The Effect of Nitrogen Fertilization on Yield and Macronutrient Concentrations in Three Cultivars of Jerusalem Artichoke (Helianthus tuberosus L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Characteristics
2.2. Cultivars
2.3. Experimental Design
2.4. Harvest
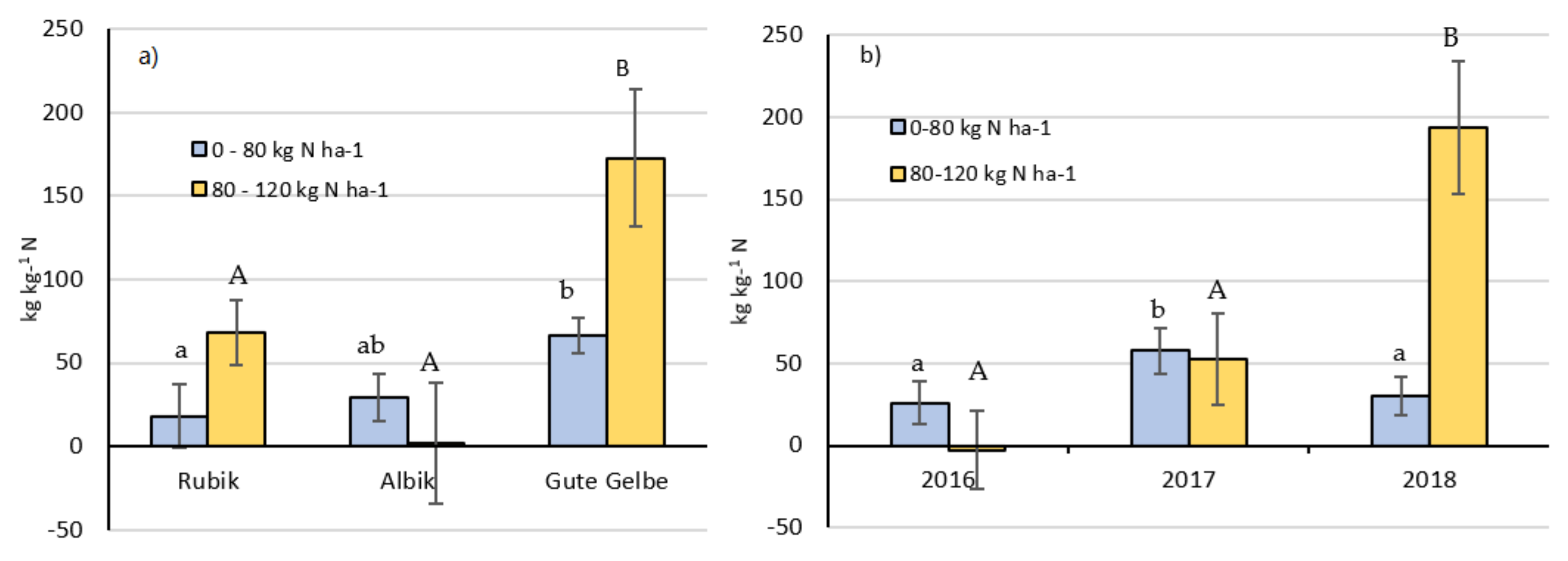
2.5. Nitrogen-Use Efficiency
- Agronomic N-use efficiency (AE, net productivity):
- 2.
- Marginal N-use efficiency (ME):
2.6. Chemical Analysis Methods
2.7. Weather Conditions
2.8. Statistical Analysis
3. Results
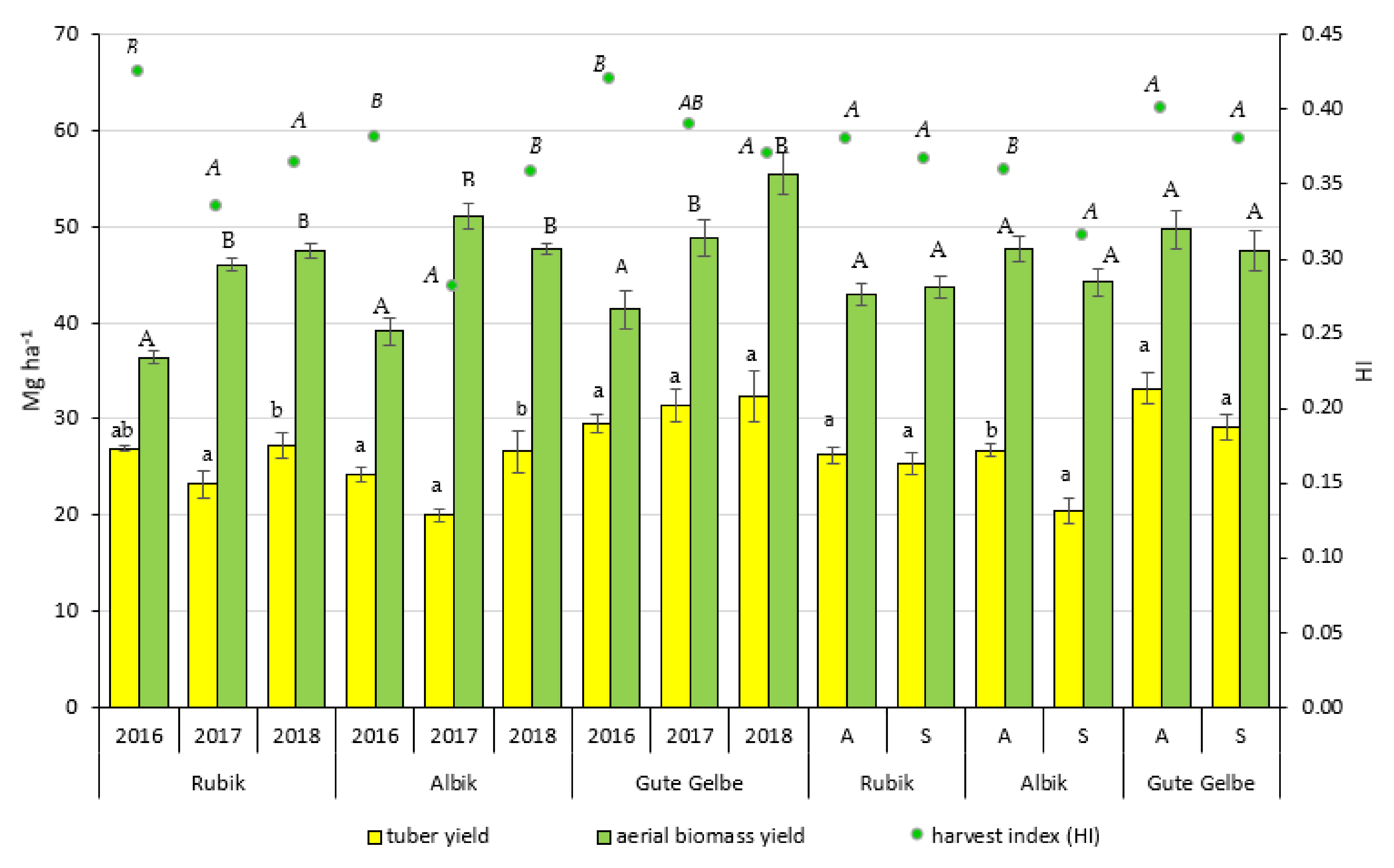
3.1. Effect of N Fertilization on Yield
3.2. Effect of N Fertilization on Agronomic N-Indices
3.3. Effect of N Fertilization on the Dry Matter Weight and Macronutrients and Na Concentration
4. Discussion
4.1. Effect of N Fertilization on Yield
4.2. Effect of N Fertilization on Agronomic N-Indices
4.3. Effect of N Fertilization on the Dry Matter Weight and Macronutrients and Na Concentration
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kays, S.J.; Nottingham, S.F. Biology and Chemistry of Jerusalem Artichoke: Helianthus tuberosus L.; CRC Press/Taylor & Francis Group: London, UK, 2008; ISBN 9781420044959. [Google Scholar]
- Breton, C.; Kiru, S.D.; Berville, A.; Anushkevich, N.Y. Breeding of Jerusalem Artichoke with the desired traits for different directions of use: Retrospective, approaches and prospects. Agric. Biol. 2017, 52, 940–951. [Google Scholar] [CrossRef][Green Version]
- Rejestrupraw.arimr.gov.pl. Available online: https://rejestrupraw.arimr.gov.pl/# (accessed on 14 July 2021). (In Polish)
- Bolibok, Ł. Energy value of the straw of selected Jerusalem artichoke cultivars. Episterme 2011, 12, 15–20. (In Polish) [Google Scholar]
- Sawicka, B. Energy value Jerusalem artichoke (Helianthus tuberosus L.) as a source of biomass. Zesz. Nauk. UP Wrocław. Rol. 2010, 97, 245–256. (In Polish) [Google Scholar]
- Liava, V.; Karkanis, A.; Danalatos, N.; Tsiropoulos, N. Cultivation Practices, Adaptability and Phytochemical Composition of Jerusalem Artichoke (Helianthus tuberosus L.): A Weed with Economic Value. Agronomy 2021, 11, 914. [Google Scholar] [CrossRef]
- Zelenkov, N.; Romanova, N.G. Jerusalem Artichoke: An Agrobiological Portrait and Prospects for Innovative Using. Available online: https://www.topinambour.ru/information/170914195656.html (accessed on 14 October 2019).
- Yang, L.; He, Q.S.; Corscadden, K.; Udenigwe, C.C. The prospects of Jerusalem artichoke in functional food ingredients and bioenergy production. Biotechnol. Rep. 2015, 5, 77–88. [Google Scholar] [CrossRef]
- Antonkiewicz, J.; Kołodziej, B.; Bielińska, E.J.; Witkowicz, R.; Tabor, S. Using Jerusalem Artichoke to Extract Heavy Metals from Municipal Sewage Sludge Amended Soil. Pol. J. Environ. Stud. 2018, 27, 513–527. [Google Scholar] [CrossRef]
- Zhang, F.; Tai, F.N.; Brestic, M. Jerusalem artichoke (Helianthus tuberosus), a medicinal salt-resistant plant has high adaptability and multiple-use values. J. Med. Plants Res. 2011, 5, 1272–1279. [Google Scholar]
