Turbidity and Chemical Oxygen Demand Reduction from Pig Slurry through a Coagulation Flocculation Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Location of the Pig Slurry Farm
2.2. Pig Slurry Sample Collection
2.3. Analytical Methods and Equipment
2.4. Physical–Chemical Characteristics of the Pig Slurry
2.5. Design of Coagulation–Flocculation Assay
2.6. Design of the Experiments and Statistical Analysis
3. Results and Discussion
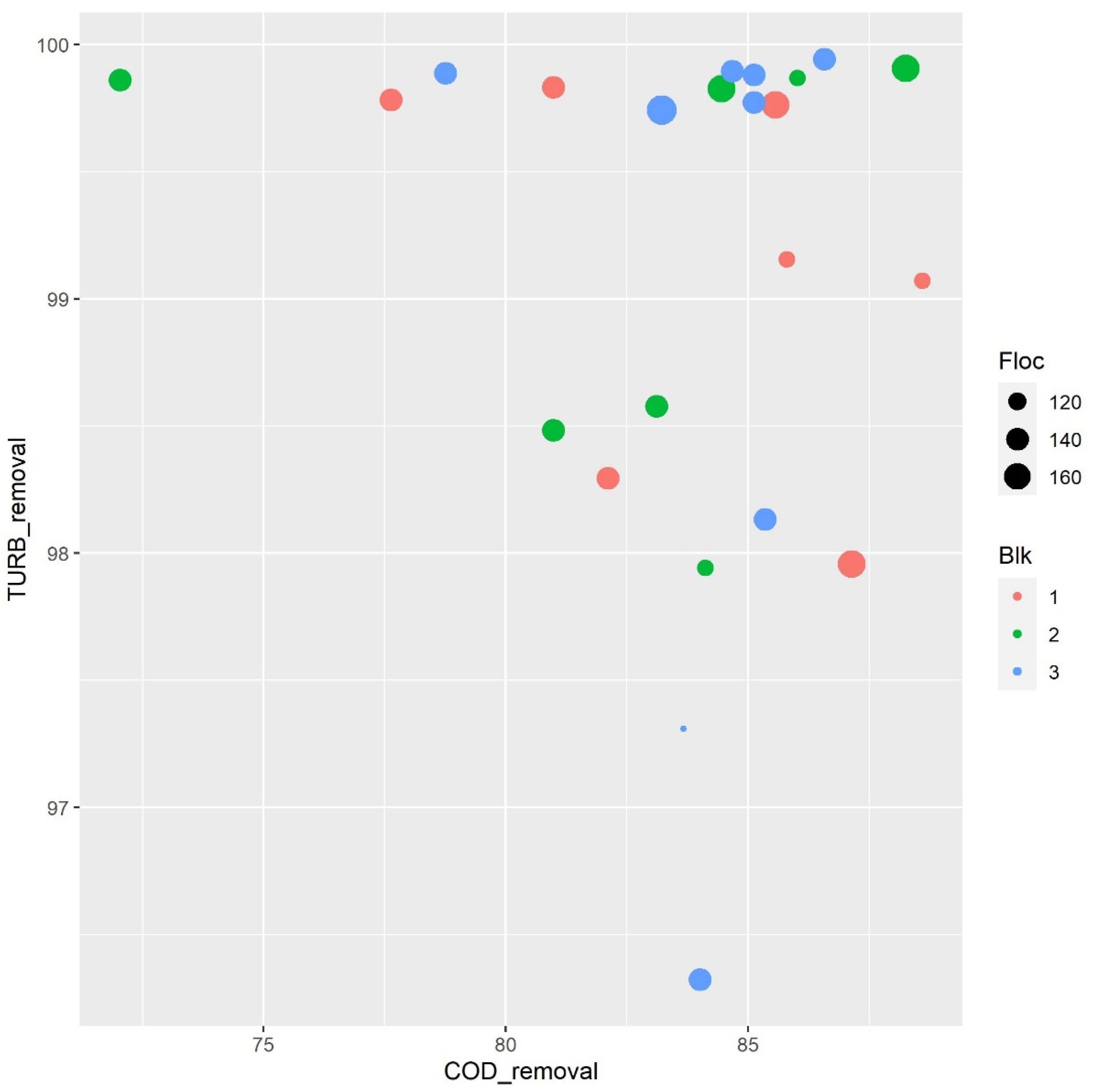
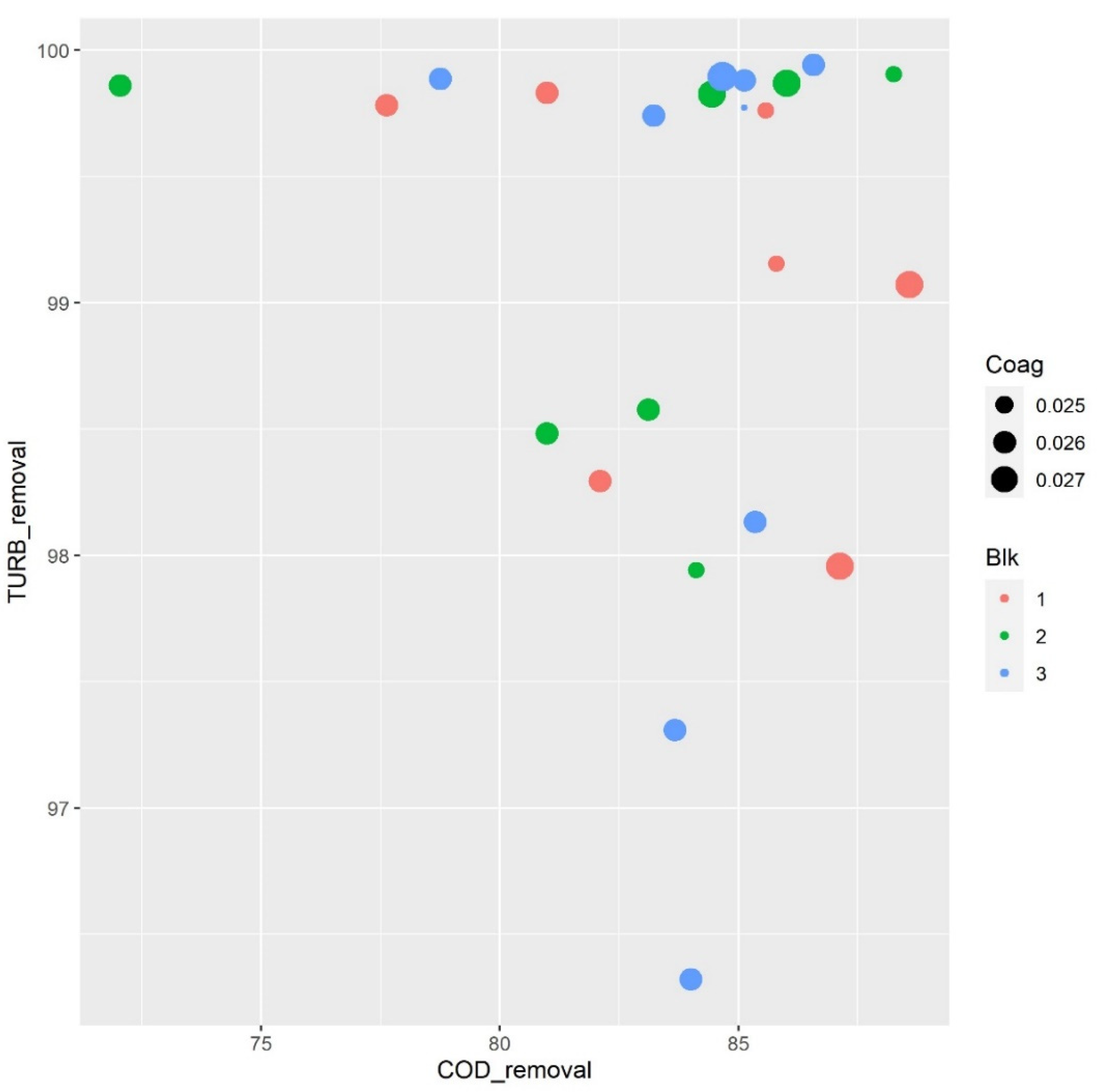
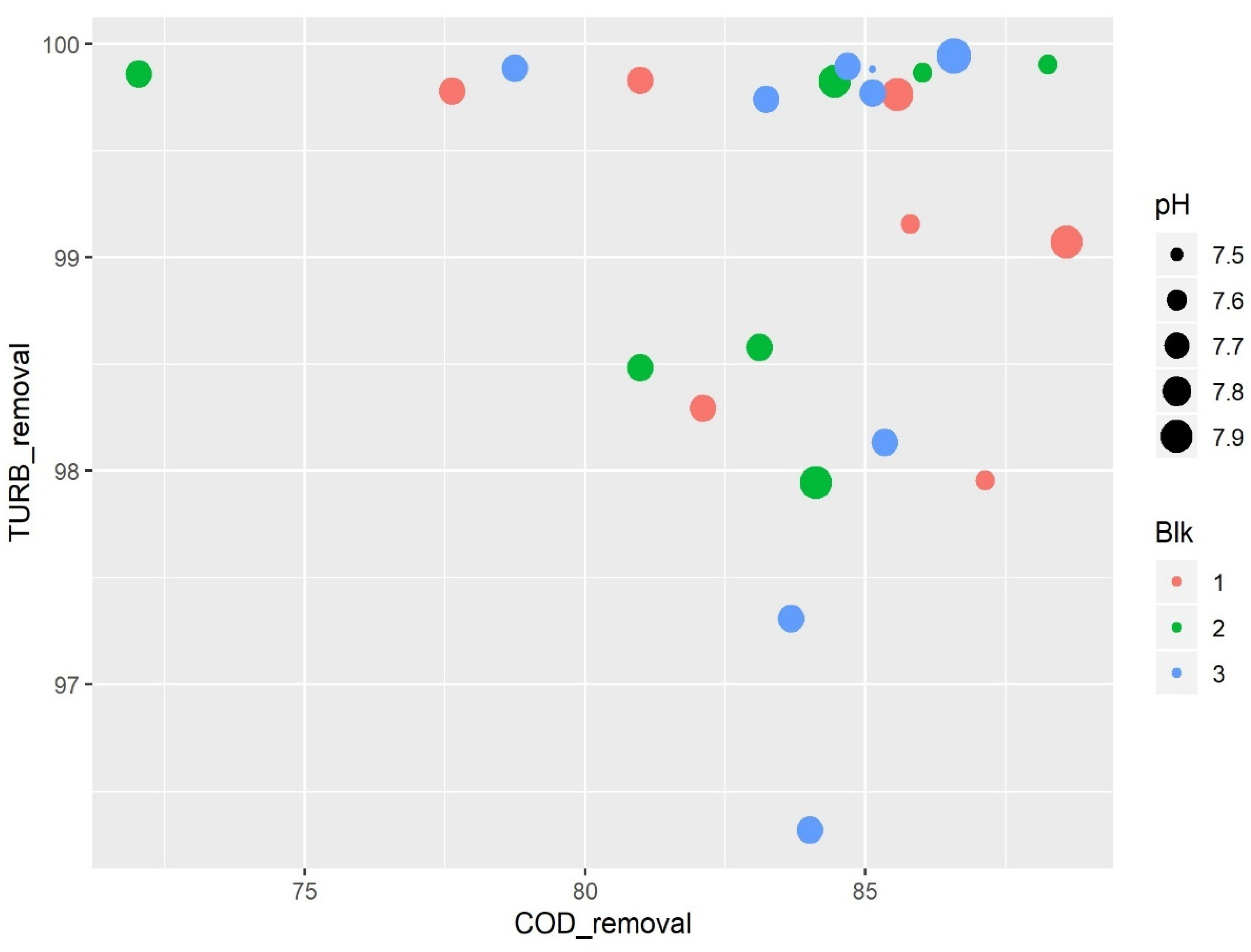
3.1. Coagulation–Flocculation Assay
3.2. Optimal Combination of Coagulant, Flocculent, and pH
| Model Term | Estimate of the Coefficient | p-Value |
|---|---|---|
| Intercept | 81.21 | <2 × 10−16 |
| x1 (Normalized coagulation) | 0.13 | 0.88 |
| x3 (Normalized pH) | −0.16 | 0.85 |
| x12 | 1.93 | 0.03 |
| x32 | 2.30 | 0.01 |
3.2.1. Effect of Flocculent and Coagulant Concentration Parameters on Turbidity and COD Removal
3.2.2. Effects of pH Parameter on Turbidity and COD Removal
3.2.3. Influence of the Two Variables, Coagulant Concentration and pH, on COD Response
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- MAPA. Sector Porcino en España. Available online: https://www.mapa.gob.es/va/ganaderia/temas/produccion-y-mercados-ganaderos/sectores-ganaderos/porcino/ (accessed on 25 June 2021).
