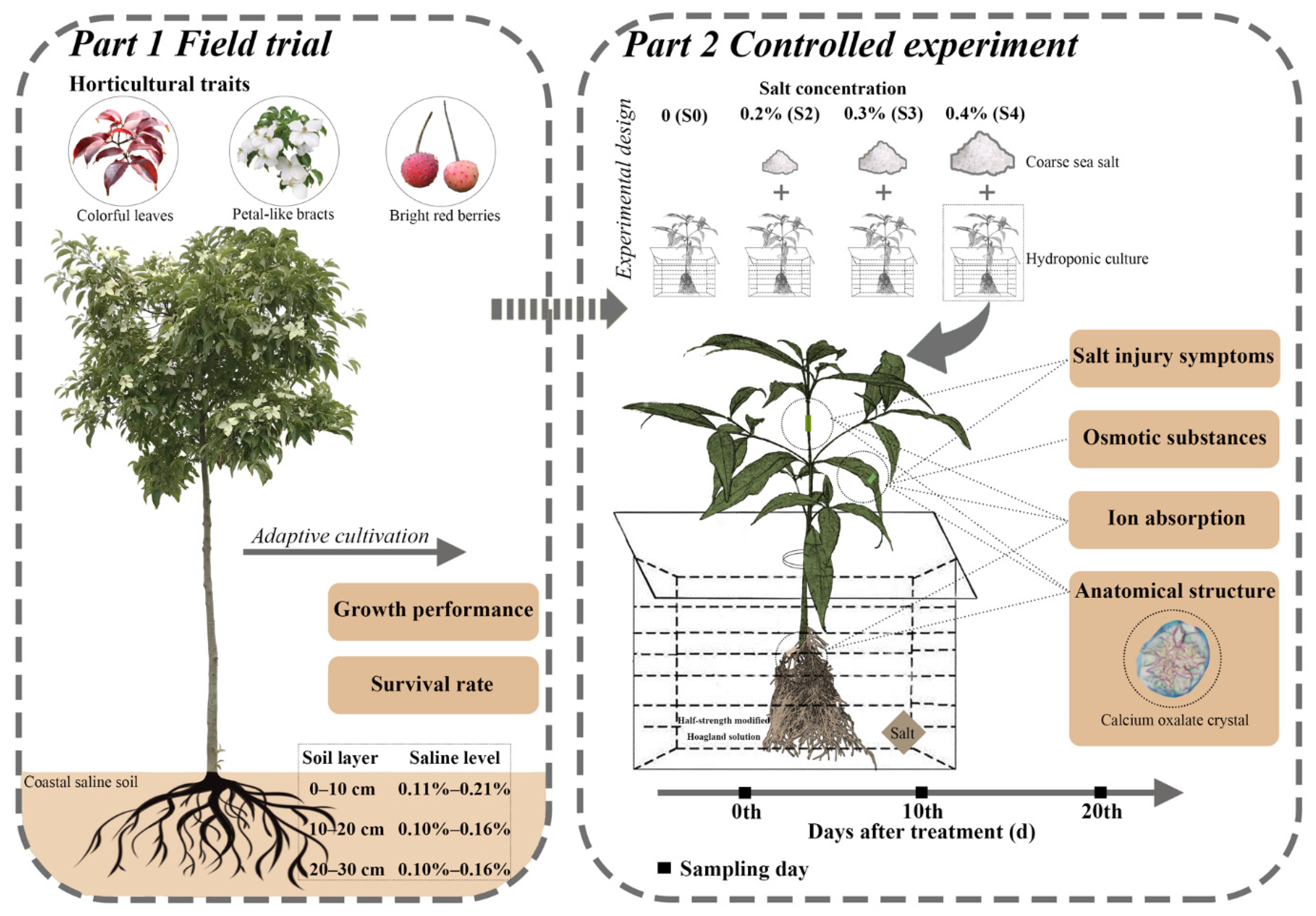
1. Introduction
Salinity is a major abiotic stress that generally negatively influences the survival, biomass production, and yield of plants [
1]. Salinity results from soil salinization and/or soil secondary salinization (basically caused by unreasonable irrigation), which mainly increase both osmotic pressure and ionic strength [
2]. When plants are exposed to salinity stress, they usually exclude or sequester sodium ions (Na
+) and chlorine ions (Cl
−) in the cytoplasm to avoid toxicity and maintain the appropriate cellular levels of potassium ions (K
+) and calcium ions (Ca
2+) necessary for ameliorating the inhibitory effects on their survival and growth [
3]. Calcium plays essential roles in preserving the structural and functional integrity of plant membranes, stabilizing cell wall structures, regulating cell osmotic pressure and ion selective transport, and enhancing the resistance of plants to environmental stress [
4].
