Single Application of Biochar Increases Fertilizer Efficiency, C Sequestration, and pH over the Long-Term in Sandy Soils of Senegal
Abstract
:1. Introduction
2. Materials and Methods
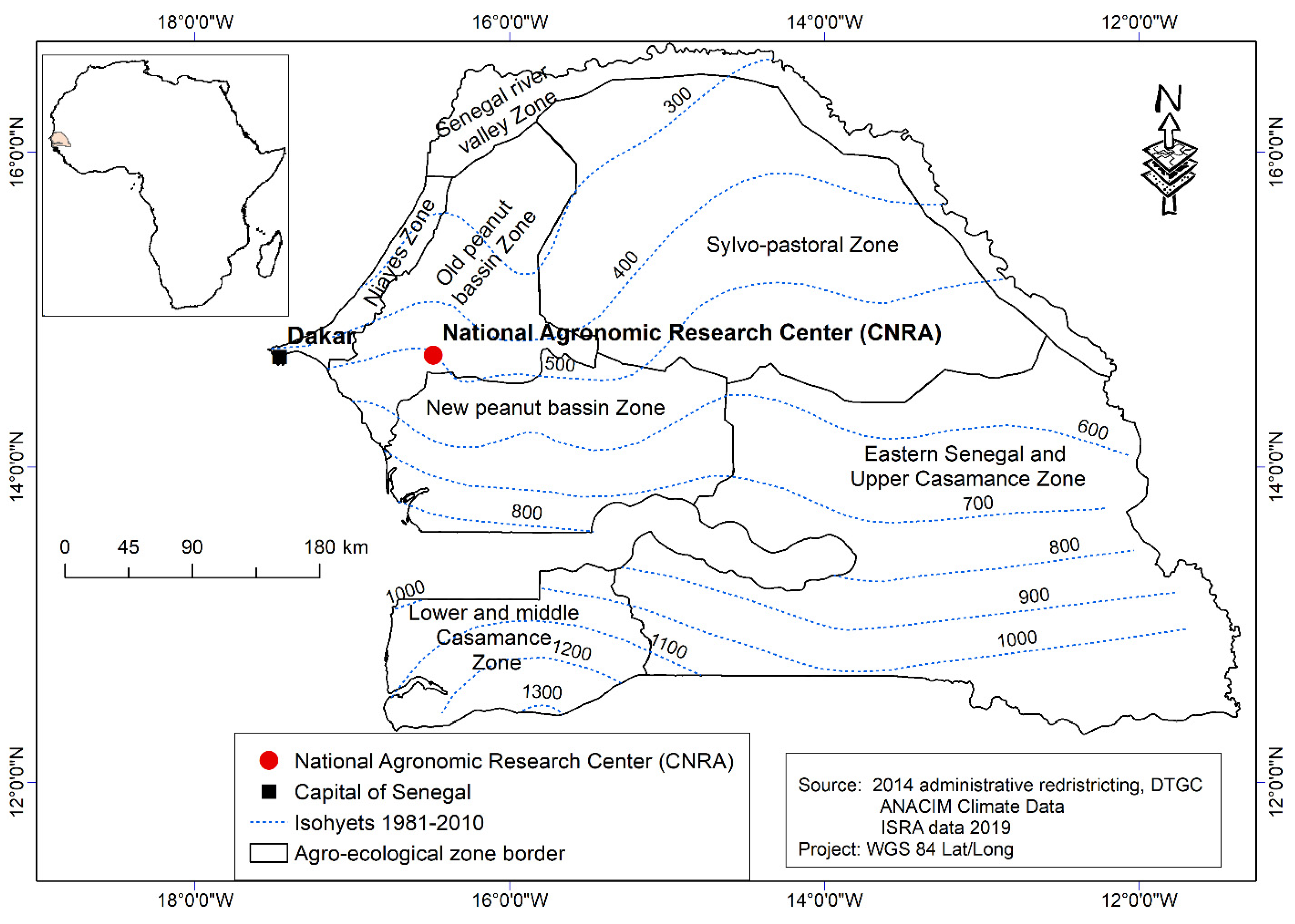
2.1. Experimental Site Description and Treatments
2.2. Plant Materials and Agronomy
2.3. Soil Analyses
2.4. Biochar Types and Characterization Methods
2.5. Millet and Peanut Grain Yield
2.6. Data Analysis
3. Results
3.1. Treatment Effects on Soil Characteristics Independent of Cropping System
3.1.1. Effect on Soil pH
3.1.2. Effect on Soil Available P
3.1.3. Effect on Soil Total N and C
3.2. Treatment Effects on Soil Characteristics and Yield under Different Cropping Systems
3.2.1. Interaction of Cropping Systems on Soil Chemical Characteristics
3.2.2. Soil Microbial Activity under Different Treatment and Cropping Systems
3.2.3. Peanut Pod Yield under the Millet–Peanut Rotation System
3.2.4. Millet Yield under the Millet Monocropping and Millet–Peanut Rotation Systems
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Affholder, F.; Poeydebat, C.; Corbeels, M.; Scopel, E.; Tittonell, P. The yield gap of major food crops in family agriculture in the tropics: Assessment and analysis through field surveys and modelling. Field Crop. Res. 2013, 143, 106–118. [Google Scholar] [CrossRef] [Green Version]
- Diagana, B.; Mankor, A.; Fall, C.S.; Guèye, A. Agriculture Durable et Réduction de la Pauvreté dans le Bassin Arachidier du Sénégal: Résultats du Modèle Analyse Tradeoffs; ISRA: Dakar, Senegal, 2008; p. 43. ISSN 0850-0711. [Google Scholar]
- Diangar, S.; Fofana, A.; Diagne, M.; Yamoah, C.F.; Dick, R.P. Pearl millet-based intercropping systems in the semiarid areas of Senegal. Afric. Crop Sci. J. 2004, 12, 133–139. [Google Scholar] [CrossRef]
- Fall, A.; Lo, M. Etude de Référence sur les Céréales: Mil, Sorgho, Maìs, et Fonio au Sénégal; CORAF/WECARD: Sénégal, 2009. [Google Scholar]
