An Efficient Ionic Liquid-Mediated Extraction and Enrichment of Isoimperatorin from Ostericum koreanum (Max.) Kitagawa
Abstract
:1. Introduction
2. Results and Discussion
2.1. Selection of IL
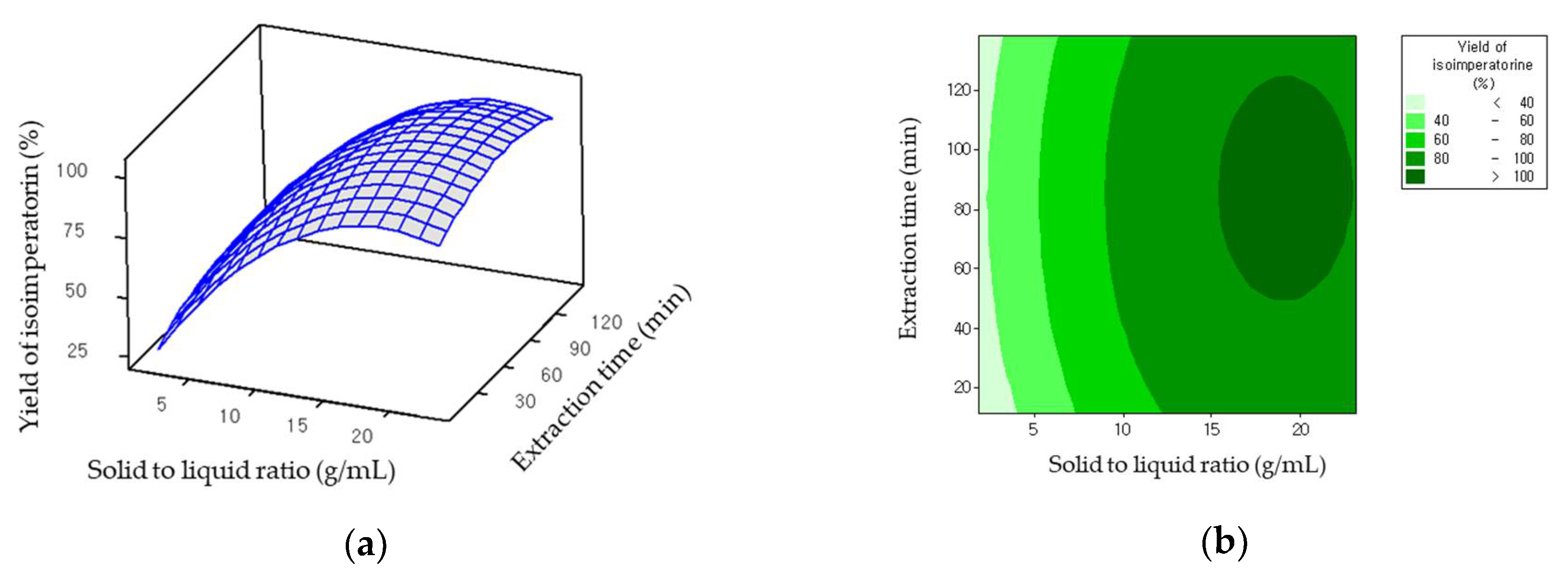
2.2. Optimization of the Variables
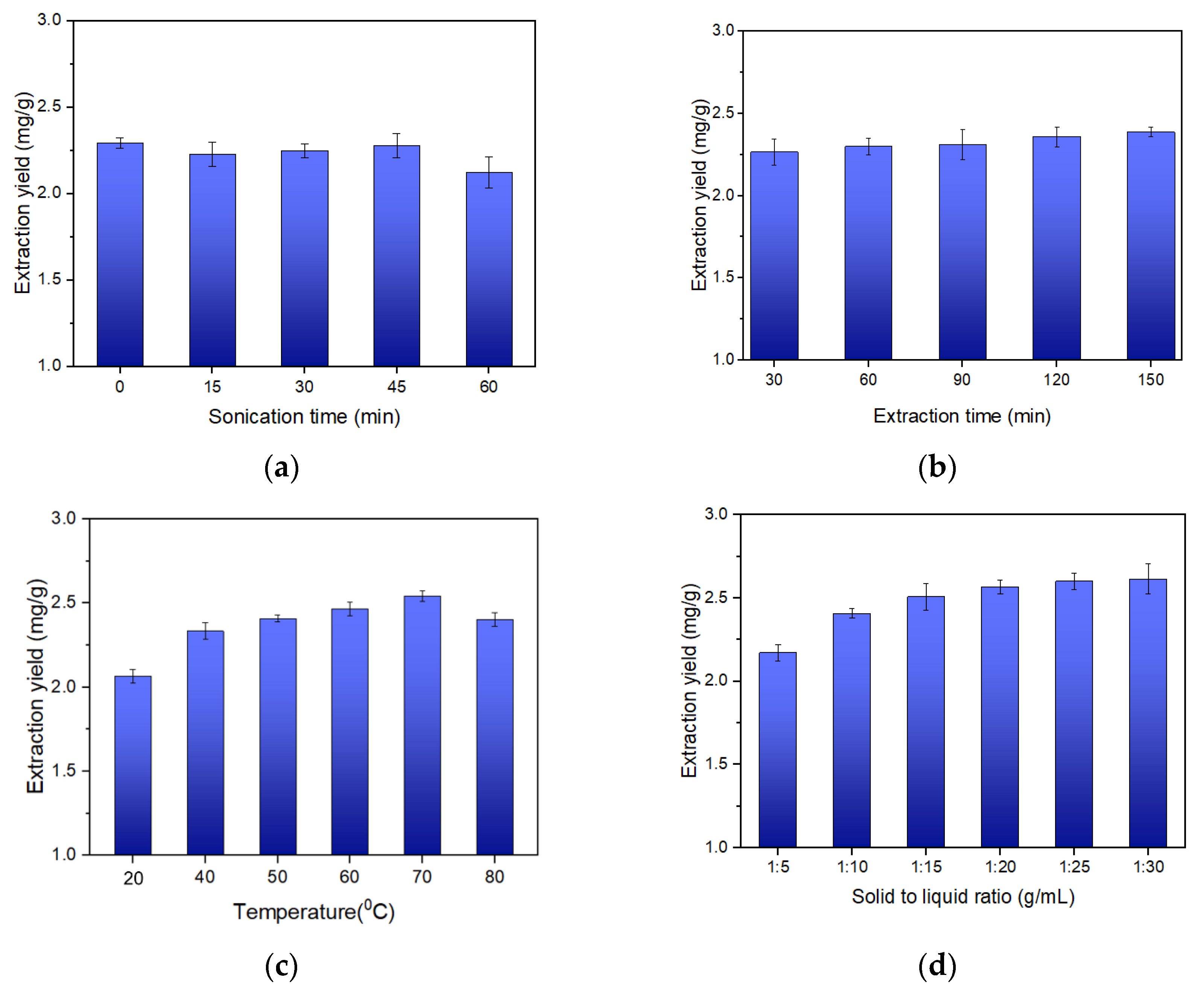
2.2.1. Effect of Sonication Time
2.2.2. Effect of Extraction Time
2.2.3. Effect of Temperature
2.2.4. Effect of Solid to Liquid Ratio
2.3. Statistical Analysis
2.4. Recovery of Isoimperatorin from the [Bmim][BF4] IL Extraction Solution
3. Materials and Methods
3.1. Reagents and Materials
3.2. Methods
3.2.1. Ionic Liquid Assisted-Extraction of Isoimperatorin
3.2.2. High-Performance Liquid Chromatography (HPLC) Analyses
3.2.3. Determination of Coumarins from the Roots of O. koreanum
3.2.4. Recovery of Isoimperatorin from the [Bmim][BF4] IL Extraction Solution
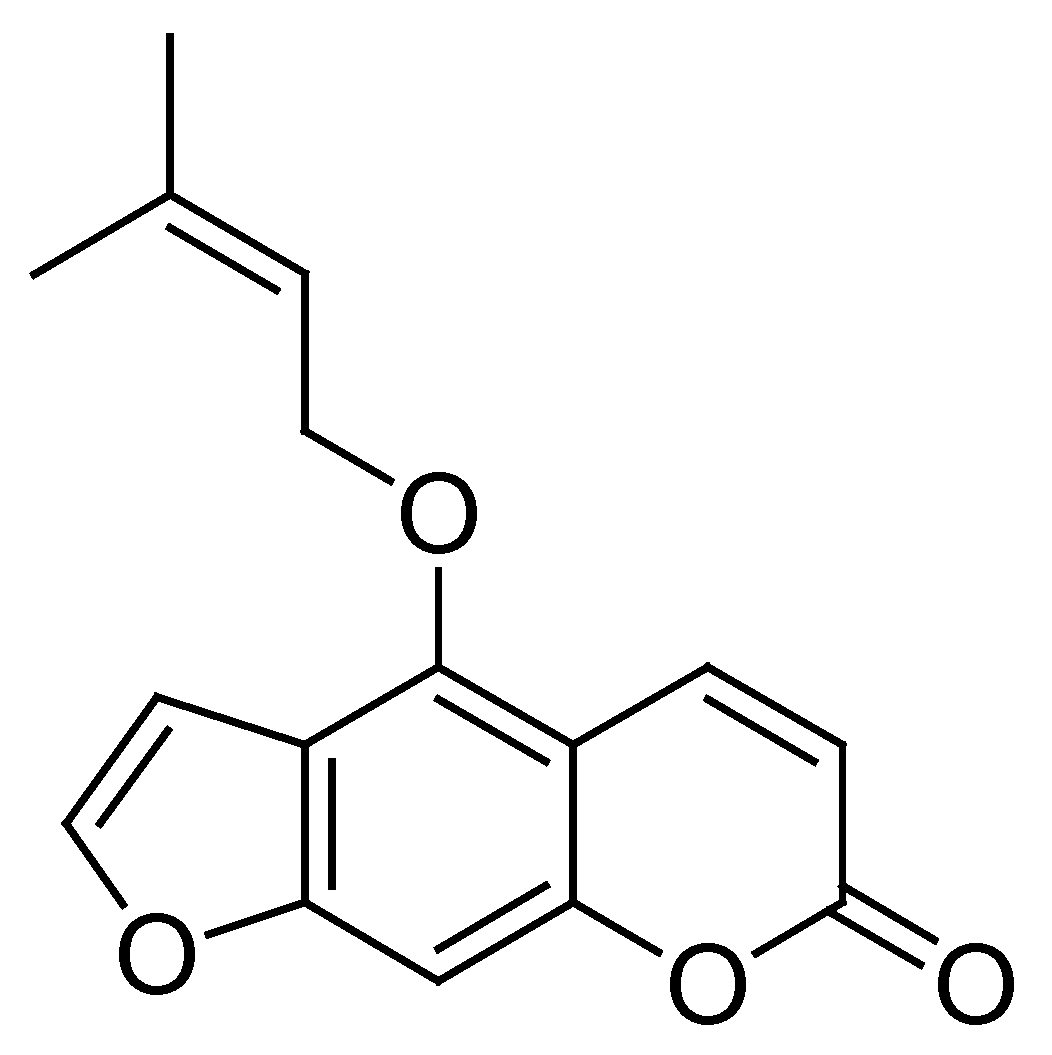
3.2.5. Structural Identification
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- Singh, S.K.; Savoy, A.W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Kujawski, W.; Fatyeyeva, K.; Kujawa, J. A review on ionic liquids-based membranes for middle and high temperature polymer electrolyte membrane fuel cells (PEM FCs). Int. J. Mol. Sci. 2021, 22, 5430. [Google Scholar] [CrossRef] [PubMed]
- Ahrenberg, M.; Beck, M.; Neise, C.; Keßler, O.; Kragl, U.; Verevkin, S.P.; Schick, C. Vapor pressure of ionic liquids at low temperatures from AC-chip-calorimetry. Phys. Chem. Chem. Phys. 2016, 18, 21381–21390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.B.; Zhang, B.; Liu, S.H.; Chen, C.C. Flammability estimation of 1-hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide. J. Loss. Prev. Process. Ind. 2020, 66, 104196. [Google Scholar] [CrossRef]
- Villanueva, M.; Coronas, A.; García, J.; Salgado, J. Thermal stability of ionic liquids for their application as new absorbents. Ind. Eng. Chem. Res. 2013, 52, 15718–15727. [Google Scholar] [CrossRef]
