Probable Factors Associated with Response to Mesenchymal Stem Cell Therapy in Stroke Patients: A Post Hoc Analysis of the STARTING-2 Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Post Hoc Analysis
2.4. Statistical Analysis
3. Results
3.1. Comparison between Good and Poor Responders
3.2. Associated Factors Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Braddom, R.L. Physical Medicine and Rehabilitation, 3rd ed.; Elsevier Saunders: Edinburgh, UK, 2007. [Google Scholar]
- Danielsson, A.; Willén, C.; Sunnerhagen, K.S. Physical Activity, Ambulation, and Motor Impairment Late after Stroke. Stroke Res. Treat. 2011, 2012, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burke, E.; Dobkin, B.H.; Noser, E.A.; Enney, L.A.; Cramer, S.C. Predictors and Biomarkers of Treatment Gains in a Clinical Stroke Trial Targeting the Lower Extremity. Stroke 2014, 45, 2379–2384. [Google Scholar] [CrossRef] [Green Version]
- Langhorne, P.; Bernhardt, J.; Kwakkel, G. Stroke rehabilitation. Lancet 2011, 377, 1693–1702. [Google Scholar] [CrossRef]
- Chopp, M.; Li, Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002, 1, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Hong, J.M.; Moon, G.J.; Lee, P.H.; Ahn, Y.H.; Bang, O.Y. A Long-Term Follow-Up Study of Intravenous Autologous Mesenchymal Stem Cell Transplantation in Patients With Ischemic Stroke. Stem Cells 2010, 28, 1099–1106. [Google Scholar] [CrossRef]
- Chung, J.W.; Chang, W.H.; Bang, O.Y.; Moon, G.J.; Kim, S.J.; Kim, S.K.; Lee, J.S.; Sohn, S.I.; Kim, Y.H. Efficacy and Safety of Intravenous Mesenchymal Stem Cells for Ischemic Stroke. Neurology 2021, 96, e1012–e1023. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, M.; Ceravolo, M.G.; Agosti, M.; Cavallini, P.; Bonassi, S.; Dall’Armi, V.; Massucci, M.; Schifini, F.; Sale, P. Clinical relevance of action observation in upper-limb stroke rehabilitation: A possible role in recovery of functional dexterity. A ran-domized clinical trial. Neurorehabilit. Neural Repair 2012, 26, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Pandian, S.; Arya, K.N.; Kumar, D. Minimal clinically important difference of the lower-extremity fugl–meyer assessment in chronic-stroke. Top. Stroke Rehabil. 2016, 23, 1–7. [Google Scholar] [CrossRef]
- Chang, W.H.; Sohn, M.K.; Lee, J.; Kim, D.Y.; Lee, S.-G.; Shin, Y.-I.; Oh, G.-J.; Lee, Y.-S.; Joo, M.C.; Han, E.Y.; et al. Predictors of functional level and quality of life at 6 months after a first-ever stroke: The KOSCO study. J. Neurol. 2016, 263, 1166–1177. [Google Scholar] [CrossRef]