Mostly, salinity stress triggers an increase in cytosolic free Ca
2+ ([Ca
2+]
cyt), so as to stimulate the Ca
2+-related salt overly sensitive (SOS) pathway to expulse excess intracellular Na
+ [
2]. The SOS pathway comprises the calcineurin B-like protein 4/SOS3 (CBL4/SOS3), CBL-interacting protein kinase 24/SOS2 (CIPK24/SOS2), and Na
+/H
+ antiporter SOS1 [
5]. Moreover, downstream [Ca
2+]
cyt could act as a second messenger to activate calcium-dependent protein kinases (CDPKs) and salt-sensing channels, and transduce the hyperosmotic signal to downstream gene transcription and protein activity [
6]. Notably, a high Ca
2+ supply is thought to be one of the most efficient approaches for improving salt tolerance by inhibiting the unidirectional influx of Na
+ and Cl
− into various organs [
4]. However, excessive Ca
2+ can combine with endogenous oxalic acid to form calcium oxalate crystals through a physical–chemical process in cells [
7]. Additionally, calcium oxalates are extensively distributed in plant organs and could become a Ca
2+ pool for multifunctional calcium regulation [
8].
Saline soil is distributed extensively in coastal, arid, and semi-arid areas of the world. Over 6% of total land area and about 30% of irrigated farmlands in the world is affected by soil salinization and/or secondary soil salinization [
9]. Contemporary soil salinization has gradually become one of the main limiting factors for plant propagation, introduction, and cultivation [
10]. Unfortunately, there are more than 34 million hm
2 of salinized regions and about 1 million hm
2 of coastal saline soil regions in China, which profoundly affects plant survival [
11], especially woody ornamental plants, resulting in less biodiversity and a fragile ecosystem in coastal regions [
12]. With the increasing demands for horticultural tree species for landscaping in the coastal area in China, more and more attractive ornamental trees have been introduced, domesticated, and cultivated. Thus, salt-tolerant species, such as
Pongamia pinnata (L.) pierre and red-osier dogwood (
Cornus sericea L. syn.
Cornus stolonifera Michx), have become potential species for afforestation in coastal saline soil regions [
13,
14].
Dogwoods belong to the genus of
Cornus subg.
syncarpea (Nakai) Q. Y. Xiang with two groups: the East Asian group and North American group [
15]. Their cultivars are widely used as ornamental plants in gardens and urban landscapes owing to attractive horticultural traits, such as petal-like bracts of red, pink, or white in the spring, bright red berries, and colorful leaves in the fall. Furthermore, more than 100 cultivars selected from the North American group have enjoyed considerable success as excellent garden ornamentals in the United States, Canada, and Mexico for over 100 years [
16,
17], although their domestication in eastern China is not entirely successful. As a group of dogwoods native to China, the East Asian group contains eight evergreen and two deciduous species, whose distributions cover most subtropical to tropical areas in China. Most importantly, these dogwoods have abundant variability in leaf and bract color [
18], especially in extensive adaptation to various habitats, i.e., moderate drought and salinity stress. Consequently, they are considered a promising species for the breeding of horticultural cultivars and elite rootstocks for grafting cultivars selected from the North American group [
19,
20].
