Effectiveness of the Influence of Selected Essential Oils on the Growth of Parasitic Fusarium Isolated from Wheat Kernels from Central Europe
Abstract
1. Introduction
2. Results
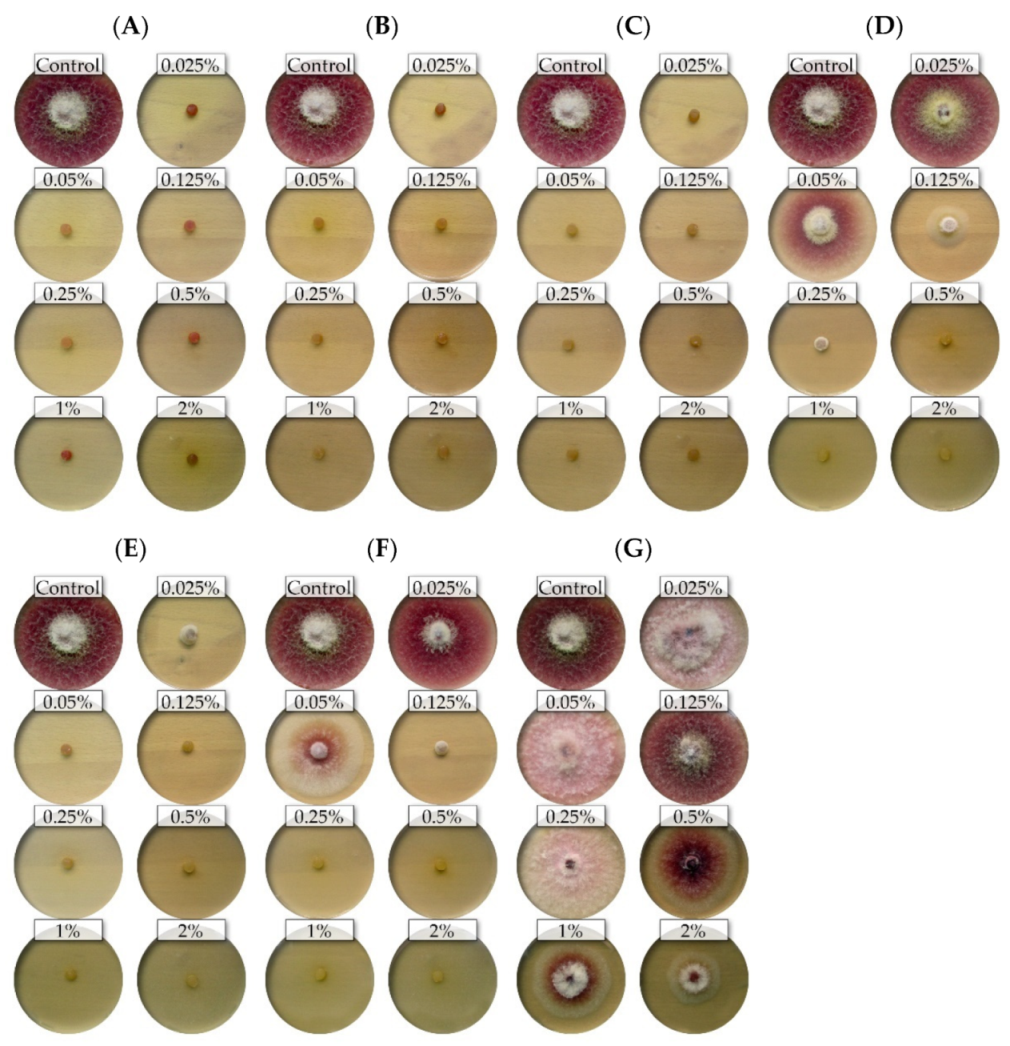
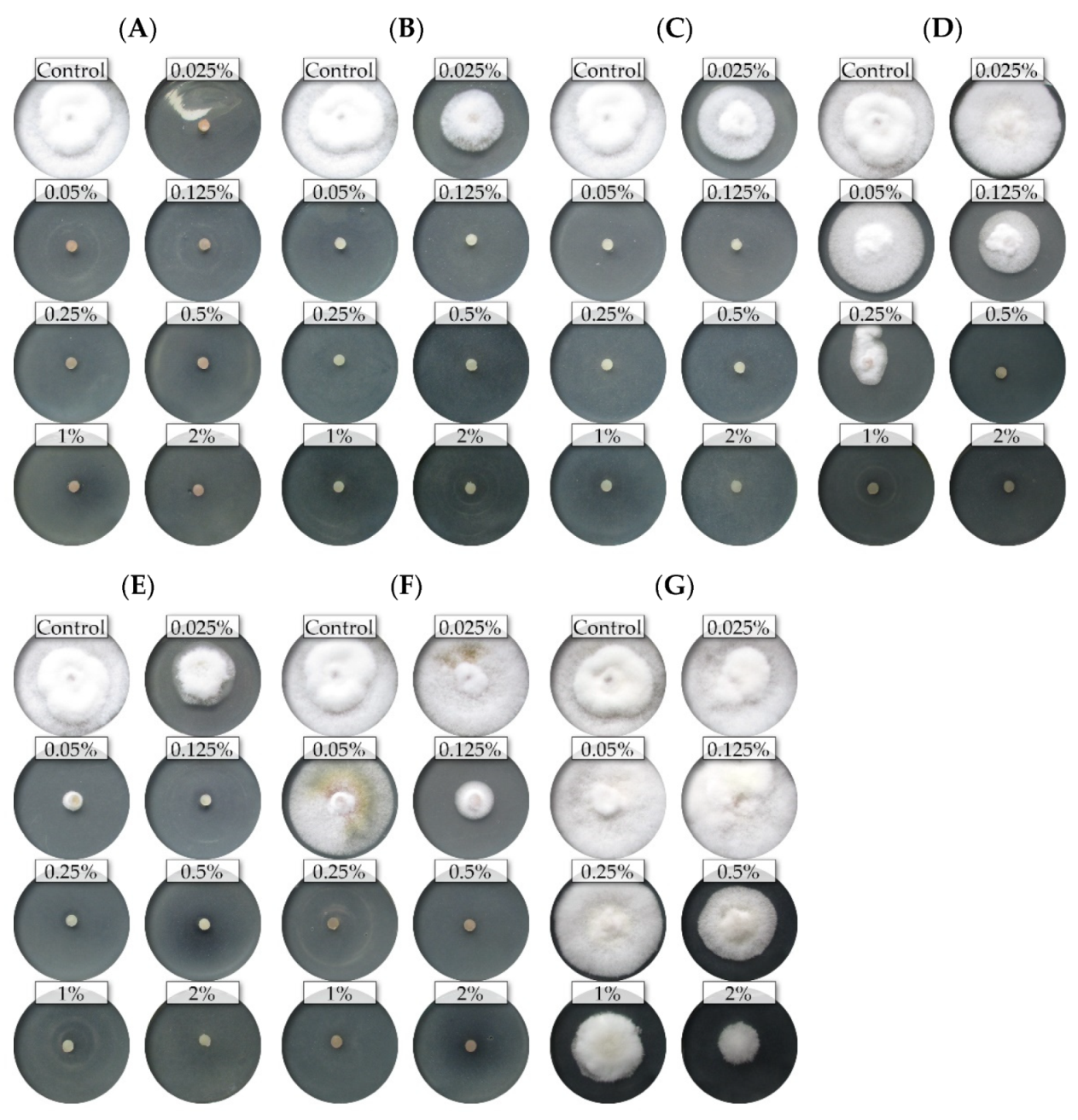
2.1. Variation in Sensitivity of Fusarium Isolates to Essential Oils
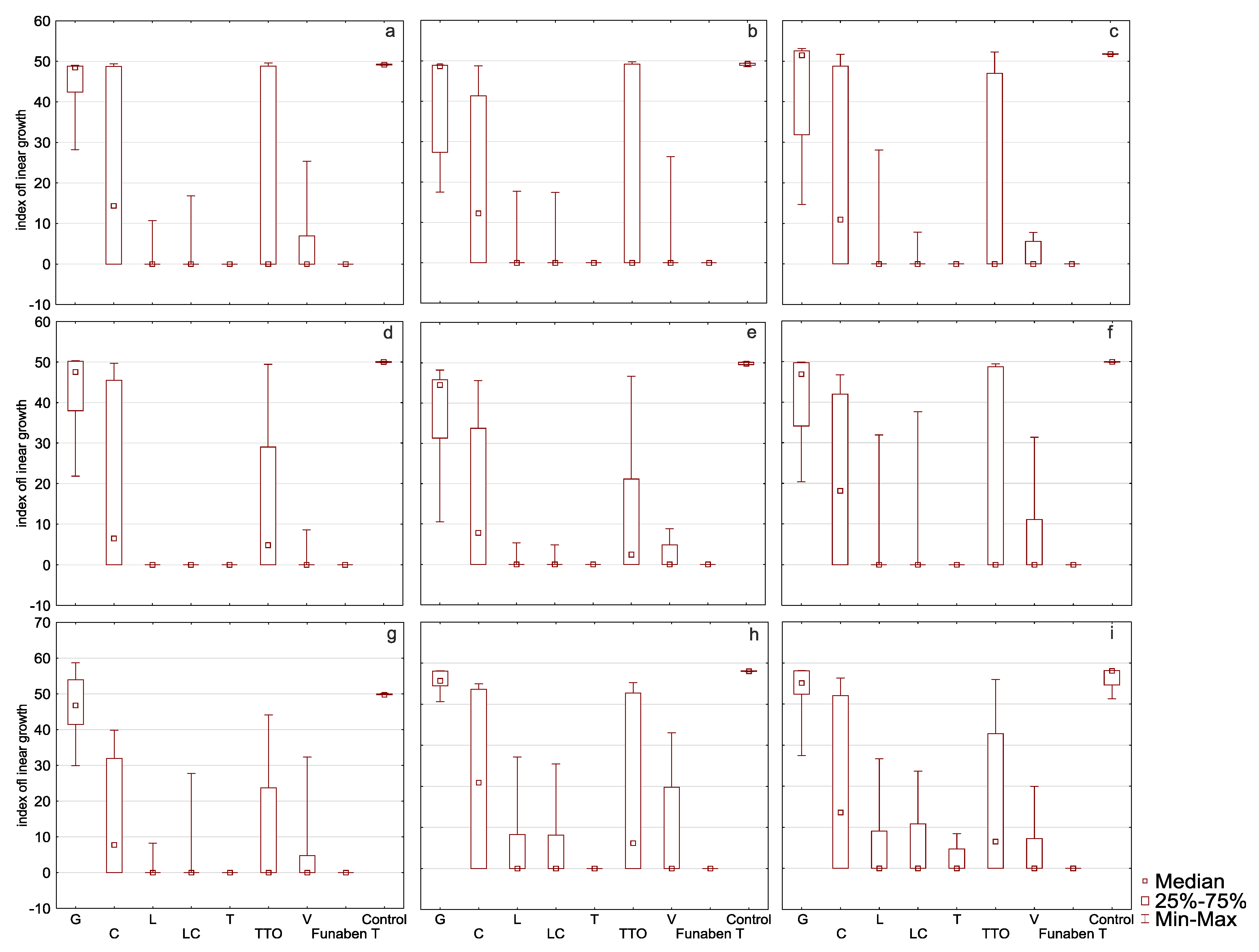
2.2. Assessment of the Effectiveness of Individual Essential Oils
3. Discussion
4. Materials and Methods
- Cultures of fungi were grown in PDA medium for 14 days at 25 °C
- Inoculum. The spore suspension of Fusarium spp. in 0.01% sterile Tween 80 were obtained from 14 days old culture. The haemocytometer Thoma was used to obtain a spore suspension of 2 × 106 CFU·cm3. Petri dishes (9 cm diameter) containing 20 × cm3 PDA medium were inoculating this spore suspension and stored at 25 °C for 14 days. Inoculum—rings with a diameter of 10 mm overgrown by mycelium.
- Inoculum was placed on the surface of the oil-modified PDA medium.
- The samples were incubated at 25 °C. Every 2 days, the diameter of developing colonies was measured until the surface of the medium in the control plates was overgrown. Tests were performed in four repetitions (n = 4). One petri dish with inoculum (disc overgrown with pathogen mycelium) was treated as a repetition
- PDA medium with the Funaben T (at concentrations of 0.125; 0.25 and 0.50%) was used as a positive control. Unmodified PDA medium (without oils) with a ring was used as a negative control
Statistical Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Krzyśko-Łupicka, T.; Sokół, S.; Piekarska-Stachowiak, A. Evaluation of Fungistatic Activity of Eight Selected Essential Oils on Four Heterogeneous Fusarium Isolates Obtained from Cereal Grains in Southern Poland. Molecules 2020, 25, 292. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, M.; Beyer, M.; Logrieco, A.; Audenaert, K.; Balmas, V.; Basler, R.; Boutigny, A.L.; Chrpová, J.; Czembor, E.; Gagkaeva, T.; et al. A European database of Fusarium graminearum and F. culmorum trichothecene genotypes. Front. Microbiol. 2016, 7, 406. [Google Scholar] [CrossRef]
- Avanço, G.B.; Ferreira, F.D.; Bomfim, N.S.; de Souza Rodrigues dos Santos, P.A.; Peralta, R.M.; Brugnari, T.; Mallmann, C.A.; Abreu Filho, B.A.; Mikcha, J.M.G.; Machinski, M. Curcuma longa L. essential oil composition, antioxidant effect, and effect on Fusarium verticillioides and fumonisin production. Food Control 2016, 73, 806–813. [Google Scholar] [CrossRef]
- Agrios, G.N. Plant Pathology, 5th ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2005; pp. 163–164. [Google Scholar]
- Somma, S.; Petruzzella, A.L.; Logrieco, A.F.; Meca, G.; Cacciola, O.S.; Moretti, A. Phylogenetic analyses of Fusarium graminearum strains from cereals in Italy, and characterisation of their molecular and chemical chemotypes. Crop Pasture Sci. 2014, 65, 52–60. [Google Scholar] [CrossRef]
- Bottalico, G. Perrone Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. Eur. J. Plant Pathol. 2002, 108, 611–624. [Google Scholar] [CrossRef]
- Vogelgsang, S.; Beyer, M.; Pasquali, M.; Jenny, E.; Musa, T.; Bucheli, T.D.; Wettstein, F.E.; Forrer, H.R. An eight-year survey of wheat shows distinctive effects of cropping factors on different Fusarium species and associated mycotoxins. Eur. J. Agron. 2019, 105, 62–77. [Google Scholar] [CrossRef]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical pesticides and human health: The urgent need for a new concept in agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef]