- Rossini, F.; Provenzano, M.E.; Kuzmanovíc, L.; Ruggeri, R. Jerusalem Artichoke (Helianthus tuberosus L.): A Versatile and Sustainable Crop for Renewable Energy Production in Europe. Agronomy 2019, 9, 528. [Google Scholar] [CrossRef]
- Gao, K.; Zhang, Z.; Zhu, T.; Coulter, J.A. The influence of flower removal on tuber yield and biomass characteristics of Helianthus tuberosus L. in a semi-arid area. Ind. Crops Prod. 2020, 150, 112374. [Google Scholar] [CrossRef]
- Fang, Y.R.; Liu, J.A.; Steiberger, Y.; Xie, G.H. Energy use efficiency and economic feasybility of Jerusalem artichoke production on arid and coastal saline lands. Ind. Crops Prod. 2018, 117, 131–139. [Google Scholar] [CrossRef]
- Izsáki, Z.; Kádi, G.N. Biomass acumulation and nutrient uptake of Jerusalem artichoke (Helianthus tuberosus L.). Am. J. Plant Sci. 2013, 4, 1629–1640. [Google Scholar] [CrossRef]
- Bogucka, B.; Pszczółkowska, A.; Okorski, A.; Jankowski, K. The Effects of Potassium Fertilization and Irrigation on the Yield and Health Status of Jerusalem Artichoke (Helianthus tuberosus L.). Agronomy 2021, 11, 234. [Google Scholar] [CrossRef]
- Soja, G.; Haunold, E. Leaf gas exchange and tuber yield in Jerusalem artichoke (Helianthus tuberosus L.) Cultivars. Field Crops Res. 1991, 26, 241–252. [Google Scholar] [CrossRef]
- Amtmann, A.; Armengaud, P. Effects of N, P, K and S on metabolism: New knowledge gained from multi-level analysis. Curr. Opin. Plant Biol. 2009, 12, 275–283. [Google Scholar] [CrossRef] [PubMed]
- He, C.H.; Wang, H.X.; Liu, W.B.; Deng, G.X. The study on neonatal pig anaemia treatment with Helianthus tuberosus. China Anim. Husb. Vet. Med. 2010, 37, 194–195. [Google Scholar]
- EL-Anany, A.M.A.; Anany, T.G. Effect of some mineral nutrients on productivity, tuber seed quality and storability of Jerusalem artichoke. Middle East J. Agric. Res. 2020, 9, 779–790. [Google Scholar] [CrossRef]
- Swanton, C.J.; Clements, D.R.; Moore, M.J.; Cavers, P.B. The biology of Canadian weeds. 101. Helianthus tuberosus L. Can. J. Plant Sci. 1992, 72, 1367–1382. [Google Scholar] [CrossRef]
- Florkiewicz, A.; Cieślik, E.; Filipiak-Florkiewicz, A. The cultivar and harvesting time influence on the chemical composition in tubers of Jeruzalem artichoke (Helianthus tuberosus L.). Żywność. Nauka. Technol. Jakość. 2007, 3, 71–81. (In Polish) [Google Scholar]
- Černiauskienė, J.; Kulaitienė, J.; Jarienė, E.; Danilčenko, H.; Žaldarienė, S.; Jeznach, M. Relationship between harvesting time and carbohydrate content of Jerusalem artichoke (Helianthus tuberosus L.) tubers. Acta Sci. Pol. Hortorum Cultus 2018, 17, 41–48. [Google Scholar] [CrossRef]
- Bogucka, B.; Jankowski, K. Jerusalem Artichoke: Quality Response to Potassium Fertilization and Irrigation in Poland. Agronomy 2020, 10, 1518. [Google Scholar] [CrossRef]
- Saengthongpinit, W.; Sajjaanantakul, T. Influence of harvest time and storage temperature on characteristics of inulin from Jerusalem artichoke (Helianthus tuberosus L.) tubers. Postharvest Biol. Technol. 2005, 37, 93–100. [Google Scholar] [CrossRef]