Although the effects of salinity stress have been widely reported in many woody species [
13,
14,
21,
22], little is known about the salt tolerance of dogwoods. We took
Cornus hongkongensis subsp.
elegans (W. P. Fang et Y. T. Hsieh) Q. Y. Xiang seedlings as the experimental materials, whose natural distribution is mainly located in the middle and lower reaches of the Yangtze River in the subtropical region of China, where extremely high temperature and high humidity short-term events occur frequently in summer. A large amount of coastal saline soil is distributed in the Yangtze–Huaihe Region north of the Yangtze River estuary and west of the Yellow Sea. This area is a potential cultivatable saline area for ornamental dogwoods because of its suitable climatic conditions. The aims of this study focused on (i) clarifying the salt tolerance capability of this species; (ii) verifying the response to increasing salt concentration on physiological levels, including osmolytes and ion absorption; and (iii) emphasizing the tissue deposition specificity of calcium oxalate crystal in response to salinity stress on an anatomical level, then speculating on the regulating role of Ca
2+ in salinized
C. elegans seedlings.
4. Discussion
Salinity stress mainly inhibits plant growth and development through osmotic stress and ion poisoning [
28]. Therefore, plants have developed a wide range of strategies to minimize the adverse effects by rebuilding osmotic and ionic homeostasis, then maintaining the physiological and biochemical stability necessary for growth and survival under saline conditions [
29,
30]. In this study, three salt concentrations (0.2%, 0.3%, and 0.4%) were assessed, according to the survey of saline levels in the field for crop production [
31] and adaptive cultivation of
C. elegans in Dafeng Forest Farm (
Table 1).
Salt damage symptoms are phenotypic phenomena following physiological responses to salt stress and are commonly used as evaluating criteria of the salt tolerance of plants. When sodium ions (Na
+) and chlorine ions (Cl
−) accumulate in leaves but are not compartmentalized in vacuoles, the leaves can be metabolically toxic so as to manifest features such as “scorched” or “burned” and slight bronzing, followed by leaf-tip death and general necrosis [
32]. In our study, the degree of salt injury symptoms in salinized seedlings of
C. elegans worsened as the stress duration extended (
Table 3). Similar symptoms have been described in many plants suffering from salt stress [
32], but the degree varies depending on the salt tolerance of the plant species [
1,
25]. In addition, the similarity of salt injury symptoms and survival rates in S2 to S0 treatments implied that
C. elegans plants could be tolerant of lower salt concentration stress (0.2%).
The survival rate of plants growing in the field was lower than that in the controlled experiment owing to multiple adverse environmental factors, such as strong coastal winds and physiological drought. However, the growth performance of the surviving plants was much better than that under the controlled experimental conditions (
Table 3 and
Table S1). The reasons may lie in the double stress in salt-treated seedlings under the controlled experimental conditions, as well as being due to the irregular rainfall and irrigation, lowering soil salt concentration, thereby alleviating salt damage in
C. elegans in the field trial. In addition, plants of
C. elegans in the field with normal blooming and fruiting (
Figure S1) revealed strong adaptability as well as valuable ornamental effects under moderate salt concentration stress (≤0.2%).