- Lopez-Ridaura, S.; van der Werf, H.; Paillat, J.M.; Le Bris, B. Environmental evaluation of transfer and treatment of excess pig slurry by life cycle assessment. J. Environ. Manag. 2009, 90, 1296–1304. [Google Scholar] [CrossRef]
- Krapac, I.G.; Dey, W.S.; Roy, W.R.; Smyth, C.A.; Storment, E.; Sargent, S.L.; Steele, J.D. Impacts of swine manure pits on groundwater quality. Environ. Pollut. 2002, 120, 475–492. [Google Scholar] [CrossRef]
- Hoeve, M.T. Life cycle assessment of pig slurry treatment technologies for nutrient redistribution in Denmark. J. Environ. Manag. 2014, 132, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Lecomte, T.; Ferrería de la Fuente, J.F.; Neuwahl, F.; Canova, M.; Pinasseau, A.; Jankov, I.; Brinkmann Serge Roudier, T.; Delgado Sancho, L. Best Available Techniques (BAT) Reference Document for Large Combustion Plants; Publications Office of the European Union: Luxembourg, 2017; ISBN 9789279743030. [Google Scholar]
- Caselles-Osorio, A.; Puigagut, J.; Segú, E.; Vaello, N.; Granés, F.; García, D.; García, J. Solids accumulation in six full-scale subsurface flow constructed wetlands. Water Res. 2007, 41, 1388–1398. [Google Scholar] [CrossRef]
- Hjorth, M.; Christensen, M.L.; Christensen, P.V. Flocculation, coagulation, and precipitation of manure affecting three separation techniques. Bioresour. Technol. 2008, 99, 8598–8604. [Google Scholar] [CrossRef] [PubMed]
- Knowles, P.; Dotro, G.; Nivala, J.; García, J. Clogging in subsurface-flow treatment wetlands: Occurrence and contributing factors. Ecol. Eng. 2011, 37, 99–112. [Google Scholar] [CrossRef]
- Pedescoll, A.; Corzo, A.; Álvarez, E.; García, J.; Puigagut, J. The effect of primary treatment and flow regime on clogging development in horizontal subsurface flow constructed wetlands: An experimental evaluation. Water Res. 2011, 45, 3579–3589. [Google Scholar] [CrossRef]
- De la Varga, D.; Díaz, M.A.; Ruiz, I.; Soto, M. Avoiding clogging in constructed wetlands by using anaerobic digesters as pre-treatment. Ecol. Eng. 2013, 52, 262–269. [Google Scholar] [CrossRef]
- Zhu, K.; Gamal El-Din, M.; Moawad, A.K.; Bromley, D. Physical and Chemical Processes for Removing Suspended Solids and Phosphorus from Liquid Swine Manure. Environ. Technol. 2004, 25, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Ndegwa, P.; ZhuJun, J.; Luo, Z. Effects of Solid Levels and Chemical Additives on Removal of Solids and Phosphorus in Swine Manure. J. Environ. Eng. 2001, 127, 1111–1115. [Google Scholar] [CrossRef]