- Da Cruz Cabral, L.; Pinto, V.F.; Patriarca, A. Application of plant derived compounds to control fungal spoilage and mycotoxin production in foods. Int. J. Food Microbiol. 2013, 166, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Danielewicz, B.; Gwiazdowski, R.; Bednarek-Bartsch, A. Influence of some selected fungicides on Fusarium genus cultures growth limitation. Prog. Plant Prot. 2013, 53, 759–761. [Google Scholar]
- Zabka, M.; Pavela, R.; Slezakova, L. Antifungal effect of Pimenta dioica essential oil against dangerous pathogenic and toxinogenic fungi. Ind. Crops Prod. 2009, 30, 250–253. [Google Scholar] [CrossRef]
- Matusinsky, P.; Zouhar, M.; Pavela, R.; Novy, P. Antifungal effect of five essential oils against important pathogenic fungi of cereals. Ind. Crops Prod. 2015, 67, 208–215. [Google Scholar] [CrossRef]
- Kumar, P.; Mishra, S.; Kumar, A.; Sharma, A. Antifungal efficacy of plant essential oils against stored grain fungi of Fusarium spp. J. Food Sci. Technol. 2016, 53, 3725–3734. [Google Scholar] [CrossRef] [PubMed]
- Gakuubi, M.M.; Maina, A.W.; Wagacha, J.M. Antifungal activity of essential oil of Eucalyptus camaldulensis Dehnh against selected Fusarium spp. Int. J. Microbiol. 2017, 7, 8761610. [Google Scholar]
- Hara, P.; Szparaga, A.; Czerwińska, E. Ecological Methods Used to Control Fungi that Cause Diseases of the Crop Plant. Annu. Set Environ. Protect. 2018, 20, 1764–1775. [Google Scholar]
- Perczak, A.; Gwiazdowska, D.; Marchwińska, K.; Juś, K.; Gwiazdowski, R.; Waśkiewicz, A. Antifungal activity of selected essential oils against Fusarium culmorum and F. graminearum and their secondary metabolites in wheat seeds. Arch. Microbiol. 2019, 201, 1085–1097. [Google Scholar] [CrossRef]
- Krzyśko-Łupicka, T.; Walkowiak, W. Evaluation of susceptibility of phytopathogenic Fusarium culmorum strain on selected essential oils. Ecol. Chem. Eng. A 2014, 21, 355–366. [Google Scholar] [CrossRef]
- Krzyśko-Łupicka, T.; Walkowiak, W.; Białoń, M. Comparison of the fungistatic activity of selected essential oils relative to Fusarium graminearum isolates. Molecules 2019, 24, 311. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.M.D.; Hirooka, E.Y.; Ferreira, F.D.; Silva, M.V.; Mossini, S.A.G.; Machinski, M.J. Effect of Zingiber officinale Roscoe essential oil in fungus control and deoxynivalenol production of Fusarium graminearum Schwabe in vitro. Food Addit. Contam Part A 2018, 35, 2168–2174. [Google Scholar] [CrossRef]
- Giamperi, L.; Bucchini, A.E.A.; Ricci, D.; Tirillini, B.; Nicoletti, M.; Rakotosaona, R.; Maggi, F. Vepris macrophylla (Baker) I. Verd Essential Oil: An Antifungal Agent against Phytopathogenic Fungi. Int. J. Mol. Sci. 2020, 21, 2776. [Google Scholar] [CrossRef]
- Regnault-Roger, C.; Vincent, C.; Arnason, J.T. Essential Oils in Insect Control: Low-Risk Products in a High-Stakes. World. Annu Rev. Entomol. 2012, 57, 405–424. [Google Scholar] [CrossRef]
- Naeini, A.; Ziglarib, T.; Shokrib, H.; Khosravi, A.R. Assessment of growth-inhibiting effect of some plant essential oils on different Fusarium isolates. J. Med. Mycol. 2010, 20, 174–178. [Google Scholar] [CrossRef]
- Mackay, T.F.C.; Stone, E.A.; Ayroles, J.F. The genetics of quantitative traits: Challenges and prospects. Nat. Rev. Genet. 2009, 10, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Waalwijk, C.; Taga, M.; Zheng, S.L.; Proctor, R.H.; Vaughan, M.M.; O’Donnell, K. Karyotype evolution in Fusarium. IMA Fungus 2018, 9, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Ayukawa, Y.; Komatsu, K.; Taga, M.; Arie, T. Cytological karyotyping of Fusarium oxysporum by the germ tube burst method (GTBM). J. Gen. Plant Pathol. 2018, 84, 254–261. [Google Scholar] [CrossRef]
