IL-4 and IL-13 Promote Proliferation of Mammary Epithelial Cells through STAT6 and IRS-1
Abstract
1. Introduction
2. Results
2.1. IL-4 and IL-13 Stimulate β-Casein Expression and Enlargement of Mammary Acini
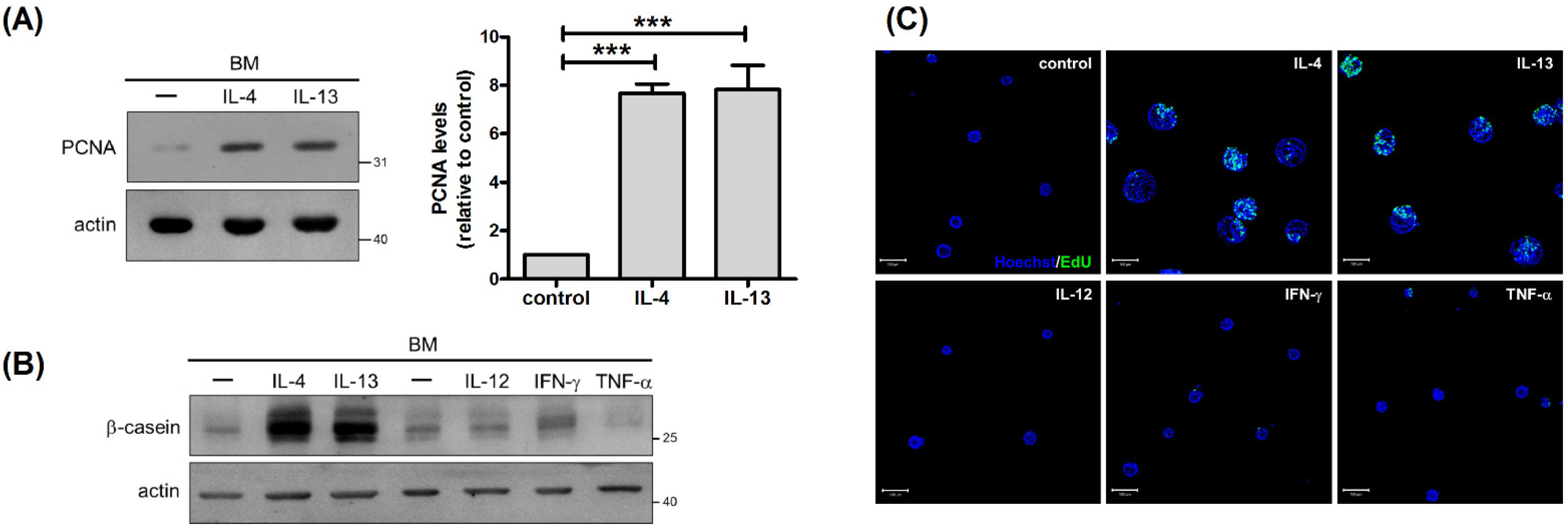
2.2. IL-4 and IL-13 Stimulate Proliferation of Mammary Cells
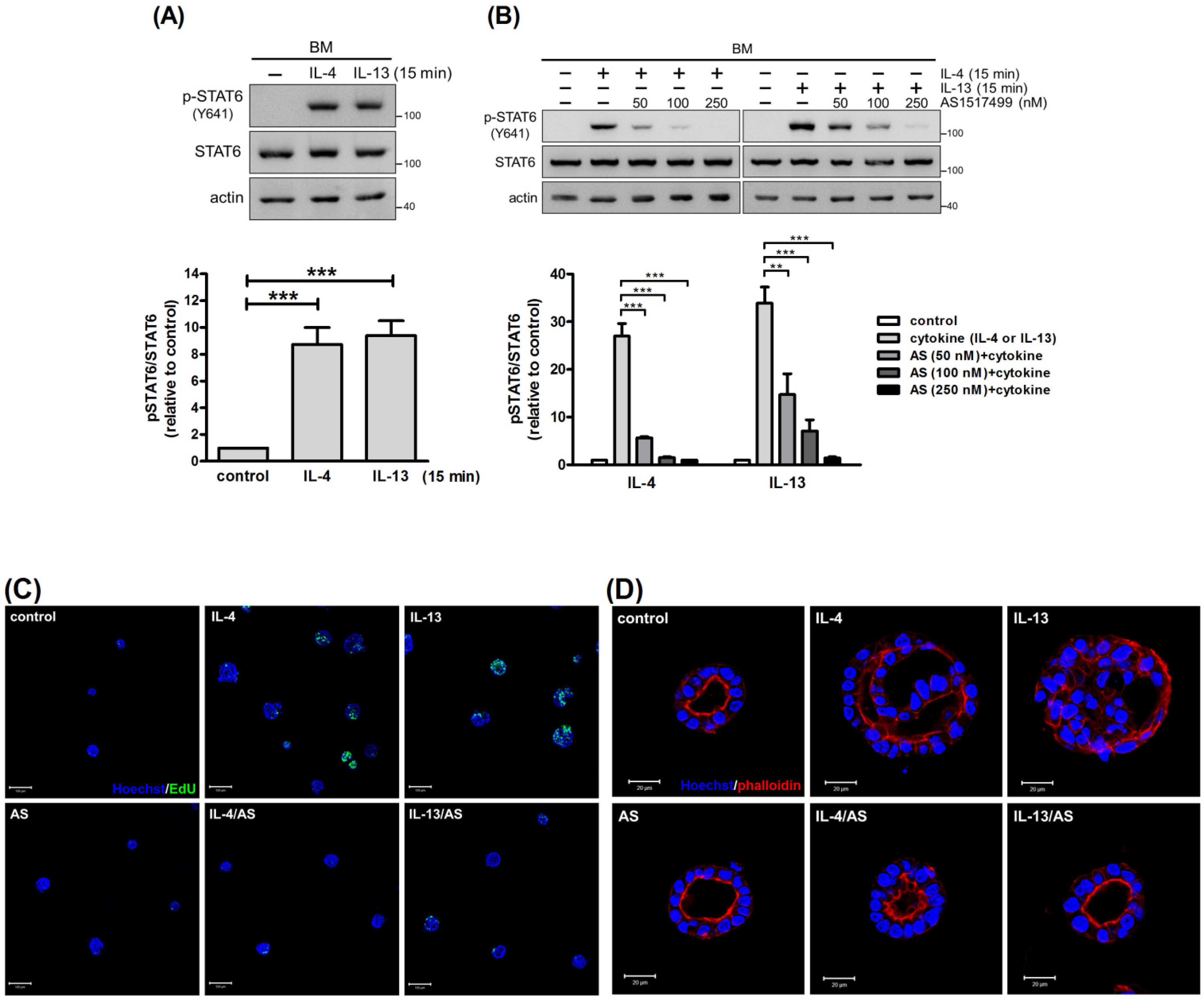
2.3. STAT6 Mediates the Pro-Proliferative Effect of IL-4 and IL-13
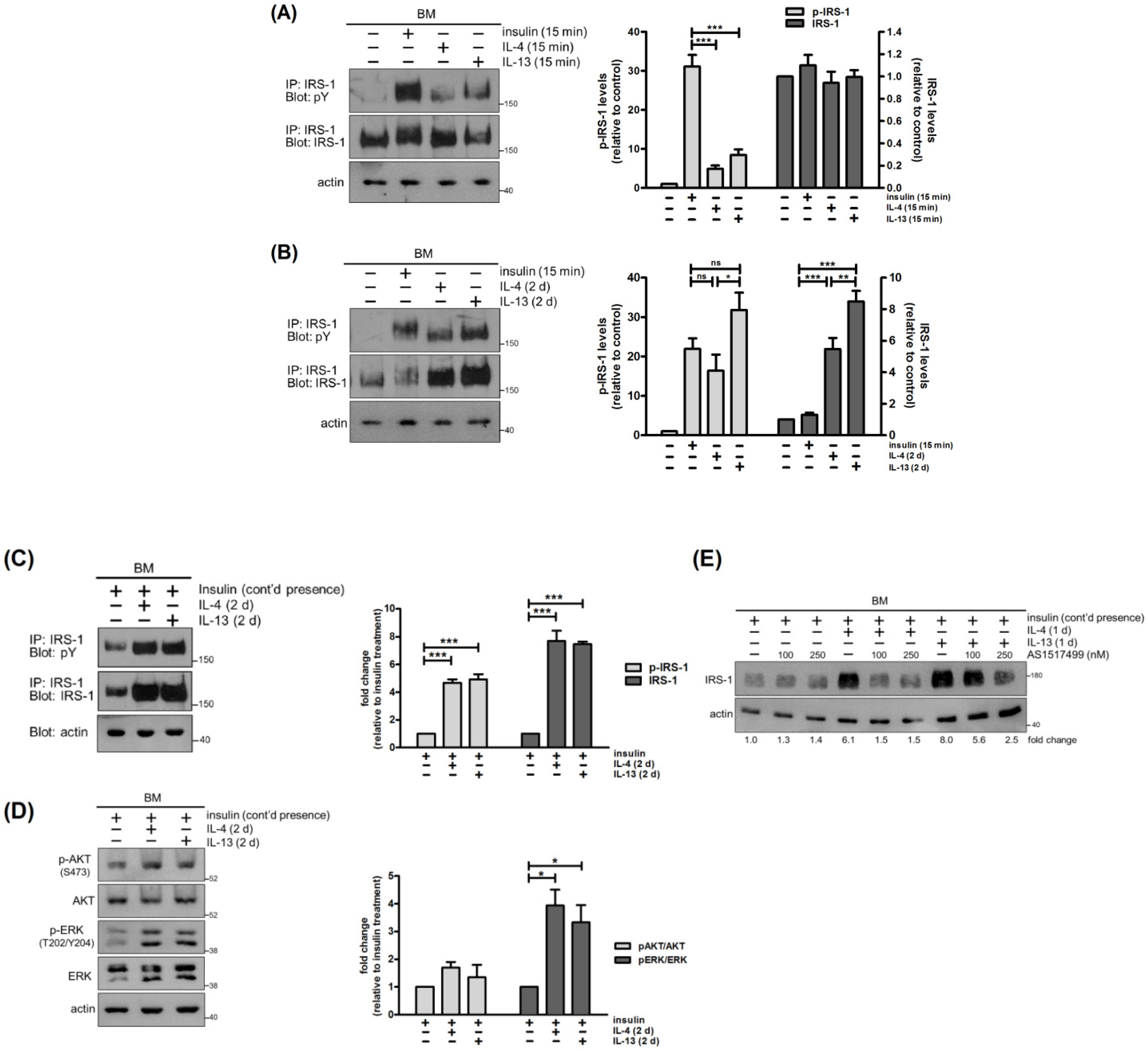
2.4. IL-4 and IL-13 Stimulate Tyrosine Phosphorylation and Expression of IRS-1
2.5. IRS-1 Is Involved in IL-4- and IL-13-Stimulated Cell Proliferation
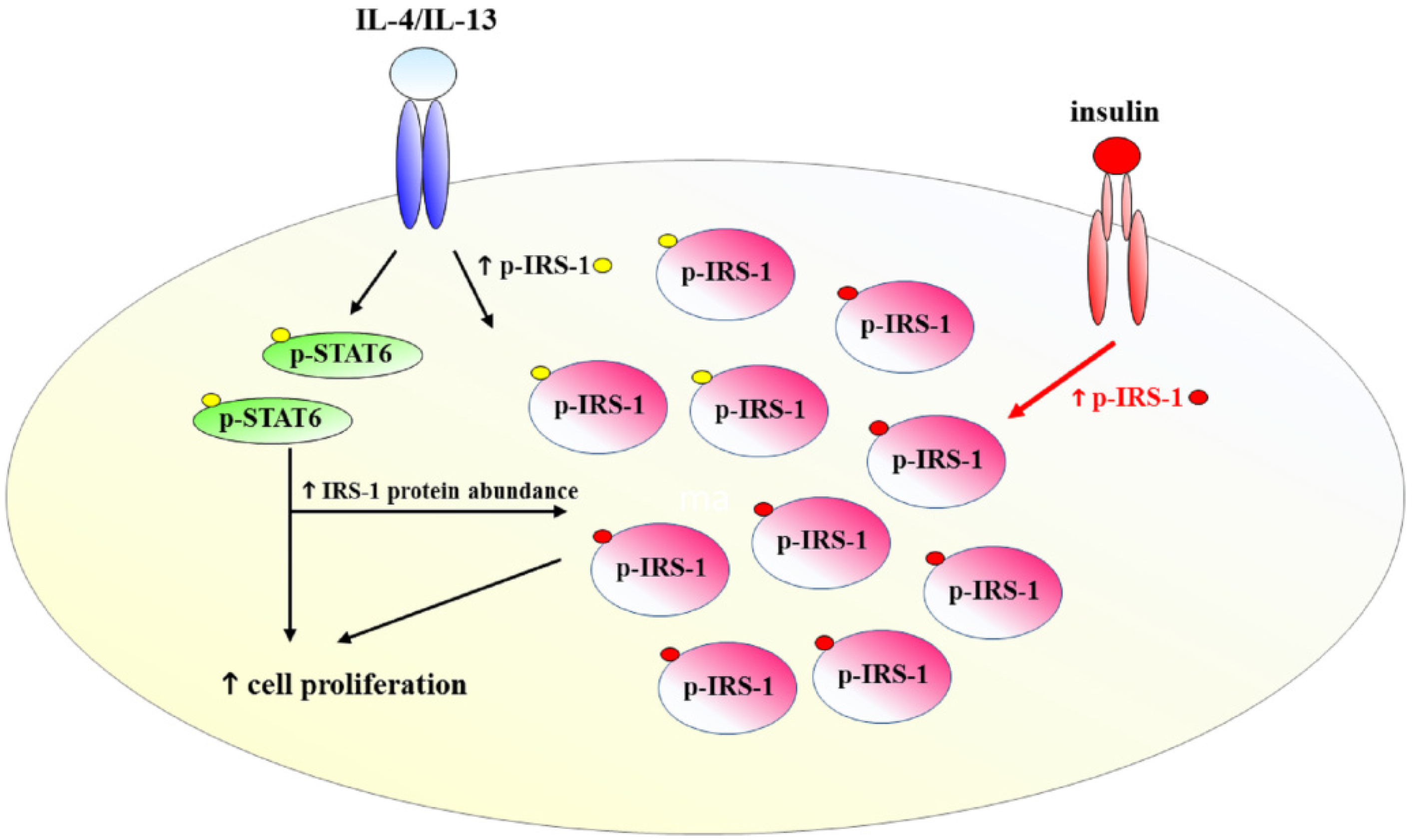
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. RNA Interference
4.4. Immunoprecipitation and Western Blot Analysis
4.5. Real-Time PCR
4.6. Cell Proliferation Assay
4.7. Immunofluorescence Microscopy
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paul, W.E.; Zhu, J. How are T(H)2-type immune responses initiated and amplified? Nat. Rev. Immunol. 2010, 10, 225–235. [Google Scholar] [CrossRef]
- Wynn, T.A. Type 2 cytokines: Mechanisms and therapeutic strategies. Nat. Rev. Immunol. 2015, 15, 271–282. [Google Scholar]
- Gandhi, N.A.; Bennett, B.L.; Graham, N.M.; Pirozzi, G.; Stahl, N.; Yancopoulos, G.D. Targeting key proximal drivers of type 2 inflammation in disease. Nat. Rev. Drug Discov. 2016, 15, 35–50. [Google Scholar] [CrossRef]
- Wills-Karp, M.; Finkelman, F.D. Untangling the complex web of IL-4- and IL-13-mediated signaling pathways. Sci. Signal. 2008, 1, pe55. [Google Scholar] [CrossRef] [PubMed]
- McCormick, S.M.; Heller, N.M. Commentary: IL-4 and IL-13 receptors and signaling. Cytokine 2015, 75, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, T.; Joshi, B.H.; Puri, R.K. IL-13 regulates cancer invasion and metastasis through IL-13Rα2 via ERK/AP-1 pathway in mouse model of human ovarian cancer. Int. J. Cancer 2012, 131, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.; Wange, W.; Cai, L.; Zhu, P.; Gao, Z.; Zheng, W. IL-13 receptor α2 stimulates human glioma cell growth and metastasis through the Src/PI3K/Akt/mTOR signaling pathway. Tumour Biol. 2016, 37, 14701–14709. [Google Scholar] [CrossRef]