- Azimi, S.C.; Shirini, F.; Pendashteh, A. Treatment of wood industry wastewater by combined coagulation–flocculation–decantation and fenton process. Water Environ. Res. 2021, 93, 433–444. [Google Scholar] [CrossRef]
- Dosta, J.; Rovira, J.; Galí, A.; Macé, S.; Mata-Álvarez, J. Integration of a Coagulation/Flocculation step in a biological sequencing batch reactor for COD and nitrogen removal of supernatant of anaerobically digested piggery wastewater. Bioresour. Technol. 2008, 99, 5722–5730. [Google Scholar] [CrossRef] [PubMed]
- Amokrane, A.; Comel, C.; Veron, J. Landfill leachates pretreatment by coagulation-flocculation. Water Res. 1997, 31, 2775–2782. [Google Scholar] [CrossRef]
- Samadi, M.T.; Saghi, M.H.; Rahmani, A.; Hasanvand, J.; Rahimi, S.; Syboney, M.S. Hamadan Landfill Leachate Treatment By Coagulation-Flocculation Process. Iran. J. Environ. Health Sci. Eng. 2010, 7, 253–258. [Google Scholar]
- Christensen, M.L.; Hjorth, M.; Keiding, K. Characterization of pig slurry with reference to flocculation and separation. Water Res. 2009, 43, 773–783. [Google Scholar] [CrossRef]
- Hjorth, M.; Jørgensen, B.U. Polymer flocculation mechanism in animal slurry established by charge neutralization. Water Res. 2012, 46, 1045–1051. [Google Scholar] [CrossRef]
- Liu, Z.; Carroll, Z.S.; Long, S.C.; Gunasekaran, S.; Runge, T. Use of cationic polymers to reduce pathogen levels during dairy manure separation. J. Environ. Manag. 2016, 166, 260–266. [Google Scholar] [CrossRef]
- Hjorth, M.; Christensen, K.V.; Christensen, M.L.; Sommer, S.G. Solid-liquid separation of animal slurry in theory and practice. A review. Agron. Sustain. Dev. 2010, 30, 153–180. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Hung, W.; Chan, G.Y.S. Physico-chemical treatments for removal of recalcitrant contaminants from landfill leachate. J. Hazard. Mater. 2006, 129, 80–100. [Google Scholar] [CrossRef] [PubMed]
- APHA. American Public Health Association. Available online: https://www.apha.org/ (accessed on 25 June 2021).
- Islam, M.M.M.; Shafi, S.; Bandh, S.A.; Shameem, N. Impact of environmental changes and human activities on bacterial diversity of lakes. In Freshwater Microbiology; Perspectives of Bacterial Dynamics in Lake Ecosystems; Academic Press: Cambridge, MA, USA, 2019; pp. 105–136. [Google Scholar] [CrossRef]
- Licciardello, F.; Aiello, R.; Alagna, V.; Iovino, M.; Ventura, D.; Cirelli, G.L. Assessment of clogging in constructed wetlands by saturated hydraulic conductivity measurements. Water Sci. Technol. 2019, 79, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Climate Data. Available online: https://es.climate-data.org/europe/espana/region-de-murcia/murcia-3214/ (accessed on 20 March 2021).
