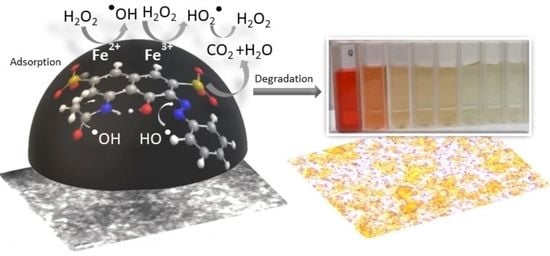
Degradation of Acid Red 1 Catalyzed by Peroxidase Activity of Iron Oxide Nanoparticles and Detected by SERS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of MIONPs
2.3. Characterization Techniques
2.4. Degradation Procedure
2.5. UV-Vis Spectroscopic Analysis
3. Results and Discussion
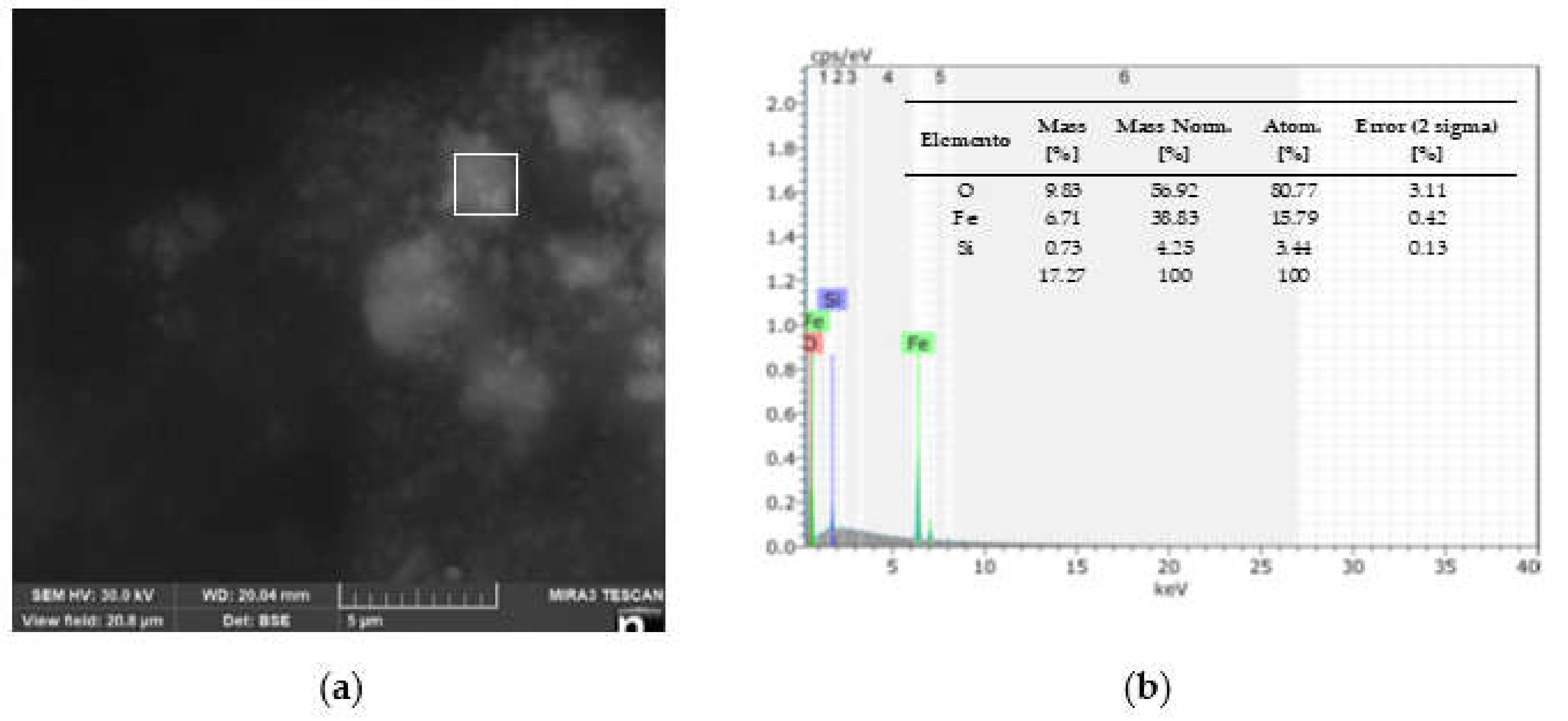
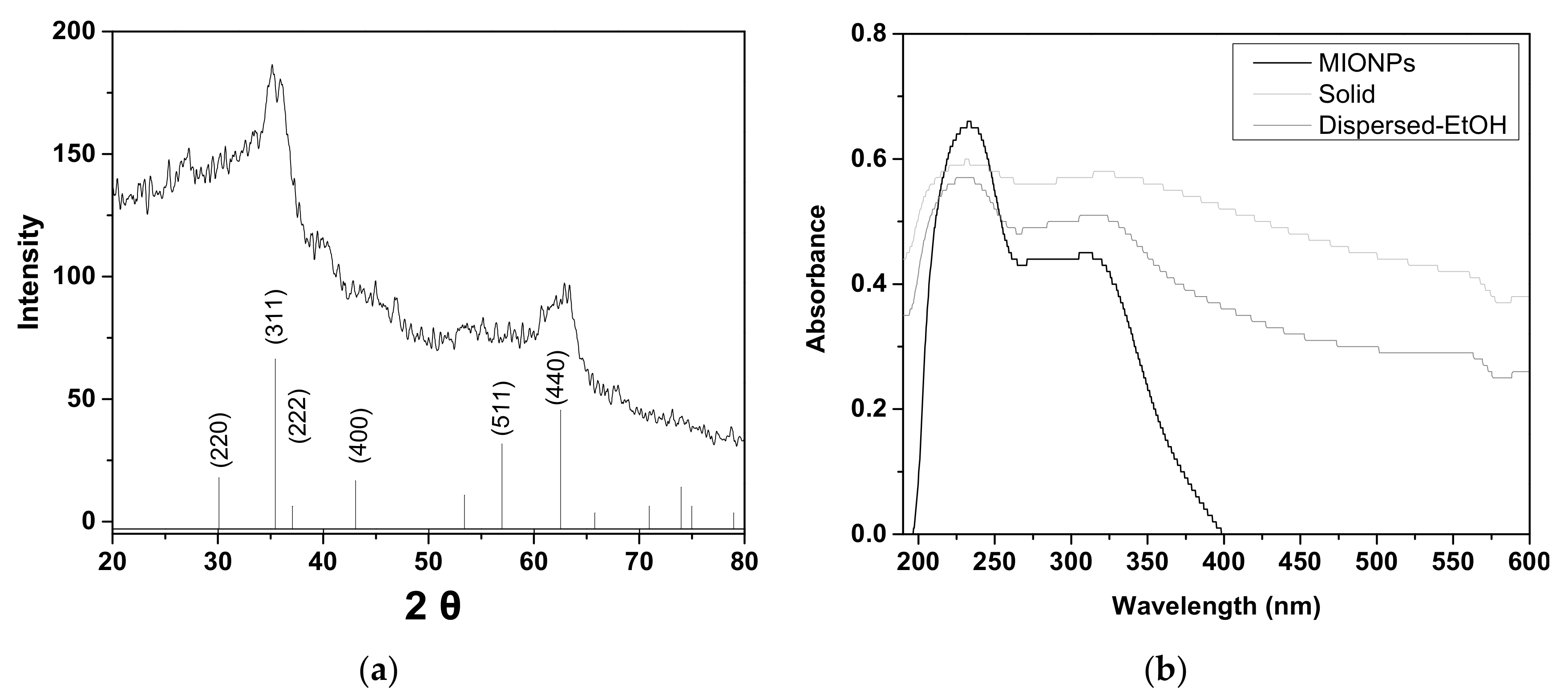
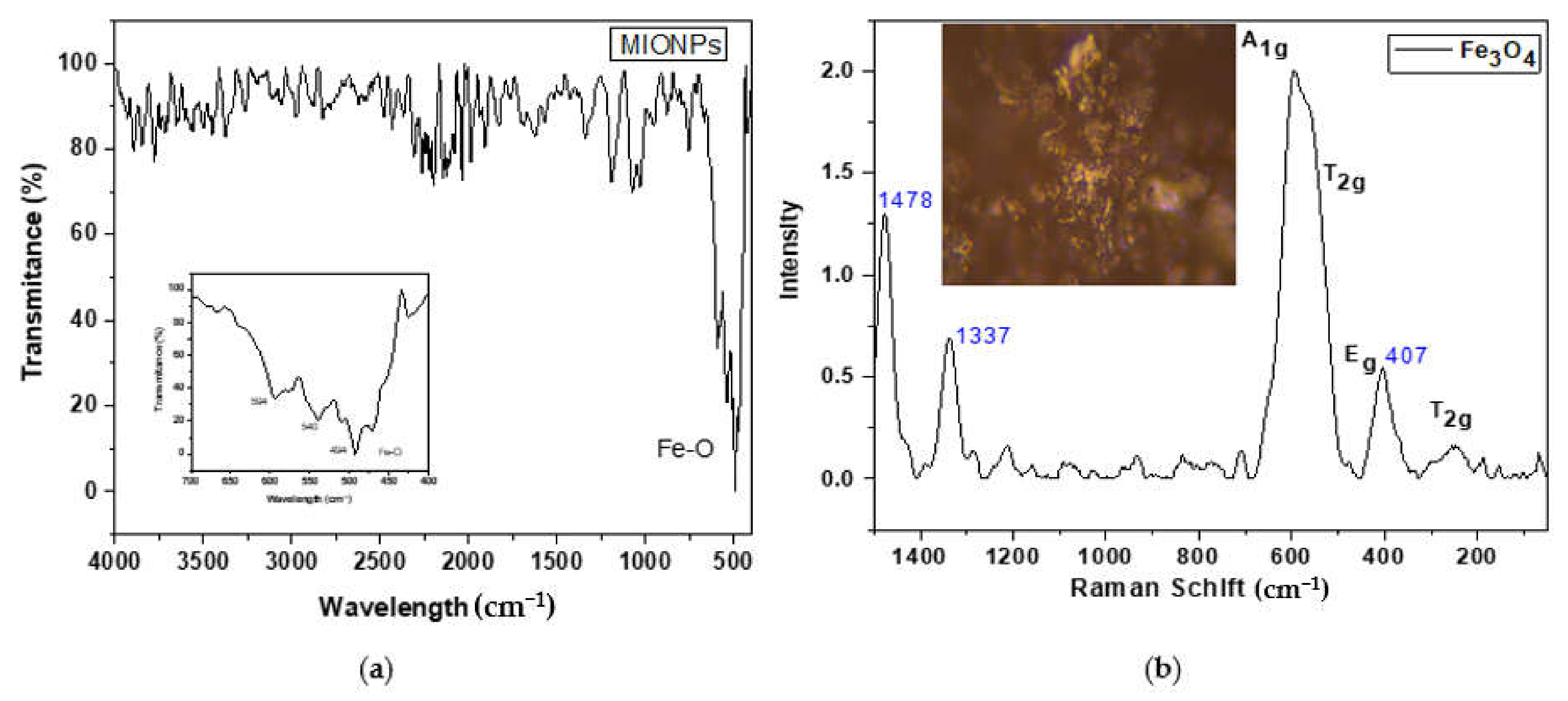
3.1. Characterization of MIONPs
3.2. Degradation Catalyzed by MIONPs
3.3. Peroxidase-like Activity of MIONPs
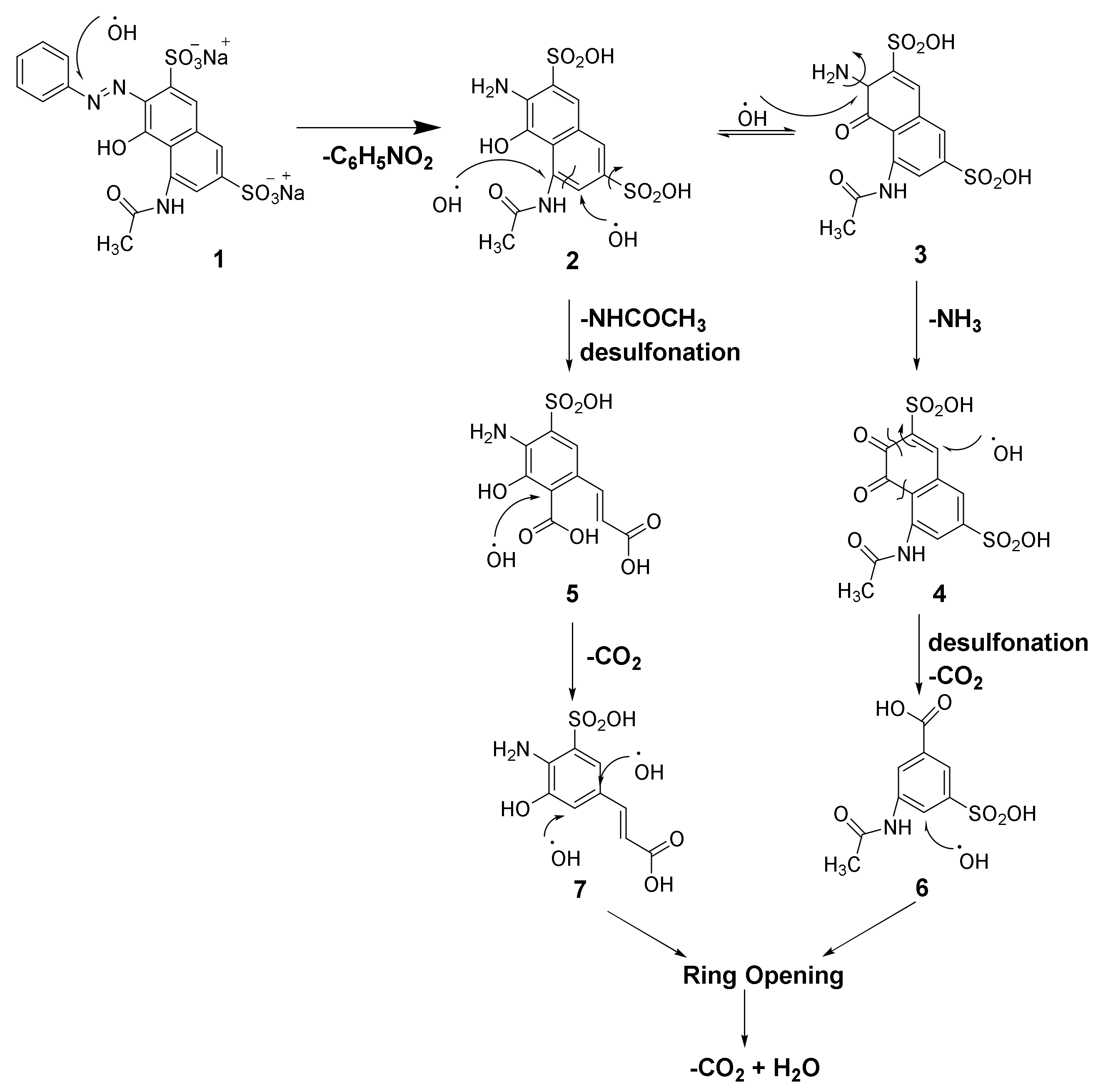
3.4. Mechanism of AR 1 Degradation
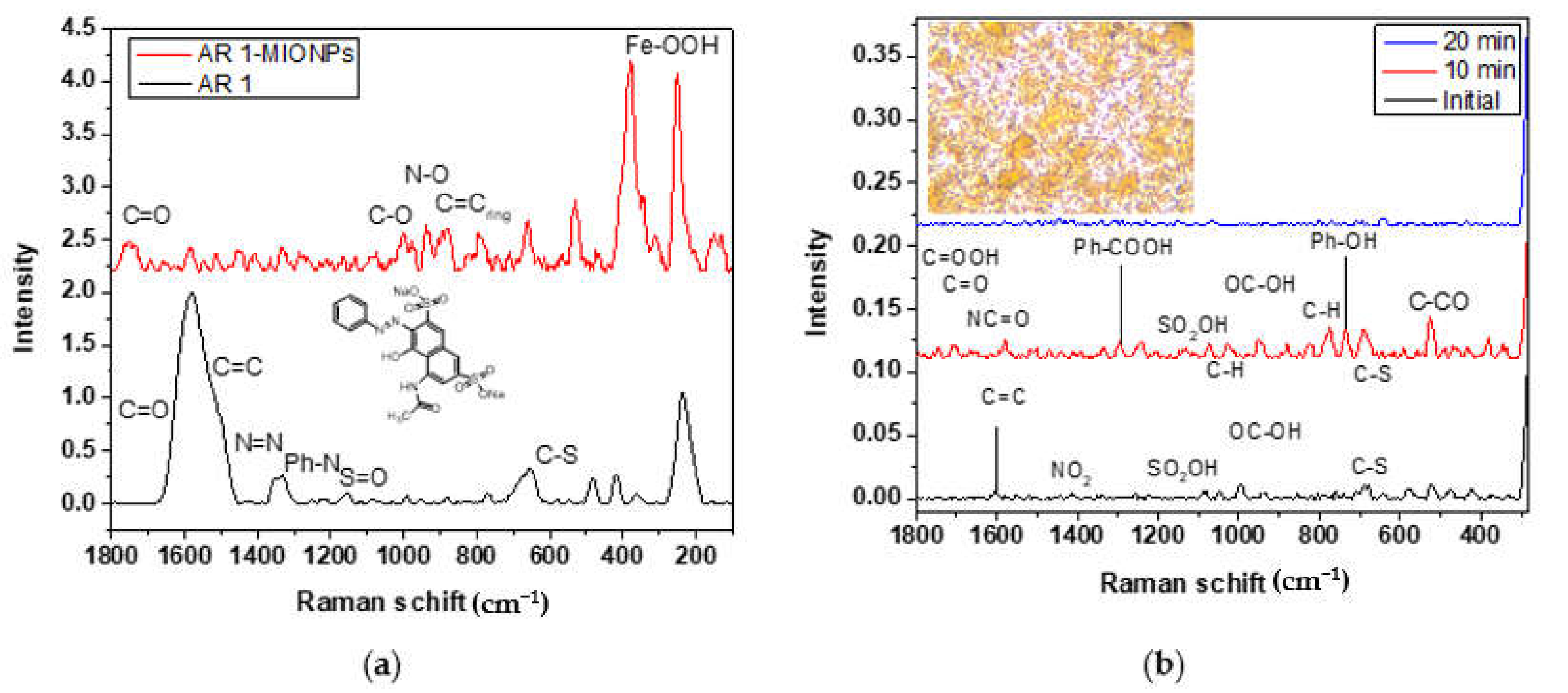
3.5. Compounds Detected by SERS in AR 1 Degradation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, K.; Arora, S. Removal of synthetic textile dyes from wastewaters: A critical review on present treatment technologies. Crit. Rev. Environ. Sci. Technol. 2011, 41, 807–878. [Google Scholar] [CrossRef]
- Shaul, G.M.; Holdsworth, T.J.; Dempsey, C.R.; Dostal, K.A. Fate of water-soluble azo dyes in the activated sludge process. Chemosphere 1991, 22, 107–119. [Google Scholar] [CrossRef]
- UNESCO. The United Nations World Water Development Report 4: Managing Water Report under Uncertainty and Risk; United Nations Educational, Scientific and Cultural Organization: Paris, France, 2012; Volume 1. [Google Scholar]