- Muschler, J.; Streuli, C.H. Cell-matrix interactions in mammary gland development and breast cancer. Cold Spring Harb. Perspect. Biol. 2010, 2, a003202. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Boudreau, A.; Bissell, M.J. Tissue architecture and function: Dynamic reciprocity via extra- and intra-cellular matrices. Cancer Metastasis Rev. 2009, 28, 167–176. [Google Scholar] [CrossRef]
- Rankin, L.C.; Artis, D. Beyond Host Defense: Emerging Functions of the Immune System in Regulating Complex Tissue Physiology. Cell 2018, 173, 554–567. [Google Scholar]
- Plaks, V.; Boldajipour, B.; Linnemann, J.R.; Nguyen, N.H.; Kersten, K.; Wolf, Y.; Casbon, A.J.; Kong, N.; van den Bijgaart, R.J.; Sheppard, D.; et al. Adaptive Immune Regulation of Mammary Postnatal Organogenesis. Dev. Cell 2015, 34, 493–504. [Google Scholar] [CrossRef]
- Moriggl, R.; Berchtold, S.; Friedrich, K.; Standke, G.J.; Kammer, W.; Heim, M.; Wissler, M.; Stöcklin, E.; Gouilleux, F.; Groner, B. Comparison of the transactivation domains of Stat5 and Stat6 in lymphoid cells and mammary epithelial cells. Mol. Cell. Biol. 1997, 17, 3663–3678. [Google Scholar] [CrossRef] [PubMed]
- Khaled, W.T.; Read, E.K.; Nicholson, S.E.; Baxter, F.O.; Brennan, A.J.; Came, P.J.; Sprigg, N.; McKenzie, A.N.; Watson, C.J. The IL-4/IL-13/Stat6 signalling pathway promotes luminal mammary epithelial cell development. Development 2007, 134, 2739–2750. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.; Watson, C.J. The spectrum of STAT functions in mammary gland development. JAK-STAT 2012, 1, 151–158. [Google Scholar]
- Kouros-Mehr, H.; Slorach, E.M.; Sternlicht, M.D.; Werb, Z. GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell 2006, 127, 1041–1055. [Google Scholar] [PubMed]
- Asselin-Labat, M.L.; Sutherland, K.D.; Barker, H.; Thomas, R.; Shackleton, M.; Forrest, N.C.; Hartley, L.; Robb, L.; Grosveld, F.G.; van der Wees, J.; et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat. Cell Biol. 2007, 9, 201–209. [Google Scholar] [CrossRef]