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; APHA: Washington, DC, USA, 2005. [Google Scholar]
- Boursier, H.; Béline, F.; Paul, E. Piggery wastewater characterisation for biological nitrogen removal process design. Bioresour. Technol. 2005, 96, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Antezana, W.; Calvet, S.; Beccaccia, A.; Ferrer, P.; García-Rebollar, P.; Cerisuelo, A. Effects of nutrition on digestion efficiency and gaseous emissions from slurry in growing pigs: III. Influence of varying the dietary level of calcium soap of palm fatty acids distillate with or without orange pulp supplementation. Anim. Feed Sci. 2015, 209, 128–136. [Google Scholar] [CrossRef]
- ProvoloL, G.; Suller, L.M. In situ determination of slurry nutrient content by electrical conductivity. Bioresour. Technol. 2007, 98, 3235–3242. [Google Scholar] [CrossRef] [PubMed]
- Yagüe, M.R.; Bosch-Serra, Á.D.; Antúnez, M.; Boixadera, J. Pig slurry and mineral fertilization strategies’ effects on soil quality: Macroaggregate stability and organic matter fractions. Sci. Total Environ. 2012, 438, 218–224. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, 10th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Sánchez, M.; González, J.L. The fertilizer value of pig slurry. I. Values depending on the type of operation. Bioresour Technol. 2005, 96, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Omotoso, S. Olusegun Influence of NPK 15-15-15 Fertilizer and Pig Manure on Nutrient Dynamics and Production of Cowpea, Vigna Unguiculata L. Walp. Am. J. Agric. For. 2014, 2, 267. [Google Scholar] [CrossRef]
- Terrero, M.A.; Muñoz, M.Á.; Faz, Á.; Gómez-López, M.D.; Acosta, J.A. Efficiency of an integrated purification system for pig slurry treatment under mediterranean climate. Agronomy 2020, 10, 208. [Google Scholar] [CrossRef]
- Kowalski, Z.; Makara, A.; Fijorek, K. Changes in the properties of pig manure slurry. Acta Biochim. Pol. 2013, 60. [Google Scholar] [CrossRef]
- EPA United States Environmental Protection Agency. Available online: https://iaspub.epa.gov/tdb/pages/treatment/treatmentOverview.do?treatmentProcessId=1934681921 (accessed on 5 February 2021).
- Stumm, V.; O’Melia, C. Stoechiometry of coagulation. In Water Works Association; John Wiley & Sons: Hoboken, NJ, USA, 1968; pp. 514–539. [Google Scholar]
- R Core Team. The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 5 February 2021).
- Lenth, R.V. Response-Surface Methods in R, Using rsm. J. Stat. Softw. 2009, 32, 1–17. [Google Scholar] [CrossRef]
- O’Melia, C.R. Review of coagulation process. J. Am. Water Works Assoc. 1969, 219–268. [Google Scholar] [CrossRef]
- Bratby, J. Coagulation and Flocculation in Water and Wastewater Treatment, 2nd ed.; IWA Publishing: London, UK, 2006. [Google Scholar]