- Keaveney, S.T.; Haines, R.S.; Harper, J.B. Ionic liquid solvents: The importance of microscopic interactions in predicting organic reaction outcomes. Pure Appl. Chem. 2017, 8, 745–757. [Google Scholar] [CrossRef] [Green Version]
- Becherini, S.; Mezzetta, A.; Chiappe, C.; Guazzelli, L. Levulinate amidinium protic ionic liquids (PILs) as suitable media for the dissolution and levulination of cellulose. New J. Chem. 2019, 43, 4554–4561. [Google Scholar] [CrossRef]
- Karmakar, A.; Mukundan, R.; Yang, P.; Batista, E.R. Solubility model of metal complex in ionic liquids from first principle calculations. RSC Adv. 2019, 9, 18506–18526. [Google Scholar] [CrossRef] [Green Version]
- Mushtaq, M.; Akram, S. Ionic Liquid for the Extraction of Plant Phenolics. In Nanotechnology-Based Industrial Applications of Ionic Liquids. Nanotechnology in the Life Sciences; Inamuddi, Abdullah, M.A., Eds.; Springer: Cham, Switzerland, 2020; pp. 81–97. [Google Scholar]
- Bogdanov, M.G.; Svinyarov, I. Ionic liquid-supported solid–liquid extraction of bioactive alkaloids. II. Kinetics, modeling and mechanism of glaucine extraction from Glaucium flavum Cr. (Papaveraceae). Sep. Purif. Technol. 2013, 103, 279–288. [Google Scholar] [CrossRef]
- Ma, W.; Lu, Y.; Hu, R.; Chen, J.; Zhang, Z.; Pan, Y. Application of ionic liquids-based microwave-assisted extraction of three alkaloids N-nornuciferine, O-nornuciferine, and nuciferine from lotus leaf. Talanta 2010, 80, 1292–1297. [Google Scholar] [CrossRef]
- Wang, L.; Bai, M.; Qin, Y.; Liu, B.; Wang, Y.; Zhou, Y. Application of ionic liquid-based ultrasonic-assisted extraction of flavonoids from bamboo leaves. Molecules 2018, 23, 2309. [Google Scholar] [CrossRef] [Green Version]
- Tan, Z.; Yi, Y.; Wang, H.; Zhou, W.; Wang, C. Extraction, preconcentration and isolation of flavonoids from Apocynum venetum L. leaves using ionic liquid-based ultrasonic-assisted extraction coupled with an aqueous biphasic system. Molecules 2016, 21, 262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.; Zhang, Y.; Han, M.; Yang, L. Aqueous ionic liquid based ultrasonic assisted extraction of eight ginsenosides from ginseng root. Ultrason. Sonochem. 2013, 20, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.A.R.; Domańska, U.; Schröder, B.; Coutinho, J.A.P.; Pinho, S.P. Selection of ionic liquids to be used as separation agents for terpenes and terpenoids. ACS Sustain. Chem. Eng. 2015, 4, 548–556. [Google Scholar] [CrossRef]
