On and Off: Epigenetic Regulation of C. albicans Morphological Switches
Abstract
1. Introduction
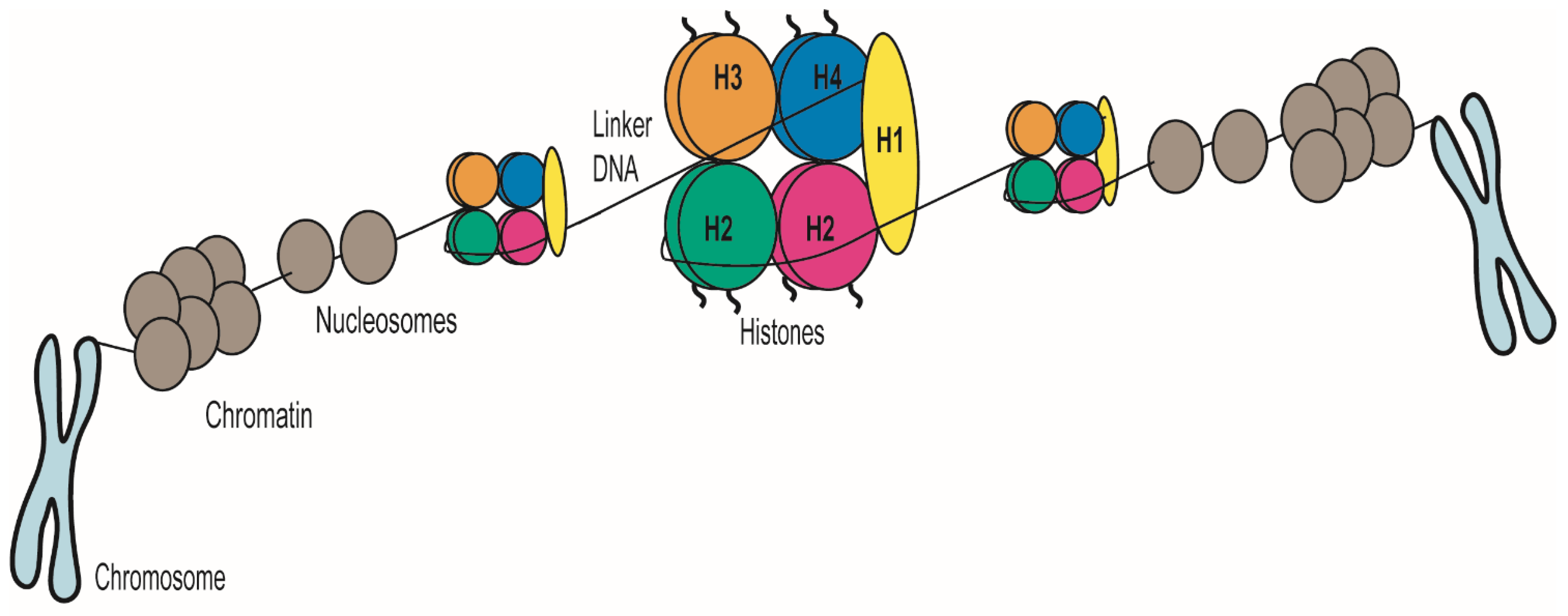
2. C. albicans’ Chromatin Structure: The Basics
3. Histone Post-Translational Modifications
4. Chromatin Remodelling Regulates Gene Expression and Chromatin Structure
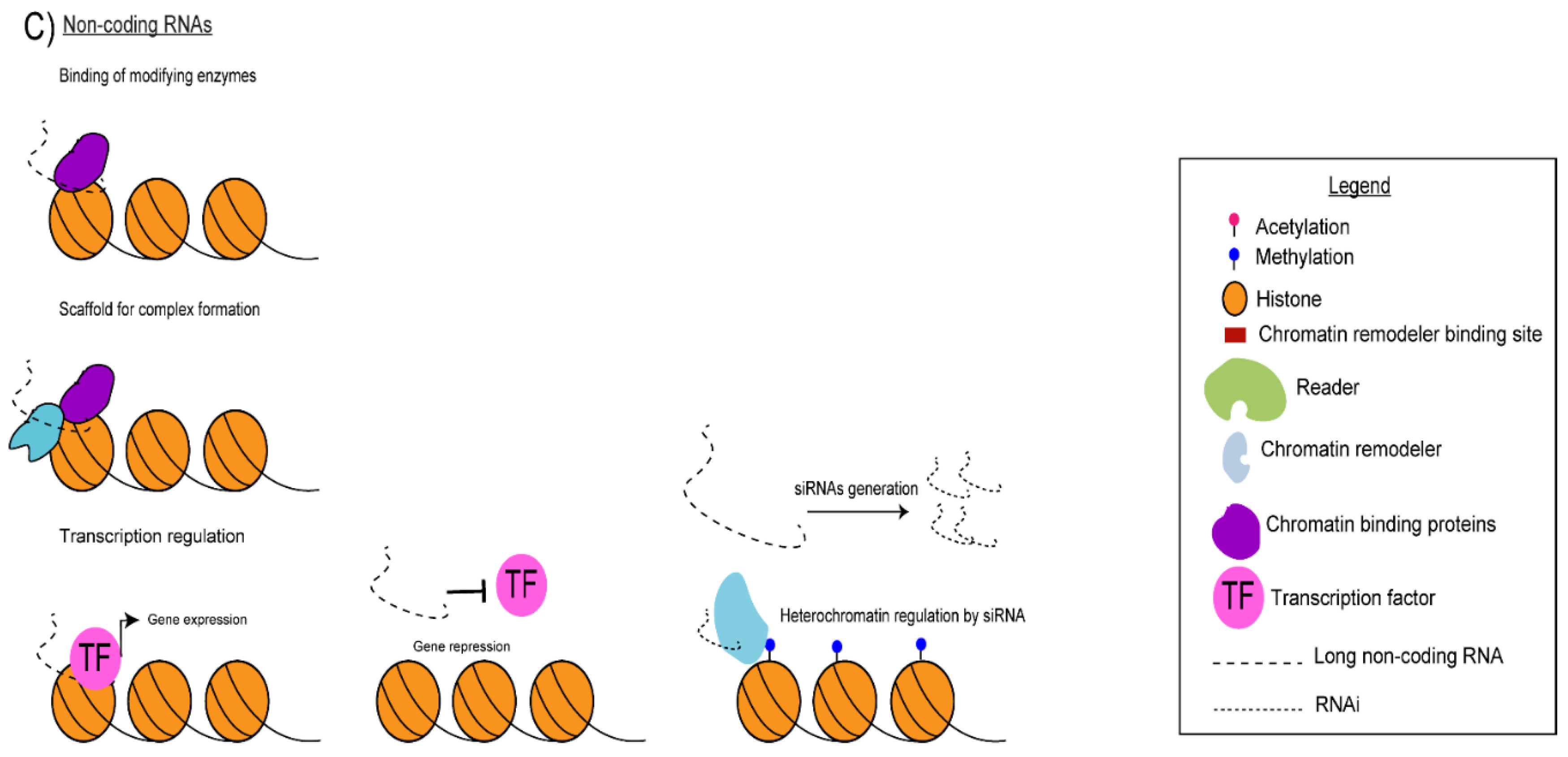
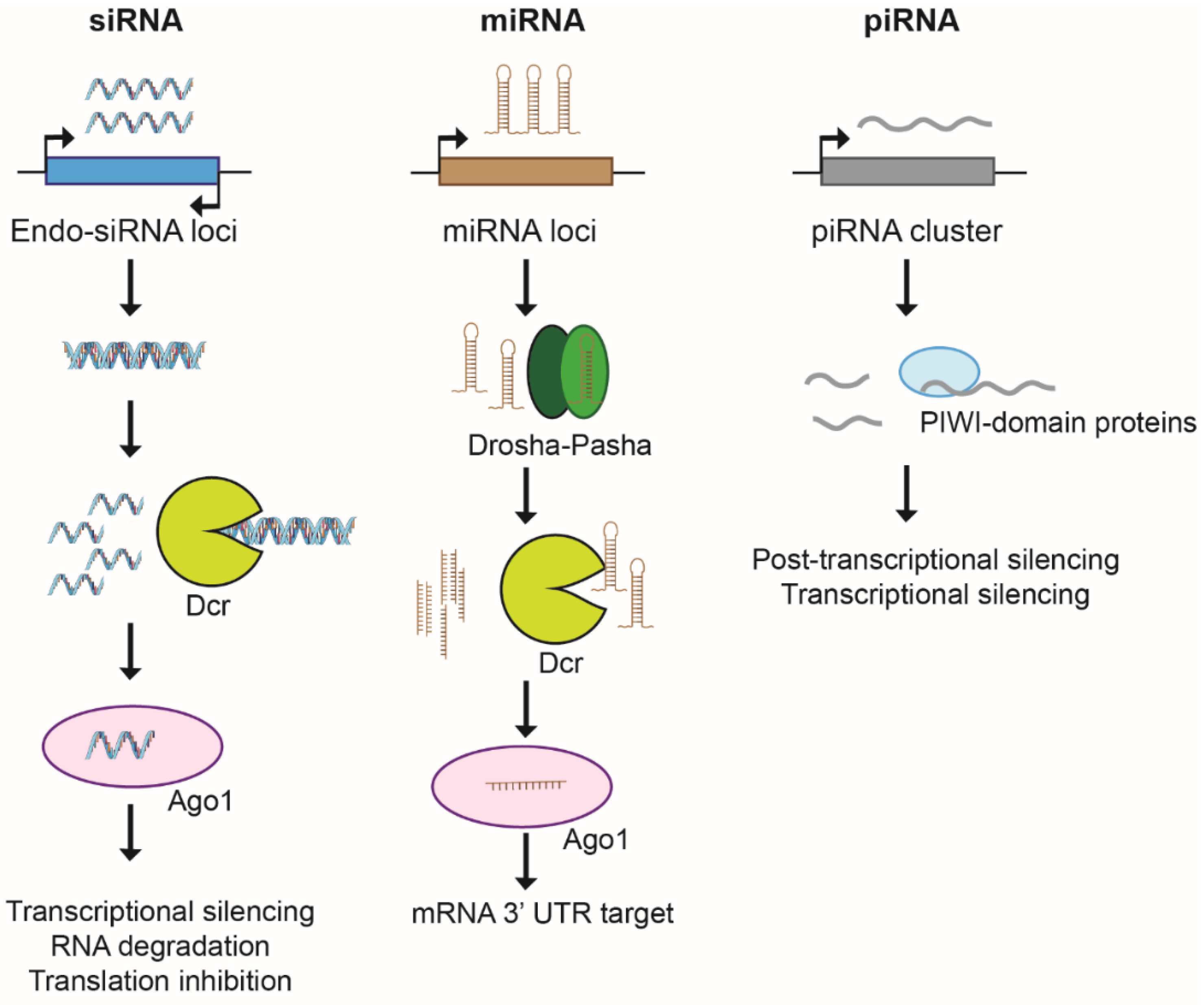
5. Non-Coding Transcription and Non-Coding RNAs
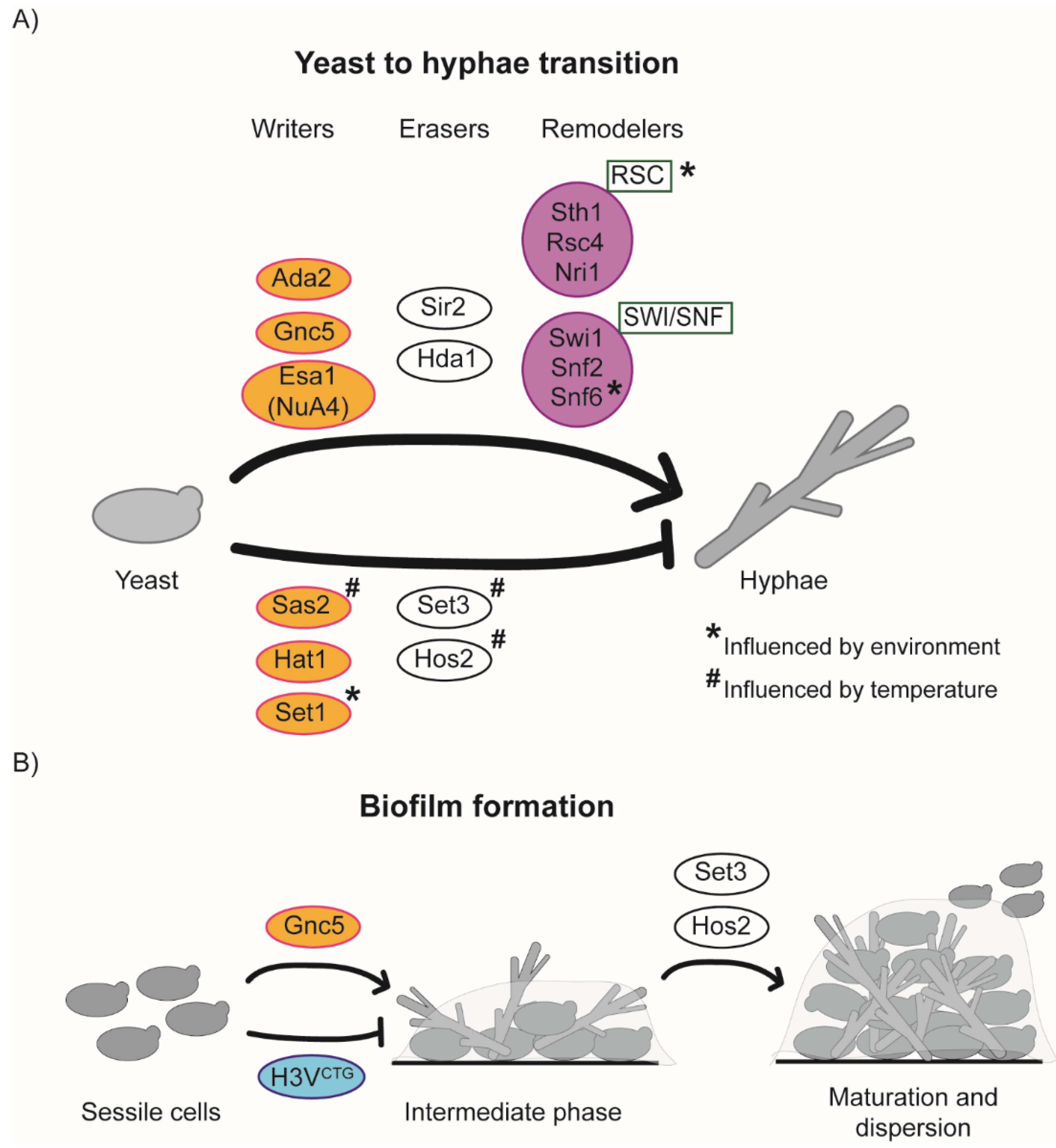
6. Chromatin-Mediated Regulation of the Yeast to Hypha Morphological Switch
7. Chromatin-Mediated Regulation of the Planktonic-Biofilm Transition
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waddington, C.H. The Epigenotype. Int. J. Epidemiol. 2012, 41, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.E.; Martienssen, R.A.; Riggs, A.D. Epigenetic Mechanisms of Gene Regulation; Cold Spring Harbor Laboratory Press: Woodbury, NY, USA, 1996. [Google Scholar]
- Bird, A. Perceptions of epigenetics. Nature 2007, 447, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Nanney, D.L. Epigenetic control systems. Proc. Natl. Acad. Sci. USA 1958, 44, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Holliday, R. Epigenetics: An overview. Dev. Genet. 1994, 15, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Aghcheh, R.K.; Kubicek, C.P. Epigenetics as an emerging tool for improvement of fungal strains used in biotechnology. Appl. Microbiol. Biotechnol. 2015, 99, 6167–6181. [Google Scholar] [CrossRef] [PubMed]
- Lappalainen, T.; Greally, J.M. Associating cellular epigenetic models with human phenotypes. Nat. Rev. Genet. 2017, 18, 441–451. [Google Scholar] [CrossRef]
- Cavalli, G.; Heard, E. Advances in epigenetics link genetics to the environment and disease. Nature 2019, 571, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden Killers: Human Fungal Infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef] [PubMed]
- Ohama, T.; Suzuki, T.; Mori, M.; Osawa, S.; Ueda, T.; Watanabe, K.; Nakase, T. Non-universal decoding of the leucine codon CUG in several Candida species. Nucleic Acids Res. 1993, 21, 4039–4045. [Google Scholar] [CrossRef]
- Krassowski, T.; Coughlan, A.Y.; Shen, X.-X.; Zhou, X.; Kominek, J.; Opulente, D.A.; Riley, R.; Grigoriev, I.V.; Maheshwari, N.; Shields, D.C.; et al. Evolutionary instability of CUG-Leu in the genetic code of budding yeasts. Nat. Commun. 2018, 9, 1887. [Google Scholar] [CrossRef] [PubMed]
- Lionakis, M.S.; Lim, J.K.; Lee, C.-C.R.; Murphy, P.M. Organ-Specific Innate Immune Responses in a Mouse Model of Invasive Candidiasis. J. Innate Immun. 2011, 3, 180–199. [Google Scholar] [CrossRef] [PubMed]
- da Silva Dantas, A.; Lee, K.K.; Raziunaite, I.; Schaefer, K.; Wagener, J.; Yadav, B.; Gow, N.A. Cell biology of Candida albicans–host interactions. Curr. Opin. Microbiol. 2016, 34, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, B.; Ferreira, C.; Alves, C.T.; Henriques, M.; Azeredo, J.; Silva, S. Vulvovaginal candidiasis: Epidemiology, microbiology and risk factors. Crit. Rev. Microbiol. 2016, 42, 905–927. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Desai, J. Candida albicans Hyphae: From Growth Initiation to Invasion. J. Fungi 2018, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Taff, H.T.; Mitchell, K.F.; Edward, J.A.; Andes, D.R. Mechanisms of Candida biofilm drug resistance. Future Microbiol. 2013, 8, 1325–1337. [Google Scholar] [CrossRef]
