Effect of Cannabis Smoke Condensate on C. albicans Growth and Biofilm Formation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Candida Strain
2.2. Preparation of the Cannabis Condensate
2.3. HPLC Analyses
2.4. Susceptibility of C. albicans to CSC Conditions
2.5. Effect of Cannabis Condensate on the Transition of C. albicans from Yeast to Hyphal Form
2.6. Effect of CSC on C. albicans Biofilm Formation
2.7. Effect of CSC on C. albicans Responses to Stressful Agents
2.8. Statistical Analyses
3. Results
3.1. Chemical Characterization of the CSC
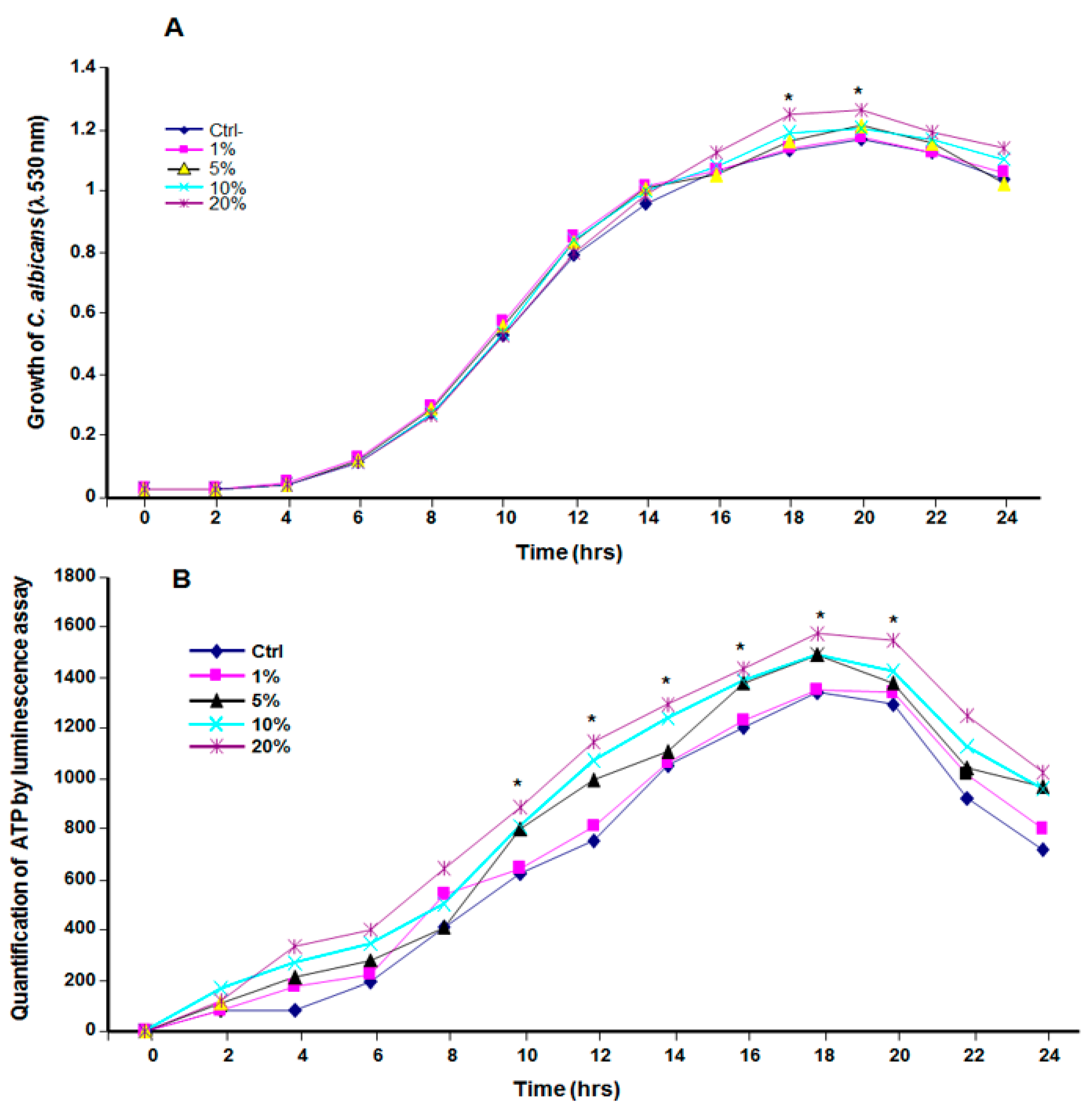
3.2. Cannabis Smoke Condensate Contributed to C. albicans Growth
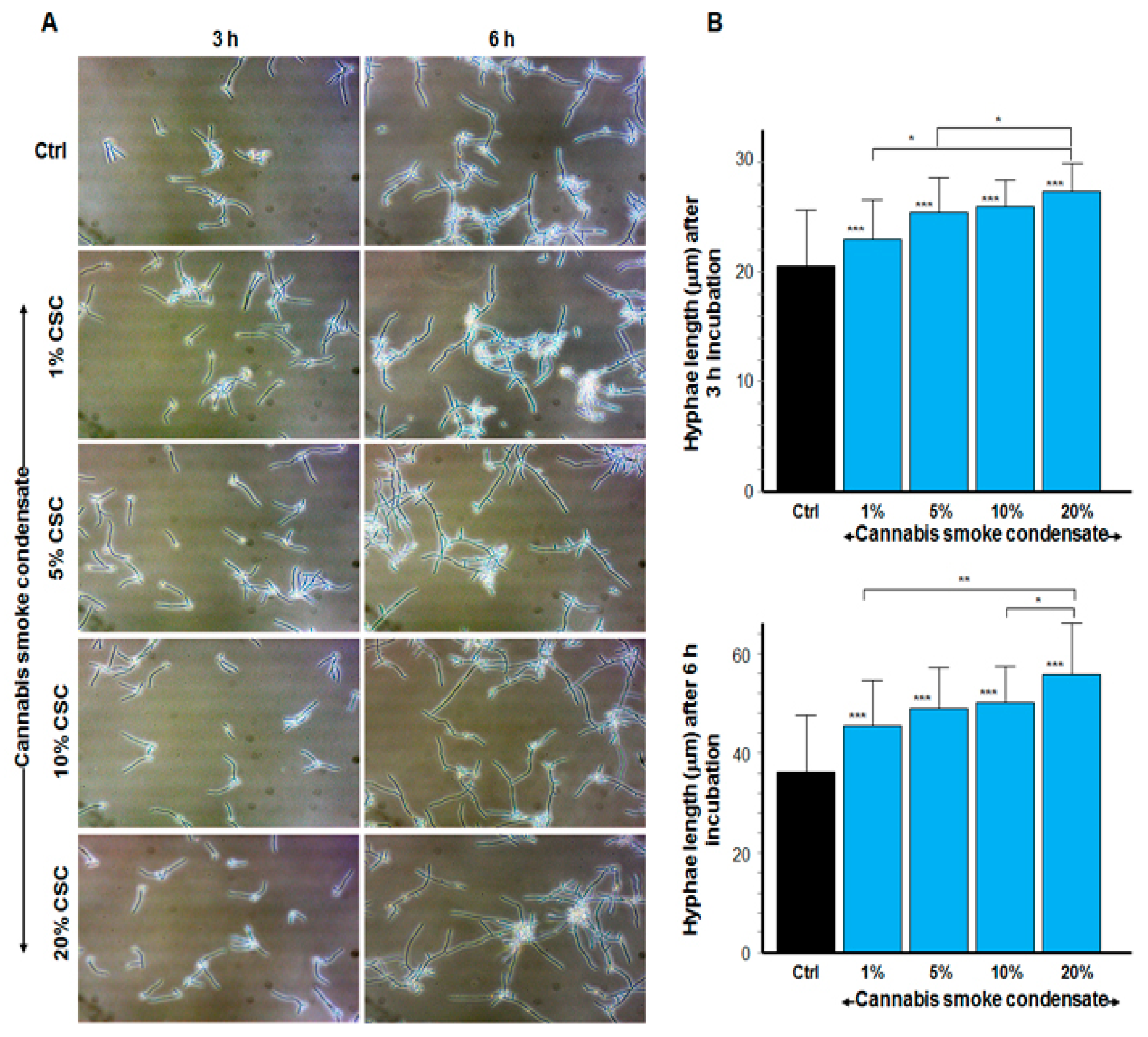