- Brillas, E.; Martínez-Huitle, C.A. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An up dated review. Appl. Catal. B Environ. 2015, 166, 603–643. [Google Scholar] [CrossRef]
- Anjaneyulu, Y.; Sreedhara Chary, N.; Suman Raj, D.S. Decolorization of industrial effluents–available methods and emerging technologies—A review. Rev. Environ. Sci. Biotechnol. 2005, 4, 245–273. [Google Scholar] [CrossRef]
- Florenza, X.; Sales Solano, A.M.; Centellas, F.; Martínez-Huitle, C.A.; Brillasa, E.; Garcia-Segura, S. Degradation of the azo dye Acid Red 1 by anodic oxidation and indirectelectrochemical processes based on Fenton’s reaction chemistry. Relationship between decolorization, mineralization and products. Electrochim. Acta 2014, 142, 276–288. [Google Scholar] [CrossRef]
- Do Vale-Júnior, E.; Da Silva, D.R.; Fajardo, A.S.; Martínez-Huitle, C.A. Treatment of an azo dye effluent by peroxi-coagulation and its comparison to traditional electrochemical advanced processes. Chemosphere 2018, 204, 548–555. [Google Scholar] [CrossRef]
- Javaid, R.; Qazi, U.Y. Catalytic Oxidation Process for the Degradation of Synthetic Dyes: An Overview. Int. J. Environ. Res. Public Health 2019, 16, 2066. [Google Scholar] [CrossRef] [Green Version]