- Du, J.Y.; Chen, M.C.; Hsu, T.C.; Wang, J.H.; Brackenbury, L.; Lin, T.H.; Wu, Y.Y.; Yang, Z.; Streuli, C.H.; Lee, Y.J. The RhoA-Rok-myosin II pathway is involved in extracellular matrix-mediated regulation of prolactin signaling in mammary epithelial cells. J. Cell. Physiol. 2012, 227, 1553–1560. [Google Scholar] [PubMed]
- Barcellos-Hoff, M.H.; Aggeler, J.; Ram, T.G.; Bissell, M.J. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development 1989, 105, 223–235. [Google Scholar]
- Mailleux, A.A.; Overholtzer, M.; Brugge, J.S. Lumen formation during mammary epithelial morphogenesis: Insights from in vitro and in vivo models. Cell Cycle 2008, 7, 57–62. [Google Scholar] [CrossRef]
- Welch, J.S.; Escoubet-Lozach, L.; Sykes, D.B.; Liddiard, K.; Greaves, D.R.; Glass, C.K. TH2 cytokines and allergic challenge induce Ym1 expression in macrophages by a STAT6-dependent mechanism. J. Biol. Chem. 2002, 277, 42821–42829. [Google Scholar] [CrossRef]
- Keegan, A.D.; Zamorano, J.; Keselman, A.; Heller, N.M. IL-4 and IL-13 Receptor Signaling From 4PS to Insulin Receptor Substrate 2: There and Back Again, a Historical View. Front. Immunol. 2018, 9, 1037. [Google Scholar] [CrossRef]
- Haeusler, R.A.; McGraw, T.E.; Accili, D. Biochemical and cellular properties of insulin receptor signalling. Nat. Rev. Mol. Cell Biol. 2018, 19, 31–44. [Google Scholar] [PubMed]
- Wang, L.M.; Myers, M.G., Jr.; Sun, X.J.; Aaronson, S.A.; White, M.; Pierce, J.H. IRS-1: Essential for insulin- and IL-4-stimulated mitogenesis in hematopoietic cells. Science 1993, 261, 1591–1594. [Google Scholar] [CrossRef] [PubMed]
- Mardilovich, K.; Pankratz, S.L.; Shaw, L.M. Expression and function of the insulin receptor substrate proteins in cancer. Cell Commun. Signal. 2009, 7, 14. [Google Scholar]
- Boucher, J.; Kleinridders, A.; Kahn, C.R. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb. Perspect. Biol. 2014, 6, a009191. [Google Scholar] [CrossRef]
- Reuveni, H.; Flashner-Abramson, E.; Steiner, L.; Makedonski, K.; Song, R.; Shir, A.; Herlyn, M.; Bar-Eli, M.; Levitzki, A. Therapeutic destruction of insulin receptor substrates for cancer treatment. Cancer Res. 2013, 73, 4383–4394. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.R.; Schwertfeger, K.L. Immune cell location and function during post-natal mammary gland development. J. Mammary Gland Biol. Neoplasia 2010, 15, 329–339. [Google Scholar] [CrossRef]