- Beekman, C.N.; Cuomo, C.A.; Bennett, R.J.; Ene, I.V. Comparative genomics of white and opaque cell states supports an epigenetic mechanism of phenotypic switching in Candida albicans. G3 GenesGenomesGenetics 2021, 11, jkab001. [Google Scholar] [CrossRef] [PubMed]
- Qasim, M.N.; Valle Arevalo, A.; Nobile, C.J.; Hernday, A.D. The Roles of Chromatin Accessibility in Regulating the Candida albicans White-Opaque Phenotypic Switch. J. Fungi 2021, 7, 37. [Google Scholar] [CrossRef]
- Luger, K.; Dechassa, M.L.; Tremethick, D.J. New insights into nucleosome and chromatin structure: An ordered state or a disordered affair? Nat. Rev. Mol. Cell Biol. 2012, 13, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Skrzypek, M.S.; Binkley, J.; Binkley, G.; Miyasato, S.R.; Simison, M.; Sherlock, G. The Candida Genome Database (CGD): Incorporation of Assembly 22, systematic identifiers and visualization of high throughput sequencing data. Nucleic Acids Res. 2017, 45, D592–D596. [Google Scholar] [CrossRef]
- Gu, M.; Naiyachit, Y.; Wood, T.J.; Millar, C.B. H2A.Z marks antisense promoters and has positive effects on antisense transcript levels in budding yeast. BMC Genom. 2015, 16, 99. [Google Scholar] [CrossRef] [PubMed]
- Martire, S.; Banaszynski, L.A. The roles of histone variants in fine-tuning chromatin organization and function. Nat. Rev. Mol. Cell Biol. 2020, 21, 522–541. [Google Scholar] [CrossRef] [PubMed]
- Zofall, M.; Fischer, T.; Zhang, K.; Zhou, M.; Cui, B.; Veenstra, T.D.; Grewal, S.I.S. Histone H2A.Z cooperates with RNAi and heterochromatin factors to suppress antisense RNAs. Nature 2009, 461, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Freire-Benéitez, V.; Price, R.J.; Buscaino, A. The Chromatin of Candida albicans Pericentromeres Bears Features of Both Euchromatin and Heterochromatin. Front. Microbiol. 2016, 7, 759. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Carey, M.; Workman, J.L. The Role of Chromatin during Transcription. Cell 2007, 128, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Strålfors, A.; Ekwall, K. Heterochromatin and Euchromatin—Organization, Boundaries, and Gene Regulation. In Reviews in Cell Biology and Molecular Medicine; American Cancer Society: Atlanta, GA, USA, 2011; ISBN 978-3-527-60090-8. [Google Scholar]
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Calo, E.; Wysocka, J. Modification of enhancer chromatin: What, how and why? Mol. Cell 2013, 49, 825–837. [Google Scholar] [CrossRef]
- Wang, X.; Cairns, M.J.; Yan, J. Super-enhancers in transcriptional regulation and genome organization. Nucleic Acids Res. 2019, 47, 11481–11496. [Google Scholar] [CrossRef]
- Price, R.J.; Weindling, E.; Berman, J.; Buscaino, A. Chromatin Profiling of the Repetitive and Non-repetitive Genomes of the Human Fungal Pathogen Candida albicans. mBio 2019, 10, e01376-19. [Google Scholar] [CrossRef] [PubMed]