- Hodges, B.C.; Cates, E.L.; Kim, J.-H. Challenges and prospects of advanced oxidation water treatment processes using catalytic nanomaterials. Nat. Nanotechn. 2018, 13, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Munoz, M.; de Pedro, Z.M.; Casas, J.A.; Rodriguez, J.J. Preparation of magnetite-based catalysts and their application in heterogeneous Fenton oxidation–A review. Appl. Catalys. B Environ. 2015, 176, 249–265. [Google Scholar] [CrossRef] [Green Version]
- Zubir, N.A.; Yacou, C.; Motuzas, J.; Zhang, X.; Diniz da Costa, J.C. Structural and functional investigation of graphene oxide–Fe3O4 nanocomposites for the heterogeneous Fenton-like reaction. Sci. Rep. 2014, 4, 4594. [Google Scholar] [CrossRef] [Green Version]
- Maharjan, A.; Dikshit, P.K.; Gupta, A.; Kim, B.S. Catalytic activity of magnetic iron oxide nanoparticles for hydrogen peroxide decomposition: Optimization and characterization. J. Chem. Technol. Biotechnol. 2020, 95, 2495–2508. [Google Scholar] [CrossRef]
- Wang, N.; Zhu, L.; Wang, M.; Wang, D.; Tang, H. Sono-enhanced degradation of dye pollutants with the use of H2O2 activated by Fe3O4 magnetic nanoparticles as peroxidase mimetic. Ultrason. Sonochem. 2010, 17, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Robles, H. Tannic Acid. In Encyclopedia of Toxicology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 474–475. [Google Scholar] [CrossRef]