- Danilčenko, H.; Jariené, E.; Gajewski, M.; Sawicka, B.; Kulaitiene, J.; Cerniauskiene, J. Changes in amino acids content in tubers of jerusalem artichoke (Helianthus tuberosus L.) cultivars during storage. Acta Sci. Pol. Hortorum Cultus 2013, 12, 97–105. [Google Scholar]
- Skiba, D.; Sawicka, B. The influence of the genetic properties and the content of selected minerals in tubers of Helianthus tuberosus L. Herbalism 2016, 1, 128–137. (In Polish) [Google Scholar] [CrossRef]
- Góral, S. Jerusalem artichoke—Helianthus tuberosus. In New Crops for Food, Industrial and as Sources Renewable Energy; Warsaw University of Life Sciences Press: Warsaw, Poland, 1996; pp. 76–86. (In Polish) [Google Scholar]
- Žaldarienė, S.; Kulaitienė, J.; Černiauskienė, J. The quality comparison of different Jerusalem artichoke (Helianthus tuberosus L.) cultivars tubers. Žemės Ūkio Mokslai 2012, 19, 268–272. [Google Scholar] [CrossRef][Green Version]
- Harmankaya, M.; Al Juhaimi, F.; Özcan, M.M. Mineral Contents of Jerusalem Artichoke (Helianthus tuberosus L.) Growing Wild in Turkey. Anal. Lett. 2012, 45, 2269–2275. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports 106; FAO: Roma, Italy, 2015; p. 188. [Google Scholar]
- Polish Standard PN-ISO 10390. Soil Quality—Determination of Soil pH; Polish Committee for Standardization: Warsaw, Poland, 1997; p. 15. (In Polish) [Google Scholar]
- Polish Standard PN-R-04023. Chemical analysis of agricultural soils. In Determination of Available Phosphorus Content in Mineral Soils; Polish Committee for Standardization: Warsaw, Poland, 1996; p. 12. (In Polish)
- Polish Standard PN-R-04022(1996)+Az1:2002. Chemical analysis of agricultural soils. In Determination of Available Potassium Content in Mineral Soils; Polish Committee for Standardization: Warsaw, Poland, 1996; p. 4. (In Polish)
- Polish Standard PN-R-04020:1994+Az1:2004. Chemical analysis of agricultural soils. In Determination of Available Magnesium Content in Mineral Soils; Polish Committee for Standardization: Warsaw, Poland, 1994; p. 4. (In Polish)
- Navarre, R.; Pavek, M. The Potato: Botany, Production and Uses; CABI: Boston, MA, USA, 2014; p. 370. [Google Scholar]
- Dobermann, A. Nitrogen Use Efficiency: Measurement and Management. In Fertilizer Best Management Practices; Krauss, A., Isherwood, K., Heffer, P., Eds.; IFA: Paris, France, 2007; pp. 1–28. [Google Scholar]
- Ostrowska, A.; Gawliński, S.; Szczubiałka, Z. Methods of Analysis and Assessment of Soil and Plant Properties; Institute of Environmental Protection: Warsaw, Poland, 1991; pp. 334–340. (In Polish) [Google Scholar]
- Khan, A.H. Improvement of Total Sulphur Measurement Techniques for Management of Reactive Mine Tailings. Master’s Thesis, The University of Western Ontario, London, ON, Canada, 2012; p. 855. [Google Scholar]
- Statsoft Inc. Statistica (Data Analysis Software System), 10th ed.; Statsoft Inc.: Tulsa, OK, USA, 2011; Available online: http:/www.statsoft.com (accessed on 1 April 2020).