- Gaudreau, K.; Gibson, R.B. Sustainability assessment of the agricultural and energy systems of Senegal. Energies 2015, 8, 3503–3528. [Google Scholar] [CrossRef] [Green Version]
- Manley, R. Etude de la Dynamique de Quelques Compartiments Organiques sur un Terroir Agropastoral de Haute CASAMANCE, Sénégal. Rapport D’avancement de Première Année de Thèse; ORSTOM: Paris, France, 1997; p. 52. [Google Scholar]
- Vayssières, J.; Thévenot, A.; Vigne, M.; Cano, M.; Broc, A.; Bellino, R.; Diacono, E.; De Laburthe, B.; Bochu, J.L.; Tillard, E.; et al. Evaluation des inefficiences zootechnique et environnementale pour intensifier écologiquement les systèmes d’élevage tropicaux. Revue Élev. Méd. Vét. Pays Trop. 2012, 64, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Manlay, R.J.; Cadet, P.; Thioulouse, J.; Chotte, J.-L. Relationships between abiotic and biotic soil properties during fal- low periods in the sudanian zone of Senegal. Appl. Soil Ecol. 2000, 14, 89–101. [Google Scholar] [CrossRef]
- Sarr, M.; Agbogba, C.E.; Russell-Smith, A.; Masse, D. Effects of soil faunal activity and woody shrubs on water infiltration rates in a semi-arid fallow of Senegal. Appl. Soil Ecol. 2001, 16, 283–290. [Google Scholar] [CrossRef]
- Traoré, H.; Barro, A.; Yonli, D.; Stewart, Z.P.; Prasad, P.V.V. Water conservation methods and cropping systems for increased productivity and economic resilience in Burkina Faso. Water 2020, 12, 976. [Google Scholar] [CrossRef] [Green Version]
- Diatta, A.A.; Ndour, N.; Manga, A.; Sambou, B.; Faye, C.S.; Diatta, L.; Dieng, S.D. Ecosystem services of Cordyla pinnata (Lepr. ex A. Rich.) Milne-Redh agroforestry parkland of Senegal’s South Peanut Basin. Int. J. Biol. Chem. Sci. 2016, 10, 2511–2525. [Google Scholar] [CrossRef] [Green Version]
- The World Bank. Population Growth (Annual %). 2018. Available online: https://data.worldbank.org/indicator/SP.POP.GROW?locations=SN (accessed on 24 January 2019).