Salinity stress induces a set of common responses in plants owing to the osmotic stress signal [
13]. Osmoregulation can be executed through the accumulation of osmolytes such as proline (Pro), soluble sugar, and soluble protein, which are compatible with cell metabolism in an adverse environment [
29,
33,
34]. The Pro concentrations in
C. elegans seedlings on the 20th day of stress were almost twofold greater than the concentrations on day 0 and day 10 of culture, regardless of no/salt treatments, respectively (
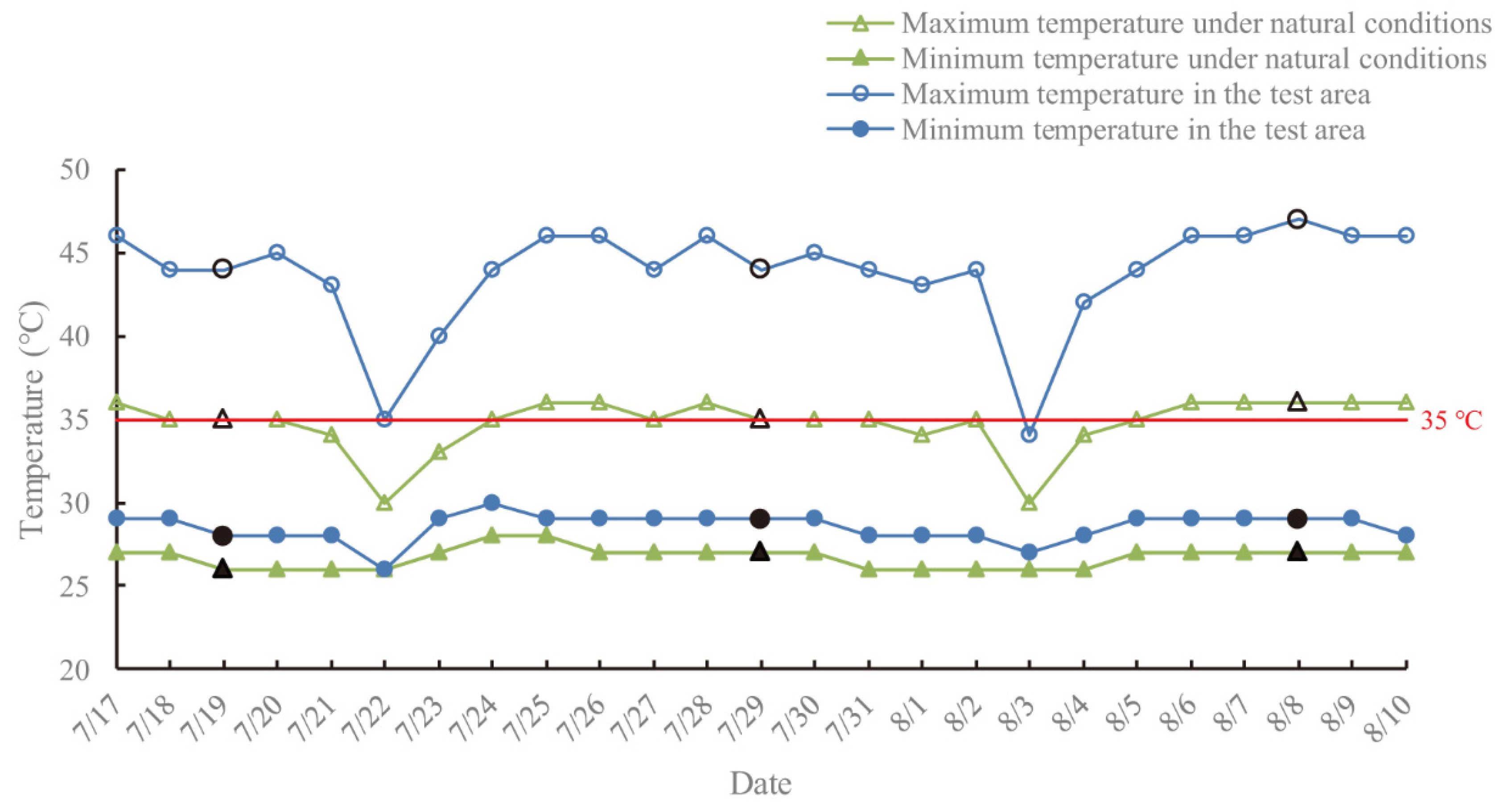
Figure 4a), demonstrating that the rise in concentration resulted from dual stress, i.e., salt and heat (
Figure 2). This speculation was also supported by the high Pro concentration in CK on the 20th day of culture.