3.3. CSC Increased the Size of C. albicans Hyphae
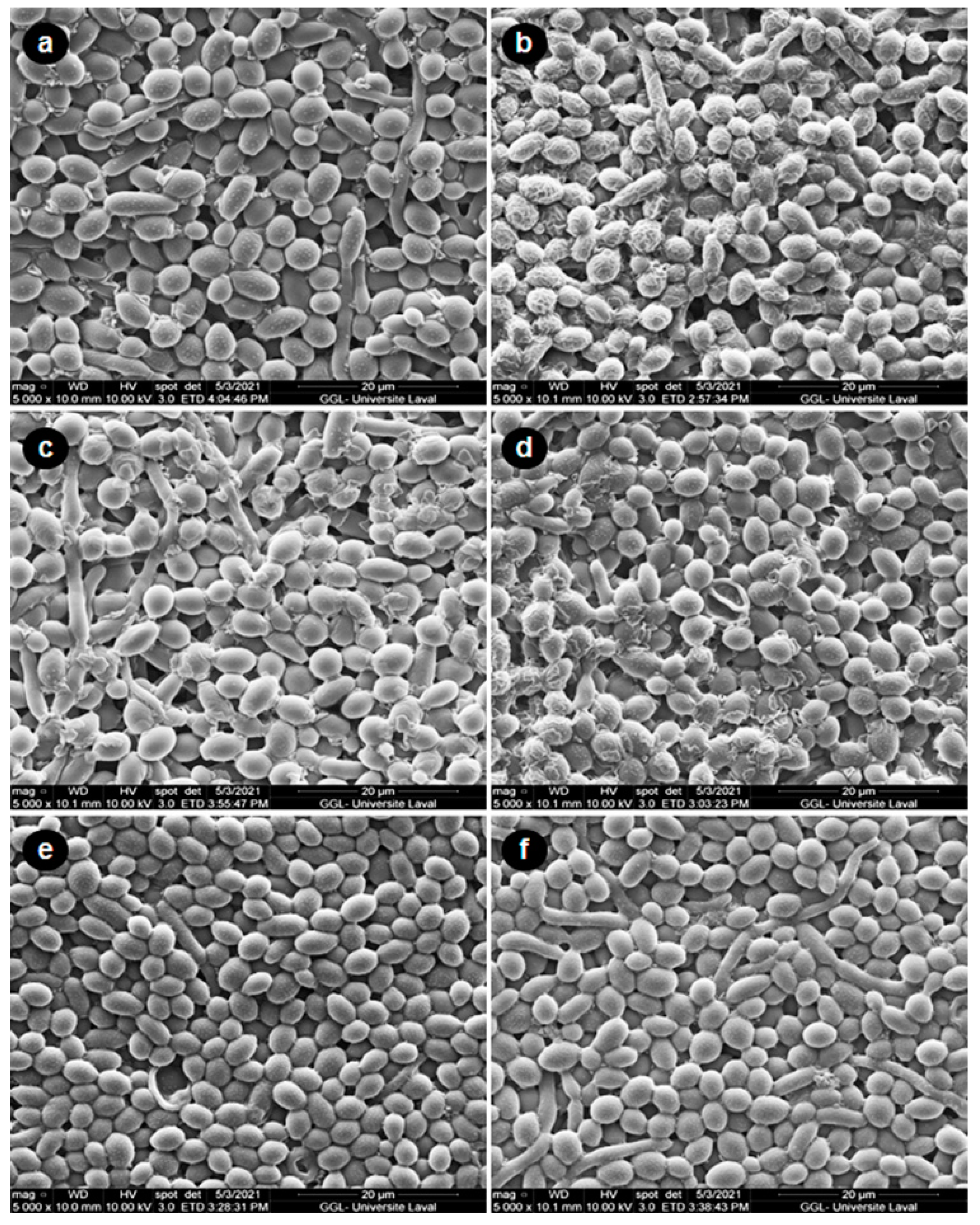
3.4. CSC Increased C. albicans Biofilm Formation
3.5. CSC Protected C. albicans against Oxidative Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Available online: https://www.who.int/news-room/fact-sheets/detail/oral-health (accessed on 8 November 2021).