- Al-Hamadani, Y.A.J.; Yusoff, M.S.; Umar, M.; Bashir, M.J.K.; Adlan, M.N. Application of psyllium husk as coagulant and coagulant aid in semi-aerobic landfill leachate treatment. J. Hazard. Mater. 2011, 190, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Rasool, M.A.; Tavakoli, B.; Chaibakhsh, N.; Pendashteh, A.R.; Mirroshandel, A.S. Use of a plant-based coagulant in coagulation-ozonation combined treatment of leachate from a waste dumping site. Ecol. Eng. 2016, 90, 431–437. [Google Scholar] [CrossRef]
- Boughou, N.; Majdy, I.; Cherkaoui, E.; Khamar, M.; Nounah, A. Effect of pH and time on the treatment by coagulation from slaughterhouse of the city of Rabat. MATEC Web Conf. 2018, 149, 2–5. [Google Scholar] [CrossRef][Green Version]
- Irfan, M.; Butt, T.; Imtiaz, N.; Abbas, N.; Khan, R.A.; Shafique, A. The removal of COD, TSS and colour of black liquor by coagulation–flocculation process at optimized pH, settling and dosing rate. Arab. J. Chem. 2017, 10, S2307–S2318. [Google Scholar] [CrossRef]
- Tahereh Zarei, M.; Ali Asghar, E.; Hadi, E.; Mehdi, M.; Mohammad Hossein, S.; Mohammad Taghi, G.; Morteza, M.; Mohsen, P. Optimization and economic evaluation of modified coagulation–flocculation process for enhanced treatment of ceramic-tile industry wastewater. AMB Express 2018, 8, 172. [Google Scholar] [CrossRef]
- Tawakkoly, B.; Alizadehdakhel, A.; Dorosti, F. Evaluation of COD and turbidity removal from compost leachate wastewater using Salvia hispanica as a natural coagulant. Ind. Crops Prod. 2019, 137, 323–331. [Google Scholar] [CrossRef]
- Patel, H.; Vashi, R.T. Comparison of naturally prepared coagulants for removal of COD and color from textile wastewater. Glob. Nest J. 2013, 15, 522–528. [Google Scholar] [CrossRef]
- Subramonian, W.; Wu, T.Y. Effect of enhancers and inhibitors on photocatalytic sunlight treatment of methylene blue. Water. Air. Soil Pollut. 2014, 225, 1922. [Google Scholar] [CrossRef]
- Kavanagh, I.; Burchill, W.; Healy, M.G.; Fenton, O.; Krol, D.J.; Lanigan, G.J. Mitigation of ammonia and greenhouse gas emissions from stored cattle slurry using acidifiers and chemical amendments. J. Clean. Prod. 2019, 237, 117822. [Google Scholar] [CrossRef]
- Husted, S.; Husted, S. A simple model of ph in slurry. J. Agric. Sci. 1995, 124, 447–453. [Google Scholar] [CrossRef]
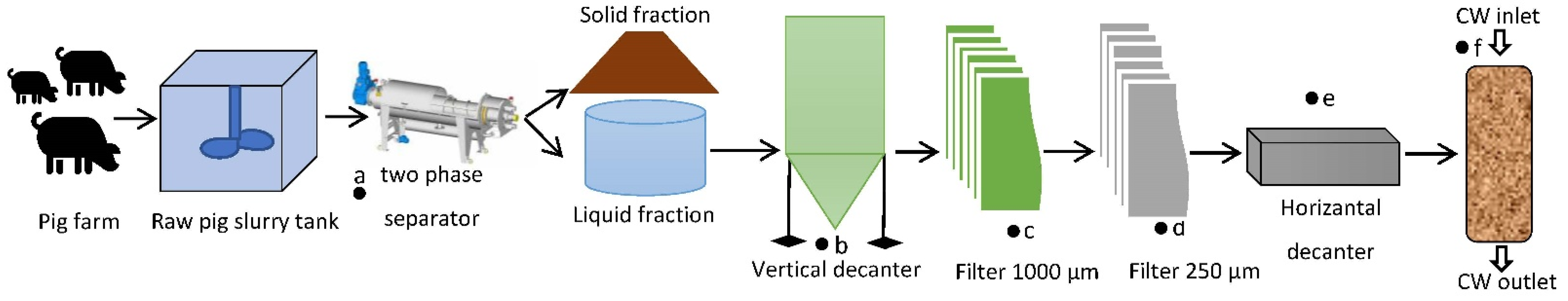

| Raw Pig Slurry | After Pretreatment (The Five-Step Process) | After Treatment with CF Process in Optimal Conditions | |
|---|---|---|---|
| pH | 7.63 | 7.88 | 6.4 |
| EC (ms cm −1) | 9.10 | 8.76 | 8 |
| SM (g L−1) | 80 | 30 | 0 |
| BOD5 (mg L−1) | 5300 | 4200 | 400 |
| COD (g L−1) | 11.2 | 8.94 | 1.29 |
| Turbidity (NTU) | 3500 | 2110 | 2.11 |
| Run Order | Coag mol L−1 | Floc µL | pH | Blk |
|---|---|---|---|---|
| 1 | 0.0248 | 164.9 | 7.90 | 1 |
| 2 | 0.0260 | 140.0 | 7.74 | 1 |
| 3 | 0.0248 | 115.1 | 7.58 | 1 |
| 4 | 0.0260 | 140.0 | 7.74 | 1 |
| 5 | 0.0272 | 115.1 | 7.90 | 1 |