- FAOSTAT. Food and Agriculture Organization-Crop Statistics. 2021. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 15 August 2021).
- Tittonell, P.; Giller, K.E. When yield gaps are poverty traps: The paradigm of ecological intensification in African smallholder agriculture. Field Crops Res. 2013, 143, 76–90. [Google Scholar] [CrossRef] [Green Version]
- Faye, A. Présentation de la Chaine de Valeur Arachide; CEPOD: Dakar, Sénégal, 2009; p. 8. [Google Scholar]
- MAE. La Culture et la Production du mil et du Sorgho au Sénégal: Bilan-Diagnostic et Perspectives; Ministere De L’agriculture Et De L’elevag: Dakar, Senegal, 2001; 130p.
- Tounkara, A.; Clermont-Dauphin, C.; Affholder, F.; Ndiaye, S.; Masse, D.; Cournac, L. Inorganic fertilizer use efficiency of millet crop increased with organic fertilizer application in rainfed agriculture on smallholdings in central Senegal. Agric. Ecosyst. Environ. 2020, 294, 106878. [Google Scholar] [CrossRef]
- Lericollais, A.; Warniez, P. Les terroirs africains, approche renouvelée par l’emploi du SIG. MappeMonde 1993, 2, 31–36. [Google Scholar]
- Wortmann, C.S.; Sones, K. (Eds.) Fertilizer Use Optimization in Sub-Saharan Africa; CABI: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Vanlauwe, B.; Kihara, J.; Chivenge, P.; Pypers, P.; Coe, R.; Six, J. Agronomic use efficiency of N fertilizer in maize-based systems in sub-Saharan Africa within the context of integrated soil fertility management. Plant Soil 2011, 339, 35–50. [Google Scholar] [CrossRef]
- Garba, M.; Serme, I.; Maman, N.; Ouattara, K.; Gonda, A.; Wortmann, C.S.; Mason, S.C. Crop response to manure plus fertilizer in Burkina Faso and Niger. Nutr. Cycl. Agroecosyst. 2018, 111, 175–188. [Google Scholar] [CrossRef]
- Sanchez, P.; Shepherd, K.D.; Soule, M.J.; Place, F.M.; Buresh, R.J.; Izac, A.-M.N.; Mokwunye, A.U.; Kwesiga, F.R.; Ndiritu, C.G.; Woomer, P.L. Soil Fertility Replenishment in Africa: An Investment in Natural Resource Capital. In Replenishing Soil Fertility in Africa; Soil Science Society of America: Madison, WI, USA; American Society of Agronomy: Madison, WI, USA, 1997; Volume 51, pp. 1–46. [Google Scholar] [CrossRef]
- Cissé, L. Etude des Effets d’apport de Matière Organique sur les Bilans Hydriques et Minéraux et la Production du mil et de l’arachide sur un sol Sableux Dégradé du Centre-Nord du Sénégal. Ph.D. Thesis, Université INPL, Nancy, France, 1986; 184p. [Google Scholar]
- Uzoh, I.M.; Igwe, C.A.; Okebalama, C.B.; Babalola, O.O. Legume-maize rotation effect on maize productivity and soil fertility parameters under selected agronomic practices in a sandy loam soil. Sci. Rep. 2019, 9, 8539. [Google Scholar] [CrossRef] [Green Version]
- Stewart, Z.P.; Pierzynski, G.M.; Middendorf, B.J.; Prasad, P.V.V. Approaches to improve soil fertility in sub-Saharan Africa. J. Exp. Bot. 2020, 71, 632–641. [Google Scholar] [CrossRef] [Green Version]
- Sultan, B.; Lalou, R.; Amadou Sanni, M.; Oumarou, A.; Soumaré, M.A. (Eds.) Les Sociétés Rurales Face aux Changements Climatiques et Environnementaux en Afrique de L’Ouest; IRD: Marseille, France, 2015; pp. 403–427. ISBN 978-2-7099-2146-6. [Google Scholar]
- Stewart, Z.P.; Middendorf, B.J.; Musumba, M.; Grabowski, P.; Palm, C.; Snapp, S.; Prasad, P.V.V. Feed the Future Innovation Lab for Collaborative Research on Sustainable Intensification; Kansas State University: Manhattan, KS, USA, 2018; Available online: https://www.sitoolkit.com (accessed on 21 January 2021).