- Saksouk, N.; Simboeck, E.; Déjardin, J. Constitutive heterochromatin formation and transcription in mammals. Epigenetics Chromatin 2015, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Erlendson, A.A.; Friedman, S.; Freitag, M. A Matter of Scale and Dimensions: Chromatin of Chromosome Landmarks in the Fungi. Microbiol. Spectr. 2017, 5, 571–597. [Google Scholar] [CrossRef]
- Mishra, P.K.; Baum, M.; Carbon, J. DNA methylation regulates phenotype-dependent transcriptional activity in Candida albicans. Proc. Natl. Acad. Sci. USA 2011, 108, 11965–11970. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Egervari, G.; Wang, Y.; Berger, S.L.; Lu, Z. Regulation of chromatin and gene expression by metabolic enzymes and metabolites. Nat. Rev. Mol. Cell Biol. 2018, 19, 563–578. [Google Scholar] [CrossRef] [PubMed]
- Engel, S.R.; Dietrich, F.S.; Fisk, D.G.; Binkley, G.; Balakrishnan, R.; Costanzo, M.C.; Dwight, S.S.; Hitz, B.C.; Karra, K.; Nash, R.S.; et al. The Reference Genome Sequence of Saccharomyces cerevisiae: Then and Now. G3 GenesGenomesGenetics 2014, 4, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Wood, V.; Harris, M.A.; McDowall, M.D.; Rutherford, K.; Vaughan, B.W.; Staines, D.M.; Aslett, M.; Lock, A.; Bahler, J.; Kersey, P.J.; et al. PomBase: A comprehensive online resource for fission yeast. Nucleic Acids Res. 2012, 40, D695–D699. [Google Scholar] [CrossRef]
- The Alliance of Genome Resources Consortium; Agapite, J.; Albou, L.-P.; Aleksander, S.; Argasinska, J.; Arnaboldi, V.; Attrill, H.; Bello, S.M.; Blake, J.A.; Blodgett, O.; et al. Alliance of Genome Resources Portal: Unified model organism research platform. Nucleic Acids Res. 2020, 48, D650–D658. [Google Scholar] [CrossRef]
- Tolsma, T.O.; Hansen, J.C. Post-translational modifications and chromatin dynamics. Essays Biochem. 2019, 63, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Sabari, B.R.; Tang, Z.; Huang, H.; Yong-Gonzalez, V.; Molina, H.; Kong, H.E.; Dai, L.; Shimada, M.; Cross, J.R.; Zhao, Y.; et al. Intracellular Crotonyl-CoA Stimulates Transcription through p300-Catalyzed Histone Crotonylation. Mol. Cell 2015, 58, 203–215. [Google Scholar] [CrossRef]
- Musselman, C.A.; Lalonde, M.-E.; Côté, J.; Kutateladze, T.G. Perceiving the epigenetic landscape through histone readers. Nat. Struct. Mol. Biol. 2012, 19, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Andrews, F.H.; Shinsky, S.A.; Shanle, E.K.; Bridgers, J.B.; Gest, A.; Tsun, I.K.; Krajewski, K.; Shi, X.; Strahl, B.D.; Kutateladze, T.G. The Taf14 YEATS domain is a reader of histone crotonylation. Nat. Chem. Biol. 2016, 12, 396–398. [Google Scholar] [CrossRef]
- Becker, P.B.; Hörz, W. ATP-Dependent Nucleosome Remodeling. Annu. Rev. Biochem. 2002, 71, 247–273. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, M.; Imam, N.; Verma, K.; Patel, A.K. Chromatin remodelers: We are the drivers!! Nucleus 2016, 7, 388–404. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Nogales, E.; Ciferri, C. Structure and Function of SWI/SNF Chromatin Remodeling Complexes and Mechanistic Implications for Transcription. Prog. Biophys. Mol. Biol. 2010, 102, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Du, Y.; Xiao, W. Yeast chromatin remodeling complexes and their roles in transcription. Curr. Genet. 