- Fitzpatrick, D.A. Horizontal gene transfer in fungi. FEMS Microbiol. Lett. 2012, 329, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.J.; van der Does, H.C.; Borkovich, K.A.; Coleman, J.J.; Daboussi, M.J.; Di Pietro, A.; Dufresne, M.; Freitag, M.; Grabherr, M.; Henrissat, B.; et al. Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature 2010, 464, 367–373. [Google Scholar] [CrossRef]
- Tuveson, R.W.; Garber, E.D. Genetics of phytopathogenic fungi. IV. Experimentally induced alterations in nuclear ratios of heterocaryons of Fusarium oxysporium F. Pisi. Genetics 1961, 46, 485–492. [Google Scholar] [CrossRef]
- Teunissen, H.A.S.; Verkooijen, J.; Cornelissen, B.J.C.; Haring, M.A. Genetic exchange of avirulence determinants and extensive karyotype rearrangements in parasexual recombinants of Fusarium oxysporum. Mol. Gen. Genom. 2002, 268, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Láday, M.; Mulè, G.; Moretti, A.; Hamari, Z.; Juhász, Á.; Szécsi, Á.; Logrieco, A. Mitochondrial DNA variability in Fusarium proliferatum (Gibberella intermedia). Eur. J. Plant Pathol. 2004, 110, 563–571. [Google Scholar] [CrossRef]
- Brankovics, B.; van Dam, P.; Rep, M.; Sybren de Hoog, G.; van der Lee, T.A.J.; Waalwijk, C.; van Diepeningen, A.D. Mitochondrial genomes reveal recombination in the presumed asexual Fusarium oxysporum species complex. BMC Genom. 2017, 18, 735. [Google Scholar] [CrossRef]
- Kumar, S.; Rai, S.; Maurya, D.K.; Kashyap, P.L.; Srivastava, A.K.; Anandaraj, M. Cross-species transferability of microsatellite markers from Fusarium oxysporum for the assessment of genetic diversity in Fusarium udum. Phytoparasitica 2013, 41, 615–622. [Google Scholar] [CrossRef]
- Duggal, A.; Dumas, M.T.; Jeng, R.S.; Hubbes, M. Ribosomal variation in six species of Fusarium. Mycopathologia 1997, 140, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Suga, H.; Kageyama, K.; Shimizu, M.; Hyakumachi, M. A natural mutation involving both pathogenicity and perithecium formation in the Fusarium graminearum species complex. G3 Genes Genomes Genet. 2016, 6, 3883–3892. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zehraoui, E.; Atanasoff-Kardjalieff, A.K.; Strauss, J.; Studt, L.; Ponts, N. Effect of H2A.Z deletion is rescued by compensatory mutations in Fusarium graminearum. PLoS Genet. 2020, 16, e1009125. [Google Scholar] [CrossRef] [PubMed]
- Magdama, F.; Monserrate-Maggi, L.; Serrano, L.; Onofre, J.G.; del Mar Jiménez-Gasco, M. Genetic diversity of Fusarium oxysporum f. sp. cubense, the Fusarium wilt pathogen of banana, in Ecuador. Plants 2020, 9, 1133. [Google Scholar] [CrossRef]
- Halpern, H.C.; Qi, P.; Kemerait, R.C.; Brewer, M.T. Genetic diversity and population structure of races of Fusarium oxysporum causing cotton wilt. G3 Genes Genomes Genet. 2020, 10, 3261–3269. [Google Scholar] [CrossRef]
- Groenewald, S.; van den Berg, N.; Marasas, W.F.O.; Viljoen, A. Biological, physiological and pathogenic variation in a genetically homogenous population of Fusarium oxysporum f. sp. cubense. Australas. Plant Pathol. 2006, 35, 401–409. [Google Scholar] [CrossRef]
- Covo, S. Genomic Instability in Fungal Plant Pathogens. Genes 2020, 11, 421. [Google Scholar] [CrossRef] [PubMed]
- Rampersad, S.N. Pathogenomics and Management of Fusarium Diseases in Plants. Pathogens 2020, 9, 340. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual, 1st ed.; Blackwell Scientific Publications: Hoboken, NJ, USA, 2006; 400p. [Google Scholar]
- Kalemba, D.; Kunicka, A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef]