- Ji, S.; Wang, Y.; Su, Z.; He, D.; Du, Y.; Guo, M.; Yang, D.; Tang, D. Ionic liquids-ultrasound based efficient extraction of flavonoid glycosides and triterpenoid saponins from licorice. RSC Adv. 2018, 8, 13989–13996. [Google Scholar] [CrossRef] [Green Version]
- Kiyonga, A.N.; Hong, G.; Kim, H.S.; Suh, Y.-G.; Jung, K. Facile and rapid isolation of oxypeucedanin hydrate and byakangelicin from Angelica dahurica by using [Bmim]Tf2 ionic liquid. Molecules 2021, 26, 830. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Spronen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Ionic liquids and deep eutectic solvents in natural products research: Mixtures of solids as extraction solvents. J. Nat. Prod. 2013, 76, 2162–2173. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; Silva, F.A.; Quental, M.V.; Mondal, D.; Freire, M.G.; Coutinho, J.A.P. Ionic-liquid-mediated extraction and separation processes for bioactive compounds: Past, present, and future trends. Chem. Rev. 2017, 117, 6984–7052. [Google Scholar] [CrossRef]
- Kroon, M.C.; van Spronsen, J.; Peters, C.J.; Sheldon, R.A.; Witkamp, G.-J. Recovery of pure products from ionic liquids using supercritical carbon dioxide as a co-solvent in extractions or as an anti-solvent in precipitations. Green Chem. 2006, 8, 246–249. [Google Scholar] [CrossRef]
- Chen, F.; Mo, K.; Liu, Z.; Yang, F.; Hou, K.; Li, S.; Zu, Y.; Yang, L. Ionic liquid-based vacuum microwave-assisted extraction followed by Macroporous resin enrichment for the separation of the three glycosides Salicin, Hyperin and Rutin from Populus Bark. Molecules 2014, 19, 9689–9711. [Google Scholar] [CrossRef] [Green Version]