Similarly, the soluble sugar concentration (SSC) significantly increased in response to high salinity stress (≥0.3%) at the termination of the experiment (
Figure 4b), parallel to the findings for
Medicago truncatula Gaertn. (barrel medic) [
35]. In addition, the unimodal pattern, with the maximum of soluble protein concentration (SPC) in all salt-treated seedlings reached on the 10th day (
Figure 4c) was similar to that in
Camptotheca acuminata Decne. var.
acuminata [
36]. Thus, we supposed the osmoregulation mode in
C. elegans under salt and heat stress to be the earliest accumulation of soluble protein and was the onset of osmoregulation, followed by soluble sugar, and Pro as the later osmolytes.
Usually, excess Na
+ and Cl
− ions are absorbed along with water entering the plant when it is exposed to a high salt concentration for a long time, causing cytosol ion homeostasis imbalance at the cellular level [
37]. In this study, Na
+ was mainly isolated in the root to prevent it from being transported to the leaves when the salt concentration ≤ 0.2%. However, Na
+ ions were transferred to the leaves when the salt concentration ≥ 0.3% (
Figure 5a), and corresponding salt damage symptoms in the seedlings also provided strong support (
Table 3).
As an exclusion strategy under the salinity field, high potassium/sodium (K
+/Na
+) and calcium/sodium (Ca
2+/Na
+) ion ratios may enhance salt tolerance in some crops [
38]. The great increase in K
+ concentration was induced by the salt culture, but was far behind the increase in Na
+ concentration in
C. elegans (
Figure 5b). Commonly, an increase in Ca
2+ concentration induced by a high-salt environment is thought to be more powerful because of its function as a secondary messenger in many biological systems [
3,
21,
39]. However, the high Ca
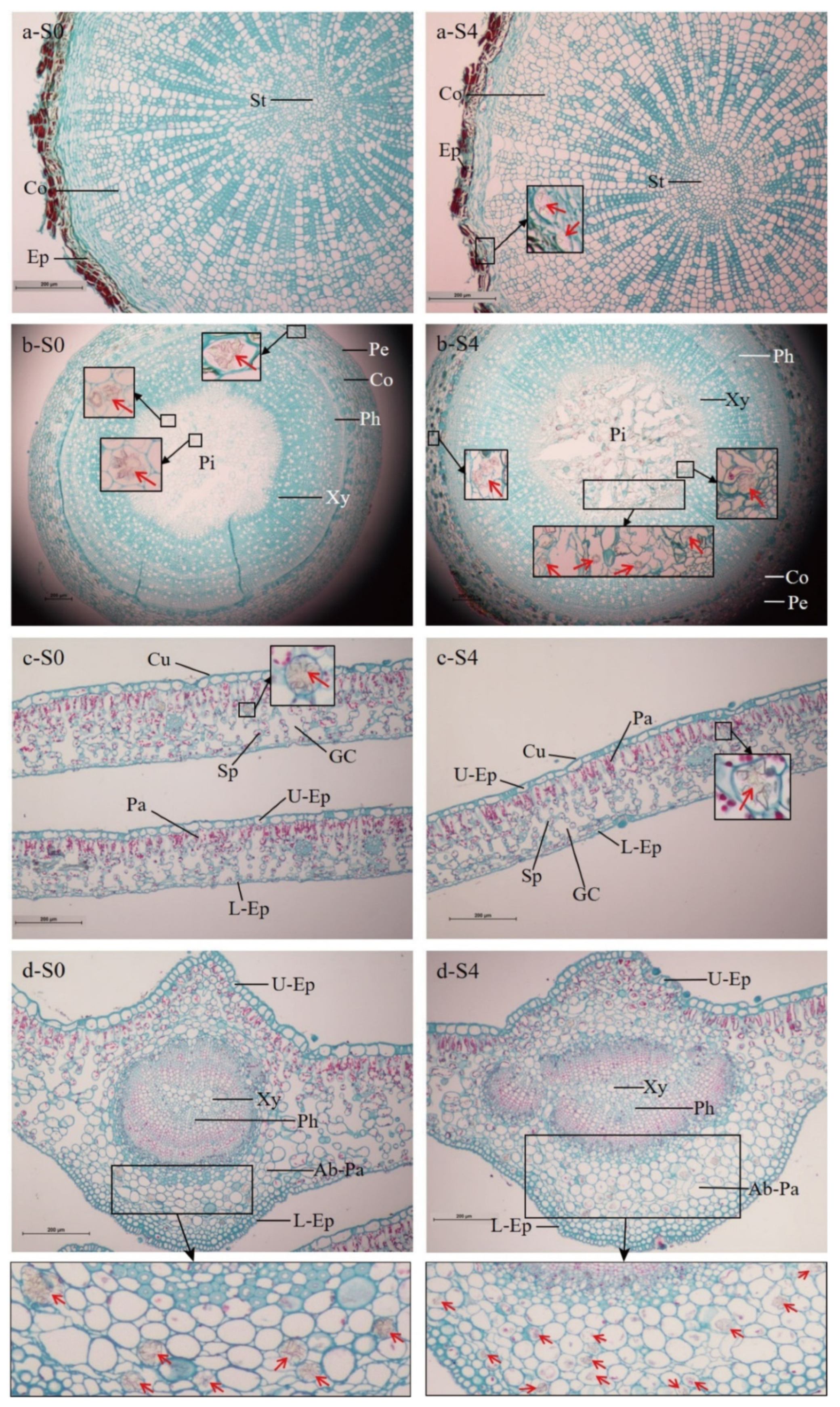
2+ level background in the leaves (as in S0,
Figure 6c-S0 and
Figure 5d-S0) and abrupt increase in Na