- Richardson, M.; Lass-Flörl, C. Changing epidemiology of systemic fungal infections. Clin. Microbiol. Infect. 2008, 14 (Suppl. 4), 5–24. [Google Scholar] [CrossRef] [Green Version]
- Peña, D.E.R.; Innocentini, L.M.A.R.; Saraiva, M.C.P.; Lourenço, A.G.; Motta, A.C.F. Oral candidiasis prevalence in human immunodeficiency virus-1 and pulmonary tuberculosis coinfection: A systematic review and meta-analysis. Microb Pathog. 2021, 150, 104720. [Google Scholar] [CrossRef] [PubMed]
- Soysa, N.S.; Ellepola, A.N. The impact of cigarette/tobacco smoking on oral candidosis: An overview. Oral Dis. 2005, 11, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Darling, M.R.; Arendorf, T.M.; Coldrey, N.A. Effect of cannabis use on oral candidal carriage. J. Oral. Pathol. Med. 1990, 19, 319–321. [Google Scholar] [CrossRef]
- Gates, P.; Jaffe, A.; Copeland, J. Cannabis smoking and respiratory health: Consideration of the literature. Respirology 2014, 19, 655–662. [Google Scholar] [CrossRef]
- World Drug Report 2018. 2018. Available online: https://www.unodc.org/wdr2018/ (accessed on 8 November 2021).
- Pacula, R.L.; Smart, R. Medical Marijuana and Marijuana Legalization. Annu. Rev. Clin. Psychol. 2017, 13, 397–419. [Google Scholar] [CrossRef]
- Santos-Álvarez, I.; Pérez-Lloret, P.; González-Soriano, J.; Pérez-Moreno, M. An approach to the evaluation of the potency of cannabis resin in Madrid: A health hazard? Adicciones 2021, 1630. [Google Scholar] [PubMed]
- Tashkin, D.P.; Baldwin, G.C.; Sarafian, T.; Dubinett, S.; Roth, M.D. Respiratory and immunologic consequences of marijuana smoking. J. Clin. Pharmacol. 2002, 42, 71S–81S. [Google Scholar] [CrossRef]
- Cho, C.M.; Hirsch, R.; Johnstone, S. General and oral health implications of cannabis use. Aust. Dent. J. 2005, 50, 70–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moir, D.; Rickert, W.S.; Levasseur, G.; Larose, Y.; Maertens, R.; White, P.; Desjardins, S. A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions. Chem. Res. Toxicol. 2008, 21, 494–502. [Google Scholar] [CrossRef]
- Lee, M.L.; Novotny, M.; Bartle, K.D. Gas chromatography/mass spectrometric and nuclear magnetic resonance spectrometric studies of carcinogenic polynuclear aromatic hydrocarbons in tobacco and marijuana smoke condensates. Anal. Chem. 1976, 48, 405–416. [Google Scholar] [CrossRef]
- Underner, M.; Urban, T.; Perriot, J.; de Chazeron, I.; Meurice, J.C. Cannabis et cancer bronchique [Cannabis smoking and lung cancer]. Rev. Mal. Respir. 2014, 31, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.C.; Tashkin, D.P.; Djahed, B.; Rose, J.E. Pulmonary hazards of smoking marijuana as compared with tobacco. N. Engl. J. Med. 1988, 318, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Perfetti, T.A.; Garg, R.; Hansch, C. IARC carcinogens reported in cigarette mainstream smoke and their calculated log P values. Food Chem. Toxicol. 2003, 41, 807–817. [Google Scholar] [CrossRef]
- Jones, R.T. Cardiovascular system effects of marijuana. J. Clin. Pharmacol. 2002, 42, 58S–63S. [Google Scholar] [CrossRef]