- Skiba, D.; Sawicka, B. Effect of mineral fertilization on the yield and shape of tubers of Helianthus tuberosus. Agron. Sci. 2017, 72, 155–166. (In Polish) [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Sousa, L.; Cabanas, J.E.; Arrobas, M. Tuber yield and leaf mineral composition of Jerusalem artichoke (Helianthus tuberosus L.) Grown under different cropping practices. Span. J. Agric. Res. 2007, 5, 545–553. [Google Scholar] [CrossRef][Green Version]
- Baldini, M.; Danuso, F.; Monti, A.; Amaducci, M.T.; Stevanato, P.; Mastro, G. Chichory and Jerusalem artichoke productivity in different areas of Italy, in relation to water availability and time of harvest. Ital. J. Agron. Riv. Agron. 2006, 2, 291–307. [Google Scholar] [CrossRef]
- Losavio, N.; Lamascese, N.; Vonella, A.V. Water requirements and nitrogen fertilization in Jerusalem artichoke (Helianthus tuberosus l.) grown under mediterranean conditions. Acta Hort. 1997, 449, 205–209. [Google Scholar] [CrossRef]
- Schittenhelm, S. Agronomic performance of root chicory, Jerusalem artichoke and sugarbeet in stress and non-stress environment. Crop Sci. 1999, 39, 1815–1823. [Google Scholar] [CrossRef]
- Sun, X.E.; Meng, X.F.; Liu, Z.P.; Long, X.H. Effects of nitrogen and phosphorus interaction on the tuber yield and its quality of Jerusalem artichoke. Chin. J. Ecol. 2013, 32, 363–367. [Google Scholar]
- El-Araby, S.M. Effect of biofertilization under nitrogen fertilization rates on growth, yield and chemical constituents of jerusalem artichoke tubers. Adv. Agric. Res. 2004, 9, 55–68. [Google Scholar]
- Stolarski, M.; Krzyzaniak, M.; Warmiński, K.; Tworkowski, J.; Szczukowski, S. Perennial herbaceous crops as a feedstock for energy and industrial purposes: Organic and mineral fertilizers versus biomass yield and efficient nitrogen utilization. Ind. Crops Prod. 2017, 107, 244–259. [Google Scholar] [CrossRef]
- Gao, K.; Zhu, T.; Wang, Q. Nitrogen fertilization, irrigation, and harvest times affect biomass and energy value of Helianthus tuberosus L. J. Plant Nutr. 2016, 39, 1906–1914. [Google Scholar] [CrossRef]
- Prośba-Białczyk, U. Productivity of jerusalem artichoke (Helianthus tuberosus L.) cultivated without fertilization). Fragm. Agron. 2007, 24, 106–112. (In Polish) [Google Scholar]
- Szpunar-Krok, E.; Bobrecka-Jamro, D.; Grochowska, S.; Buczek, J. Yield of the aboveground parts and tubers of Jerusalem artichoke (Helianthus tuberosus L.) depending on plant density. Acta Sci. Pol. Agric. 2016, 15, 69–78. [Google Scholar]
- Long, X.H.; Mehta, S.K.; Liu, Z.P. Effect of NO3− -N enrichment on seawater stress tolerance of Jerusalem artichoke (Helianthus tuberosus). Pedosphere 2008, 18, 113–123. [Google Scholar] [CrossRef]
- Pimsaen, W.; Jogloy, S.; Suriharn, B.; Kesmala, T.; Pensuk, V.; Patanothai, A. Genotype by Environment (GxE) Interactions for Yield Components of Jerusalem Artichoke (Helianthus tuberosus L.). Asian J. Plant Sci. 2010, 9, 11–19. [Google Scholar] [CrossRef][Green Version]
- Matias, J.; Gonzales, J.; Cabanillas, J.; Royano, L. Influence of NPK fertilisation and harvest date on agronomic performance of Jerusalem artichoke crop in the Guadiana Basin (Southwestern Spain). Ind. Crops Prod. 2013, 48, 191–197. [Google Scholar] [CrossRef]
- Cui, Z.L.; Zhang, F.S.; Chen, X.P.; Miao, Y.X.; Li, J.L.; Shi, L.W.; Xu, J.F.; Ye, Y.L.; Liu, C.S.; Yang, Z.P.; et al. On-farm estimation of indigenous nutrient supply for site-specific nitrogen management in the North China plain. Nutr. Cycl. Agroecosys. 2008, 81, 37–47. [Google Scholar] [CrossRef]
- Grzebisz, W.; Čermák, P.; Rroco, E.; Szczepaniak, W.; Potarzycki, J.; Füleky, G. Potassium impact on nitrogen use efficiency in potato—A case study from the Central-East Europe. Plant Soil Environ. 2017, 63, 422–427. [Google Scholar] [CrossRef]