2020, 66, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Biggar, S.R.; Crabtree, G.R. Continuous and widespread roles for the Swi-Snf complex in transcription. EMBO J. 1999, 18, 2254–2264. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.L.; Herskowitz, I. Characterization of the yeast SWI1, SWI2, and SWI3 genes, which encode a global activator of transcription. Cell 1992, 68, 573–583. [Google Scholar] [CrossRef]
- Sudarsanam, P.; Cao, Y.; Wu, L.; Laurent, B.C.; Winston, F. The nucleosome remodeling complex, Snf/Swi, is required for the maintenance of transcription in vivo and is partially redundant with the histone acetyltransferase, Gcn5. EMBO J. 1999, 18, 3101–3106. [Google Scholar] [CrossRef] [PubMed]
- Martens, J.A.; Winston, F. Evidence that Swi/Snf directly represses transcription in S. cerevisiae. Genes Dev. 2002, 16, 2231–2236. [Google Scholar] [CrossRef]
- Monahan, B.J.; Villén, J.; Marguerat, S.; Bähler, J.; Gygi, S.P.; Winston, F. Fission yeast SWI/SNF and RSC complexes show compositional and functional differences from budding yeast. Nat. Struct. Mol. Biol. 2008, 15, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Lorch, Y.; Kornberg, R.D. Chromatin-remodeling and the initiation of transcription. Q. Rev. Biophys. 2015, 48, 465–470. [Google Scholar] [CrossRef]
- Balachandra, V.K.; Verma, J.; Shankar, M.; Tucey, T.M.; Traven, A.; Schittenhelm, R.B.; Ghosh, S.K. The RSC (Remodels the Structure of Chromatin) complex of Candida albicans shows compositional divergence with distinct roles in regulating pathogenic traits. PLoS Genet. 2020, 16, e1009071. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Cao, F.; Nie, X.; Liu, H.; Chen, J. The Swi/Snf chromatin remodeling complex is essential for hyphal development in Candida albicans. FEBS Lett. 2006, 580, 2615–2622. [Google Scholar] [CrossRef]
- Guan, Z.; Liu, H. Overlapping Functions between SWR1 Deletion and H3K56 Acetylation in Candida albicans. Eukaryot. Cell 2015, 14, 578–587. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kaikkonen, M.U.; Lam, M.T.Y.; Glass, C.K. Non-coding RNAs as regulators of gene expression and epigenetics. Cardiovasc. Res. 2011, 90, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Till, P.; Mach, R.L.; Mach-Aigner, A.R. A current view on long non-coding RNAs in yeast and filamentous fungi. Appl. Microbiol. Biotechnol. 2018, 102, 7319–7331. [Google Scholar] [CrossRef] [PubMed]
- Paturi, S.; Deshmukh, M.V. A Glimpse of "Dicer Biology" Through the Structural and Functional Perspective. Front. Mol. Biosci. 2021, 8, 643657. [Google Scholar] [CrossRef] [PubMed]
- Smialowska, A.; Djupedal, I.; Wang, J.; Kylsten, P.; Swoboda, P.; Ekwall, K. RNAi mediates post-transcriptional repression of gene expression in fission yeast Schizosaccharomyces pombe. Biochem. Biophys. Res. Commun. 2014, 444, 254–259. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Izumi, N.; Shoji, K.; Suzuki, Y.; Katsuma, S.; Tomari, Y. Zucchini consensus motifs determine the mechanism of pre-piRNA production. Nature 2020, 578, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Fejes Tóth, K.; Aravin, A.A. piRNA Biogenesis in Drosophila melanogaster. Trends Genet. 2017, 33, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Batki, J.; Schnabl, J.; Wang, J.; Handler, D.; Andreev, V.I.; Stieger, C.E.; Novatchkova, M.; Lampersberger, L.; Kauneckaite, K.; Xie, W.; et al. The nascent RNA binding complex SFiNX licenses piRNA-guided heterochromatin formation. Nat. Struct. Mol. Biol. 2019, 26, 720–731. [Google Scholar] [CrossRef]