- Gouon-Evans, V.; Rothenberg, M.E.; Pollard, J.W. Postnatal mammary gland development requires macrophages and eosinophils. Development 2000, 127, 2269–2282. [Google Scholar] [CrossRef] [PubMed]
- Lilla, J.N.; Werb, Z. Mast cells contribute to the stromal microenvironment in mammary gland branching morphogenesis. Dev. Biol. 2010, 337, 124–133. [Google Scholar] [PubMed]
- Lilla, J.N.; Joshi, R.V.; Craik, C.S.; Werb, Z. Active plasma kallikrein localizes to mast cells and regulates epithelial cell apoptosis, adipocyte differentiation, and stromal remodeling during mammary gland involution. J. Biol. Chem. 2009, 284, 13792–13803. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Lyons, T.; Monks, J.; Lucia, M.S.; Wilson, R.S.; Hines, L.; Man, Y.G.; Borges, V.; Schedin, P. Alternatively activated macrophages and collagen remodeling characterize the postpartum involuting mammary gland across species. Am. J. Pathol. 2010, 176, 1241–1255. [Google Scholar] [CrossRef]
- Degnim, A.C.; Brahmbhatt, R.D.; Radisky, D.C.; Hoskin, T.L.; Stallings-Mann, M.; Laudenschlager, M.; Mansfield, A.; Frost, M.H.; Murphy, L.; Knutson, K.; et al. Immune cell quantitation in normal breast tissue lobules with and without lobulitis. Breast Cancer Res. Treat. 2014, 144, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Betts, C.B.; Pennock, N.D.; Caruso, B.P.; Ruffell, B.; Borges, V.F.; Schedin, P. Mucosal Immunity in the Female Murine Mammary Gland. J. Immunol. 2018, 201, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Hitchcock, J.R.; Hughes, K.; Harris, O.B.; Watson, C.J. Dynamic architectural interplay between leucocytes and mammary epithelial cells. FEBS J. 2020, 287, 250–266. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Li, J.; Zhang, J.; Chaurasiya, S.; Strom, A.; Wang, H.; Liao, W.T.; Cavallaro, F.; Denz, P.; Bernard, V.; et al. Oncogenic KRAS-Driven Metabolic Reprogramming in Pancreatic Cancer Cells Utilizes Cytokines from the Tumor Microenvironment. Cancer Discov. 2020, 10, 608–625. [Google Scholar] [PubMed]