- Du, X.; Xu, Y.; Qin, L.; Lu, X.; Liu, Q.; Bai, Y. Simple and Rapid Spectrophotometric Determination of Titanium on Etched Aluminum Foils. Am. J. Anal. Chem. 2014, 5, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.; Wen, X.; Wang, Q. Solvothermal in situ synthesis of Fe3O4-multiwalled carbon nanotubes with enhanced heterogeneous Fenton-like activity. Mater. Res. Bull. 2012, 47, 3369–3376. [Google Scholar] [CrossRef]
- Cheera, P.; Karlapudi, S.; Sellola, G.; Ponneri, V. A facile green synthesis of spherical Fe3O4 magnetic nanoparticles and their effect on degradation of methylene blue in aqueous solution. J. Mol. Liq. 2016, 221, 993–998. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Mazumder, N.; Mondal, S.; Panigrahi, K.; Banerjee, A.; Das, D.; Sarkar, S.; Roy, D.; Kumar Chattopadhyay, K. Size-modulation of functionalized Fe3O4: Nanoscopic customization to devise resolute piezoelectric nanocomposites. Dalton Trans. 2020, 49, 7872. [Google Scholar] [CrossRef]
- Ali, S.; Khan, S.A.; Yamani, Z.H.; Qamar, M.T.; Morsy, M.A.; Sarfraz, S. Shape and size-controlled superparamagnetic iron oxidenanoparticles using various reducing agents and their relaxometric properties by Xigo acorn area. Appl. Nanosci. 2019, 9, 479–489. [Google Scholar] [CrossRef]