- Sellam, A.; Hogues, H.; Askew, C.; Tebbji, F.; van Het Hoog, M.; Lavoie, H.; Kumamoto, C.A.; Whiteway, M.; Nantel, A. Experimental annotation of the human pathogen Candida albicans coding and non-coding transcribed regions using high-resolution tiling arrays. Genome Biol. 2010, 11, R71. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.A.; Vyas, V.K.; Weinberg, D.E.; Drinnenberg, I.A.; Bartel, D.P.; Fink, G.R. Candida albicans Dicer (CaDcr1) is required for efficient ribosomal and spliceosomal RNA maturation. Proc. Natl. Acad. Sci. USA 2012, 109, 523–528. [Google Scholar] [CrossRef]
- Fuchs, B.B.; Eby, J.; Nobile, C.J.; El Khoury, J.B.; Mitchell, A.P.; Mylonakis, E. Role of filamentation in Galleria mellonella killing by Candida albicans. Microbes Infect. 2010, 12, 488–496. [Google Scholar] [CrossRef]
- Noble, S.M.; Gianetti, B.A.; Witchley, J.N. Candida albicans cell-type switching and functional plasticity in the mammalian host. Nat. Rev. Microbiol. 2017, 15, 96–108. [Google Scholar] [CrossRef]
- Murad, A.M.A. NRG1 represses yeast-hypha morphogenesis and hypha-specific gene expression in Candida albicans. EMBO J. 2001, 20, 4742–4752. [Google Scholar] [CrossRef] [PubMed]
- Braun, B.R.; Johnson, A.D. TUP1, CPH1 and EFG1 make independent contributions to filamentation in candida albicans. Genetics 2000, 155, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, M.; Thompson, D.S.; Lazzell, A.; Carlisle, P.L.; Pierce, C.; Monteagudo, C.; López-Ribot, J.L.; Kadosh, D. UME6, a Novel Filament-specific Regulator of Candida albicans Hyphal Extension and Virulence. Mol. Biol. Cell 2008, 19, 1354–1365. [Google Scholar] [CrossRef]
- Shapiro, R.S.; Robbins, N.; Cowen, L.E. Regulatory Circuitry Governing Fungal Development, Drug Resistance, and Disease. Microbiol. Mol. Biol. Rev. 2011, 75, 213–267. [Google Scholar] [CrossRef] [PubMed]
- Ernst, J.F. Transcription factors in Candida albicans—Environmental control of morphogenesis. Microbiology 2000, 146, 1763–1774. [Google Scholar] [CrossRef] [PubMed]
- Dunker, C.; Polke, M.; Schulze-Richter, B.; Schubert, K.; Rudolphi, S.; Gressler, A.E.; Pawlik, T.; Prada Salcedo, J.P.; Niemiec, M.J.; Slesiona-Künzel, S.; et al. Rapid proliferation due to better metabolic adaptation results in full virulence of a filament-deficient Candida albicans strain. Nat. Commun. 2021, 12, 3899. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, S.G.; Shafiq, A.; Hakim, S.T.; Anjum, Y.; Kazm, S.U. Effect of Growth Media, pH and Temperature on Yeast to Hyphal Transition in Candida albicans. Open J. Med. Microbiol. 2013, 3, 185–192. [Google Scholar] [CrossRef]