- Morone, G.; Iosa, M.; Paolucci, T.; Muzzioli, L.; Paolucci, S. Relationship Between Body Mass Index and Rehabilitation Outcomes in Subacute Stroke With Dysphagia. Am. J. Phys. Med. Rehabil. 2019, 98, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Stinear, C.M. Prediction of motor recovery after stroke: Advances in biomarkers. Lancet Neurol. 2017, 16, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Stinear, C. Prediction of recovery of motor function after stroke. Lancet Neurol. 2010, 9, 1228–1232. [Google Scholar] [CrossRef]
- Jang, S.Y.; Shin, Y.-I.; Kim, D.Y.; Sohn, M.K.; Lee, J.; Lee, S.-G.; Oh, G.-J.; Lee, Y.-S.; Joo, M.C.; Han, E.Y.; et al. Effect of obesity on functional outcomes at 6 months post-stroke among elderly Koreans: A prospective multicentre study. BMJ Open 2015, 5, e008712. [Google Scholar] [CrossRef] [PubMed]
- Sacco, R.L.; DeRosa, J.T.; Haley, J.E.C.; Levin, B.; Ordronneau, P.; Phillips, S.J.; Rundek, T.; Snipes, R.G.; Thompson, J.L.P. Glycine antagonist in neuroprotection for patients with acute stroke: GAIN Americas: A randomized controlled trial. JAMA 2001, 285, 1719–1728. [Google Scholar] [CrossRef]
- Kim, Y.; Chang, W.; Bang, O.; Kim, S.; Park, Y.; Lee, P. Long-term effects of rTMS on motor recovery in patients after subacute stroke. J. Rehabil. Med. 2010, 42, 758–764. [Google Scholar] [CrossRef] [Green Version]
- Bang, O.Y.; Lee, J.S.; Lee, P.H.; Lee, G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann. Neurol. 2005, 57, 874–882. [Google Scholar] [CrossRef]
- Savitz, S.I.; Misra, V.; Kasam, M.; Juneja, H.; Cox, C.S., Jr.; Alderman, S.; Aisiku, I.; Kar, S.; Gee, A.; Grotta, J.C. Intravenous autologous bone marrow mononuclear cells for ischemic stroke. Ann. Neurol. 2011, 70, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Stinear, C.M.; Lang, C.E.; Zeiler, S.; Byblow, W.D. Advances and challenges in stroke rehabilitation. Lancet Neurol. 2020, 19, 348–360. [Google Scholar] [CrossRef]
- Viale, L.; Catoira, N.P.; Di Girolamo, G.; González, C.D. Pharmacotherapy and motor recovery after stroke. Expert Rev. Neurother. 2017, 18, 65–82. [Google Scholar] [CrossRef]
- Hill, W.D.; Hess, D.C.; Martin-Studdard, A.; Carothers, J.J.; Zheng, J.; Hale, D.; Maeda, M.; Fagan, S.C.; Carroll, J.E.; Conway, S.J. SDF-1 (CXCL12) Is Upregulated in the Ischemic Penumbra Following Stroke: Association with Bone Marrow Cell Homing to Injury. J. Neuropathol. Exp. Neurol. 2004, 63, 84–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bang, O.Y.; Kim, E.H.; Cha, J.M.; Moon, G.J. Adult Stem Cell Therapy for Stroke: Challenges and Progress. J. Stroke 2016, 18, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Narbonne, P. The effect of age on stem cell function and utility for therapy. Cell Med. 2018, 10, 2155179018773756. [Google Scholar] [CrossRef] [Green Version]