- Wortmann, C.; Amede, T.; Bekunda, M.; Kome, C.; Masikati, P.; Ndungu-Magiroi, K.; Snapp, S.; Stewart, Z.P.; Westgate, M.E.; Zida, Z. Improvement of smallholder farming systems in Africa. Agron. J. 2020, 112, 5325–5333. [Google Scholar] [CrossRef]
- Malyan, S.K.; Kumar, S.S.; Fagodiya, R.K.; Ghosh, P.; Kumar, A.; Singh, R.; Singh, L. Biochar for environmental sustainability in the energy-water-agroecosystem nexus. Renew. Sustain. Energy Rev. 2021, 149, 111379. [Google Scholar] [CrossRef]
- Mona, S.; Malyan, S.K.; Saini, N.; Deepak, B.; Pugazhendhi, A.; Kumar, S.S. Towards sustainable agriculture with carbon sequestration, and greenhouse gas mitigation using algal biochar. Chemosphere 2021, 275, 129856. [Google Scholar] [CrossRef]
- Khaled, A.; Schoenau, R. Addition of biochar to a sandy desert soil: Effect on crop growth, water retention and selected properties. Agronomy 2019, 9, 327. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Husk, B.; Major, J. Le biochar comme amendement du sol au Québec: Résultats agronomiques de quatre ans d’essais terrain. CRAAQ J. Inf. Sci. 2012, 30–31. Available online: https://www.agrireseau.net/agroenvironnement/documents/Major.pdf (accessed on 14 August 2021).
- Mukherjee, A.; Lal, R. Biochar impacts on soil physical properties and greenhouse gas emissions. Agronomy 2013, 3, 313–339. [Google Scholar] [CrossRef] [Green Version]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. A review of biochar and its use and function in soil. Adv. Agron. 2010, 105, 47–82. [Google Scholar] [CrossRef]
- Biederman, L.A.; Harpole, S.W. Biochar and Managed Perennial Ecosystems: Testing for Synergy in Ecosystem Function and Biodiversity. Iowa State Research Farm Progress Reports. 2013. Available online: http://lib.dr.iastate.edu/farms_reports/1990 (accessed on 15 August 2021).