- Awgchew, H.; Gebremedhin, H.; Taddesse, G.; Alemu, D. Influence of Nitrogen Rate on Nitrogen use Efficiency and Quality of Potato (Solanum tuberosum L.) Varieties at Debre Berhan, Central Highlands of Ethiopia. Int. J. Soil Sci. 2017, 12, 10–17. [Google Scholar] [CrossRef]
- Niemiec, M.; Szeląg-Sikora, A.; Cupiał, M. Evaluation of the Efficiency of Celeriac Fertilization with the Use of Slow-acting Fertilizers. Agric. Agric. Sci. Procedia 2015, 7, 177–183. [Google Scholar] [CrossRef]
- Biel, W.; Jaroszewska, A.; Zapałowska, A.; Łysoń, E.; Hury, G. Influence of fertilisation with ash from conifers biomass and sewage sludge on selected nutrients in tubers of Jerusalem artichoke (Helianthus tuberosus L.). Acta Agroph. 2017, 24, 17–28. (In Polish) [Google Scholar]
- Barta, J.; Patkai, G. Chemical composition and storability of Jerusalem artichoke tubers. Acta Aliment. Hung. 2007, 36, 257–267. [Google Scholar] [CrossRef]
- Danilčenko, H.; Jariene, E.; Slepetiene, A.; Sawicka, B.; Zaldariene, S. The distribution of bioactive compounds in the tubers of organically grown Jerusalem artichoke (Helianthus tuberosus L.) during the growing period. Acta Sci. Pol. Hortorum Cultus 2017, 16, 97–107. [Google Scholar] [CrossRef][Green Version]
- Bach, V.; Kidmose, U.; Bjørn, G.K.; Edelenbos, M. Effects of harvest time and variety on sensory quality and chemical composition of Jerusalem artichoke (Helianthus tuberosus) tubers. Food Chem. 2012, 133, 82–89. [Google Scholar] [CrossRef]
- Praznik, W.; Cieślik, E.; Filipiak, A. The influence of harvest time on the content of nutritional components in tubers of Jerusalem artichoke (Helianthus tuberosus L.). In Proceedings of the Seventh Seminar on Inulin, Louvain, Belgium, 22–23 January 1998; pp. 154–157. [Google Scholar]
- Catană, L.; Catană, M.; Iorga, E.; Lazăr, A.G.; Lazăr, M.A.; Teodorescu, R.I.; Asănică, A.C.; Belc, N.; Iancu, A. Valorification of Jerusalem Artichoke Tubers (Helianthus tuberosus) for Achieving of Functional Ingredient with High Nutritional Value. “Agric. Life Life Agric.” Conf. Proc. 2018, 1, 276–283. [Google Scholar] [CrossRef][Green Version]
- Terzić, S.; Atlagić, J. Nitrogen and sugar content variability in tubers of Jerusalem artichoke (Helianthus tuberosus). Genetika 2009, 41, 289–295. [Google Scholar] [CrossRef]
- Feleafel, M.N. Effect of ntirogen and potassium fertilization and their interactions on growth, yield and quality of Jerusalem artichoke. J. Agric. Environ. Sci. Alex. Univ. Egypt 2004, 3, 59–74. [Google Scholar]
- Seiler, G.J. Protein and mineral concentrations in tubers of selected genotypes of wild and cultivated Jerusalem artichoke (Helianthus tuberosus, Asteraceae). Econ. Bot. 1990, 44, 322–335. [Google Scholar] [CrossRef]
- Cieślik, E.; Gębusia, A.; Florkiewicz, A.; Mickowska, B. The content of protein and of amino acids in Jerusalem artichoke tubers (Helianthus tuberosus L.) of red variety Rote Zonenkugel. Acta Sci. Pol. Technol. Aliment. 2011, 10, 433–441. [Google Scholar] [PubMed]
- Sawicka, B.; Kalembasa, S. Fluctuation of Protein Nitrogen Level in Tubers of Helianthus tuberosus L. Caused by Varying Levels of Nitrogen Fertilisation. Ecol. Chem. Eng. A 2013, 20, 213–223. [Google Scholar] [CrossRef]
- De Santis, D.; Frangipane, M.T. Evaluation of chemical composition and sensory profile in Jerusalem artichoke (Helianthus tuberosus L.) tubers: The effect of clones and cooking conditions. Int. J. Gastro. Food Sci. 2018, 11, 25–30. [Google Scholar] [CrossRef]
- Cieślik, E. The content of minerals in tubers of new varieties of Jerusalem artichoke (Helianthus tuberosus L.). Zesz. Nauk. AR Krak. 1998, 10, 23–30. (In Polish) [Google Scholar]
- Barta, J.; Fodor, P.; Torok, S.Z.; Vukov, K. Mineral components and micro-elements in Jerusalem artichoke tubers grow in Hungary. Acta Aliment. Hung. 1990, 19, 41–46. [Google Scholar]
