Titania Nanotube Architectures Synthesized on 3D-Printed Ti-6Al-4V Implant and Assessing Vancomycin Release Protocols
Abstract
:1. Introduction
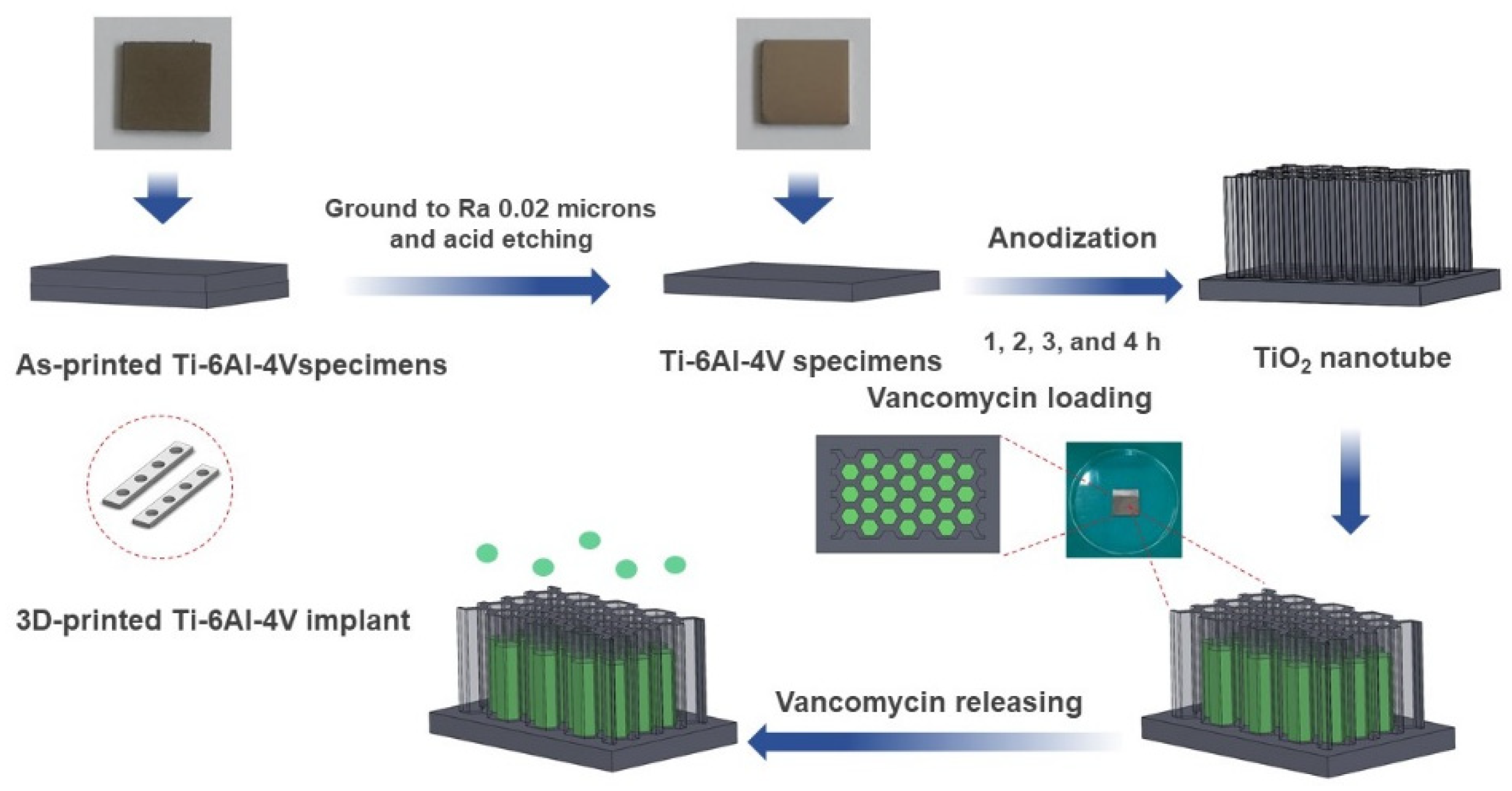
2. Materials and Methods
2.1. Materials and Anodization
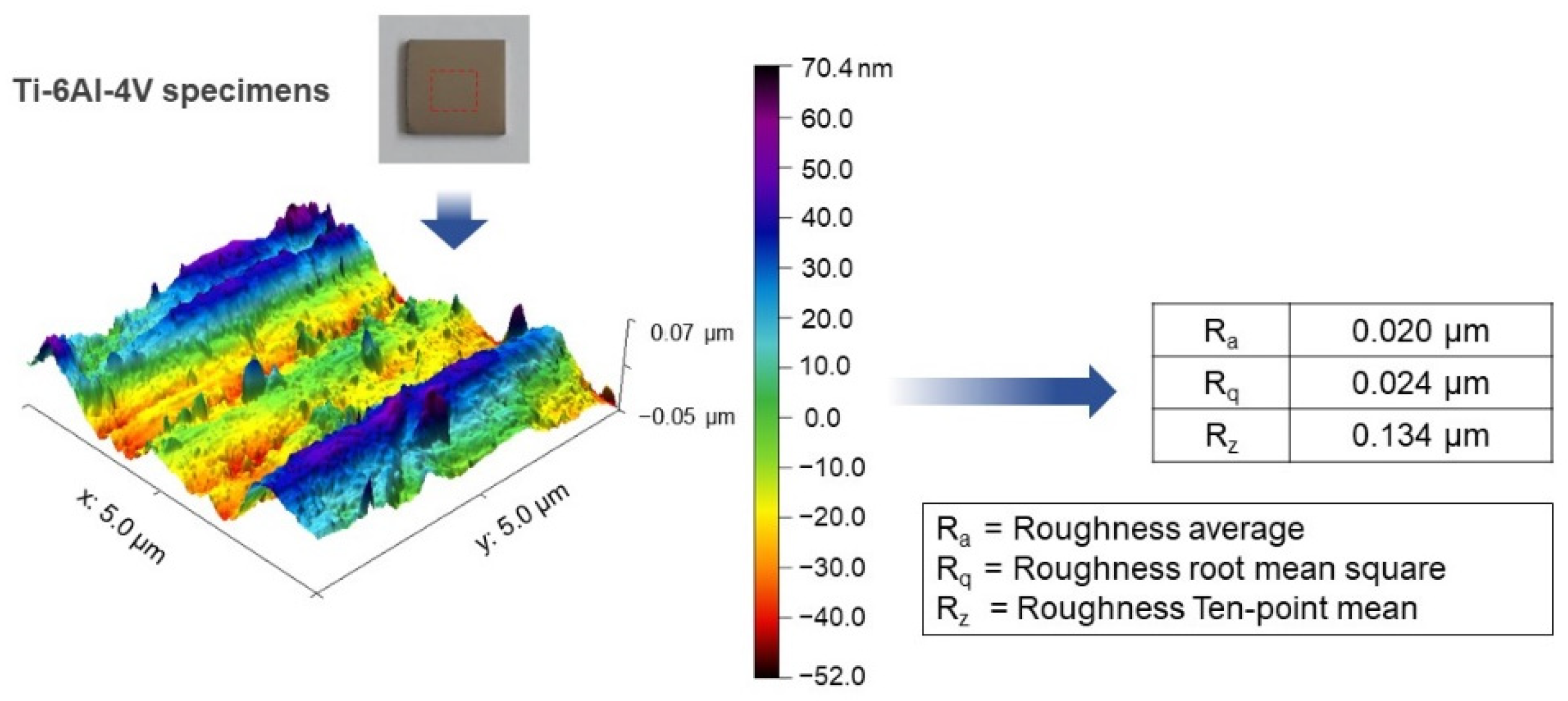
2.2. Titania Nanotubes Characterization
2.3. Drug Loading
2.4. Drug Releasing Analysis
3. Results
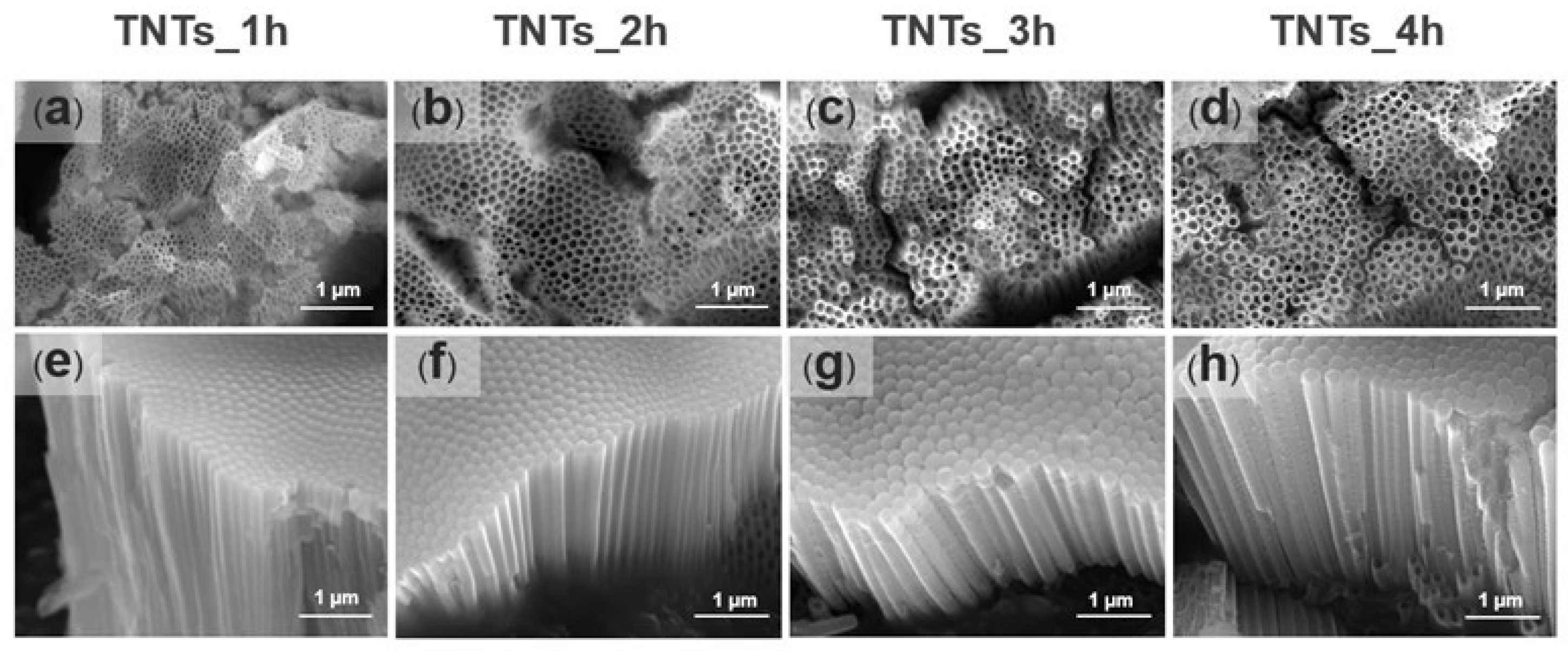
3.1. Morphology of TiO2 Nanotube Layer
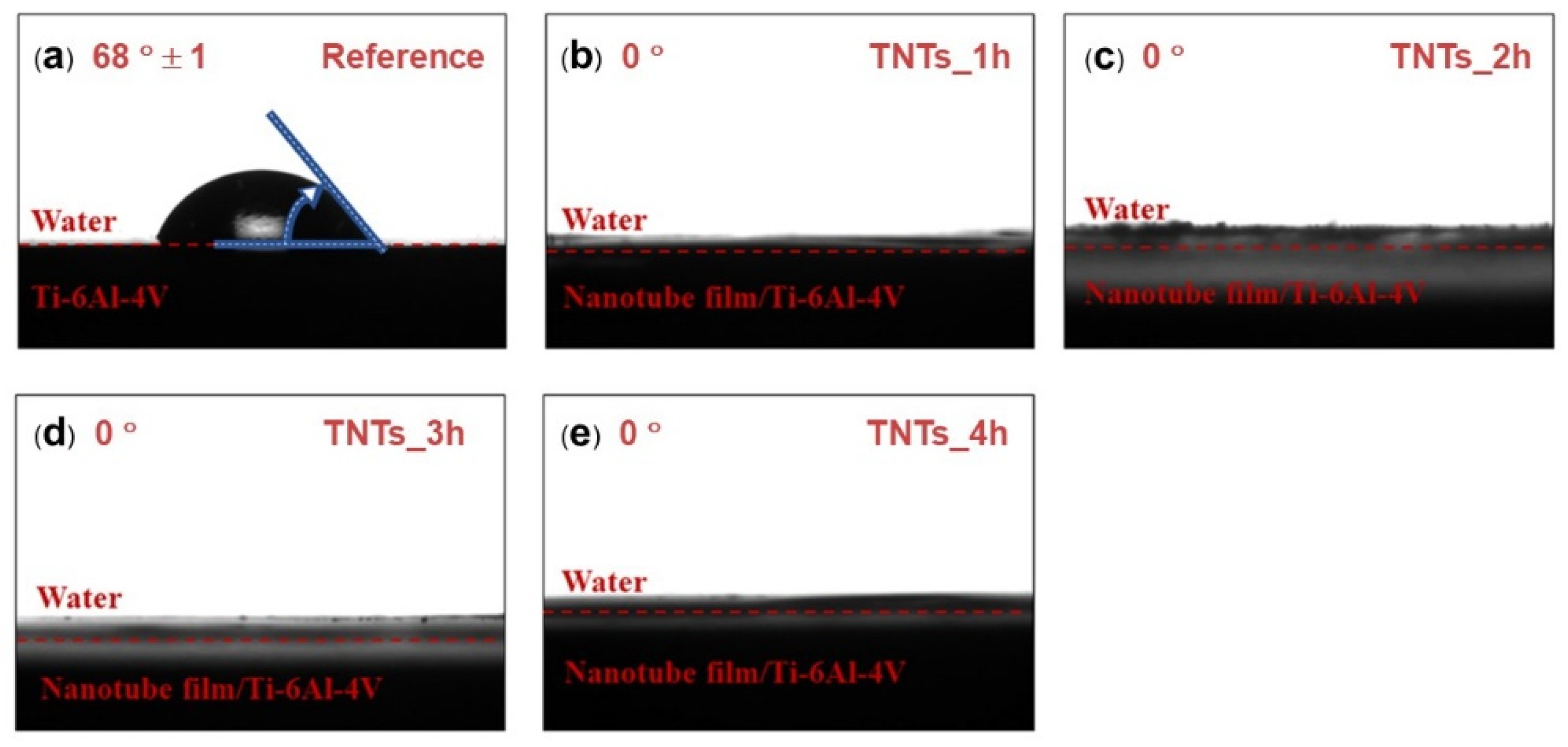
3.2. Hydrophilicity of TNTs Surface
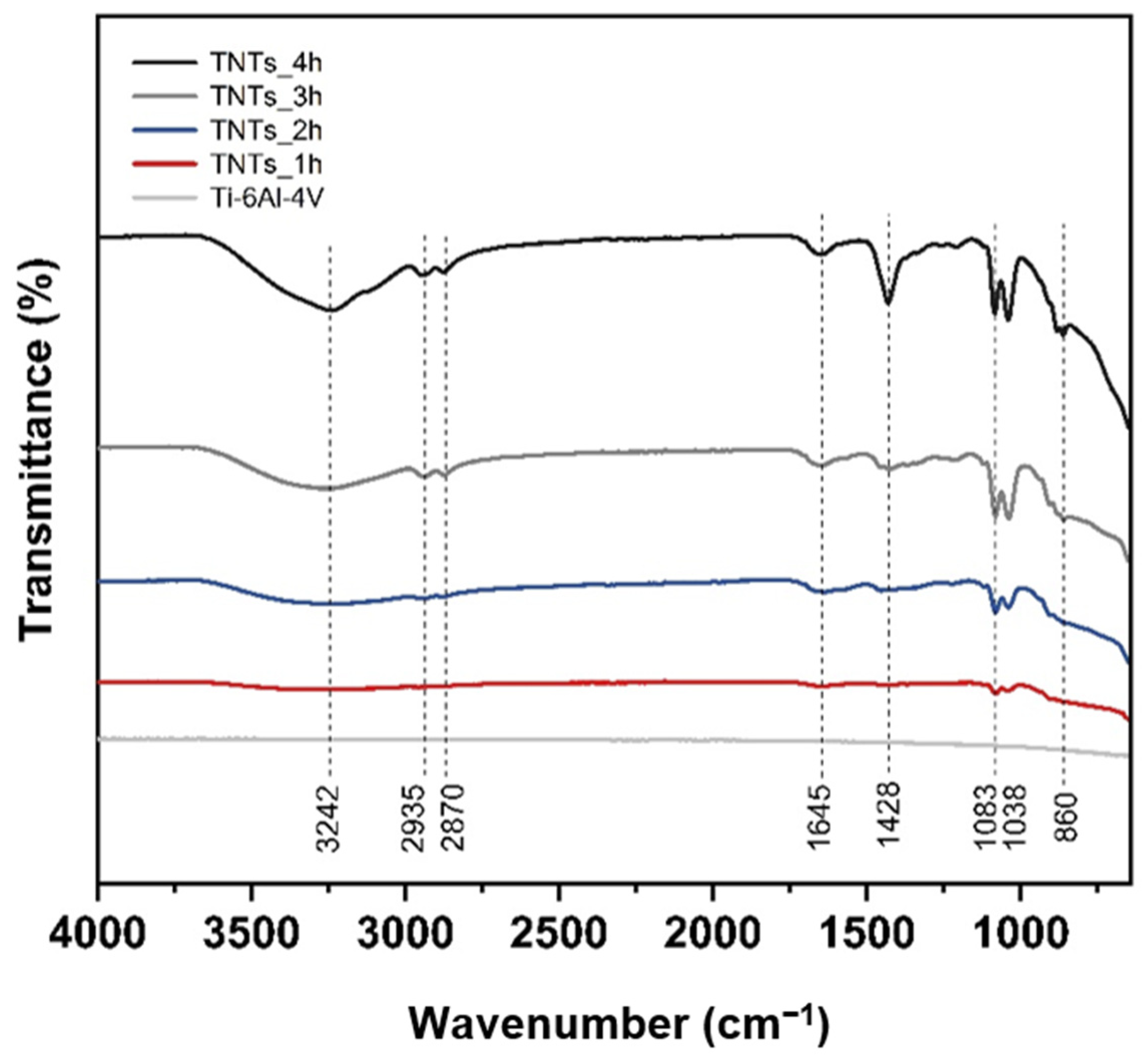
3.3. Functional Group of Nanotubes Film
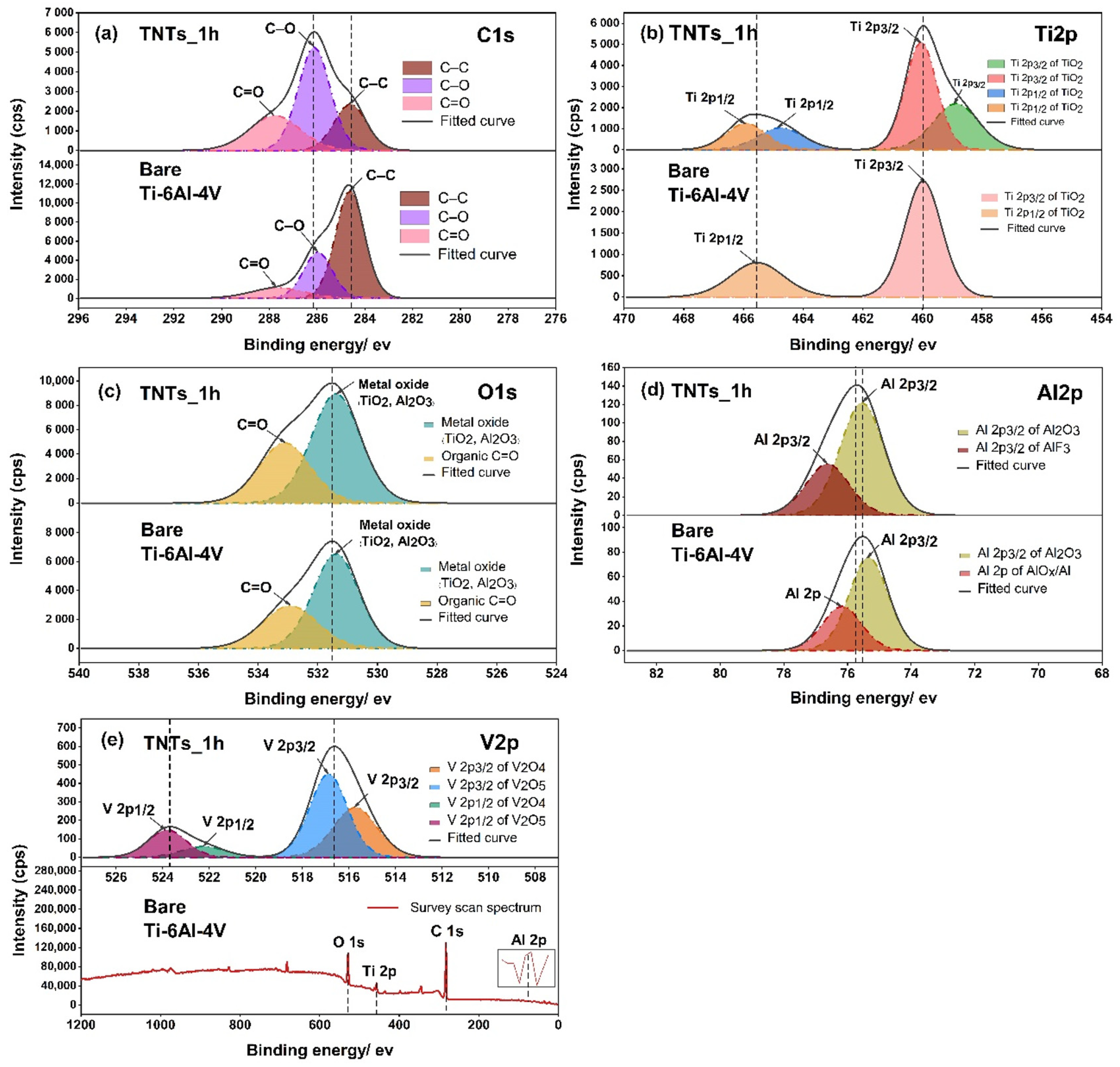
3.4. Oxide Species Analysis
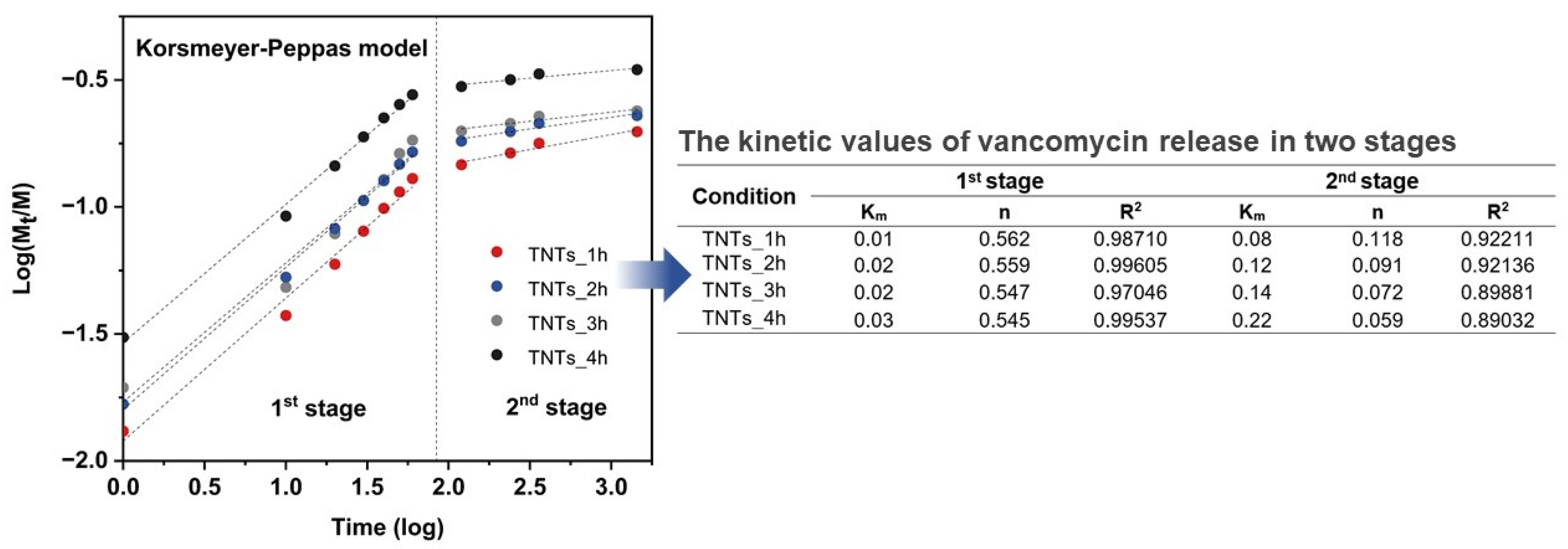
3.5. Antibacterial Drug Release Behavior
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goharian, A.; Abdullah, M.R. Bioinert Metals (Stainless Steel, Titanium, Cobalt Chromium). In Trauma Plating Systems; Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 115–142. [Google Scholar]
- Tantavisut, S.; Lohwongwatana, B.; Khamkongkaeo, A.; Tanavalee, A.; Tangpornprasert, P.; Ittiravivong, P. In vitro biocompatibility of novel titanium-based amorphous alloy thin film in human osteoblast like cells. Chulalongkorn Med. J. 2019, 63, 89–93. [Google Scholar]
- Tantavisut, S.; Lohwongwatana, B.; Khamkongkaeo, A.; Tanavalee, A.; Tangpornprasert, P.; Ittiravivong, P. The novel toxic free titanium-based amorphous alloy for biomedical application. J. Mater. Res. Technol. 2018, 7, 248–253. [Google Scholar] [CrossRef]