- Jeffery, S.; Verheijen, F.; Velde, M.; Bastos, A. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Spokas, K.A.; Cantrell, K.B.; Novak, J.M.; Archer, D.W.; Ippolito, J.A.; Collins, H.P.; Boateng, A.A.; Lima, I.M.; Lamb, M.C.; McAloon, A.J.; et al. Biochar: A synthesis of its agronomic impact beyond carbon sequestration. J. Environ. Qual. 2011, 41, 973–989. [Google Scholar] [CrossRef]
- Akolgo, G.A.; Kemausuor, F.; Awafo, E.A.; Amankwah, E.; Atta-Darkwa, T.; Essandoh, E.O.; Bart-Plange, A.; Branco de Freitas Maia, C.M. Biochar a soil amendment tool: Effects on soil properties and yield of maize and cabbage in Brong-Ahafo Region Ghana. Open J. Soil Sci. 2020, 10, 91–108. [Google Scholar] [CrossRef] [Green Version]
- Jeffery, S.; Abalos, D.; Prodana, M.; Bastos, A.C.; Van Groenigen, J.W.; Hungate, B.A.; Verheijen, F. Biochar boosts tropical but not temperate crop yields. Environ. Res. Lett. 2017, 12, 053001. [Google Scholar] [CrossRef]
- Cornelissen, G.; Martinsen, V.; Shitumbanuma, V.; Alling, V.; Breedveld, G.D.; Rutherford, D.W.; Sparrevik, M.; Hale, S.E.; Obia, A.; Mulder, J. Biochar effect on maize yield and soil characteristics in five conservation farming sites in Zambia. Agronomy 2013, 3, 256–274. [Google Scholar] [CrossRef] [Green Version]
- Asirifi, I.; Werner, S.; Heinze, S.; Saba, C.K.; Lawson, I.Y.; Marschner, B. Short-term effect of biochar on microbial biomass, respiration and enzymatic activities in wastewater irrigated soils in urban agroecosystems of the West African savannah. Agronomy 2021, 11, 271. [Google Scholar] [CrossRef]
- Nyambo, P.; Taeni, T.; Chiduza, C.; Araya, T. Effects of maize residue biochar amendments on soil properties and soil loss on acidic Hutton soil. Agronomy 2018, 8, 256. [Google Scholar] [CrossRef] [Green Version]
- Tyron, E.H. Effect of charcoal on certain physical, chemical and biological properties of forest soils. Ecol. Monogr. 1948, 18, 81–115. [Google Scholar] [CrossRef]
- Shneour, E.A. Oxidation of graphitic carbon in certain soils. Science 1966, 151, 991–992. [Google Scholar] [CrossRef] [PubMed]
- Spokas, K.; Reicosky, D. Impacts of sixteen different biochars on soil greenhouse gas production. Ann. Environ. Sci. 2009, 3, 179–193. [Google Scholar]
- Ousmane, A.; Goudiaby, K.; Diedhiou, S.; Diatta, Y.; Adiane, A.; Diouf, P.; Fall, S.; Dalanda, M.; Ndoye, I. Soil properties and groundnut (Arachis hypogea L.) responses to intercropping with Eucalyptus camaldulensis Dehn and amendment with its biochar. J. Mater. Environ. Sci. 2020, 11, 220–229. [Google Scholar]
- Diatta, A.A. Effects of Biochar Application on Soil Fertility and Pearl Millet (Pennisetum glaucum L.) Yield. Master’ Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2016; p. 147. Available online: http://hdl.handle.net/10919/80944 (accessed on 8 October 2021).
- Van Zwieten, L.; Kimber, S.; Morris, S.; Chan, K.Y.; Downie, A.; Rust, J.; Joseph, S.; Cowie, A. Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 2010, 327, 235–246. [Google Scholar] [CrossRef]
- MEB. Le Biocharbon, Quelles Opportunités Pour le Sénégal. Rapport d’étude du Ministère de l’Energie et des Biocarburants; BTG Biomass Technology Group BV: Durgerdamstraat, The Netherlands, 2009; p. 12. [Google Scholar]
- Mathieu, C.; Pieltain, F. Analyse Chimique des Sols: Méthodes Choisies; Edition Tec & Doc: Montpellier, France, 2003; 387p. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–37. [Google Scholar] [CrossRef]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis, 2nd ed.; Part 2; Page, A.L., Miller, R.H., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Kjeldahl, J.A. New method for the determination of nitrogen in organic matter. Z. Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef] [Green Version]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis: Part 3 Chemical Methods; American Society of Agronomy: Madison, WI, USA, 1996; Volume 5, pp. 961–1010. [Google Scholar] [CrossRef]
- Chopart, J.L. Etude au Champ des Systèmes Racinaires des Principales Cultures Pluviales au Sénégal (Arachide Mil-Sorgho-Riz Pluvial); Thèse I.N.P.: Toulouse, France, 1980; p. 162. [Google Scholar]
- Adam, G.; Duncan, H. Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biol. Biochem. 2001, 33, 943–951. [Google Scholar] [CrossRef] [Green Version]