- Dearth, R.K.; Cui, X.; Kim, H.J.; Kuiatse, I.; Lawrence, N.A.; Zhang, X.; Divisova, J.; Britton, O.L.; Mohsin, S.; Allred, D.C.; et al. Mammary tumorigenesis and metastasis caused by overexpression of insulin receptor substrate 1 (IRS-1) or IRS-2. Mol. Cell. Biol. 2006, 26, 9302–9314. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Chen, J.; Baserga, R. Nuclear insulin receptor substrate-1 activates promoters of cell cycle progression genes. Oncogene 2008, 27, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Venmar, K.T.; Kimmel, D.W.; Cliffel, D.E.; Fingleton, B. IL4 receptor α mediates enhanced glucose and glutamine metabolism to support breast cancer growth. Biochim. Biophys. Acta 2015, 1853, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Booth, B.W.; Sandifer, T.; Martin, E.L.; Martin, L.D. IL-13-induced proliferation of airway epithelial cells: Mediation by intracellular growth factor mobilization and ADAM17. Respir. Res. 2007, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Wodsedalek, D.J.; Paddock, S.J.; Wan, T.C.; Auchampach, J.A.; Kenarsary, A.; Tsaih, S.W.; Flister, M.J.; O’Meara, C.C. IL-13 promotes in vivo neonatal cardiomyocyte cell cycle activity and heart regeneration. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H24–H34. [Google Scholar] [CrossRef]
- Wei, L.H.; Yang, Y.; Wu, G.; Ignarro, L.J. IL-4 and IL-13 upregulate ornithine decarboxylase expression by PI3K and MAP kinase pathways in vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 2008, 294, C1198–C1205. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Suzuki, A.; Leland, P.; Joshi, B.H.; Puri, R.K. Targeting of IL-4 and IL-13 receptors for cancer therapy. Cytokine 2015, 75, 79–88. [Google Scholar] [PubMed]
- Shi, J.; Song, X.; Traub, B.; Luxenhofer, M.; Kornmann, M. Involvement of IL-4, IL-13 and Their Receptors in Pancreatic Cancer. Int. J. Mol. Sci. 2021, 22, 2998. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Traub, B.; Shi, J.; Kornmann, M. Possible Roles of Interleukin-4 and -13 and Their Receptors in Gastric and Colon Cancer. Int. J. Mol. Sci. 2021, 22, 727. [Google Scholar] [CrossRef]