- Romine, J.; Gao, X.; Xu, X.-M.; So, K.F.; Chen, J. The proliferation of amplifying neural progenitor cells is impaired in the aging brain and restored by the mTOR pathway activation. Neurobiol. Aging 2015, 36, 1716–1726. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Why are MSCs therapeutic? New data: New insight. J. Pathol. 2009, 217, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Manganas, L.N.; Zhang, X.; Li, Y.; Hazel, R.D.; Smith, S.D.; Wagshul, M.E.; Henn, F.; Benveniste, H.; Djurić, P.M.; Enikolopov, G.; et al. Magnetic Resonance Spectroscopy Identifies Neural Progenitor Cells in the Live Human Brain. Science 2007, 318, 980–985. [Google Scholar] [CrossRef] [Green Version]
- Burke, D.T.; Al-Adawi, S.; Bell, R.B.; Easley, K.; Chen, S.; Burke, D.P. Effect of Body Mass Index on Stroke Rehabilitation. Arch. Phys. Med. Rehabil. 2014, 95, 1055–1059. [Google Scholar] [CrossRef] [PubMed]
- Oesch, L.; Tatlisumak, T.; Arnold, M.; Sarikaya, H. Obesity paradox in stroke—Myth or reality? A systematic review. PLoS ONE 2017, 12, e0171334. [Google Scholar] [CrossRef]
- Kalichman, L.; Alperovitch-Najenson, D.; Treger, I. The impact of patient’s weight on post-stroke rehabilitation. Disabil. Rehabil. 2015, 38, 1684–1690. [Google Scholar] [CrossRef] [PubMed]
- Freeman, C.; Blough, A.; Rotich, D.; Curl, A.; Eickmeyer, S.M. The obesity paradox may not lead to functional gains in stroke patients undergoing acute inpatient rehabilitation. PM R. 2021. [Google Scholar] [CrossRef]
- Huang, S.-L.; Chen, B.-B.; Hsueh, I.-P.; Jeng, J.-S.; Koh, C.-L.; Hsieh, C.-L. Prediction of lower extremity motor recovery in persons with severe lower extremity paresis after stroke. Brain Inj. 2018, 32, 627–633. [Google Scholar] [CrossRef]
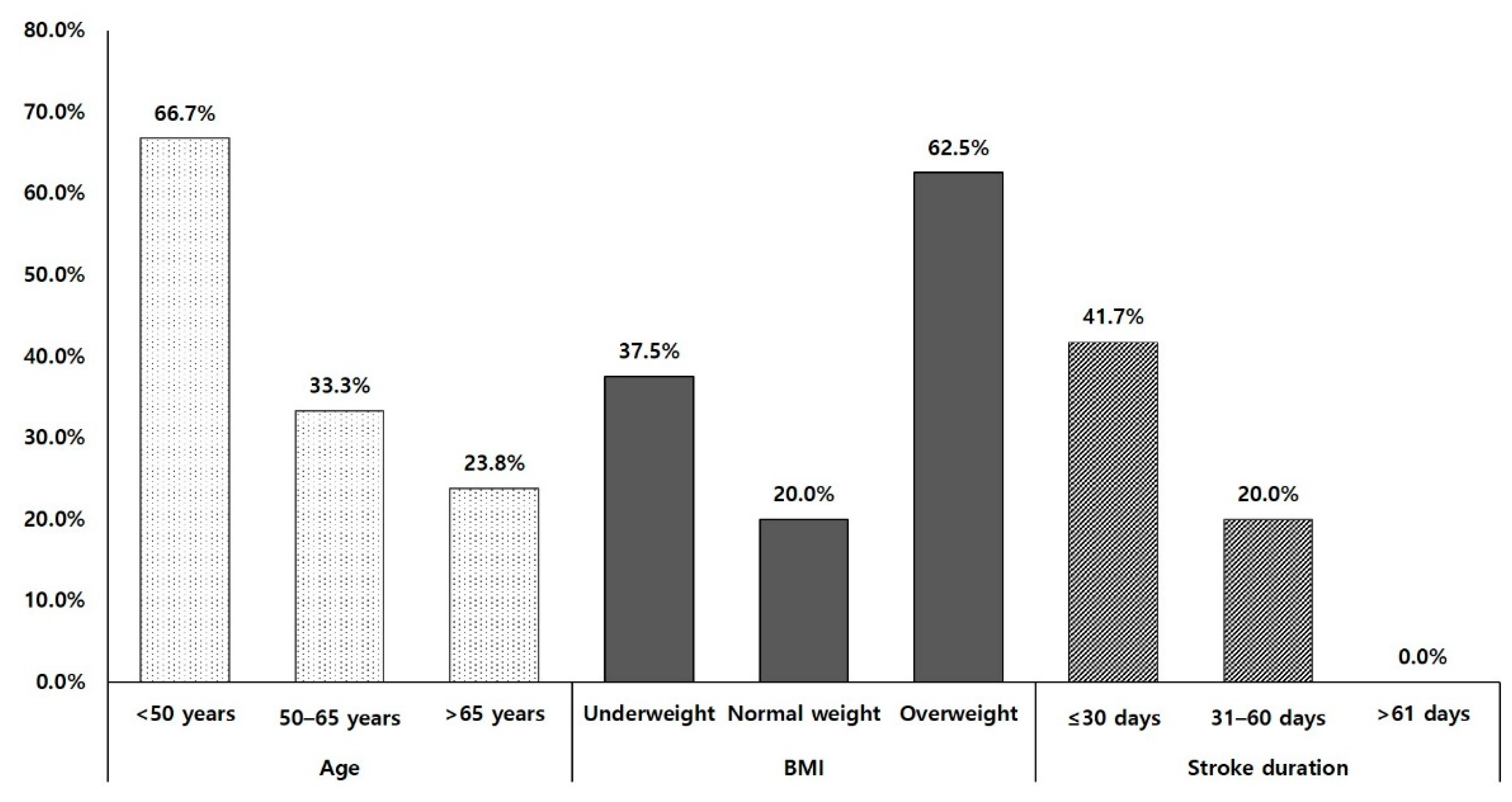
| Characteristics | Subgroup | Good Responders (n = 12) | Poor Responders (n = 24) | p Value |
|---|---|---|---|---|
| Age, mean (SEM) | 60.7 (4.8) | 66.6 (2.0) | 0.48 | |
| <50 years, n (%) | 4 (33.3%) | 2 (8.3%) | 0.182 | |
| 50–65 years, n (%) | 3 (25.0%) | 6 (25.0%) | ||
| >65 years, n (%) | 5 (41.7%) | 16 (66.7%) | ||
| Sex | Male, n (%) | 6 (50.0%) | 9 (37.5%) | 0.499 |
| Female, n (%) | 6 (50.0%) | 15 (62.5%) | ||
| BMI, mean (SEM) | 24.5 (1.3) | 22.2 (0.6) | 0.115 | |
| Underweight, n (%) | 3 (25.0%) | 5 (20.8%) | 0.106 | |
| Normal weight, n (%) | 4 (33.3%) | 16 (66.7%) | ||
| Overweight, n (%) | 5 (41.7%) | 3 (12.5%) | ||