- Darouiche, R.O. Treatment of Infections Associated with Surgical Implants. N. Engl. J. Med. 2004, 350, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, A.; Trueman, P. Economic burden of surgical site infections within the episode of care following joint replacement. J. Orthop. Surg. Res. 2019, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fang, L.; Wang, S.; Chen, Y.; Ma, H.; Zhao, H.; Xie, Z. Antibiotic treatment regimens for bone infection after debridement: A study of 902 cases. BMC Musculoskelet. Disord. 2020, 21, 215. [Google Scholar] [CrossRef]
- Losic, D.; Aw, M.S.; Santos, A.; Gulati, K.; Bariana, M. Titania nanotube arrays for local drug delivery: Recent advances and perspectives. Expert Opin. Drug Deliv. 2014, 12, 103–127. [Google Scholar] [CrossRef]
- Van Vugt, T.A.G.; Arts, J.; Geurts, J.A.P. Antibiotic-Loaded Polymethylmethacrylate Beads and Spacers in Treatment of Orthopedic Infections and the Role of Biofilm Formation. Front. Microbiol. 2019, 10, 1626. [Google Scholar] [CrossRef]
- Filipović, U.; Dahmane, R.G.; Ghannouchi, S.; Zore, A.; Bohinc, K. Bacterial adhesion on orthopedic implants. Adv. Colloid Interface Sci. 2020, 283, 102228. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Geng, Z.; Li, Z.; Cui, Z.; Zhu, S.; Liang, Y.; Liu, Y.; Wang, R.; Yang, X. Controlled release behaviour and antibacterial effects of antibiotic-loaded titania nanotubes. Mater. Sci. Eng. C 2016, 62, 105–112. [Google Scholar] [CrossRef]
- Decha-Umphai, D.; Chunate, H.-T.; Phetrattanarangsi, T.; Boonchuduang, T.; Choosri, M.; Puncreobutr, C.; Lohwongwatana, B.; Khamwannah, J. Effects of post-processing on microstructure and adhesion strength of TiO2 nanotubes on 3D-printed Ti-6Al-4V alloy. Surf. Coat. Technol. 2021, 421, 127431. [Google Scholar] [CrossRef]
- Kunrath, M.F.; Hübler, R.; Shinkai, R.; Teixeira, E. Application of TiO2 Nanotubes as a Drug Delivery System for Biomedical Implants: A Critical Overview. ChemistrySelect 2018, 3, 11180–11189. [Google Scholar] [CrossRef]