- Numpaque, M.A.; Oviedo, L.A.; Gil, J.H.; García, C.M.; Durango, D.L. Thymol and carvacrol: Biotransformation and antifungal activity against the plant pathogenic fungi Colletotrichum acutatum and Botryodiplodia theobromae. Trop. Plant Pathol. 2011, 36, 3–13. [Google Scholar] [CrossRef]
- Zuzarte, M.; Vale-Silva, L.; Gonçalves, M.J.; Cavaleiro, C.; Vaz, S.; Canhoto, J.; Pinto, E.; Salgueiro, L. Antifungal activity of phenolic-rich Lavandula multifida L. essential oil. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1359–1366. [Google Scholar] [CrossRef]
- Ochoa-Velasco, C.E.; Navarro-Cruz, A.R.; Vera-López, O.; Palou, E.; Avila-Sosa, R. Growth modelling to control (in vitro) Fusarium verticillioides and Rhizopus stolonifer with thymol and carvacrol. Rev. Argent. Microbiol. 2018, 50, 70–74. [Google Scholar] [CrossRef]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboodyb, M.S.; Vijayakumarb, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, S.; Du, S.; Chen, S.; Sun, H. Antifungal activity of thymol and carvacrol against postharvest pathogens Botrytis cinerea. J. Food Sci. Technol. 2019, 56, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Prakash, B.; Kedia, A.; Mishra, P.K.; Dubey, N.K. Plant essential oils as food preservatives to control moulds, mycotoxin contamination and oxidative deterioration of agri-food commodities—Potentials and challenges. Food Control 2015, 47, 381–391. [Google Scholar] [CrossRef]
- Kalagatur, N.K.; Dhamodaran, N.; Siddaiah, C.; Mudili, V.; Sreepathi, M.H. Antifungal and Zearalenone Inhibitory Activity of Ocimum sanctum L. Essential Oil on Fusarium graminearum Determined by UHPLC and RT-qPCR. Bio-Protocol 2016, 6, e1893. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Feo, V.D. Essential Oils and Antifungal Activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef]
- Perczak, A.; Gwiazdowska, D.; Gwiazdowski, R.; Juś, K.; Marchwińska, K.; Waśkiewicz, A. The Inhibitory Potential of Selected Essential Oils on Fusarium spp. Growth and Mycotoxins Biosynthesis in Maize Seeds. Pathogens 2020, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Bermejo, D.; Angelov, I.; Vicente, G.; Stateva, R.P.; Rodriguez García-Risco, M.; Reglero, G.; Ibañez, E.; Fornari, T. Extraction of thymol from different varieties of thyme plantsusing green solvents. J. Sci. Food Agric. 2015, 95, 2901–2907. [Google Scholar] [CrossRef]
- Gavarić, N.; Smole Možina, S.; Kladar, N.; Bozin, B. Chemical Profile, Antioxidant and Antibacterial Activity of Thyme and Oregano Essential Oils, Thymol and Carvacrol and Their Possible Synergism. J. Essent. Oil Bear. Plants 2015, 18, 1013–1021. [Google Scholar] [CrossRef]
- Marchese, A.; Arciola, C.R.; Coppo, E.; Barbieri, R.; Barreca, D.; Chebaibi, S.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M.; Daglia, M. The natural plant compound carvacrol as an antimicrobial and anti-biofilm agent: Mechanisms, synergies and bio-inspired anti-infective materials. Biofouling 2018, 6, 630–656. [Google Scholar] [CrossRef]
- Barrera-Necha, L.L.; Garduno-Pizana, C.; Garcia-Barrera, L.J. In vitro Antifungal Activity of Essential Oils and Their Compounds on Mycelial Growth of Fusarium oxysporum f. sp. gladioli (Massey) Snyder and Hansen. Plant Pathol. J. 2009, 8, 17–21. [Google Scholar] [CrossRef]
- Seseni, L.; Regnier, T.; Roux van der Merwe, M.P.; Mogale, E.; Badenhorst, J. Control of Fusarium spp. causing damping off of pine seedlings by means of selected essential oils. Ind. Crops Prod. 2015, 76, 329–332. [Google Scholar] [CrossRef]
- Ćosić, J.; Vrandečić, K.; Postić, J.; Jurković, D.; Ravlić, M. In vitro antifungal activity of essential oils on growth of phytopathogenic fungi. Poljoprivreda 2010, 16, 25–28. [Google Scholar]