- Bray, R.H.; Kurtz, L.T. Determination of total organic and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–45. [Google Scholar] [CrossRef]
- Toth, S.J.; Prince, A.L. Estimation of cation-exchange capacity and exchangeable Ca, K, and Na contents of soils by flame photometer techniques. Soil Sci. 1949, 67, 439–446. [Google Scholar] [CrossRef]
- Guppy, C.N.; Menzies, N.W.; Moody, P.W.; Blamey, F.P.C. Competitive sorption reactions between phosphorus organic matter in soil: A review. Aust. J. Soil Res. 2005, 43, 189–202. [Google Scholar] [CrossRef]
- Ndour, Y.B. Statut Organique et Microbiologique des sols dans des Systèmes Agroforestiers et à Jachère du Sénégal. Mémoire de Diplôme d’Etudes Approfondies en Sciences de l’Environnement; Faculté des Sciences et Techniques, Institut des Sciences de l’Environnement, Université Cheikh Anta Diop de Dakar: Dakar, Sénégal, 1998; p. 63. [Google Scholar]
- Häring, V.; Manka’abusi, D.; Akoto-Danso, E.K.; Werner, S.; Atiah, K.; Steiner, C.; Lompo, D.J.P.; Adiku, S.; Buerkert, A.; Marschn, B. Effects of biochar, waste water irrigation and fertilization on soil properties in West African urban agriculture. Sci. Rep. 2017, 7, 10738. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, L.; Lesueur, D.; Robin, A.; Robain, H.; Wiriyakitnateekul, W.; Bräu, L. Impact of biochar application dose on soil microbial communities associated with rubber trees in North East Thailand. Sci. Total Environ. 2019, 689, 970–979. [Google Scholar] [CrossRef] [Green Version]
- Brassard, P.; Godbout, S.; Raghavan, V. Soil biochar amendment as a climate change mitigation tool: Key parameters and mechanisms involved. J. Environ. Manag. 2016, 181, 484–497. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef] [Green Version]
- Vanlauwe, B.; Descheemaeker, K.; Giller, K.E.; Huising, J.; Merckx, R.; Nziguheba, G.; Wendt, J.; Zingore, S. Integrated soil fertility management in sub-Saharan Africa: Unravelling local adaptation. Soil 2015, 491–508. [Google Scholar] [CrossRef] [Green Version]
- Pieri, C. Fertilité des Terres de Savanes. Bilan de Trente ans de Recherche et de Développement Agricoles au Sud du Sahara; CIRAD-IRAT: Montpellier, France, 1989; p. 444. Available online: https://agritrop.cirad.fr/375686/ (accessed on 14 August 2021).
- Zhao, Z.; Zhang, C.; Li, F.; Gao, S.; Zhang, J. Effect of compost and inorganic fertilizer on organic carbon and activities of carbon cycle enzymes in aggregates of an intensively cultivated Vertisol. PLoS ONE 2020, 15, e0229644. [Google Scholar] [CrossRef] [PubMed]
- Rasool, R.; Kukal, S.S.; Hira, G.S. Soil organic carbon and physical properties as affected by long-term application of FYM and inorganic fertilizers in maize–wheat system. Soil Tillage Res. 2008, 101, 31–36. [Google Scholar] [CrossRef]
- Thuita, M.; Pieter, P.; Herrmann, L.; Okalebo, R.J.; Othieno, C.; Muema, E.; Lesueur, D. Commercial rhizobial inoculants significantly enhance growth and nitrogen fixation of a promiscuous soybean variety in Kenyan soils. Biol. Fertil. Soil. 2011, 48, 87–96. [Google Scholar] [CrossRef]
- Alikhani, H.A.; Saleh-Rastin, N.; Antoun, H. Phosphate solubilization activity of rhizobia native to Iranian soils. In First International Meeting on Microbial Phosphate Solubilization; Velázquez, E., Rodríguez-Barrueco, C., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 35–41. [Google Scholar]
- Faye, A.; Dalpé, Y.; Ndung’u-Magiroi, K.; Jefwa, J.; Ndoye, I.; Diouf, M.; Lesueur, D. Evaluation of commercial arbuscular mycorrhizal inoculants. Can. J. Plant. Sci. 2013, 93, 1201–1208. [Google Scholar] [CrossRef]
- Faye, A.; Stewart, Z.P.; Ndung’u-Magiroi, K.; Diouf, M.; Ndoye, I.; Diop, T.; Dalpé, Y.; Prasad, P.V.V.; Lesueur, D. Testing of commercial inoculants to enhance P uptake and grain yield of promiscuous soybean in Kenya. Sustainability 2020, 12, 3803. [Google Scholar] [CrossRef]
- Stewart, Z.P.; Pierzynski, G.M.; Middendorf, B.J.; Prasad, P.V.V. Sub-Saharan Africa Soil Fertility Prioritization Report: III. Combined Summary. Feed the Future Innovation Lab for Collaborative Research on Sustainable Intensification; Kansas State University: Manhattan, KS, USA, 2017; Available online: https://www.k-state.edu/siil/resources/soilfertility/index.html (accessed on 7 October 2021).