- Kunrath, M.F.; Leal, B.F.; Hübler, R.; Oliveira, S.; Teixeira, E. Antibacterial potential associated with drug-delivery built TiO2 nanotubes in biomedical implants. AMB Express 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Riahi, Z.; Seyedkhani, S.A.; Sadrnezhaad, S.K. Electrophoretic encapsulation for slow release of vancomycin from perpendicular TiO2 nanotubes grown on Ti6Al4V electrodes. Mater. Res. Express 2019, 6, 125424. [Google Scholar] [CrossRef]
- Yang, Z.; Wei, X.; Gao, W.; Cao, P. Anodization of NiTi alloy in an ethylene glycol electrolyte. Surf. Coat. Technol. 2014, 252, 142–147. [Google Scholar] [CrossRef]
- Hammed, M.G.; Hassan, A.A. Enhancement of the Structural and Optical Properties of (PVA-PANI) Polymer Blend By Addition of CuI Nanoparticles. IOP Conf. Ser. Mater. Sci. Eng. 2020, 928, 072157. [Google Scholar] [CrossRef]
- Fleischman, A.N.; Austin, M.S. Local Intra-wound Administration of Powdered Antibiotics in Orthopaedic Surgery. J. Bone Jt. Infect. 2017, 2, 23–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamlekhan, A.; Butt, A.; Patel, S.; Royhman, D.; Takoudis, C.; Sukotjo, C.; Yuan, J.; Jursich, G.; Mathew, M.; Hendrickson, W.; et al. Fabrication of Anti-Aging TiO2 Nanotubes on Biomedical Ti Alloys. PLoS ONE 2014, 9, e96213. [Google Scholar] [CrossRef]
- Das, K.; Bose, S.; Bandyopadhyay, A. Surface modifications and cell–materials interactions with anodized Ti. Acta Biomater. 2007, 3, 573–585. [Google Scholar] [CrossRef]
- De Dicastillo, C.L.; Patiño, C.; Galotto, M.J.; Palma, J.L.; Alburquenque, D.; Escrig, J. Novel Antimicrobial Titanium Dioxide Nanotubes Obtained through a Combination of Atomic Layer Deposition and Electrospinning Technologies. Nanomaterials 2018, 8, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- León, A.; Reuquen, P.; Garín, C.; Segura, R.; Vargas, P.; Zapata, P.; Orihuela, P.A. FTIR and Raman Characterization of TiO2 Nanoparticles Coated with Polyethylene Glycol as Carrier for 2-Methoxyestradiol. Appl. Sci. 2017, 7, 49. [Google Scholar] [CrossRef]
- Nada, A.; Moustafa, Y.M.M.; Hamdy, A. Improvement of titanium dioxide nanotubes through study washing effect on hydrothermal. Br. J. Environ. Sci. 2014, 2, 29–40. [Google Scholar]
- Nam, C.T.; Yang, W.-D.; Duc, L.M. Study on photocatalysis of TiO2 nanotubes prepared by methanol-thermal synthesis at low temperature. Bull. Mater. Sci. 2013, 36, 779–788. [Google Scholar] [CrossRef] [Green Version]
- Vasconcelos, D.C.L.; Costa, V.C.; Nunes, E.H.M.; Sabioni, A.C.S.; Gasparon, M.; Vasconcelos, W.L. Infrared Spectroscopy of Titania Sol-Gel Coatings on 316L Stainless Steel. Mater. Sci. Appl. 2011, 2, 1375–1382. [Google Scholar] [CrossRef] [Green Version]
- Sarraf, M.; Sukiman, N.L.; Bushroa, A.R.; Nasiri-Tabrizi, B.; Dabbagh, A.; Abu Kasim, N.H.; Basirun, W.J. In vitro bioactivity and corrosion resistance enhancement of Ti-6Al-4V by highly ordered TiO2 nanotube arrays. J. Aust. Ceram. Soc. 2019, 55, 187–200. [Google Scholar] [CrossRef]
- Saharudin, K.A.; Sreekantan, S.; Aziz, S.N.Q.A.A.; Hazan, R.; Lai, C.W.; Mydin, R.B.S.; Mat, I. Surface Modification and Bioactivity of Anodic Ti6Al4V Alloy. J. Nanosci. Nanotechnol. 2013, 13, 1696–1705. [Google Scholar] [CrossRef] [PubMed]
- Bharti, B.; Kumar, S.; Lee, H.-N.; Kumar, R. Formation of oxygen vacancies and Ti3+ state in TiO2 thin film and enhanced optical properties by air plasma treatment. Sci. Rep. 2016, 6, 32355. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Liu, Q.; Zou, J.; Li, D.; Yuan, S.; Wang, Z.; Tang, B. Surface damage mitigation of Ti6Al4V alloy via thermal oxidation for oil and gas exploitation application: Characterization of the microstructure and evaluation of the surface performance. RSC Adv. 2017, 7, 13517–13535. [Google Scholar] [CrossRef] [Green Version]