- Brochot, A.; Guilbot, A.; Haddioui, L.; Roques, C. Antibacterial, antifungal, and antiviral effects of three essential oil blends. Microbiol. Open 2017, 6, e00459. [Google Scholar] [CrossRef]
- Manganyi, M.C.; Regnier, T.; Olivier, E.I. Antimicrobial activities of selected essential oils against Fusarium oxysporum isolates and their biofilms. S. Afr. J. Bot. 2015, 99, 115–121. [Google Scholar] [CrossRef]
- Simić, A.; Soković, M.D.; Ristić, M.; Grujić-Jovanović, S.; Vukojević, J.; Marin, P.D. The chemical composition of some Lauraceae essential oils and their antifungal activities. Phytother. Res. 2004, 18, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, V.M.; Conti, R.; Araújo, J.M.; Souza-Motta, C.M. Endophytic fungi from the medicinal plant Lippia sidoides Cham. and their antimicrobial activity. Symbiosis 2011, 53, 89–95. [Google Scholar] [CrossRef]
- Maggi, F.; Randriana, R.F.; Rasoanaivo, P.; Nicoletti, M.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Petrelli, D.; Vitali, L.A.; Papa, F.; et al. Chemical composition and in vitro biological activities of the essential oil of Veprys macrophylla (Baker) I. Verd. Endemic to Madagascar. Chem. Biodivers. 2013, 10, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Dudai, N.; Weinstein, Y.; Krup, M.; Rabinski, T.; Ofir, R. Citral is a new inducer of caspase-3 in tumor cell lines. Planta Med. 2005, 71, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Tajidin, N.E.; Ahmad, S.H.; Rosenani, A.B.; Azimah, H.; Munirah, M. Chemical composition and citral content in lemongrass (Cymbopogon citratus) essential oil at three maturity stages. Afr. J. Biotechnol. 2012, 11, 2685–2693. [Google Scholar] [CrossRef]
- Herman, A.; Tambor, K.; Herman, A. Linalool Affects the Antimicrobial Efficacy of Essential Oils. Curr. Microbiol. 2016, 72, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Thielmann, J.; Muranyi, P. Review on the chemical composition of Litsea cubeba essential oils and the bioactivity of its major constituents citral and limonene. J. Essent. Oil Res. 2019, 31, 361–378. [Google Scholar] [CrossRef]
- Sadowska, K.; Łukaszewska-Skrzypniak, N.; Wojczyńska, J.; Stępniewska-Jarosz, S.; Tyrakowska, M.; Rataj-Guranowska, M. Evaluation of susceptibility of potential rape pathogens to selected essential oils. Prog. Plant Prot. 2017, 57, 201–205. [Google Scholar] [CrossRef]
- Morcia, C.; Malanati, M.; Terzi, V. In vitro activity of terpinen-4-ol, eugenol, carvone, 1,8-cineole (eucalyptol) and thymol against mycotoxigenic plant pathogens. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2012, 29, 415–422. [Google Scholar] [CrossRef][Green Version]
- Jamiołkowska, A. Laboratory effect of azoxystrobin (Amistar 250 SC) and grapefruit extract (Biosept 33 SL) on growth of fungi colonizing zucchini plants. Acta Sci. Pol. Hortorum Cultus 2011, 10, 245–257. [Google Scholar]
- Hashem, M.; Moharam, A.M.; Zaied, A.A.; Saleh, F.E.M. Efficacy of essential oils in the control of cumin root rot disease caused by Fusarium spp. Crop Prot. 2010, 29, 1111–1117. [Google Scholar] [CrossRef]
- Macias, F.A.; Marin, D.; Oliveros-Bastidas, A.; Varela, R.M.; Simonet, A.M.; Carrera, C.; Molinillo, J.M. Allelopathy as a new strategy for sustainable ecosystems development. Biol. Sci. Space 2003, 17, 18–23. [Google Scholar] [CrossRef]
- Li, Z.H.; Wang, Q.; Ruan, X.; Pan, C.D.; Jiang, D.A. Phenolics and plant allelopathy. Molecules 2010, 15, 8933–8952. [Google Scholar] [CrossRef] [PubMed]
- Jabran, K.; Mahajan, G.; Sardana, V.; Chauhan, B.S. Allelopathy for weed control in agricultural systems. Crop Prot. 2015, 72, 57–65. [Google Scholar] [CrossRef]