| 6 | 0.0272 | 164.9 | 7.58 | 1 |
| 7 | 0.0260 | 140.0 | 7.74 | 1 |
| 1 | 0.0272 | 115.1 | 7.58 | 2 |
| 2 | 0.0260 | 140.0 | 7.74 | 2 |
| 3 | 0.0260 | 140.0 | 7.74 | 2 |
| 4 | 0.0272 | 164.9 | 7.90 | 2 |
| 5 | 0.0260 | 140.0 | 7.74 | 2 |
| 6 | 0.0248 | 164.9 | 7.58 | 2 |
| 7 | 0.0248 | 115.1 | 7.90 | 2 |
| 1 | 0.0260 | 180.0 | 7.74 | 3 |
| 2 | 0.0240 | 140.0 | 7.74 | 3 |
| 3 | 0.0260 | 140.0 | 7.74 | 3 |
| 4 | 0.0280 | 140.0 | 7.74 | 3 |
| 5 | 0.0260 | 140.0 | 7.74 | 3 |
| 6 | 0.0260 | 140.0 | 7.74 | 3 |
| 7 | 0.0260 | 140.0 | 8.00 | 3 |
| 8 | 0.0260 | 100.0 | 7.74 | 3 |
| 9 | 0.0260 | 140.0 | 7.48 | 3 |
| Run Order | Coag mol L −1 | Floc µL | pH | % COD Removal | % TURB Removal |
|---|---|---|---|---|---|
| 1 | 0.024 | 164.94 | 7.90 | 85.57 | 99.76 |
| 2 | 0.026 | 140 | 7.74 | 82.10 | 98.29 |
| 3 | 0.024 | 115.06 | 7.57 | 85.79 | 99.15 |
| 4 | 0.026 | 140 | 7.74 | 80.98 | 99.83 |
| 5 | 0.027 | 115.06 | 7.90 | 88.59 | 99.07 |
| 6 | 0.027 | 164.94 | 7.57 | 87.13 | 97.95 |
| 7 | 0.026 | 140 | 7.74 | 77.62 | 99.78 |
| 1 | 0.027 | 115.06 | 7.57 | 86.01 | 99.86 |
| 2 | 0.026 | 140 | 7.74 | 83.10 | 98.57 |
| 3 | 0.026 | 140 | 7.74 | 72.03 | 99.86 |
| 4 | 0.027 | 164.94 | 7.90 | 84.45 | 99.82 |
| 5 | 0.026 | 140 | 7.74 | 80.98 | 98.48 |
| 6 | 0.024 | 164.94 | 7.57 | 88.25 | 99.90 |
| 7 | 0.024 | 115.06 | 7.90 | 84.11 | 97.94 |
| 1 | 0.026 | 179.99 | 7.74 | 83.22 | 99.74 |
| 2 | 0.024 | 140 | 7.74 | 85.12 | 99.77 |
| 3 | 0.026 | 140 | 7.74 | 85.34 | 98.13 |
| 4 | 0.028 | 140 | 7.74 | 84.67 | 99.89 |
| 5 | 0.026 | 140 | 7.74 | 84.00 | 96.32 |
| 6 | 0.026 | 140 | 7.74 | 78.74 | 99.88 |
| 7 | 0.026 | 140 | 7.99 | 86.57 | 99.94 |
| 8 | 0.026 | 100 | 7.74 | 83.66 | 97.30 |
| 9 | 0.026 | 140 | 7.48 | 85.12 | 99.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El bied, O.; Kessler, M.; Terrero, M.A.; Fechtali, T.; Cano, A.F.; Acosta, J.A. Turbidity and Chemical Oxygen Demand Reduction from Pig Slurry through a Coagulation Flocculation Process. Agronomy 2021, 11, 2158. https://doi.org/10.3390/agronomy11112158
El bied O, Kessler M, Terrero MA, Fechtali T, Cano AF, Acosta JA. Turbidity and Chemical Oxygen Demand Reduction from Pig Slurry through a Coagulation Flocculation Process. Agronomy. 2021; 11(11):2158. https://doi.org/10.3390/agronomy11112158
Chicago/Turabian StyleEl bied, Oumaima, Mathieu Kessler, Martire Angélica Terrero, Taoufiq Fechtali, Angel Faz Cano, and José A. Acosta. 2021. "Turbidity and Chemical Oxygen Demand Reduction from Pig Slurry through a Coagulation Flocculation Process" Agronomy 11, no. 11: 2158. https://doi.org/10.3390/agronomy11112158
APA StyleEl bied, O., Kessler, M., Terrero, M. A., Fechtali, T., Cano, A. F., & Acosta, J. A. (2021). Turbidity and Chemical Oxygen Demand Reduction from Pig Slurry through a Coagulation Flocculation Process. Agronomy, 11(11), 2158. https://doi.org/10.3390/agronomy11112158