- Zhang, X.; Niu, Y.; Meng, X.; Li, Y.; Zhao, J. Structural evolution and characteristics of the phase transformations between α-Fe2O3, Fe3O4 and γ-Fe2O3 nanoparticles under reducing and oxidizing atmospheres. Cryst. Eng. Comm. 2013, 15, 8166–8172. [Google Scholar] [CrossRef]
- Bibi, I.; Nazar, N.; Ata, S.; Sultan, M.; Ali, A.; Abbas, A.; Jilani, K.; Kamal, S.; Sarim, F.M.; Khan, M.I.; et al. Green synthesis of iron oxide nanoparticles using pomegranate seeds extract and photocatalytic activity evaluation for the degradation of textile dye. J. Mater. Res. Technol. 2019, 8, 6115–6124. [Google Scholar] [CrossRef]
- Razack, S.A.; Suresh, A.; Sriram, S.; Ramakrishnan, G.; ·Sadanandham, S.; Veerasamy, M.; Nagalamadaka, R.B.; ·Sahadevan, R. Green synthesis of iron oxide nanoparticles using Hibiscus rosa-sinensis for fortifying wheat biscuits. SN Appl. Sci. 2020, 2, 898. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Zhu, W.; Zhang, M.; Lin, Y.; Xu, N.; Chen, F.; Wang, D.; Chen, Z. Adsorption and Synergetic Fenton-like Degradation of Methylene Blue by a Novel Mesoporous α-Fe2O3/SiO2 at Neutral pH. Ind. Eng. Chem. Res. 2018, 57, 5539–5549. [Google Scholar] [CrossRef]
- Stoia, M.; Istratie, R.; Păcurariu, C. Investigation of magnetite nanoparticles stability in air by thermal analysis and FTIR spectroscopy. J. Therm. Anal. Calorim. 2016, 125, 1185–1198. [Google Scholar] [CrossRef]