+ concentration in salted treatments did not cause an effective increment in the Ca
2+/Na
+ ratio in salt-stressed
C. elegans seedlings (
Figure 5d). Even so, a high salt concentration still drove higher calcium oxalate crystal formation (
Figure 6-S4,
Table 7) to relieve salt stress.
As previous reports have stated, calcium oxalate crystals can serve as a strong localized Ca
2+ sink in regulating bulk Ca
2+ levels, maintaining ionic equilibrium and enhancing stress tolerance in plants, such as salt stress [
8]. Indeed, in
C. elegans, massive calcium oxalate crystals were mainly deposited in the parenchyma cells, the palisade tissues and their junction with spongy tissues of the mesophyll, and were particularly enriched in the main vein (
Figure 6). The pattern of calcium oxalate crystal accumulation along the veins is common in numerous plants. It is supposed that such precipitation in the cells surrounding the veins could prevent Ca
2+ from accumulating around the chlorenchyma cells, which would affect cellular function [
8], e.g., photosynthesis.
Under adverse conditions, plants must gradually evolve certain specific morphological structural modifications [
27]. As we observed in the stressed seedlings, the looseness and disintegration of cortical cells in the root and stem (
Figure 6a,b), and looseness in the palisade and spongy tissues, as well as the enlargement of the gas chamber of the leaves (
Figure 6c), demonstrated that the loss of intracellular water causes cells to shrink. On the other hand, the loose arrangement in the palisade and spongy tissues in the leaves enlarged their areas, and combined with enlargement of the leaf gas chamber, these modifications could improve CO
2 utilization [
40].
Consequently, the results of Ca
2+ distribution and anatomic structure changes in the leaves coincided with the results provided by Lu et al. [
20], who observed the presence of normal photosynthetic characteristics in
C. elegans seedlings treated with 0.2% salt stress.