- Darling, M.R.; Arendorf, T.M. Review of the effects of cannabis smoking on oral health. Int. Dent. J. 1992, 42, 19–22. [Google Scholar]
- Faustino, I.S.P.; González-Arriagada, W.A.; Cordero-Torres, K.; Lopes, M.A. Candidiasis of the tongue in cannabis users: A report of 2 cases. Gen. Dent. 2020, 68, 66–68. [Google Scholar] [PubMed]
- Darling, M.R.; Arendorf, T.M. Effects of cannabis smoking on oral soft tissues. Community Dent. Oral. Epidemiol. 1993, 21, 78–81. [Google Scholar] [CrossRef]
- Keboa, M.T.; Enriquez, N.; Martel, M.; Nicolau, B.; Macdonald, M.E. Oral Health Implications of Cannabis Smoking: A Rapid Evidence Review. J. Can. Dent. Assoc. 2020, 86, k2. [Google Scholar] [PubMed]
- Semlali, A.; Killer, K.; Alanazi, H.; Chmielewski, W.; Rouabhia, M. Cigarette smoke condensate increases C. albicans adhesion, growth, biofilm formation, and EAP1, HWP1 and SAP2 gene expression. BMC Microbiol. 2014, 14, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alanazi, H.; Park, H.J.; Chakir, J.; Semlali, A.; Rouabhia, M. Comparative study of the effects of cigarette smoke and electronic cigarettes on human gingival fibroblast proliferation, migration and apoptosis. Food Chem. Toxicol. 2018, 118, 390–398. [Google Scholar] [CrossRef]
- Theberge, S.; Semlali, A.; Alamri, A.; Leung, K.P.; Rouabhia, M. C. albicans growth, transition, biofilm formation, and gene expression modulation by antimicrobial decapeptide KSL-W. BMC Microbiol. 2013, 13, 246. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/research-data/canadian-cannabis-survey-2020-summary.html (accessed on 8 November 2021).
- Saville, S.P.; Lazzell, A.L.; Monteagudo, C.; Lopez-Ribot, J.L. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot. Cell 2003, 2, 1053–1060. [Google Scholar] [CrossRef] [Green Version]
- Romo, J.A.; Pierce, C.G.; Chaturvedi, A.K.; Lazzell, A.L.; McHardy, S.F.; Saville, S.P.; Lopez-Ribot, J.L. Development of Anti-Virulence Approaches for Candidiasis via a Novel Series of Small-Molecule Inhibitors of Candida albicans Filamentation. mBio 2017, 8, e01991-17. [Google Scholar] [CrossRef] [Green Version]
- Felk, A.; Kretschmar, M.; Albrecht, A.; Schaller, M.; Beinhauer, S.; Nichterlein, T.; Sanglard, D.; Korting, H.C.; Schäfer, W.; Hube, B. Candida albicans hyphal formation and the expression of the Efg1-regulated proteinases Sap4 to Sap6 are required for the invasion of parenchymal organs. Infect. Immun. 2002, 70, 3689–3700. [Google Scholar] [CrossRef] [Green Version]
- Versteeg, P.A.; Slot, D.E.; van der Velden, U.; van der Weijden, G.A. Effect of cannabis usage on the oral environment: A review. Int. J. Dent. Hyg. 2008, 6, 315–320. [Google Scholar] [CrossRef]
- Joshi, S.; Ashley, M. Cannabis: A joint problem for patients and the dental profession. Br. Dent. J. 2016, 220, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Blumstein, G.W.; Parsa, A.; Park, A.K.; McDowell, B.L.; Arroyo-Mendoza, M.; Girguis, M.; Adler-Moore, J.P.; Olson, J.; Buckley, N.E. Effect of Delta-9-tetrahydrocannabinol on mouse resistance to systemic Candida albicans infection. PLoS ONE 2014, 9, e103288. [Google Scholar] [CrossRef] [PubMed]