- Pierzynski, G.M.; Middendorf, B.J.; Stewart, Z.P.; Prasad, P.V.V. Sub-Saharan Africa Soil Fertility Prioritization Report: I. Survey Results. Feed the Future Innovation Lab for Collaborative Research on Sustainable Intensification; Kansas State University: Manhattan, KS, USA, 2017; Available online: https://www.k-state.edu/siil/resources/soilfertility/index.html (accessed on 7 October 2021).
- Middendorf, B.J.; Pierzynski, G.M.; Stewart, Z.P.; Prasad, P.V.V. Sub-Saharan Africa Soil Fertility Prioritization Report. II. Summit Results. Feed the Future Innovation Lab for Collaborative Research on Sustainable Intensification; Kansas State University: Manhattan, KS, USA, 2017; Available online: https://www.k-state.edu/siil/resources/soilfertility/index.html (accessed on 7 October 2021).
- Wortmann, C.; Stewart, Z.P. Nutrient management for sustainable food crop intensification in the tropical savannas in Africa. Agron. J. 2021. [Google Scholar] [CrossRef]








| Treatments | Composition | kg N ha−1 Applied |
|---|---|---|
| T1 | Absolute control (no amendment, no fertilizer) | 0 |
| T2 | Positive control (Recommended 150 kg ha−1 NPK + 100 kg ha−1 Urea) | 68.5 |
| T3 | 5 t ha−1 of rice husk biochar alone | 10 |
| T4 | 10 t ha−1 rice husk biochar alone | 20 |
| T5 | 5 t ha−1 of Typha australis biochar + 5 t ha−1 cattle manure | 75 |
| T6 | 10 t ha−1 of Typha australis biochar + 5 t ha−1 cattle manure | 85 |
| T7 | 75 kg ha−1 NPK + 50 kg ha−1 Urea + 5 t ha−1 of rice husk biochar | 44.25 |
| T8 | 50 kg ha−1 NPK + 33 kg ha−1 Urea + 5 t ha−1 rice husk biochar + 5 t ha−1 cattle manure | 97.68 |
| T9 | 50 kg ha−1 NPK + 33 kg ha−1 Urea + 5 t ha−1 rice husk biochar | 32.68 |
| T10 | 50 kg ha−1 NPK + 33 kg ha−1 Urea + 5 t ha−1 of Typha australis Biochar + 5 t ha−1 cattle manure | 97.68 |
| Parameters | Rice Husk | Typha australis |
|---|---|---|
| pH | 8.9 | 10.9 |
| Organic C (%) | 0.1 | 0.9 |
| Total N (%) | 0.2 | 0.2 |
| Organic Matter (%) | 2 | 3 |
| Ca (cmol kg−1) | 19.8 | 22.9 |
| Mg (cmol kg−1) | 6.8 | 7.4 |
| K (cmol kg−1) | 2.6 | 3.1 |
| Na (cmol kg−1) | 1.8 | 1.5 |
| Total Exchangeable Basis (T.E.B.) | 31 | 34.9 |
| Available P (ppm) | 4.1 | 3.2 |
| Available K (ppm) | 107.4 | 268.8 |
| Nutrients | Availability |
| N (NO3) (mg kg−1) | 499.4 |
| N (NH4) (mg kg−1) | 100.5 |
| Total N (%) | 1.3 |