- Bartolomé, R.A.; Martín-Regalado, Á.; Jaén, M.; Zannikou, M.; Zhang, P.; de Los Ríos, V.; Balyasnikova, I.V.; Casal, J.I. Protein Tyrosine Phosphatase-1B Inhibition Disrupts IL13Rα2-Promoted Invasion and Metastasis in Cancer Cells. Cancers 2020, 12, 500. [Google Scholar]
- Gaggianesi, M.; Turdo, A.; Chinnici, A.; Lipari, E.; Apuzzo, T.; Benfante, A.; Sperduti, I.; Di Franco, S.; Meraviglia, S.; Lo Presti, E.; et al. IL4 Primes the Dynamics of Breast Cancer Progression via DUSP4 Inhibition. Cancer Res. 2017, 77, 3268–3279. [Google Scholar] [CrossRef]
- Lee, A.V.; Zhang, P.; Ivanova, M.; Bonnette, S.; Oesterreich, S.; Rosen, J.M.; Grimm, S.; Hovey, R.C.; Vonderhaar, B.K.; Kahn, C.R.; et al. Developmental and hormonal signals dramatically alter the localization and abundance of insulin receptor substrate proteins in the mammary gland. Endocrinology 2003, 144, 2683–2694. [Google Scholar]
- Sykes, L.; MacIntyre, D.A.; Yap, X.J.; Teoh, T.G.; Bennett, P.R. The Th1:th2 dichotomy of pregnancy and preterm labour. Mediat. Inflamm. 2012, 2012, 967629. [Google Scholar]
- Porter, H.A.; Perry, A.; Kingsley, C.; Tran, N.L.; Keegan, A.D. IRS1 is highly expressed in localized breast tumors and regulates the sensitivity of breast cancer cells to chemotherapy, while IRS2 is highly expressed in invasive breast tumors. Cancer Lett. 2013, 338, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Chao, R.; Li, D.; Yue, Z.; Huang, C.; Kou, Y.; Zhou, Q.; Gao, Y.; Hasegawa, T.; Guo, J.; Li, M. Interleukin-4 Restores Insulin Sensitivity in Insulin-Resistant Osteoblasts by Increasing the Expression of Insulin Receptor Substrate 1. Biochemistry 2020, 85, 334–343. [Google Scholar] [CrossRef]
- Rütti, S.; Howald, C.; Arous, C.; Dermitzakis, E.; Halban, P.A.; Bouzakri, K. IL-13 improves beta-cell survival and protects against IL-1beta-induced beta-cell death. Mol. Metab. 2016, 5, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Panno, M.L.; Mauro, L.; Marsico, S.; Bellizzi, D.; Rizza, P.; Morelli, C.; Salerno, M.; Giordano, F.; Andò, S. Evidence that the mouse insulin receptor substrate-1 belongs to the gene family on which the promoter is activated by estrogen receptor alpha through its interaction with Sp1. J. Mol. Endocrinol. 2006, 36, 91–105. [Google Scholar] [PubMed]
