 | Esculetin | 9.2 ± 2.8 | 71.4 ± 2.1 * |
| 11 | Daphnetin | 13.3 ± 3.0 | 97.4 ± 0.7 * |
| 12 | Psoralen | 13.1 ± 0.8 | 57.1 ± 8.8 |
| 13 | Xanthotol | 8.5 ± 5.4 | 80.1 ± 2.5 * |
| 14 | Xanthotoxin | 6.3 ± 5.0 | 36.3 ± 5.4 * |
| 15 | Imperatorin | 6.5 ± 2.9 * | 19.1 ± 2.8 * |
| 16 | Isoimperatorin | 4.1 ± 2.0 * | 23.9 ± 6.3 * |
| 17 | Bergapten | 4.7 ± 2.4 * | 21.9 ± 1.6 * |
| 18 | Isopimpinellin | 0.3 ± 1.6 * | 22.5 ± 5.1 * |
| Positive Control | Thiodiazole Copper | 12.2 ± 1.7 | 63.6 ± 2.9 |
| Coumarins | MIC (mg/L) | MBC (mg/L) |
|---|---|---|
| Coumarin | 384 | 512 |
| Umbelliferone | 256 | 384 |
| Esculetin | 192 | 192 |
| Daphnetin | 64 | 64 |
| Coumarins | 12 h | 24 h | ||||
|---|---|---|---|---|---|---|
| Toxicity Regression Equations | IC50 (mg/L) | R Value | Toxicity Regression Equations | IC50 (mg/L) | R Value | |
| Coumarin | Y = 1.0293X + 3.1841 | 57.11 | 0.9330 | Y = 1.2294X + 2.1747 | 198.64 | 0.9783 |
| Umbelliferone | Y = 1.501X + 2.6435 | 37.15 | 0.9820 | Y = 1.8022X + 1.4204 | 96.88 | 0.9683 |
| Esculetin | Y = 2.0185X + 2.1871 | 24.75 | 0.9712 | Y = 2.1808X + 1.0057 | 67.85 | 0.9807 |
| Daphnetin | Y = 2.0489X + 3.0719 | 8.73 | 0.9863 | Y = 3.2992X + 0.7286 | 23.98 | 0.9809 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Ding, W.; Xu, Y.; Wu, D.; Li, S.; Chen, J.; Guo, B. New Insights into the Antibacterial Activity of Hydroxycoumarins against Ralstonia solanacearum. Molecules 2016, 21, 468. https://doi.org/10.3390/molecules21040468
Yang L, Ding W, Xu Y, Wu D, Li S, Chen J, Guo B. New Insights into the Antibacterial Activity of Hydroxycoumarins against Ralstonia solanacearum. Molecules. 2016; 21(4):468. https://doi.org/10.3390/molecules21040468
Chicago/Turabian StyleYang, Liang, Wei Ding, Yuquan Xu, Dousheng Wu, Shili Li, Juanni Chen, and Bing Guo. 2016. "New Insights into the Antibacterial Activity of Hydroxycoumarins against Ralstonia solanacearum" Molecules 21, no. 4: 468. https://doi.org/10.3390/molecules21040468
APA StyleYang, L., Ding, W., Xu, Y., Wu, D., Li, S., Chen, J., & Guo, B. (2016). New Insights into the Antibacterial Activity of Hydroxycoumarins against Ralstonia solanacearum. Molecules, 21(4), 468. https://doi.org/10.3390/molecules21040468