- Naumkin, A.V.; Kraut-Vass, A.; Powell, C.J. NIST X-ray Photoelectron Spectroscopy Database. In Measurement Services Division of the National Institute of Standards and Technology (NIST) Technology Services; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2008. [Google Scholar]
- Gulati, K.; Aw, M.S.; Losic, D. Drug-eluting Ti wires with titania nanotube arrays for bone fixation and reduced bone infection. Nanoscale Res. Lett. 2011, 6, 571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmer, J.; Palmer, T.; Babu, S.; Specht, E. In situ observations of lattice expansion and transformation rates of α and β phases in Ti–6Al–4V. Mater. Sci. Eng. A 2005, 391, 104–113. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Wang, L. Structure and properties of nitrogen incorporated in TiO2nanotubes array. Mater. Res. Express 2014, 1, 025040. [Google Scholar] [CrossRef]
- Jordanovová, V.; Losertová, M.; Štencek, M.; Lukášová, T.; Martynková, G.S.; Peikertová, P. Microstructure and Properties of Nanostructured Coating on Ti6Al4V. Materials 2020, 13, 708. [Google Scholar] [CrossRef] [Green Version]
- Mor, G.; Varghese, O.K.; Paulose, M.; Mukherjee, N.; Grimes, C.A. Fabrication of tapered, conical-shaped titania nanotubes. J. Mater. Res. 2003, 18, 2588–2593. [Google Scholar] [CrossRef]
- Soares, T.A.; Mozaffari, H.; Reinecke, H. Generation of microstructures on a Ti–6Al–4V substrate through anodization. Surf. Coat. Technol. 2015, 278, 64–70. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef] [Green Version]