- Wei, M.; Liu, B.; Gu, Q.; Su, L.; Yu, Y.; Zhu, Z. Stat6 cooperates with Sp1 in controlling breast cancer cell proliferation by modulating the expression of p21(Cip1/WAF1) and p27 (Kip1). Cell. Oncol. 2013, 36, 79–93. [Google Scholar]
- Baserga, R. The insulin receptor substrate-1: A biomarker for cancer? Exp. Cell Res. 2009, 315, 727–732. [Google Scholar] [CrossRef]
- Hakuno, F.; Fukushima, T.; Yoneyama, Y.; Kamei, H.; Ozoe, A.; Yoshihara, H.; Yamanaka, D.; Shibano, T.; Sone-Yonezawa, M.; Yu, B.C.; et al. The Novel Functions of High-Molecular-Mass Complexes Containing Insulin Receptor Substrates in Mediation and Modulation of Insulin-Like Activities: Emerging Concept of Diverse Functions by IRS-Associated Proteins. Front. Endocrinol. 2015, 6, 73. [Google Scholar] [CrossRef]
- Chang, Y.H.; Ho, K.T.; Lu, S.H.; Huang, C.N.; Shiau, M.Y. Regulation of glucose/lipid metabolism and insulin sensitivity by interleukin-4. Int. J. Obes. 2012, 36, 993–998. [Google Scholar] [CrossRef]
- Stanya, K.J.; Jacobi, D.; Liu, S.; Bhargava, P.; Dai, L.; Gangl, M.R.; Inouye, K.; Barlow, J.L.; Ji, Y.; Mizgerd, J.P.; et al. Direct control of hepatic glucose production by interleukin-13 in mice. J. Clin. Investig. 2013, 123, 261–271. [Google Scholar]
- Ricardo-Gonzalez, R.R.; Red Eagle, A.; Odegaard, J.I.; Jouihan, H.; Morel, C.R.; Heredia, J.E.; Mukundan, L.; Wu, D.; Locksley, R.M.; Chawla, A. IL-4/STAT6 immune axis regulates peripheral nutrient metabolism and insulin sensitivity. Proc. Natl. Acad. Sci. USA 2010, 107, 22617–22622. [Google Scholar] [CrossRef]
- Binnemars-Postma, K.; Bansal, R.; Storm, G.; Prakash, J. Targeting the Stat6 pathway in tumor-associated macrophages reduces tumor growth and metastatic niche formation in breast cancer. FASEB J. 2018, 32, 969–978. [Google Scholar]
- Ruffell, B.; Affara, N.I.; Coussens, L.M. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012, 33, 119–126. [Google Scholar] [CrossRef]