- Wang, X.; Chang, P.; Ding, J.; Chen, J. Distinct and Redundant Roles of the Two MYST Histone Acetyltransferases Esa1 and Sas2 in Cell Growth and Morphogenesis of Candida albicans. Eukaryot. Cell 2013, 12, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Hnisz, D.; Majer, O.; Frohner, I.E.; Komnenovic, V.; Kuchler, K. The Set3/Hos2 Histone Deacetylase Complex Attenuates cAMP/PKA Signaling to Regulate Morphogenesis and Virulence of Candida albicans. PLOS Pathog. 2010, 6, e1000889. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Rusche, L.N. Genetic Analysis of Sirtuin Deacetylases in Hyphal Growth of Candida albicans. mSphere 2021, 6, e00053-2. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Su, C.; Mao, X.; Raniga, P.P.; Liu, H.; Chen, J. Efg1-mediated Recruitment of NuA4 to Promoters Is Required for Hypha-specific Swi/Snf Binding and Activation in Candida albicans. Mol. Biol. Cell 2008, 19, 4260–4272. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Su, C.; Wang, A.; Liu, H. Hyphal Development in Candida albicans Requires Two Temporally Linked Changes in Promoter Chromatin for Initiation and Maintenance. PLoS Biol. 2011, 9, e1001105. [Google Scholar] [CrossRef]
- Lu, Y.; Su, C.; Solis, N.V.; Filler, S.G.; Liu, H. Synergistic Regulation of Hyphal Elongation by Hypoxia, CO2, and Nutrient Conditions Controls the Virulence of Candida albicans. Cell Host Microbe 2013, 14, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Kadosh, D.; Lopez-Ribot, J.L. Candida albicans: Adapting to Succeed. Cell Host Microbe 2013, 14, 483–485. [Google Scholar] [CrossRef] [PubMed]
- Shivarathri, R.; Tscherner, M.; Zwolanek, F.; Singh, N.K.; Chauhan, N.; Kuchler, K. The Fungal Histone Acetyl Transferase Gcn5 Controls Virulence of the Human Pathogen Candida albicans through Multiple Pathways. Sci. Rep. 2019, 9, 9445. [Google Scholar] [CrossRef]
- Chang, P.; Fan, X.; Chen, J. Function and subcellular localization of Gcn5, a histone acetyltransferase in Candida albicans. Fungal Genet. Biol. 2015, 81, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Nobile, C.J.; Fox, E.P.; Hartooni, N.; Mitchell, K.F.; Hnisz, D.; Andes, D.R.; Kuchler, K.; Johnson, A.D. A Histone Deacetylase Complex Mediates Biofilm Dispersal and Drug Resistance in Candida albicans. mBio 2014, 5, e01201-14. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Q.; Zeng, L.; Huang, X. Protein Acetylation/Deacetylation: A Potential Strategy for Fungal Infection Control. Front. Microbiol. 2020, 11, 574736. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Sui, M.; Li, M.; Wang, J.; Meng, Y.; Sun, T.; Liang, Q.; Suo, C.; Gao, X.; et al. Fungal acetylome comparative analysis identifies an essential role of acetylation in human fungal pathogen virulence. Commun. Biol. 2019, 2, 154. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Verma, J.; Vidan, N.; Wang, Y.; Tucey, T.M.; Lo, T.L.; Harrison, P.F.; See, M.; Swaminathan, A.; Kuchler, K.; et al. The YEATS Domain Histone Crotonylation Readers Control Virulence-Related Biology of a Major Human Pathogen. Cell Rep. 2020, 31, 107528. [Google Scholar] [CrossRef]
- Tebbji, F.; Chen, Y.; Sellam, A.; Whiteway, M. The Genomic Landscape of the Fungus-Specific SWI/SNF Complex Subunit, Snf6, in Candida albicans. mSphere 2017, 2, e00497-17. [Google Scholar] [CrossRef]