- Indira, K.; Mudali, U.K.; Nishimura, T.; Rajendran, N. A Review on TiO2 Nanotubes: Influence of Anodization Parameters, Formation Mechanism, Properties, Corrosion Behavior, and Biomedical Applications. J. Bio-Tribo-Corros. 2015, 1, 28. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Peng, Q.; Chen, R.; Wen, Y.; Shan, B. Correlation between Hydrophilicity and Surface Aggregation in Anodized TiO2 Nanotube Arrays. Phys. Procedia 2013, 48, 220–227. [Google Scholar] [CrossRef] [Green Version]
- Lu, R.; Wang, C.; Wang, X.; Wang, Y.; Wang, N.; Chou, J.; Li, T.; Zhang, Z.; Ling, Y.; Chen, S. Effects of hydrogenated TiO2 nanotube arrays on protein adsorption and compatibility with osteoblast-like cells. Int. J. Nanomed. 2018, 13, 2037–2049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomrungpiyathan, T.; Luenam, S.; Lohwongwatana, B.; Sirichativapee, W.; Nabudda, K.; Puncreobutr, C. A custom-made distal humerus plate fabricated by selective laser melting. Comput. Methods Biomech. Biomed. Eng. 2021, 24, 585–596. [Google Scholar] [CrossRef]
- Martinez-Marquez, D.; Gulati, K.; Carty, C.P.; Stewart, R.A.; Ivanovski, S. Determining the relative importance of titania nanotubes characteristics on bone implant surface performance: A quality by design study with a fuzzy approach. Mater. Sci. Eng. C 2020, 114, 110995. [Google Scholar] [CrossRef]
- Shah, U.; Deen, K.; Asgar, H.; Rahman, Z.; Haider, W. Understanding the mechanism of TiO2 nanotubes formation at low potentials (≤8 V) through electrochemical methods. J. Electroanal. Chem. 2017, 807, 228–234. [Google Scholar] [CrossRef]
- Ionita, D.; Bajenaru-Georgescu, D.; Totea, G.; Mazare, A.; Schmuki, P.; Demetrescu, I. Activity of vancomycin release from bioinspired coatings of hydroxyapatite or TiO2 nanotubes. Int. J. Pharm. 2017, 517, 296–302. [Google Scholar] [CrossRef]