| NIHSS at baseline, mean (SEM) | 11.1 (1.6) | 12.3 (1.0) | 0.511 | |
| ≤8, n (%) | 4 (33.3%) | 5 (20.8%) | 0.813 | |
| 9–15, n (%) | 5 (41.7%) | 12 (50.0%) | ||
| ≥16, n (%) | 3 (25.0%) | 7 (29.2%) | ||
| Stroke duration, mean (SEM) | 20.2 (3.4) | 28.0 (4.8) | 0.762 | |
| ≤30 days, n (%) | 10 (83.3%) | 14 (58.3%) | 0.4 | |
| 31–60 days, n (%) | 2 (16.7%) | 8 (33.3%) | ||
| >61 days, n (%) | 0 (0.0%) | 2 (8.3%) | ||
| FMA-LL at baseline, mean (SEM) | 7.3 (2.0) | 9.0 (1.4) | 0.156 | |
| MEPs response | Yes | 1 (8.3%) | 7 (29.2%) | 0.224 |
| No | 11 (91.7%) | 17 (70.8%) |
| Potential Relating Factors | Good Responders | |||
|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | |||
| Exp(β) (95% CI) | p Value | Exp(β) (95% CI) | p Value | |
| Age group | 0.425 (0.167–1.083) | 0.073 | 0.264 (0.070–0.994) | 0.049 * |
| Sex | 1.447 (0.410–6.767) | 0.475 | NS | NS |
| BMI group | 2.400 (0.396–14.556) | 0.341 | NS | NS |
| NIHSS group at baseline | 0.727 (0.277–1.910) | 0.518 | NS | NS |
| Stroke duration group | 0.294 (0.060–1.440) | 0.131 | 0.067 (0.006–0.786) | 0.031 * |
| FMA-LL at baseline | 0.959 (0.856–1.076) | 0.478 | NS | NS |
| MEPs response | 0.221 (0.024–2.050) | 0.184 | 0.056 (0.002–1.643) | 0.095 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, W.H.; Lee, J.; Chung, J.-W.; Kim, Y.-H.; Bang, O.Y.; The STARTING-2 Collaborators. Probable Factors Associated with Response to Mesenchymal Stem Cell Therapy in Stroke Patients: A Post Hoc Analysis of the STARTING-2 Trial. J. Pers. Med. 2021, 11, 1137. https://doi.org/10.3390/jpm11111137
Chang WH, Lee J, Chung J-W, Kim Y-H, Bang OY, The STARTING-2 Collaborators. Probable Factors Associated with Response to Mesenchymal Stem Cell Therapy in Stroke Patients: A Post Hoc Analysis of the STARTING-2 Trial. Journal of Personalized Medicine. 2021; 11(11):1137. https://doi.org/10.3390/jpm11111137
Chicago/Turabian StyleChang, Won Hyuk, Jungsoo Lee, Jong-Won Chung, Yun-Hee Kim, Oh Young Bang, and The STARTING-2 Collaborators. 2021. "Probable Factors Associated with Response to Mesenchymal Stem Cell Therapy in Stroke Patients: A Post Hoc Analysis of the STARTING-2 Trial" Journal of Personalized Medicine 11, no. 11: 1137. https://doi.org/10.3390/jpm11111137
APA StyleChang, W. H., Lee, J., Chung, J.-W., Kim, Y.-H., Bang, O. Y., & The STARTING-2 Collaborators. (2021). Probable Factors Associated with Response to Mesenchymal Stem Cell Therapy in Stroke Patients: A Post Hoc Analysis of the STARTING-2 Trial. Journal of Personalized Medicine, 11(11), 1137. https://doi.org/10.3390/jpm11111137