- Pukkila-Worley, R.; Peleg, A.Y.; Tampakakis, E.; Mylonakis, E. Candida albicans Hyphal Formation and Virulence Assessed Using a Caenorhabditis elegans Infection Model. Eukaryot. Cell 2009, 8, 1750–1758. [Google Scholar] [CrossRef] [PubMed]
- Bruno, V.M.; Wang, Z.; Marjani, S.L.; Euskirchen, G.M.; Martin, J.; Sherlock, G.; Snyder, M. Comprehensive annotation of the transcriptome of the human fungal pathogen Candida albicans using RNA-seq. Genome Res. 2010, 20, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Rai, L.S.; Singha, R.; Brahma, P.; Sanyal, K. Epigenetic determinants of phenotypic plasticity in Candida albicans. Fungal Biol. Rev. 2018, 32, 10–19. [Google Scholar] [CrossRef]
- Nobile, C.J.; Fox, E.P.; Nett, J.E.; Sorrells, T.R.; Mitrovich, Q.M.; Hernday, A.D.; Tuch, B.B.; Andes, D.R.; Johnson, A.D. A Recently Evolved Transcriptional Network Controls Biofilm Development in Candida albicans. Cell 2012, 148, 126–138. [Google Scholar] [CrossRef]
- Fox, E.P.; Bui, C.K.; Nett, J.E.; Hartooni, N.; Mui, M.C.; Andes, D.R.; Nobile, C.J.; Johnson, A.D. An expanded regulatory network temporally controls Candida albicans biofilm formation. Mol. Microbiol. 2015, 96, 1226–1239. [Google Scholar] [CrossRef] [PubMed]
- Mancera, E.; Nocedal, I.; Hammel, S.; Gulati, M.; Mitchell, K.F.; Andes, D.R.; Nobile, C.J.; Butler, G.; Johnson, A.D. Evolution of the complex transcription network controlling biofilm formation in Candida species. eLife 2021, 10, e64682. [Google Scholar] [CrossRef] [PubMed]
- Gulati, M.; Nobile, C.J. Candida albicans biofilms: Development, regulation, and molecular mechanisms. Microbes Infect. 2016, 18, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Rai, L.S.; Singha, R.; Sanchez, H.; Chakraborty, T.; Chand, B.; Bachellier-Bassi, S.; Chowdhury, S.; d’Enfert, C.; Andes, D.R.; Sanyal, K. The Candida albicans biofilm gene circuit modulated at the chromatin level by a recent molecular histone innovation. PLoS Biol. 2019, 17, e3000422. [Google Scholar] [CrossRef] [PubMed]



| C. albicans Gene and Known Function Candida Genome [21] | S. cerevisiae Ortholog SGD [36] | S. pombe Ortholog Pombase [37] | Human Ortholog Alliance of Genome Resources [38] | |
|---|---|---|---|---|
| Histone Acetyltransferase | ESA1 (orf19.5416) NuA4 HAT complex acts on H4K5, H4K12 | ESA1 (YOR244W) (alias: TAS1, KAT5) | MST1 (SPAC637.12c) | TIP60 (alias: KAT5) |
| SAS2 (orf19.2087) SAS HAT complex acts on H4K16 | SAS2 (YMR127C) (alias: KAT8) | MST2 (SPAC17G8.13c) | ||
| GCN5 (orf19.705) SAGA/ADA complex | GCN5 (YGR252W) (alias: ADA4, SW19, AAS104, KAT2) | GCN5 (SPAC1952.05) | KAT2B (alias: CAF) | |
| KAT2A (alias: GCN5) | ||||
| ADA2 (orf19.2331) SAGA/ADA complex acts on H3K9 | ADA2 (YDR448W) (alias: SWI8) | ADA2 (SPCC24B10.08c) | TADA2B (alias: ADA2B) | |
| TADA2A (alias: ADA2A) | ||||
| RTT109 (orf19.7491) acts on H3K56 | RTT109 (YLL002W) (alias: KIM2, REM50, KAT11) | rtt109 (SPBC342.06c) | No ortholog | |
| YNG2 (orf19.878) (alias: NBN1) NuA4 HAT complex acts on nucleosomal H4 | YNG2 (YHR090C) (alias: EAF4, NBN1) | png1 (SPAC3G9.08) | ING3 (alias: Eaf4, ING2) | |