- Ekholm, P.H.; Reinivuo, P.; Mattila, H.; Pakkala, J.; Koponen, A.; Happonen, J.; Hellström Ovaskainen, M.L. Changes in the mineral and trace element contents of cereals, fruits and vegetables in Finland. J. Food Comp. Anal. 2007, 20, 487–495. [Google Scholar] [CrossRef]
- Afoakwah, N.A.; Dong, Y.; Zhao, Y.; Xiong, Z.; Owusu, J.; Wang, Y.; Zhang, J. Characterization of Jerusalem artichoke (Helianthus tuberosus L.) powder and its application in emulsion-type sausage. LWT—Food Sci. Technol. 2015, 64, 74–81. [Google Scholar] [CrossRef]
- Danilčenko, H.; Jariené, E.; Gajewski, M.; Cerniauskiene, J.; Kulaitiene, J.; Sawicka, B.; Aleknaviciene, P. Accumulation of elements in some organically grown alternative horticultural crops in Lithuania. Acta Sci. Pol. Hortorum Cultus 2011, 10, 23–31. [Google Scholar]
- Sawicka, B.; Danilčenko, H.; Jariene, E.; Skiba, D.; Rachoń, L.; Barbaś, P.; Pszczółkowski, P. Nutritional Value of Jerusalem Artichoke Tubers (Helianthus tuberosus L.) Grown in Organic System under Lithuanian and Polish Conditions. Agriculture 2021, 11, 440. [Google Scholar] [CrossRef]
- Jarienė, E.; Jeznach, M.; Danilčenko, H.; Žaldarienė, S.; Tarasevičienė, Ž.; Wawrzyniak, A.; Tul-Krzyszczuk, A. Distribution of macronutrients in organically grown Jerusalem artichoke (Helianthus tuberosus L.) tubers throughout the growing period. J. Element. 2016, 21, 1315–1325. [Google Scholar] [CrossRef]
- Sawicka, B.; Kalembasa, D.; Skiba, D. Variability in macroelement content in the aboveground part of Helianthus tuberosus L. at different nitrogen fertilization levels. Plant Soil Environ. 2015, 61, 158–163. [Google Scholar] [CrossRef]
- Fu, T.; Liu, Z.; Yang, Y.; Xie, G.H. Accumulation and concentration of nitrogen, phosphorus and potassium in Jerusalem artichoke in a semi-arid region. Ital. J. Agron. 2018, 13, 185–193. [Google Scholar] [CrossRef]


| Year | Date of Fertilizer Application | Planting Date | Harvest Date | ||
|---|---|---|---|---|---|
| Aerial Biomass | Tubers (Autumn) | Tubers (Spring) | |||
| 2016 | 25.04 | 25.04 | 07.11 | 08.11 | 14.03.2017 |
| 2017 | 19.04 | 20.04 | 20.10 | 08.11 | 27.03.2018 |
| 2018 | 19.04 | 20.04 | 12.10 | 16.10 | 25.03.2019 |
| Month | Average Monthly Temperatures (T) °C | Monthly Precipitation (P) mm | ||||||
|---|---|---|---|---|---|---|---|---|
| 2016/17 | 2017/18 | 2018/19 | 1981–2010 | 2016/17 | 2017/18 | 2018/19 | 1981–2010 | |
| April | 7.4 | 5.7 | 10.8 | 7.7 | 28.8 | 59.1 | 33.5 | 33.3 |
| May | 13.7 | 12.1 | 15.7 | 13.5 | 56.9 | 25.1 | 25.0 | 58.5 |
| June | 17.1 | 15.7 | 17.2 | 16.1 | 69.3 | 74.5 | 53.7 | 80.4 |
| July | 18.1 | 16.8 | 19.7 | 18.7 | 130.4 | 107.6 | 141.0 | 74.2 |
| August | 17.1 | 17.4 | 19.2 | 17.9 | 70.4 | 63.1 | 44.6 | 59.4 |
| September | 13.6 | 12.8 | 14.5 | 12.8 | 21.1 | 168.1 | 20.3 | 56.9 |
| October | 6.1 | 8.7 | 8.7 | 8.0 | 104.3 | 114.9 | 84.7 | 42.6 |
| November | 2.4 | 3.9 | 3.3 | 2.9 | 84.5 | 42.4 | 16.0 | 44.8 |
| December | 0.8 | 1.8 | 0.9 | −0.9 | 41.1 | 35.2 | 58.8 | 38.2 |
| January | −3.4 | −0.4 | −2.5 | −2.4 | 20.2 | 41.5 | 43.5 | 36.4 |
| February | −1.4 | −4.6 | 1.8 | −1.7 | 47.6 | 3.1 | 31.5 | 24.2 |
| March | 4.0 | −1.3 | 3.9 | 1.8 | 45.3 | 10.4 | 47.2 | 32.9 |
| Average/Total | 8.1 | 7.4 | 9.4 | 7.9 | 721.2 | 745.0 | 599.8 | 581.8 |
| Source of Variation | df | Tuber Yield | Aerial Biomass Yield | HI | |||
|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | ||
| Year (Y) | 2 | 83.8 | <0.0001 | 190 | <0.0001 | 577 | <0.0001 |
| Error 1 | 6 | ||||||
| Harvest date (HD) | 1 | 311 | <0.0001 | 21.0 | 0.0038 | 362 | <0.0001 |
| Y × HD | 2 | 605 | <0.0001 | 0.24 | 0.7944 | 1099 | <0.0001 |
| Error 2 | 6 | ||||||
| N fertilizer rate (NR) | 2 | 422 | <0.0001 | 122 | <0.0001 | 118 | <0.0001 |
| Y × NR | 4 | 83.3 | <0.0001 | 46.5 | <0.0001 | 115 | <0.0001 |