- Chamritski, I.; Burns, G. Infrared- and Raman-Active Phonons of Magnetite, Maghemite, and Hematite: A Computer Simulation and Spectroscopic Study. J. Phys. Chem. B 2005, 109, 11. [Google Scholar] [CrossRef]
- De Faria, D.L.A.; Venaüncio Silva, S.; De Oliveira, M.T. Raman Microspectroscopy of Some Iron Oxides and Oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Shebanova, O.N.; Lazor, P. Raman spectroscopic study of magnetite (FeFe2O4): A new assignment for the vibrational spectrum. J. Sol. Stat. Chem. 2003, 174, 424–430. [Google Scholar] [CrossRef]
- Guin, D.; Manorama, S.V. Room temperature synthesis of monodispersed iron oxide nanoparticles. Mater. Lett. 2008, 62, 3139–3142. [Google Scholar] [CrossRef]
- Herrera-Becerra, R.; Rius, J.L.; Zorrilla, C. Tannin biosynthesis of iron oxide nanoparticles. Appl. Phy. A 2010, 100, 453–459. [Google Scholar] [CrossRef]
- Morgan, B.; Lahav, O. The effect of pH on the kinetics of spontaneous Fe(II) oxidation by O2 in aqueous solution–basic principles and a simple heuristic description. Chemosphere 2007, 68, 2080–2084. [Google Scholar] [CrossRef] [PubMed]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced Oxidation Processes for Organic Contaminant Destruction Based on the Fenton Reaction and Related Chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Shoniya, T.; Sreekanth, R.; Sijumon, V.A.; Usha, K.A.; Aravinda, K.C.T. Oxidative degradation of Acid Red 1 in aqueous medium. Chem. Eng. J. 2014, 244, 473–482. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Xu, Z.; Bian, Y.; Niu, Y.; Zhang, Y.; Wang, Z. Flexible and smart fibers decorated with Ag nanoflowers for highly active surface-enhanced Raman scattering detection. J. Raman Spectrosc. 2019, 50, 1468–1476. [Google Scholar] [CrossRef]
- Jun, B.-M.; Kim, Y.; Yoon, Y.; Yea, Y.; Park, C.M. Enhanced sonocatalytic degradation of recalcitrant organic contaminants using a magnetically recoverable Ag/Fe-loaded activated biochar composite. Ceram. Intern. 2020, 46, 22521–22531. [Google Scholar] [CrossRef]
- Lv, Q.; Li, G.; Sun, H.; Kong, L.; Lu, H.; Gao, X. Preparation of magnetic core/shell structured c-Fe2O3@Ti-tmSiO2 and its application for the adsorption and degradation of dyes. Microporous Mesoporous Mater. 2014, 186, 7–13. [Google Scholar] [CrossRef]




| Organic Dyes [Concentration] | Catalyst | Conditions | Degradation And Time | Reference |
|---|---|---|---|---|
| RhB (10 mg/L) | Fe3O4 NPs (10–15 nm) | Cat = 0.5 g/L, H2O2 40 mM pH = 5, T = 25 °C, UI = 20 kHz | 90% 1 h | [13] |
| RhB (10 mg/L) | Ag3PO4–Fe3O4@AB (10 nm) | Cat = 0.1 g/L, H2O2 = 1.5 mM pH = 3.5, T = 25 °C, UI = 40–970 kHz | 99.3% 1 h | [35] |
| AO 7 (35 mg/L) | GO–Fe3O4 NPs Fe3O4 NPs (10 nm) | Cat = 0.2 g/L, H2O2 = 22 mM pH = 3, T = 25 °C | 98%, 3 h ~80% | [11] |