- Lapkin, A.A.; Plucinski, P.K.; Cutler, M. Comparative assessment of technologies for extraction of artemisinin. J. Nat. Prod. 2006, 69, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, T.; Kumar, A. Efficient extraction of agarose from red Algae using ionic liquids. Green Sustain. Chem. 2014, 4, 190–201. [Google Scholar] [CrossRef] [Green Version]
- Davey, R.; Garside, J. Form Molecules to Crystallizers: An Introduction to Crystallization; Chapters 2 and 3; Oxford University Press: Oxford, UK, 2000; pp. 9–24. [Google Scholar]
- Kiyonga, A.N.; An, J.H.; Lee, K.Y.; Lim, C.; Suh, Y.G.; Chin, Y.W.; Jung, K. Rapid and efficient separation of decursin and Decursinol angelate from Angelica gigas Nakai using ionic liquid, (BMIm)BF4, combined with crystallization. Molecules 2019, 24, 2390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, Y.S.; In, K.K.; Kim, C.M. Chemical constituents from the roots of Ostericum koreanum. Kor. J. Pharmacogn. 2000, 31, 284–287. [Google Scholar]
- Shin, S. In vitro effects of essential oils from Ostericum koreanum against antibiotic resistant Salmonella spp. Arch. Pharm. Res. 2005, 28, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Kim, H.J.; Lee, S.J.; Choi, H.Y.; Jin, C.; Lee, Y.S. A new chromone, 11-hydroxy-sec-Oglucosylhamaudol from Ostericum koreanum. Chem. Pharm. Bull. 2007, 55, 1065–1066. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.K.; Woo, W.S. Coumarin constituents from the roots of Angelica koreana Max. Korea J. Pharmacogn. 1982, 13, 10–13. [Google Scholar]
- Jung, H.W.; Mahesh, R.; Park, J.H.; Boo, Y.C.; Park, K.M.; Park, Y.K. Bisabolangelone isolated from Ostericum koreanum inhibits the production of inflammatory mediators by down-regulation of NF-kappaB and ERK MAP kinase activity in LPS-stimulated RAW264.7 cells. Int. Immunopharmacol. 2010, 10, 155–162. [Google Scholar] [CrossRef]
- Mahesh, R.; Jung, H.W.; Park, J.H.; Park, Y.-K. In vitro antioxidant capacity and neuronal cell toxicity of roots of Ostericum koreanum Maximowicz. E-J. Chem. 2011, 8, 1451–1455. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kim, Y.S.; Ryu, S.Y. Antiproliferative effect of furanocoumarins from the root of Angelica dahurica on cultured human tumor cell lines. Phytother. Res. 2007, 21, 288–290. [Google Scholar] [CrossRef]