- Yao, M.; Ventura, P.B.; Jiang, Y.; Rodriguez, F.J.; Wang, L.; Perry, J.S.A.; Yang, Y.; Wahl, K.; Crittenden, R.B.; Bennett, M.L.; et al. Astrocytic trans-Differentiation Completes a Multicellular Paracrine Feedback Loop Required for Medulloblastoma Tumor Growth. Cell 2020, 180, 502–520. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Liu, C.; Chen, F.K.; Feng, Z.P.; Jia, L.; Liu, P.J.; Yang, Z.X.; Hou, F.; Deng, Z.Y. M2-like tumour-associated macrophage-secreted IGF promotes thyroid cancer stemness and metastasis by activating the PI3K/AKT/mTOR pathway. Mol. Med. Rep. 2021, 24, 604. [Google Scholar] [PubMed]
- Lee, Y.J.; Streuli, C.H. Extracellular matrix selectively modulates the response of mammary epithelial cells to different soluble signaling ligands. J. Biol. Chem. 1999, 274, 22401–22408. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.-J.; Wang, S.-H.; Wu, C.-C.; Su, Y.-A.; Chiang, C.-Y.; Lai, C.-H.; Wang, T.-H.; Cheng, T.-L.; Kuo, J.-Y.; Hsu, T.-C.; et al. IL-4 and IL-13 Promote Proliferation of Mammary Epithelial Cells through STAT6 and IRS-1. Int. J. Mol. Sci. 2021, 22, 12008. https://doi.org/10.3390/ijms222112008
Wu W-J, Wang S-H, Wu C-C, Su Y-A, Chiang C-Y, Lai C-H, Wang T-H, Cheng T-L, Kuo J-Y, Hsu T-C, et al. IL-4 and IL-13 Promote Proliferation of Mammary Epithelial Cells through STAT6 and IRS-1. International Journal of Molecular Sciences. 2021; 22(21):12008. https://doi.org/10.3390/ijms222112008
Chicago/Turabian StyleWu, Wan-Ju, Sue-Hong Wang, Chun-Chi Wu, Yi-An Su, Chin-Yin Chiang, Ching-Hong Lai, Tsung-Hsiang Wang, Tsung-Lin Cheng, Jia-Yu Kuo, Tsai-Ching Hsu, and et al. 2021. "IL-4 and IL-13 Promote Proliferation of Mammary Epithelial Cells through STAT6 and IRS-1" International Journal of Molecular Sciences 22, no. 21: 12008. https://doi.org/10.3390/ijms222112008
APA StyleWu, W.-J., Wang, S.-H., Wu, C.-C., Su, Y.-A., Chiang, C.-Y., Lai, C.-H., Wang, T.-H., Cheng, T.-L., Kuo, J.-Y., Hsu, T.-C., Lin, T.-H., & Lee, Y.-J. (2021). IL-4 and IL-13 Promote Proliferation of Mammary Epithelial Cells through STAT6 and IRS-1. International Journal of Molecular Sciences, 22(21), 12008. https://doi.org/10.3390/ijms222112008