| ING2 ± (alias: ING1L) | ||||
| ING4 ± | ||||
| ING5 ± | ||||
| NAT4 (orf19.4664) | NAT4 (YMR069W) | naa40 (SPCC825.04c) | NAA40 (alias: NAT11, PATT1) | |
| HAT1 (orf19.779) | HAT1 (YPL001W) (alias: KAT1) | hat1 (SPAC139.06) | HAT1 (alias: KAT1) | |
| SAS3 (orf19.2540) | SAS3 (YBL052C) (alias: KAT6) | MST2 (SPAC17G8.13c) | KAT7 (alias: HBO1, MYST-2) | |
| KAT6A ± (alias: MYST-3) | ||||
| KAT6B ± (alias: MYST-4) | ||||
| KAT8 ± (alias: MYST-1) | ||||
| Histone Deacetylase | HDA1 (orf19.2606) in a complex with Hda2 and Hda3 | HDA1 (YNL021W) | clr3 (SPBC800.03) | HDAC10 (alias: HD10) |
| HDAC6 (alias: HD6) | ||||
| SET3 (orf19.7221) SET3 HDAC complex with Hos2, Snt1 and Sif2 | SET3 (YKR029C) | set3 (SPAC22E12.11c) | KMT2E * | |
| SET4 (YJL105W) (SET3 paralog) | SETD5 * | |||
| RPD3 (orf19.2834) | RPD3 (YNL330C) | clr6 (SPBC36.05c) | HDAC1 (alias: KDAC1, RPD3) | |
| RPD31 (orf19.6801) | HDAC2 (alias: KDAC2, RPD3) | |||
| SIR2 (orf19.1992) (alias: SIR21) | HST1 (YOL068C) (SIR2 paralog) | sir2 (SPBC16D10.07c) | SIRT1 (alias: SIR2) | |
| SIR2 (YDL042C) | ||||
| HST1 (orf19.4761) (alias: SIR22) | HST1 (YOL068C) | |||
| SIR2 (YDL042C) | ||||
| HST2 (orf19.2580) | HST2 (YPL015C) | hst2 (SPCC132.02) | SIRT3 | |
| SIRT2 | ||||
| HST3 (orf19.1934) Acts on H3K56 | HST3 (YOR025W) | hst4 (SPAC1783.04c) | No ortholog | |
| Histone Methyltransferase | SET1 (orf19.6009) Acts on H3K4 | SET1 (YHR119W) (alias: KMT2) | set1 (SPCC306.04c) | SETD1B (alias: KMT2G) |
| SETD1A (alias: KMT2F) | ||||
| Histone Demethylase | RPH1 (orf19.2743) | RPH1 (YER169W) | jmj3 (SPBC83.07) | KDM4A |
| KDM4B | ||||
| KDM4C | ||||
| KDM4D | ||||
| KDM4E | ||||
| Chromatin Remodeler | SWR1 (orf19.1871) SWR1 complex | SWR1 (YDR334W) | swr1 (SPAC11E3.01c) | SRCAP (alias: SWR1) |
| SWI1 (orf19.5657) SWI/SNF complex | SWI1 (YPL016W) (alias: ADR6) | sol1 (SPBC30B4.04c) | ARID5A (alias: MRF1) | |
| ARID5B (alias: MRF2) | ||||
| SNF2 (orf19.1526) SWI/SNF complex | SNF2 (YOR290C) | snf21 (SPAC1250.01) | SMARCA2 | |
| SMARCA4 | ||||
| STH1 (orf19.239) RSC complex | STH1 (YIL126W) | SMARCA2 | ||
| SMARCA4 | ||||
| RNA interference | DCR1 (orf19.3796) | No ortholog | dcr1 (SPCC188.13c) | DICER1 |
| AGO1 (orf19.2903) RISC complex | No ortholog | ago1 (SPCC736.11) | PIWIL1 | |
| PIWIL2 | ||||
| PIWIL3 | ||||
| PIWIL4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iracane, E.; Vega-Estévez, S.; Buscaino, A. On and Off: Epigenetic Regulation of C. albicans Morphological Switches. Pathogens 2021, 10, 1463. https://doi.org/10.3390/pathogens10111463
Iracane E, Vega-Estévez S, Buscaino A. On and Off: Epigenetic Regulation of C. albicans Morphological Switches. Pathogens. 2021; 10(11):1463. https://doi.org/10.3390/pathogens10111463
Chicago/Turabian StyleIracane, Elise, Samuel Vega-Estévez, and Alessia Buscaino. 2021. "On and Off: Epigenetic Regulation of C. albicans Morphological Switches" Pathogens 10, no. 11: 1463. https://doi.org/10.3390/pathogens10111463
APA StyleIracane, E., Vega-Estévez, S., & Buscaino, A. (2021). On and Off: Epigenetic Regulation of C. albicans Morphological Switches. Pathogens, 10(11), 1463. https://doi.org/10.3390/pathogens10111463