- Tong, K.; Xin, C.; Chen, W. Isoimperatorin induces apoptosis of the SGC-7901 human gastric cancer cell line via the mitochondria-mediated pathway. Oncol. Lett. 2017, 13, 518–524. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.B.; Gao, H.R.; Ren, Y.J.; Fang, F.X.; Tian, H.T.; Gao, Z.J.; Song, W.; Huang, S.M.; Zhao, A.F. Effects of isoimperatorin on proliferation and apoptosis of human gastric carcinoma cells. Oncol. Lett. 2018, 15, 7993–7998. [Google Scholar] [CrossRef]
- Tan, N.; Yazıcı-Tütüniş, S.; Bilgin, M.; Tan, E.; Miski, M. Antibacterial activities of prenylated coumarins from the roots of Prangos hulusii. Molecules 2017, 22, 1098. [Google Scholar] [CrossRef]
- Guo, N.; Wu, J.; Fan, J.; Yuan, P.; Shi, Q.; Jin, K.; Cheng, W.; Zhao, X.; Zhang, Y.; Li, W.; et al. In vitro activity of isoimperatorin, alone and in combination, against Mycobacterium tuberculosis. Lett. Appl. Microbiol. 2014, 58, 344–349. [Google Scholar] [CrossRef]
- Wijerathne, C.U.; Seo, C.-S.; Song, J.-W.; Park, H.-S.; Moon, O.-S.; Won, Y.-S.; Kwon, H.-J.; Son, H.-Y. Isoimperatorin attenuates airway inflammation and mucus hypersecretion in an ovalbumin-induced murine model of asthma. Inter. Immunopharmacol. 2017, 49, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jia, M.; Ma, Y.; Li, X. Pharmacological effect of four linear furocoumarins in Radix Angelicae dahuricae. Nat. Prod. Res. Dev. 2010, 22, 485–489. [Google Scholar]
- Znati, M.; Jannet, H.B.; Cazaux, S.; Souchard, J.P.; Skhiri, F.H.; Bouajila, J. Antioxidant, 5-Lipoxygenase Inhibitory and Cytotoxic Activities of Compounds Isolated from the Ferula lutea Flowers. Molecules 2014, 19, 16959–16975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pokharel, Y.R.; Han, E.H.; Kim, J.Y.; Oh, S.J.; Kim, S.K.; Woo, E.-R.; Jeong, H.G.; Kang, K.W. Potent protective effect of isoimperatorin against aflatoxin B1-inducible cytotoxicity in H4IIE cells: Bifunctional effects on glutathione S-transferase and CYP1A. Carcinogenesis 2006, 27, 2483–2490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassanein, E.H.M.; Sayed, A.M.; Hussein, O.E.; Mahmoud, A.M. Coumarins as modulators of the Keap1/Nrf2/ARE signaling pathway. Oxid. Med. Cell Longev. 2020, 2020, 1675957. [Google Scholar] [CrossRef] [Green Version]