| Total C (%) | 13.0 |
| C:N ratio | 10.2 |
| Total P (g kg−1) | 6.5 |
| Available P (g kg−1) | 1.4 |
| Ash (%) | 74.2 |
| Ca (g kg−1) | 17.0 |
| Mg (g kg−1) | 2.6 |
| Na (g kg−1) | 1.5 |
| K (g kg−1) | 2.1 |
| Total N Reference (2012): 0.21b (%0) | Total C Reference (2012): 1.84e (%) | |||||
|---|---|---|---|---|---|---|
| 2013 | 2015 | 2017 | ||||
| Treatments | N (%0) | C (%) | N (%0) | C (%) | N (%0) | C (%) |
| T1 | 0.14b | 1.9e | 0.3a | 1.81e | 0.34a | 1.74e |
| T2 | 0.26b | 2.11e | 0.19bc | 2.73d | 0.19bc | 2.83d |
| T3 | 0.36ab | 2.98ab | 0.19bc | 2.65d | 0.21bc | 2.68d |
| T4 | 0.31ab | 2.79bc | 0.19bc | 2.66d | 0.20bc | 2.61d |
| T5 | 0.4a | 3.4a | 0.23bc | 2.71d | 0.25bc | 2.89d |
| T6 | 0.23b | 2.55bcd | 0.22b | 2.71d | 0.21b | 2.81d |
| T7 | 0.31b | 2.65bcd | 0.17c | 2.60d | 0.20b | 2.3d |
| T8 | 0.15b | 2.59bcd | 0.19bc | 2.73d | 0.21b | 2.83d |
| T9 | 0.23ab | 2.73bc | 0.19bc | 2.68d | 0.22b | 2.72d |
| T10 | 0.12b | 2.3cde | 0.16c | 2.68d | 0.17b | 2.7d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faye, A.; Stewart, Z.P.; Diome, K.; Edward, C.-T.; Fall, D.; Ganyo, D.K.K.; Akplo, T.M.; Prasad, P.V.V. Single Application of Biochar Increases Fertilizer Efficiency, C Sequestration, and pH over the Long-Term in Sandy Soils of Senegal. Sustainability 2021, 13, 11817. https://doi.org/10.3390/su132111817
Faye A, Stewart ZP, Diome K, Edward C-T, Fall D, Ganyo DKK, Akplo TM, Prasad PVV. Single Application of Biochar Increases Fertilizer Efficiency, C Sequestration, and pH over the Long-Term in Sandy Soils of Senegal. Sustainability. 2021; 13(21):11817. https://doi.org/10.3390/su132111817
Chicago/Turabian StyleFaye, Aliou, Zachary P. Stewart, Khady Diome, Calys-Tagoe Edward, Dioumacor Fall, Désiré Komla Kyky Ganyo, Tobi Moriaque Akplo, and P. V. Vara Prasad. 2021. "Single Application of Biochar Increases Fertilizer Efficiency, C Sequestration, and pH over the Long-Term in Sandy Soils of Senegal" Sustainability 13, no. 21: 11817. https://doi.org/10.3390/su132111817
APA StyleFaye, A., Stewart, Z. P., Diome, K., Edward, C.-T., Fall, D., Ganyo, D. K. K., Akplo, T. M., & Prasad, P. V. V. (2021). Single Application of Biochar Increases Fertilizer Efficiency, C Sequestration, and pH over the Long-Term in Sandy Soils of Senegal. Sustainability, 13(21), 11817. https://doi.org/10.3390/su132111817