- Feng, W.; Zheng, X. Essential oils to control Alternaria alternata in vitro and in vivo. Food Control 2007, 18, 1126–1130. [Google Scholar] [CrossRef]
- Riccioni, L.; Orzeli, L. Activity of tea tree (Melaleuca alternifolia, Cheel) and thyme (Thymus vulgaris, Linnaeus.) essential oil against some pathogenic seed borne fungi. J. Essent. Oil Res. 2011, 23, 43–47. [Google Scholar] [CrossRef]
- Białoń, M.; Krzyśko-Łupicka, T.; Nowakowska-Bogdan, E.; Wieczorek, P. Chemical composition of two different lavender essential oils and their effect on facial skin microbiota. Molecules 2019, 18, 3270. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Chen, J.; Zheng, X.; Liu, Q. Thyme oil to control Alternaria alternata in vitro and in vivo as fumigant and contact treatments. Food Control 2011, 22, 78–81. [Google Scholar] [CrossRef]
- Wagle, B.; Budathoki, U. Antifungal Activities of Essential Oils and Crude Extracts of Some Aromatic Plants against Fusarium Rot of Trichosanthes dioica. Nepal J. Sci. Technol. 2013, 13, 97–102. [Google Scholar] [CrossRef]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef]
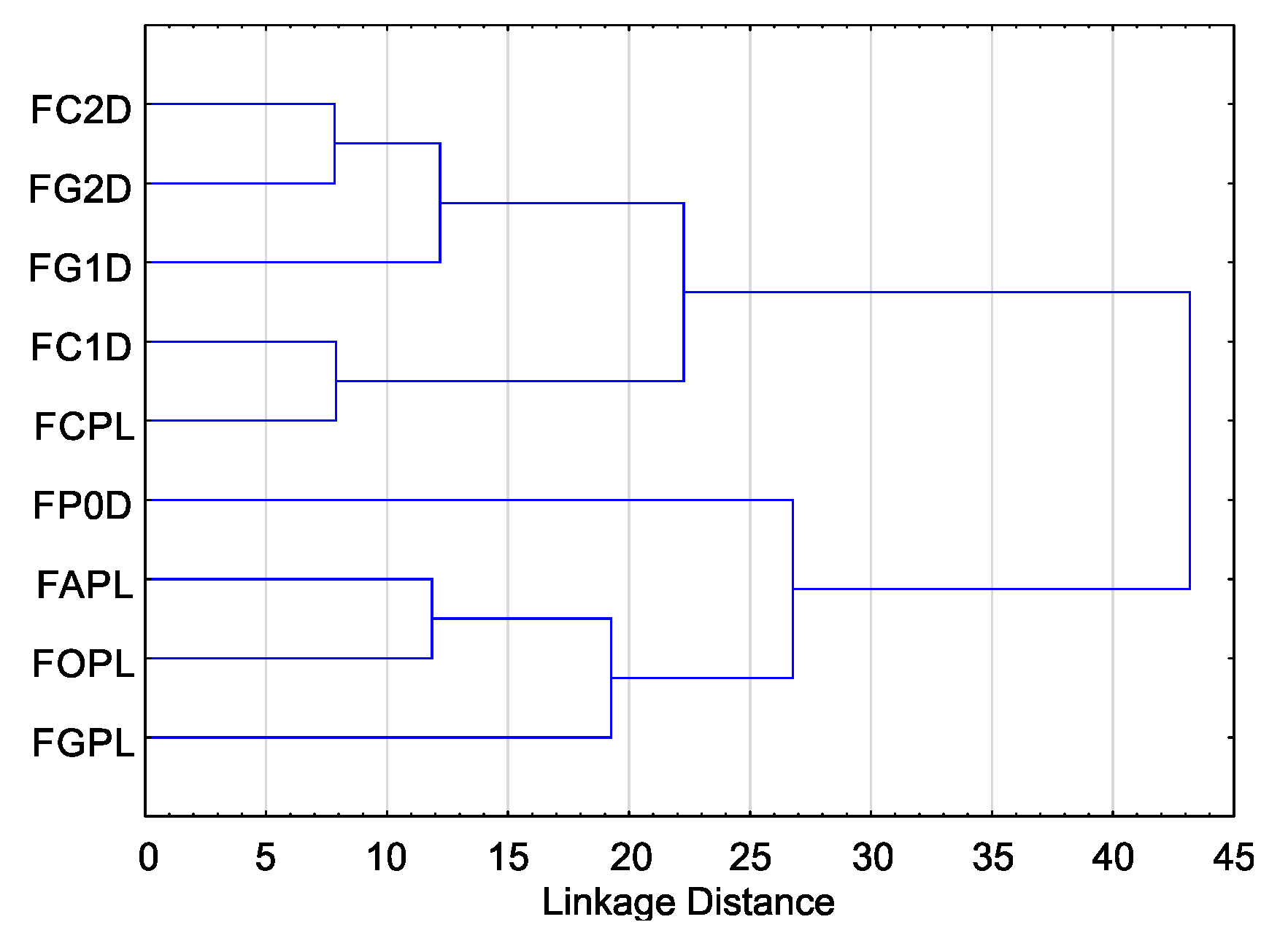
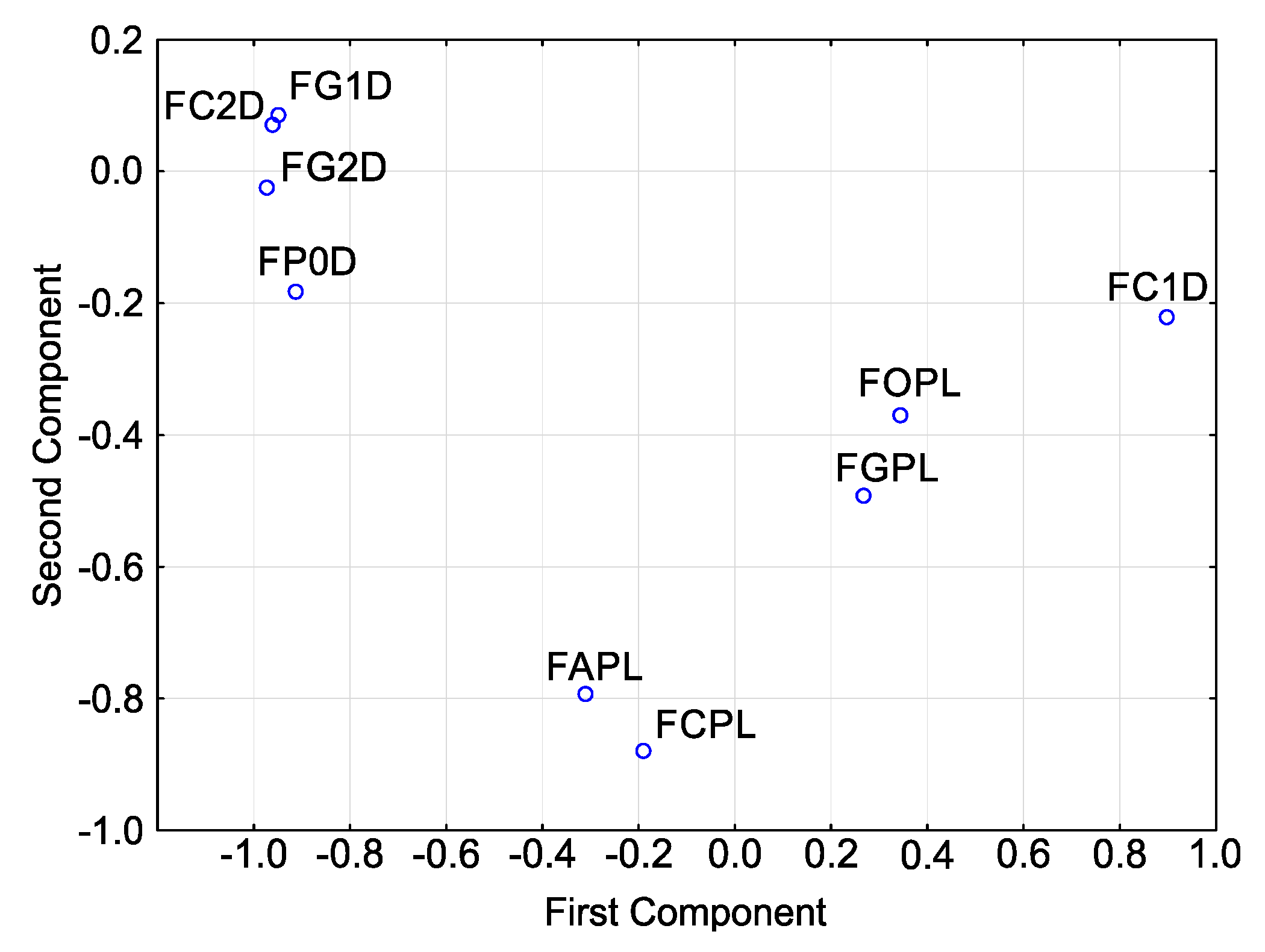

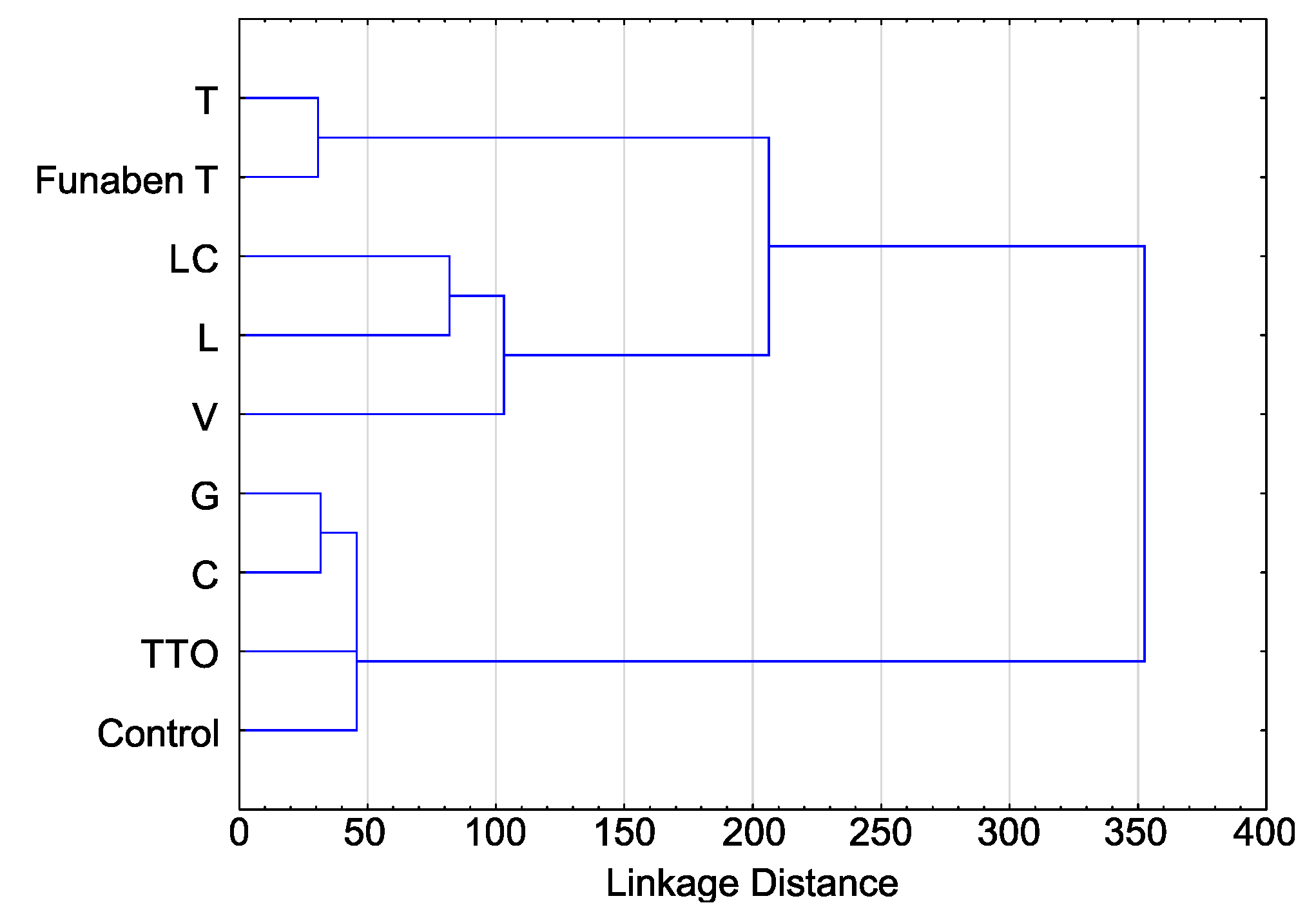
| Isolates | H (8, 212) | p |
|---|---|---|
| FAPL | 124.68 | 0.00 * |
| FCPL | 123.34 | 0.00 * |
| FC1D | 145.2 | 0.00 * |
| FC2D | 114.44 | 0.00 * |
| FGPL | 131.64 | 0.00 * |
| FG1D | 120.18 | 0.00 * |
| FG2D | 115.97 | 0.00 * |
| FOPL | 111.1 | 0.00 * |
| FP0D | 111.5 | 0.00 * |
| Oil | Isolate | N | Mean | SD | Isolate | N | Mean | SD | Isolate | N | Mean | SD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grapefruit | 28 | 44.57 | 6.72 | 28 | 43.13 | 8.98 | 28 | 46.87 | 8.04 | |||
| Cajeput | 28 | 22.52 | 22.82 | 28 | 17.00 | 20.65 | 27 | 15.75 | 16.13 | |||
| Lemongrass | 28 | 0.75 | 2.37 | 28 | 0.00 | 0.00 | 28 | 1.06 | 2.68 | |||
| Litsea cubeba | 28 | 1.48 | 4.00 | 28 | 0.00 | 0.00 | 28 | 1.71 | 5.53 | |||
| Thyme | FC2D | 28 | 0.00 | 0.00 | FC1D | 28 | 0.00 | 0.00 | FGPL | 29 | 0.00 | 0.00 |
| Tea tree | 28 | 16.17 | 21.85 | 28 | 13.31 | 18.69 | 28 | 10.97 | 15.52 | |||
| Verbena | 28 | 3.62 | 6.66 | 28 | 0.98 | 2.49 | 28 | 4.04 | 8.55 | |||
| Control | 12 | 49.18 | 0.13 | 12 | 50.02 | 0.11 | 12 | 49.86 | 0.19 | |||
| Funaben T | 4 | 0.00 | 0.00 | 4 | 0.00 | 0.00 | 4 | 0.00 | 0.00 | |||
| Grapefruit | 28 | 40.51 | 11.98 | 27 | 38.19 | 11.65 | 28 | 45.81 | 2.4 | |||
| Cajeput | 28 | 20.36 | 20.88 | 28 | 15.06 | 17.71 | 27 | 21.06 | 18.65 | |||
| Lemongrass | 28 | 2.44 | 6.10 | 28 | 0.76 | 1.89 | 28 | 4.65 | 8.55 | |||
| Litsea cubeba | 28 | 2.42 | 6.04 | 28 | 0.68 | 1.70 | 28 | 4.66 | 8.54 | |||
| Thyme | FG2D | 28 | 0.00 | 0.00 | FCPL | 27 | 0.00 | 0.00 | FAPL | 28 | 0.00 | 0.00 |
| Tea tree | 28 | 15.76 | 22.04 | 28 | 11.32 | 16.57 | 28 | 17.12 | 19.38 | |||
| Verbena | 28 | 3.73 | 9.31 | 28 | 1.79 | 3.00 | 28 | 6.8 | 11.19 | |||
| Control | 12 | 49.15 | 0.40 | 12 | 49.83 | 0.33 | 12 | 48.03 | 0.09 | |||
| Funaben T | 4 | 0.00 | 0.00 | 4 | 00.00 | 0.00 | 4 | 00.00 | 0.00 | |||
| Grapefruit | 28 | 42.59 | 13.22 | 28 | 40.69 | 10.43 | 28 | 43.52 | 6.34 | |||
| cajeput | 28 | 21.09 | 22.36 | 28 | 19.58 | 19.22 | 28 | 19.75 | 18.75 | |||
| Lemongrass | 28 | 2.96 | 8.08 | 28 | 4.5 | 11.22 | 28 | 5.09 | 9.06 | |||
| Litsea cubeba | 28 | 1.10 | 2.74 | 28 | 5.36 | 13.36 | 28 | 4.71 | 8.07 | |||
| Thyme | FG1D | 28 | 0.00 | 0.00 | FP0D | 28 | 0.00 | 0.00 | FOPL | 28 | 1.77 | 2.94 |
| Tea tree | 28 | 16.3 | 22.5 | 28 | 16.79 | 21.84 | 28 | 13.9 | 17.36 | |||