| MB (120−300 mg/L) | α-Fe2O3/SiO2 | Cat = 1 g/L, H2O2 = 2 M pH = 3, T = 25 °C | 99% 60 h | [23] |
| MB (50 mg/L) RhB (25 mg/L)) | α-Fe2O3@Ti-tmSiO2 | Cat = 0.25 g/L, H2O2 = 53 mM pH = 4, T = 50 °C | 100%, 5.5 h 100%, 9h | [36] |
| AR 1 (200 mg/L) | Fe3O4 NPs (3.2 nm) | Cat = 4 g/L, H2O2 = 2 mM pH = 3.5, T = 25 °C | 97.4% 1 h | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-Vélez, E.; Martínez, H.; Castillo, F. Degradation of Acid Red 1 Catalyzed by Peroxidase Activity of Iron Oxide Nanoparticles and Detected by SERS. Nanomaterials 2021, 11, 3044. https://doi.org/10.3390/nano11113044
Vázquez-Vélez E, Martínez H, Castillo F. Degradation of Acid Red 1 Catalyzed by Peroxidase Activity of Iron Oxide Nanoparticles and Detected by SERS. Nanomaterials. 2021; 11(11):3044. https://doi.org/10.3390/nano11113044
Chicago/Turabian StyleVázquez-Vélez, Edna, Horacio Martínez, and Fermín Castillo. 2021. "Degradation of Acid Red 1 Catalyzed by Peroxidase Activity of Iron Oxide Nanoparticles and Detected by SERS" Nanomaterials 11, no. 11: 3044. https://doi.org/10.3390/nano11113044
APA StyleVázquez-Vélez, E., Martínez, H., & Castillo, F. (2021). Degradation of Acid Red 1 Catalyzed by Peroxidase Activity of Iron Oxide Nanoparticles and Detected by SERS. Nanomaterials, 11(11), 3044. https://doi.org/10.3390/nano11113044