- Rajtar, B.; Skalicka-Woźniak, K.; Świątek, Ł.; Stec, A.; Boguszewska, A.; Polz-Dacewicz, M. Antiviral effect of compounds derived from Angelica archangelica L. on Herpes simplex virus-1 and Coxsackievirus B3 infections. Food Chem. Toxicol. 2017, 109, 1026–1031. [Google Scholar] [CrossRef]
- Lai, Y.; Han, T.; Zhan, S.; Jiang, Y.; Liu, X.; Li., G. Antiviral activity of Isoimperatorin against influenza a virus in vitro and its inhibition of neuraminidase. Front. Pharmacol. 2021, 12, 657826. [Google Scholar] [CrossRef]
- Lee, M.K.; Ling, J.H.; Chun, M.-H.; Jeong, J.-H.; Na, Y.-C.; Lee, K.-W.; Jung, J.H.; Hong, J. Simultaneous determination of biological marker compounds in Ostericum koreanum by HPLC method and discrimination by principal component analysis. Bull. Korean Chem. Soc. 2008, 29, 2465. [Google Scholar]
- Kim, S.; Kim, K.Y.; Han, C.S.; Ki, K.S.; Min, K.J.; Zhang, X.; Whang, W.K. Simultaneous analysis of six major compounds in Osterici Radix and Notopterygii Rhizoma et Radix by HPLC and discrimination of their origins from chemical fingerprint analysis. Arch. Pharm. Res. 2012, 35, 691–699. [Google Scholar] [CrossRef]
- Raza, H.; Abbas, Q.; Hassan, M.; Eo, S.-H.; Ashraf, Z.; Kim, D.; Phull, A.R.; Kim, S.J.; Kang, S.K.; Seo, S.-Y. Isolation, characterization, and in silico, in vitro and in vivo antiulcer studies of isoimperatorin crystallized from Ostericum koreanum. Pharm. Biol. 2016, 55, 218–226. [Google Scholar] [CrossRef] [Green Version]
- Passos, H.; Freire, M.G.; Coutinho, J.A.P. Ionic liquid solutions as extractive solvents for value-added compounds from biomass. Green Chem. 2014, 16, 4786–4815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.C.; Liu, C.M.; Li, J.; Qi, Y.J.; Li, Y.C.; Li, S.N. Development of ‘ultrasound-assisted dynamic extraction’ and its combination with CCC and CPC for simultaneous extraction and isolation of phytochemicals. Ultrason. Sonochem. 