| Verbena | 28 | 1.90 | 3.10 | 28 | 6.02 | 11.09 | 28 | 3.1 | 5.39 | |||
| Control | 12 | 51.72 | 0.08 | 12 | 49.93 | 0.07 | 12 | 46.39 | 3.41 | |||
| Funaben T | 4 | 0.00 | 0.00 | 4 | 0.00 | 0.00 | 4 | 0.00 | 0.00 |
| Monoterpenes | Monoterpenoids | Sesquiterpenes | Sesquiterpenoids | Other Chemical Compounds |
|---|---|---|---|---|
| 0.41 | −0.64 | −0.03 | 0.47 | 0.66 |
| b1 | SE of b1 | b | SE of b | t (1754) | p | |
|---|---|---|---|---|---|---|
| Intercept | 736.24 | 73.69 | 9.99 | 0.00 * | ||
| Monoterpenes | −5.42 | 0.56 | −6.87 | 0.71 | −9.62 | 0.00 * |
| Monoterpenoids | −8.49 | 0.85 | −7.55 | 0.76 | −9.94 | 0.00 * |
| Sesquiterpenes | −1.46 | 0.13 | −6.65 | 0.59 | −11.09 | 0.00 * |
| Sesquiterpenoids | 0.02 | 0.06 | 0.45 | 1.19 | 0.38 | 0.70 |
| Other chemical compounds | −4.24 | 0.45 | −7.08 | 0.76 | −9.32 | 0.00 * |
| Concentration | −0.21 | 0.016 | −6.21 | 0.46 | −13.40 | 0.00 * |
| Compound | RI | Etheric Oils | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Lit * | Cal * | T | L | LC | V | TTO | C | G | |
| Monoterpenes | |||||||||
| Tricyclene | 923 | 920 | 0.17 ± 0.01 | 0.44 ± 0.08 | 0 | 0 | |||
| α-thujene | 928 | 928 | 0.44 ± 0.05 | 0.83 ± 0.07 | |||||
| α-pinene | 936 | 933 | 2.75 ± 0.09 | 0.49 ± 0.11 | 2.86 ± 0.16 | 0 | 3.42 ± 0.06 | 5.37 ± 0.01 | 3.27 ± 0.01 |
| Camphene | 950 | 947 | 1.93 ± 0.07 | 3.71 ± 0.06 | 0.58 ± 0.04 | 0.80 ± 0.02 | 0 | ||
| β-pinene | 978 | 974 | 0.65 ± 0.02 | 0 | 3.95 ± 0.08 | 1.08 ± 0.05 | 0.81 ± 0.02 | 3.93 ± 0.15 | |
| β-myrcene | 989 | 991 | 2.44 ± 0.03 | 0.38 ± 0.11 | 3.01 ± 0.07 | 5.32 ± 0.01 | |||
| α-phellandrene | 1004 | 1002 | 0.87 ± 0.03 | 0.05 ± 0.02 | 0.15 ± 0.08 | ||||
| Sabinene (4,10-thujene) | 1004 | 1009 | 0.27 ± 0.04 | 0.17 ± 0.03 | 1.56 ± 0.03 | ||||
| 3-carene | 1011 | 1005 | 17.04 ± 0.15 | ||||||
| α-terpinene | 1017 | 1018 | 2.32 ± 0.10 | 10.29 ± 0.09 | |||||
| p-cymene | 1024 | 1020 | 3.62 ± 0.03 | ||||||
| Limonene | 1029 | 1026 | 15.15 ± 0.18 | 20.94 ± 0.13 | 34.63 ± 0.73 | ||||
| γ-terpinene | 1060 | 1061 | 8.10 ± 0.07 | 2.02 ± 0.07 | 0.37 ± 0.04 | 0.78 ± 0.01 | |||
| Terpinolene | 1087 | 1087 | 0.45 ± 0.01 | 3.87 ± 0.05 | 0.27 ± 0.02 | 0.08 ± 0.01 | |||
| β-patchulene | 1457 | 1455 | 0.16 ± 0.04 | ||||||
| Sum monoterpenes | 34.38 | 4.64 | 28.33 | 8.73 | 37.12 | 12.95 | 45.64 | ||
| Monoterpenoids | |||||||||
| α and β citral (geranial and neral) | - | - | 68.94 ± 0.10 | 61.72 ± 0.43 | 36.00 ± 0.08 | ||||
| Trifluorolavandulol | 1999 | 2.19 ± 0.07 | |||||||
| Eucalyptol | 1031 | 1027 | 13.46 ± 0.17 | 13.90 ± 0.15 | 18.50 ± 0.05 | ||||
| Linalool oxide | 1065 | 1064 | 0.12 ± 0.03 | ||||||
| Linalool | 1099 | 1105 | 8.90 ± 0.18 | 5.73 ± 0.22 | 2.58 ± 0.04 | 8.53 ± 0.01 | 11.19 ± 0.17 | 4.83 ± 0.039 | |
| 1-terpineol | 1137 | 1135 | 1.19 ± 0.05 | 1.39 ± 0.06 | 0.47 ± 0.01 | 0.87 ± 0.10 | |||
| p-menth-3-en-9-ol | 1141 | 1140 | 0.71 ± 0.02 | ||||||
| Camphor | 1143 | 1141 | 4.62 ± 0.03 | ||||||
| Verbenol | 1145 | 1145 | 0.18 ± 0.014 | ||||||
| β-citronellal | 1154 | 1152 | 1.87 ± 0.13 | 0.42 ± 0.0.03 | |||||
| Borneol | 1166 | 1168 | 3.07 ± 0.09 | 2.93 ± 0.07 | 1.32 ± 0.02 | ||||
| 1-terpinen-4-ol | 1177 | 1181 | 4.51 ± 0.05 | 38.24 ± 0.38 | 4.41 ± 0.35 | 0.09 ± 0.003 | |||
| α-terpineol | 1190 | 1197 | 1.14 ± 0.09 | 1.02 ± 0.06 | 18.26 ± 150 | 6.88 ± 0.04 | 36.57 ± 0.21 | 1.83 ± 0.048 | |
| α-pinene oxide | 1197 | 1195 | 0.51 ± 0.029 | ||||||
| cis-geraniol | 1238 | 1234 | 0.55 ± 0.046 | ||||||
| β citral (neral) | 1242 | 1231 | 0.92 ± 0.058 | ||||||
| trans-geraniol | 1255 | 1252 | 0.45 ± 0.04 | ||||||
| Linalyl acetate | 1255 | 1260 | 0.93 ± 0.06 | 1.87 ± 0.028 | |||||
| Geranial | 1270 | 1269 | 1.36 ± 0.022 | ||||||