- Fraimow, H.S. Systemic Antimicrobial Therapy in Osteomyelitis. Semin. Plast. Surg. 2009, 23, 090–099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honda, H.; Doern, C.D.; Michael-Dunne, W.; Warren, D.K. The impact of vancomycin susceptibility on treatment outcomes among patients with methicillin resistant Staphylococcus aureusbacteremia. BMC Infect. Dis. 2011, 11, 335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moseke, C.; Hage, F.; Vorndran, E.; Gbureck, U. TiO2 nanotube arrays deposited on Ti substrate by anodic oxidation and their potential as a long-term drug delivery system for antimicrobial agents. Appl. Surf. Sci. 2012, 258, 5399–5404. [Google Scholar] [CrossRef]
- He, X.; Zhou, Z.; Han, Z.; Zeng, Y.; Chen, X.; Su, J. Mechanism of Controlled Release of Vancomycin from Crumpled Graphene Oxides. ACS Omega 2019, 4, 12252–12258. [Google Scholar] [CrossRef] [Green Version]
- Kärger, J.; Ruthven, D.M. Diffusion in nanoporous materials: Fundamental principles, insights and challenges. New J. Chem. 2016, 40, 4027–4048. [Google Scholar] [CrossRef] [Green Version]


| Condition | Anodization Time (h) | Nanotube Size (nm) | |
|---|---|---|---|
| Pore Diameter | Length | ||
| TNTs_1h | 1 | 53 ± 15 * | 1976 ± 56 |
| TNTs_2h | 2 | 108 ± 19 * | 1938 ± 75 |
| TNTs_3h | 3 | 93 ± 20 * | 2629 ± 145 * |
| TNTs_4h | 4 | 114 ± 16 * | 2492 ± 77 * |
| Sum of Squares | df | Mean Square | F | p | |
|---|---|---|---|---|---|
| Between Groups | 453,951.984 | 3 | 151,317.328 | 488.539 | 0.000 |
| Within Groups | 246,548.565 | 796 | 309.734 | - | - |
| Total | 700,500.549 | 799 | - | - | - |
| Sum of Squares | df | Mean Square | F | p | |
|---|---|---|---|---|---|