2015, 26, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.-L.; Wen, G.; Yuan, M.-Y.; Gao, F. Ultrasound-assisted extraction of total flavonoids from corn silk and their antioxidant activity. J. Chem. 2016, 2016, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Adam, F.; Abert-Vian, M.; Peltier, G.; Chemat, F. “Solvent-free” ultrasound-assisted extraction of lipids from fresh microalgae cells: A green, clean and scalable process. Bioresour. Technol. 2012, 114, 457–465. [Google Scholar] [CrossRef]
- Mason, T.J. Chemistry with Ultrasound; Elsevier Applied Science: New York, NY, USA, 1990. [Google Scholar]
- Mason, T.J. Practical Sonochemistry: User’s Guide to Applications in Chemistry and Chemical Engineering; Ellis Horwood Ltd.: New York, NY, USA, 1992. [Google Scholar]
- Chen, Y.; Yang, S.Y.; Li, N.; Zhang, L.J.; Qian, Y. Ultrasound-assisted extraction of imperatorin and Isoimperatorin from roots of Angelica dahurica. Adv. Mater. Res. 2012, 550–553, 1845–1851. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, K.H.; Lee, D.G.; Lee, S. The protective activity of linear furanocoumarins from Angelica dahurica against glucose-mediated protein damage. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 355–358. [Google Scholar] [CrossRef]
- Cho, J.Y.; Lee, J.; Park, J.; Park, M.H. Isolation of inhibitory components on tumor necrosis factor-a production from Angelica koreana. Yakhak Hoeji 1998, 42, 125–131. [Google Scholar]


| Run | X1 (min) | X2 (g/mL) | Concentration (ug) | Concentration (%) | |
|---|---|---|---|---|---|
| Predicted | Experimental | Experimental | |||
| 1 | 30 | 1:5 | 1.35 | 1.29 | 48.88 |
| 2 | 30 | 1:20 | 2.52 | 2.45 | 93.02 |
| 3 | 120 | 1:5 | 1.45 | 1.38 | 52.31 |
| 4 | 120 | 1:20 | 2.65 | 2.57 | 97.62 |
| 5 | 75 | 1:2 | 0.95 | 1.02 | 38.86 |
| 6 | 75 | 1:23 | 2.61 | 2.68 | 102.05 |
| 7 | 11.5 | 1:12.5 | 2.12 | 2.19 | 83.31 |
| 8 | 140 | 1:12.5 | 2.29 | 2.37 | 90.09 |
| 9 | 75 | 1:12.5 | 2.45 | 2.39 | 90.97 |
| 10 | 75 | 1:12.5 | 2.45 | 2.40 | 91.24 |