| Thymol | 1290 | 1298 | 45.75 ± 0.18 | ||||||
| α-terpinyl acetate | 1347 | 0.23 ± 0.021 | |||||||
| Nerol acetate | 1363 | 1366 | 1.68 ± 0.01 | ||||||
| Geraniol acetate | 1380 | 1385 | 2.26 ± 0.045 | ||||||
| Sum momoterpenoids | 60.98 | 79.99 | 68.58 | 87.18 | 59.02 | 71.66 | 17.69 | ||
| Sesquiterpenes | |||||||||
| α-cubebene | 1351 | 1350 | 0.52 ± 0.04 | 0.37 ± 0.004 | |||||
| α-longipinene | 1352 | 1350 | 0.67 ± 0.08 | ||||||
| ylangene | 1370 | 1370 | 0.51 ± 0.01 | ||||||
| β-cubebene | 1387 | 1390 | 0.49 ± 0.028 | ||||||
| β-elemene | 1388 | 1387 | 0.14 ± 0.05 | ||||||
| Longifolene | 1407 | 1408 | 1.12 ± 0.02 | ||||||
| α-gurjunene | 1409 | 1410 | 0.23 ± 0.02 | 1.19 ± 0.09 | |||||
| caryophyllene | 1419 | 1423 | 4.31 ± 0.02 | 3.76 ± 0.012 | 2.45 ± 0.018 | 0.55 ± 0.06 | 0.28 ± 0.003 | 2.60 ± 0.19 | 0.98 ± 0.061 |
| α-caryophyllene | 1420 | 1408 | 0.33 ± 0.03 | 0.45 ± 0.01 | 0.20 ± 0.004 | 1.70 ± 0.03 | 0.14 ± 0.014 | ||
| β-gurjunene | 1431 | 1430 | 1.15 ± 0.05 | 1.23 ± 0.05 | 0.57 ± 0.03 | ||||
| (+)aromadendrene | 1441 | 1440 | 0.94 ± 0.10 | ||||||
| γ-elemene | 1449 | 1445 | 0.05 ± 0.01 | ||||||
| Allo-aromadendrene | 1460 | 1458 | 0.23 ± 0.03 | 3.63 ± 0.06 | |||||
| γ-muurolene | 1476 | 1478 | 0.12 ± 0.06 | ||||||
| Germacene D | 1481 | 1496 | 0 | 0.18 ± 0.01 | |||||
| (+)-valencene | 1491 | 1499 | 0 | 0.14 ± 0.09 | |||||
| β-selinene | 1493 | 1490 | 1.62 ± 0.03 | ||||||
| γ-cadinene | 1513 | 1517 | 4.83 ± 0.10 | ||||||
| σ-cadinene | 1523 | 1526 | 0.83 ± 0.04 | 0.48 ± 0.009 | |||||
| Cadinene | 1533 | 1530 | 0.37 ± 0.05 | ||||||
| Sum sesquiterpenes | 4.64 | 10.86 | 2.65 | 1.67 | 3.86 | 12.90 | 2.83 | ||
| Sesquiterpenoids | |||||||||
| trans-nerolidol | 1524 | 1522 | 0.02 ± 0.006 | ||||||
| elemol | 1536 | 1540 | 0.05 ± 0.01 | ||||||
| Caryophyllene oxide | 1581 | 1572 | 1.14 ± 0.03 | 0.44 ± 0.05 | 0.42 ± 0.08 | 0.23 ± 0.003 | |||
| Guaiol | 1589 | 1590 | 0.55 ± 0.05 | ||||||
| Eudesmol | 1616 | 1611 | 1.52 ± 0.03 | ||||||
| Farnesol | 1722 | 1718 | 0.05 ± 0.011 | ||||||
| Nootkatone | 1813 | 1818 | 1.37 ± 0.069 | ||||||
| Farnesyl acetate | 1818 | 1820 | 0.03 ± 0.002 | ||||||
| Sum sesquiterpenoids | 1.14 | 0.44 | 2.49 | 1.75 | |||||
| Sum other chemical compounds | 3.37 | 26.81 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzyśko-Łupicka, T.; Sokół, S.; Sporek, M.; Piekarska-Stachowiak, A.; Walkowiak-Lubczyk, W.; Sudoł, A. Effectiveness of the Influence of Selected Essential Oils on the Growth of Parasitic Fusarium Isolated from Wheat Kernels from Central Europe. Molecules 2021, 26, 6488. https://doi.org/10.3390/molecules26216488
Krzyśko-Łupicka T, Sokół S, Sporek M, Piekarska-Stachowiak A, Walkowiak-Lubczyk W, Sudoł A. Effectiveness of the Influence of Selected Essential Oils on the Growth of Parasitic Fusarium Isolated from Wheat Kernels from Central Europe. Molecules. 2021; 26(21):6488. https://doi.org/10.3390/molecules26216488
Chicago/Turabian StyleKrzyśko-Łupicka, Teresa, Sławomir Sokół, Monika Sporek, Anna Piekarska-Stachowiak, Weronika Walkowiak-Lubczyk, and Adam Sudoł. 2021. "Effectiveness of the Influence of Selected Essential Oils on the Growth of Parasitic Fusarium Isolated from Wheat Kernels from Central Europe" Molecules 26, no. 21: 6488. https://doi.org/10.3390/molecules26216488
APA StyleKrzyśko-Łupicka, T., Sokół, S., Sporek, M., Piekarska-Stachowiak, A., Walkowiak-Lubczyk, W., & Sudoł, A. (2021). Effectiveness of the Influence of Selected Essential Oils on the Growth of Parasitic Fusarium Isolated from Wheat Kernels from Central Europe. Molecules, 26(21), 6488. https://doi.org/10.3390/molecules26216488