| HD × NR | 2 | 43.6 | <0.0001 | 60.2 | <0.0001 | 4.87 | 0.0168 |
| Y × HD × NR | 4 | 13.7 | <0.0001 | 3.80 | 0.0157 | 20.4 | <0.0001 |
| Error 3 | 24 | ||||||
| Cultivar © | 2 | 793 | <0.0001 | 120 | <0.0001 | 370 | <0.0001 |
| Y × C | 4 | 62.3 | <0.0001 | 37.5 | <0.0001 | 133 | <0.0001 |
| HD × C | 2 | 98.3 | <0.0001 | 20.4 | <0.0001 | 29.1 | <0.0001 |
| NR × C | 4 | 125 | <0.0001 | 116 | <0.0001 | 6.41 | <0.0001 |
| Y × HD × C | 4 | 58.8 | <0.0001 | 29.8 | <0.0001 | 68.4 | <0.0001 |
| Y × NR × C | 8 | 21.6 | <0.0001 | 27.9 | <0.0001 | 32.4 | <0.0001 |
| HD × NR × C | 4 | 62.4 | <0.0001 | 14.6 | <0.0001 | 36.6 | <0.0001 |
| Y × HD × NR × C | 8 | 40.9 | <0.0001 | 18.5 | <0.0001 | 16.6 | <0.0001 |
| Error 4 | 72 | ||||||
| Total | 161 | ||||||
| Year | Cultivar | N Fertilizer Rate [kg ha−1] | Tuber Yield | Aerial Biomass Yield | HI * |
|---|---|---|---|---|---|
| Mg ha−1 | |||||
| 2016 | Rubik | Control | 26.45 ± 0.50 a−d | 34.41 ± 0.79 ab | 0.43 ± 0.01 fg |
| 80 | 27.70 ± 0.59 a−d | 35.00 ± 0.83 a−c | 0.44 ± 0.02 g | ||
| 120 | 26.53 ± 0.35 a−d | 39.81 ± 0.81 a−f | 0.40 ± 0.01 d−g | ||
| Albik | Control | 24.07 ± 1.56 a−c | 43.19 ± 3.60 b−g | 0.36 ± 0.02 a−g | |
| 80 | 25.70 ± 1.43 a−d | 38.93 ± 0.90 a−e | 0.40 ± 0.03 d−g | ||
| 120 | 22.59 ± 0.63 a−c | 35.19 ± 1.29 a−c | 0.39 ± 0.01 c−g | ||
| Gute Gelbe | Control | 25.99 ± 1.89 a−d | 32.85 ± 0.90 a | 0.44 ± 0,04 g | |
| 80 | 29.36 ± 0.76 a−e | 39.74 ± 0.47 a−f | 0.42 ± 0.02 fg | ||
| 120 | 33.32 ± 0.22 c−e | 51.63 ± 2.02 g−j | 0.39 ± 0.02 c−g | ||
| 2017 | Rubik | Control | 19.21 ± 0.51 a | 43.41 ± 0.89 c−g | 0.31 ± 0.02 a−d |
| 80 | 23.48 ± 2.61 a−c | 48.78 ± 1.04 g−j | 0.32 ± 0.05 a−e | ||
| 120 | 26.91 ± 2.64 a−d | 46.04 ± 0.80 d−I | 0.37 ± 0.05 b−g | ||
| Albik | Control | 19.80 ± 1.51 ab | 52.85 ± 2.93 h−j | 0.27 ± 0.01 a | |
| 80 | 21.77 ± 0.57 a−c | 53.48 ± 1.66 h−j | 0.29 ± 0.02 a−c | ||
| 120 | 18.31 ± 0.46 a | 47.22 ± 1.24 e−j | 0.28 ± 0.02 ab | ||
| Gute Gelbe | Control | 24.28 ± 2.14 a−c | 38.15 ± 0.55 a−d | 0.39 ± 0.06 c−g | |
| 80 | 31.92 ± 1.36 b−e | 53.85 ± 1.35 h−j | 0.37 ± 0.03 b−g | ||
| 120 | 38.23 ± 1.75 de | 54.63 ± 0.61 i−k | 0.41 ± 0.03 e−g | ||
| 2018 | Rubik | Control | 26.04 ± 3.02 a−d | 45.87 ± 0.87 d−I | 0.36 ± 0.07 a−g |
| 80 | 24.92 ± 1.87 a−c | 45.32 ± 0.69 d−h | 0.35 ± 0.05 a−f | ||
| 120 | 30.85 ± 1.64 a−e | 51.45 ± 0.53 g−j | 0.37 ± 0.03 b−g | ||
| Albik | Control | 22.03 ± 2.67 a−c | 45.50 ± 0.73 d−h | 0.33 ± 0.07 a−e | |
| 80 | 25.46 ± 3.03 a−c | 48.30 ± 0.83 f−j | 0.35 ± 0.06 a−f | ||
| 120 | 32.28 ± 4.84 b−e | 49.18 ± 0.49 g−j | 0.40 ± 0.10 d−g | ||
| Gute Gelbe | Control | 25.61 ± 3.85 a−c | 55.28 ± 4.69 jk | 0.32 ± 0,03 a−e | |
| 80 | 30.54 ± 5.54 a−e | 48.66 ± 1.43 g−j | 0.39 ± 0.10 c−g | ||
| 120 | 40.99 ± 1.05 e | 62.76 ± 0.96 k | 0.40 ± 0.02 d−g | ||
| Average for year | 2016 | 26.86 ± 0.51 ab | 38.97 ± 0.89 a | 0.41 ± 0.00 b | |
| 2017 | 24.88 ± 1.00 a | 48.71 ± 0.84 b | 0.33 ± 0.01 a | ||
| 2018 | 28.75 ± 1.29 b | 50.26 ± 0.91 b | 0.36 ± 0.01 a | ||
| Factor | Dry Matter (DM) | N | P | K | Ca | Mg | S | Na | ||
|---|---|---|---|---|---|---|---|---|---|---|
| % | g kg−1 DM | |||||||||
| N fertilizer rate [kg ha−1] (n = 54) | Control | 27.38 ± 0.47 b* | 11.81 ± 0.19 a | 4.05 ± 0.08 a | 15.56 ± 0.43 a | 0.59 ± 0.02 a | 0.55 ± 0.01 a | 1.05 ± 0.03 a | 0.46 ± 0.01 a | |
| 80 | 27.33 ± 0.45 b | 12.35 ± 0.14 ab | 3.96 ± 0.10 a | 15.37 ± 0.35 a | 0.58 ± 0.03 a | 0.56 ± 0.01 a | 1.12 ± 0.03 a | 0.46 ± 0.01 a | ||
| 120 | 24.76 ± 0.62 a | 12.67 ± 0.16 b | 4.08 ± 0.08 a | 15.60 ± 0.42 a | 0.57 ± 0.03 a | 0.57 ± 0.01 a | 1.12 ± 0.03 a | 0.50 ± 0.01 a | ||
| Cultivar (n = 54) | Rubik | 27.38 ± 0.68 a | 12.29 ± 0.19 ab | 3.99 ± 0.08 a | 14.57 ± 0.31 a | 0.56 ± 0.02 a | 0.55 ± 0.01 b | 1.05 ± 0.02 a | 0.51 ± 0.01 b | |