| 11 | 75 | 1:12.5 | 2.45 | 2.42 | 92.12 |
| 12 | 75 | 1:12.5 | 2.45 | 2.55 | 96.95 |
| 13 | 75 | 1:12.5 | 1.35 | 2.47 | 93.99 |
| Degrees of Freedom | Sum of Squares | F-Value | p-Value | |
|---|---|---|---|---|
| Model | 5 | 5219.74 | 82.97 | 0.0001 |
| X1 | 1 | 38.75 | 3.08 | 0.123 |
| X2 | 1 | 3997.11 | 317.69 | 0.0001 |
| X12 | 1 | 146.14 | 11.62 | 0.011 |
| X22 | 1 | 1037.39 | 89.25 | 0.0001 |
| X1X2 | 1 | 0.34 | 0.03 | 0.873 |
| Residual | 7 | 88.07 | - | - |
| Lack of fit | 3 | 63.46 | 3.44 | 0.132 |
| Pure error | 4 | 24.61 | - | - |
| Total | 12 | 5307.81 | ||
| R2 | 98.34% | |||
| R2 -adj | 97.16% |
| Ratios | Recovery Yield of Isoimperatorin (mg) | Recovery Yield of Isoimperatorin (%) |
|---|---|---|
| 1:05 | 0.62 | 24.03 |
| 1:1 | 1.31 | 50.78 |
| 1:1.5 | 1.76 | 68.22 |
| 1:2 | 2.04 | 79.07 |
| 1:2.5 | 2.29 | 86.43 |
| 1:3 | 2.12 | 82.17 |
| Run | Weight of the Product (mg) | Recovery Yield of Isoimperatorin (mg) | Recovery Yield of Isoimperatorin (%) | Purity of Isoimperatorin (%) |
|---|---|---|---|---|
| 1 | 9.12 | 2.33 | 90.31 | 25.55 |
| 2 | 8.03 | 2.25 | 87.21 | 28.02 |
| 3 | 8.11 | 2.21 | 85.66 | 27.25 |
| Average | 8.42 | 2.26 | 87.73 | 26.94 |
| Standard deviation | 0.61 | 0.06 | 2.37 | 1.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiyonga, A.N.; Park, G.H.; Kim, H.S.; Suh, Y.-G.; Kim, T.K.; Jung, K. An Efficient Ionic Liquid-Mediated Extraction and Enrichment of Isoimperatorin from Ostericum koreanum (Max.) Kitagawa. Molecules 2021, 26, 6555. https://doi.org/10.3390/molecules26216555
Kiyonga AN, Park GH, Kim HS, Suh Y-G, Kim TK, Jung K. An Efficient Ionic Liquid-Mediated Extraction and Enrichment of Isoimperatorin from Ostericum koreanum (Max.) Kitagawa. Molecules. 2021; 26(21):6555. https://doi.org/10.3390/molecules26216555
Chicago/Turabian StyleKiyonga, Alice Nguvoko, Gyu Hwan Park, Hyun Su Kim, Young-Ger Suh, Tae Kon Kim, and Kiwon Jung. 2021. "An Efficient Ionic Liquid-Mediated Extraction and Enrichment of Isoimperatorin from Ostericum koreanum (Max.) Kitagawa" Molecules 26, no. 21: 6555. https://doi.org/10.3390/molecules26216555