| Between Groups | 3,751,794.162 | 3 | 1,250,598.054 | 140.606 | 0.000 |
| Within Groups | 320,196.327 | 36 | 8894.342 | - | - |
| Total | 4,071,990.489 | 39 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chunate, H.-t.; Khamwannah, J.; Aliyu, A.A.A.; Tantavisut, S.; Puncreobutr, C.; Khamkongkaeo, A.; Tongyam, C.; Tumkhanon, K.; Phetrattanarangsi, T.; Chanamuangkon, T.; et al. Titania Nanotube Architectures Synthesized on 3D-Printed Ti-6Al-4V Implant and Assessing Vancomycin Release Protocols. Materials 2021, 14, 6576. https://doi.org/10.3390/ma14216576
Chunate H-t, Khamwannah J, Aliyu AAA, Tantavisut S, Puncreobutr C, Khamkongkaeo A, Tongyam C, Tumkhanon K, Phetrattanarangsi T, Chanamuangkon T, et al. Titania Nanotube Architectures Synthesized on 3D-Printed Ti-6Al-4V Implant and Assessing Vancomycin Release Protocols. Materials. 2021; 14(21):6576. https://doi.org/10.3390/ma14216576
Chicago/Turabian StyleChunate, H-thaichnok, Jirapon Khamwannah, Abdul Azeez Abdu Aliyu, Saran Tantavisut, Chedtha Puncreobutr, Atchara Khamkongkaeo, Chiraporn Tongyam, Krittima Tumkhanon, Thanawat Phetrattanarangsi, Theerapat Chanamuangkon, and et al. 2021. "Titania Nanotube Architectures Synthesized on 3D-Printed Ti-6Al-4V Implant and Assessing Vancomycin Release Protocols" Materials 14, no. 21: 6576. https://doi.org/10.3390/ma14216576
APA StyleChunate, H.-t., Khamwannah, J., Aliyu, A. A. A., Tantavisut, S., Puncreobutr, C., Khamkongkaeo, A., Tongyam, C., Tumkhanon, K., Phetrattanarangsi, T., Chanamuangkon, T., Sitthiwanit, T., Decha-umphai, D., Pongjirawish, P., & Lohwongwatana, B. (2021). Titania Nanotube Architectures Synthesized on 3D-Printed Ti-6Al-4V Implant and Assessing Vancomycin Release Protocols. Materials, 14(21), 6576. https://doi.org/10.3390/ma14216576