| Albik | 26.14 ± 0.49 a | 11.85 ± 0.18 a | 3.94 ± 0.10 a | 14.99 ± 0.35 a | 0.61 ± 0.02 a | 0.52 ± 0.01 a | 0.98 ± 0.01 a | 0.44 ± 0.02 a | ||
| Gute Gelbe | 24.76 ± 0.62 a | 12.69 ± 0.14 b | 4.15 ± 0.07 a | 16.98 ± 0.45 b | 0.58 ± 0,02 a | 0.60 ± 0.01 c | 1.26 ± 0.03 b | 0.46 ± 0.02 a | ||
| Year (n = 54) | 2016 | 26.91 ± 0.71 a | 12.40 ± 0.20 a | 4.43 ± 0.08 b | 13.81 ± 0.12 a | 0.71 ± 0.01 b | 0.57 ± 0.01 a | 1.11 ± 0.02 a | 0.37 ± 0.01 a | |
| 2017 | 25.70 ± 0.36 a | 12.24 ± 0.15 a | 3.81 ± 0.07 a | 16.36 ± 0.44 b | 0.51 ± 0.02 a | 0.55 ± 0.01 a | 1.10 ± 0.03 a | 0.52 ± 0.01 b | ||
| 2018 | 26.87 ± 0.64 a | 12.19 ± 0.16 a | 3.85 ± 0.08 a | 16.37 ± 0.44 b | 0.51 ± 0.02 a | 0.55 ± 0.01 a | 1.08 ± 0.03 a | 0.52 ± 0.01 b | ||
| Harvest date (n = 81) | Autumn | 27.95 ± 0.40 b | 11.79 ± 0.16 a | 3.88 ± 0.07 a | 14.32 ± 0.20 a | 0.46 ± 0.02 a | 0.55 ± 0.01 a | 1.00 ± 0.02 a | 0.47 ± 0.01 a | |
| Spring | 25.03 ± 0.40 a | 12.76 ± 0.09 b | 4.17 ± 0.06 b | 16.70 ± 0.37 b | 0.70 ± 0.01 b | 0.56 ± 0.01 a | 1.19 ± 0.02 b | 0.47 ± 0.01 a | ||
| Factor | N | P | K | Ca | Mg | Na | S | |
|---|---|---|---|---|---|---|---|---|
| kg ha−1 | ||||||||
| N fertilizer rate [kg ha−1] (n = 54) | Control | 77.66 ± 2.47 a* | 24.80 ± 1.75 a | 101.33 ± 4.69 a | 3.86 ± 0.19 a | 3.37 ± 0.12 a | 3.00 ± 0.13 a | 7.02 ± 0.26 a |
| 80 | 86.49 ± 3.14 ab | 30.49 ± 2.14 a | 102.49 ± 4.08 a | 3.94 ± 0.18 a | 3.97 ± 0.15 b | 3.34 ± 0.15 b | 7.73 ± 0.36 ab | |
| 120 | 95.87 ± 3.91 b | 39.15 ± 3.04 b | 126.70 ± 6.06 b | 4.34 ± 0.26 a | 4.55 ± 0.20 c | 3.62 ± 0.19 c | 8.49 ± 0.39 c | |
| Cultivar (n = 54) | Rubik | 86.43 ± 2.69 ab | 27.59 ± 1.46 a | 103.08 ± 3.91 a | 3.95 ± 0.21 a | 3.93 ± 0.13 b | 3.57 ± 0.14 b | 7.41 ± 0.27 b |
| Albik | 76.40 ± 3.19 a | 24.13 ± 2.23 a | 97.36 ± 4.91 a | 3.95 ± 0.24 a | 3.31 ± 0.13 a | 2.86 ± 0.16 a | 6.29 ± 0.26 a | |
| Gute Gelbe | 97.19 ± 3.56 b | 42.71 ± 2.84 b | 130.08 ± 5.67 b | 4.24 ± 0.19 a | 4.64 ± 0.19 c | 3.52 ± 0.15 b | 9.52 ± 0.33 c | |
| Year (n = 54) | 2016 | 87.29 ± 1.83 a | 32.10 ± 1.16 ab | 97.39 ± 1.75 a | 5.03 ± 0.11 b | 4.02 ± 0.08 a | 2.64 ± 0.11 a | 7.85 ± 0.21 a |
| 2017 | 81.26 ± 3.33 a | 26.30 ± 2.37 a | 108.50 ± 4.99 ab | 3.31 ± 0.18 a | 3.71 ± 0.19 a | 3.42 ± 0.13 a | 7.33 ± 0.38 a | |
| 2018 | 91.47 ± 4.34 a | 36.03 ± 3.32 b | 124.63 ± 6.93 b | 3.80 ± 0.26 a | 4.15 ± 0.21 a | 3.89 ± 0.18 a | 8.06 ± 0.40 a | |
| Harvest date (n = 81) | Autumn | 94.51 ± 2.76 b | 31.10 ± 1.78 a | 114.79 ± 3.87 b | 3.69 ± 0.16 a | 4.41 ± 0.15 b | 3.73 ± 0.12 b | 8.02 ± 0.27 a |
| Spring | 79.65 ± 2.58 a | 26.03 ± 2.15 a | 104.25 ± 4.21 a | 4.37 ± 0.17 b | 3.50 ± 0.12 a | 2.93 ± 0.14 a | 7.43 ± 0.27 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wierzbowska, J.; Cwalina-Ambroziak, B.; Bogucka, B. The Effect of Nitrogen Fertilization on Yield and Macronutrient Concentrations in Three Cultivars of Jerusalem Artichoke (Helianthus tuberosus L.). Agronomy 2021, 11, 2161. https://doi.org/10.3390/agronomy11112161
Wierzbowska J, Cwalina-Ambroziak B, Bogucka B. The Effect of Nitrogen Fertilization on Yield and Macronutrient Concentrations in Three Cultivars of Jerusalem Artichoke (Helianthus tuberosus L.). Agronomy. 2021; 11(11):2161. https://doi.org/10.3390/agronomy11112161
Chicago/Turabian StyleWierzbowska, Jadwiga, Bożena Cwalina-Ambroziak, and Bożena Bogucka. 2021. "The Effect of Nitrogen Fertilization on Yield and Macronutrient Concentrations in Three Cultivars of Jerusalem Artichoke (Helianthus tuberosus L.)" Agronomy 11, no. 11: 2161. https://doi.org/10.3390/agronomy11112161
APA StyleWierzbowska, J., Cwalina-Ambroziak, B., & Bogucka, B. (2021). The Effect of Nitrogen Fertilization on Yield and Macronutrient Concentrations in Three Cultivars of Jerusalem Artichoke (Helianthus tuberosus L.). Agronomy, 11(11), 2161. https://doi.org/10.3390/agronomy11112161