- Szerencsés, B.; Igaz, N.; Tóbiás, Á.; Prucsi, Z.; Rónavári, A.; Bélteky, P.; Madarász, D.; Papp, C.; Makra, I.; Vágvölgyi, C.; et al. Size-dependent activity of silver nanoparticles on the morphological switch and biofilm formation of opportunistic pathogenic yeasts. BMC Microbiol. 2020, 20, 176. [Google Scholar] [CrossRef]
- Thomson, W.M.; Poulton, R.; Broadbent, J.M.; Moffitt, T.E.; Caspi, A.; Beck, J.D.; Welch, D.; Hancox, R.J. Cannabis smoking and periodontal disease among young adults. JAMA 2008, 29, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Yazdanian, M.; Armoon, B.; Noroozi, A.; Mohammadi, R.; Bayat, A.H.; Ahounbar, E.; Higgs, P.; Nasab, H.S.; Bayani, A.; Hemmat, M. Dental caries and periodontal disease among people who use drugs: A systematic review and meta-analysis. BMC Oral Health 2020, 20, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz-Katterbach, M.; Imfeld, T.; Imfeld, C. Cannabis and caries--does regular cannabis use increase the risk of caries in cigarette smokers? Schweiz. Monatsschr. Zahnmed. 2009, 119, 576–583. [Google Scholar] [PubMed]
- Anil, S.; Vellappally, S.; Hashem, M.; Preethanath, R.S.; Patil, S.; Samaranayake, L.P. Xerostomia in geriatric patients: A burgeoning global concern. J. Investig. Clin. Dent. 2016, 7, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Tarapan, S.; Matangkasombut, O.; Trachootham, D.; Sattabanasuk, V.; Talungchit, S.; Paemuang, W.; Phonyiam, T.; Chokchaitam, O.; Mungkung, O.O.; Lam-Ubol, A. Oral Candida colonization in xerostomic postradiotherapy head and neck cancer patients. Oral Dis. 2019, 25, 1798–1808. [Google Scholar] [CrossRef] [PubMed]
- Chaffee, B.W. Cannabis Use and Oral Health in a National Cohort of Adults. J. Calif. Dent. Assoc. 2021, 49, 493–501. [Google Scholar] [PubMed]


| Chemical | RT (min) | Concentration (ppm) |
|---|---|---|
| CBG | 2.98 | 47.225 |
| CBN | 4.75 | 63.125 |
| Δ9-THC | 6.03 | 1055.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tazi, N.; Pigeon, X.; Mbuyi-Boisvert, J.M.; Giret, S.; Béland, F.; Rouabhia, M. Effect of Cannabis Smoke Condensate on C. albicans Growth and Biofilm Formation. Microorganisms 2021, 9, 2348. https://doi.org/10.3390/microorganisms9112348
Tazi N, Pigeon X, Mbuyi-Boisvert JM, Giret S, Béland F, Rouabhia M. Effect of Cannabis Smoke Condensate on C. albicans Growth and Biofilm Formation. Microorganisms. 2021; 9(11):2348. https://doi.org/10.3390/microorganisms9112348
Chicago/Turabian StyleTazi, Neftaha, Xavier Pigeon, Jérôme Mulamba Mbuyi-Boisvert, Simon Giret, François Béland, and Mahmoud Rouabhia. 2021. "Effect of Cannabis Smoke Condensate on C. albicans Growth and Biofilm Formation" Microorganisms 9, no. 11: 2348. https://doi.org/10.3390/microorganisms9112348
APA StyleTazi, N., Pigeon, X., Mbuyi-Boisvert, J. M., Giret, S., Béland, F., & Rouabhia, M. (2021). Effect of Cannabis Smoke Condensate on C. albicans Growth and Biofilm Formation. Microorganisms, 9(11), 2348. https://doi.org/10.3390/microorganisms9112348






