The Protective Effect of Heme Oxygenase-1 on Liver Injury Caused by DON-Induced Oxidative Stress and Cytotoxicity
Abstract
:1. Introduction
2. Results
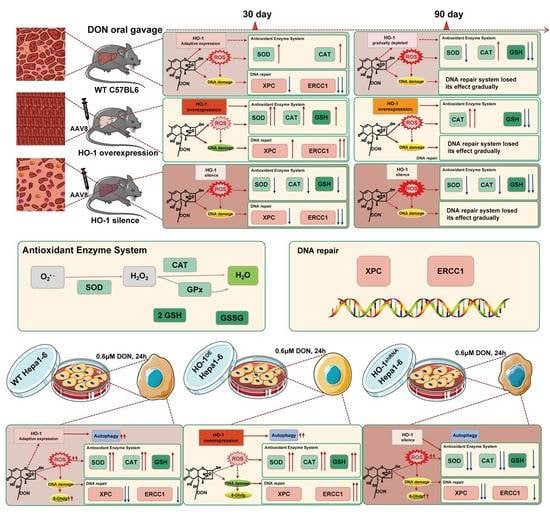
2.1. HO-1 Can Reduce Liver Damage Caused by Low-Dose DON Exposure
2.1.1. HO-1 Alleviated Liver Inflammation Induced by Low-Dose DON
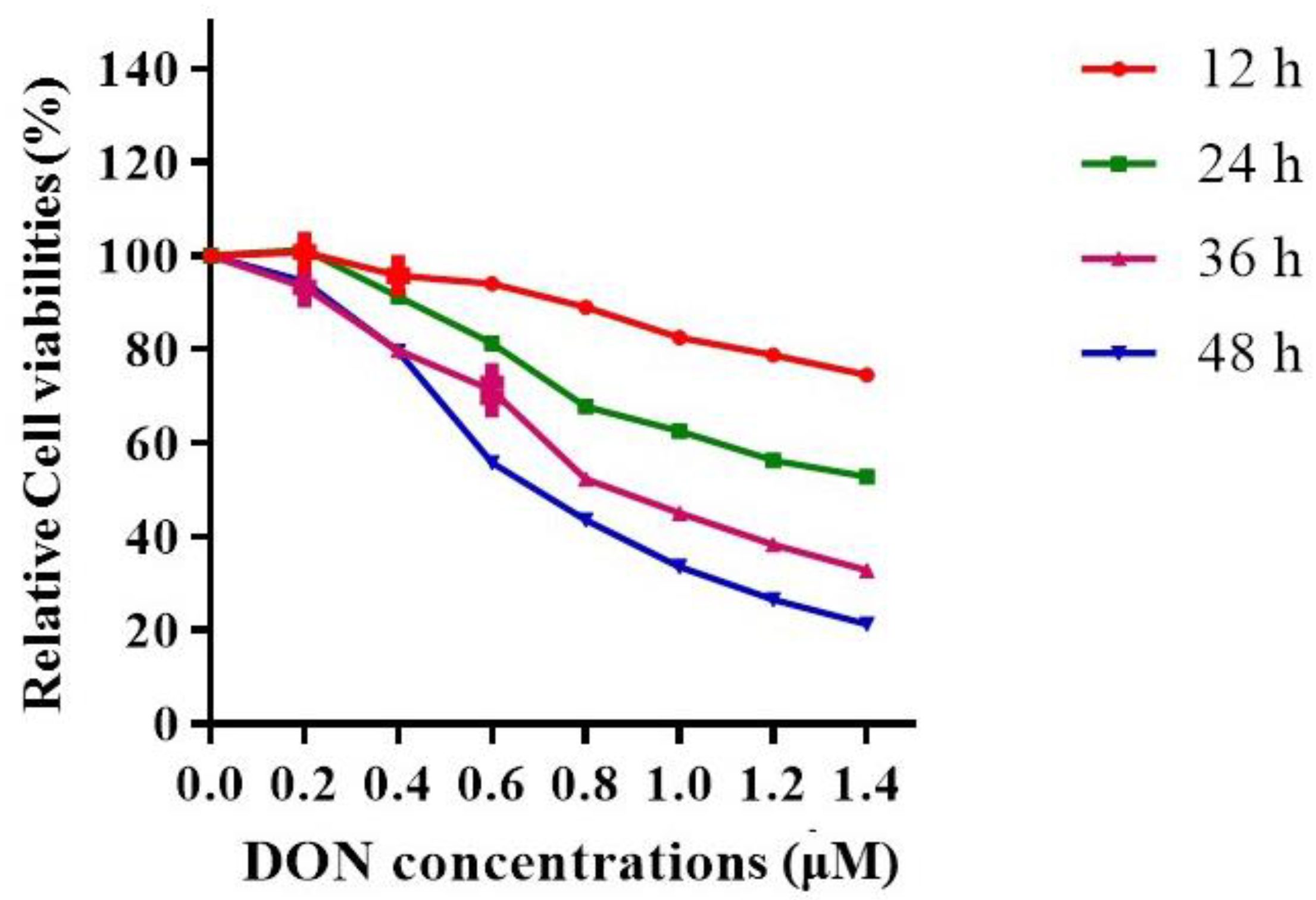
2.1.2. The Effects of DON Exposure Time and Dose on Hepa 1–6 Cell Viabilities
2.1.3. HO-1 Inhibited the Levels of ROS and 8-OHdG Induced by DON
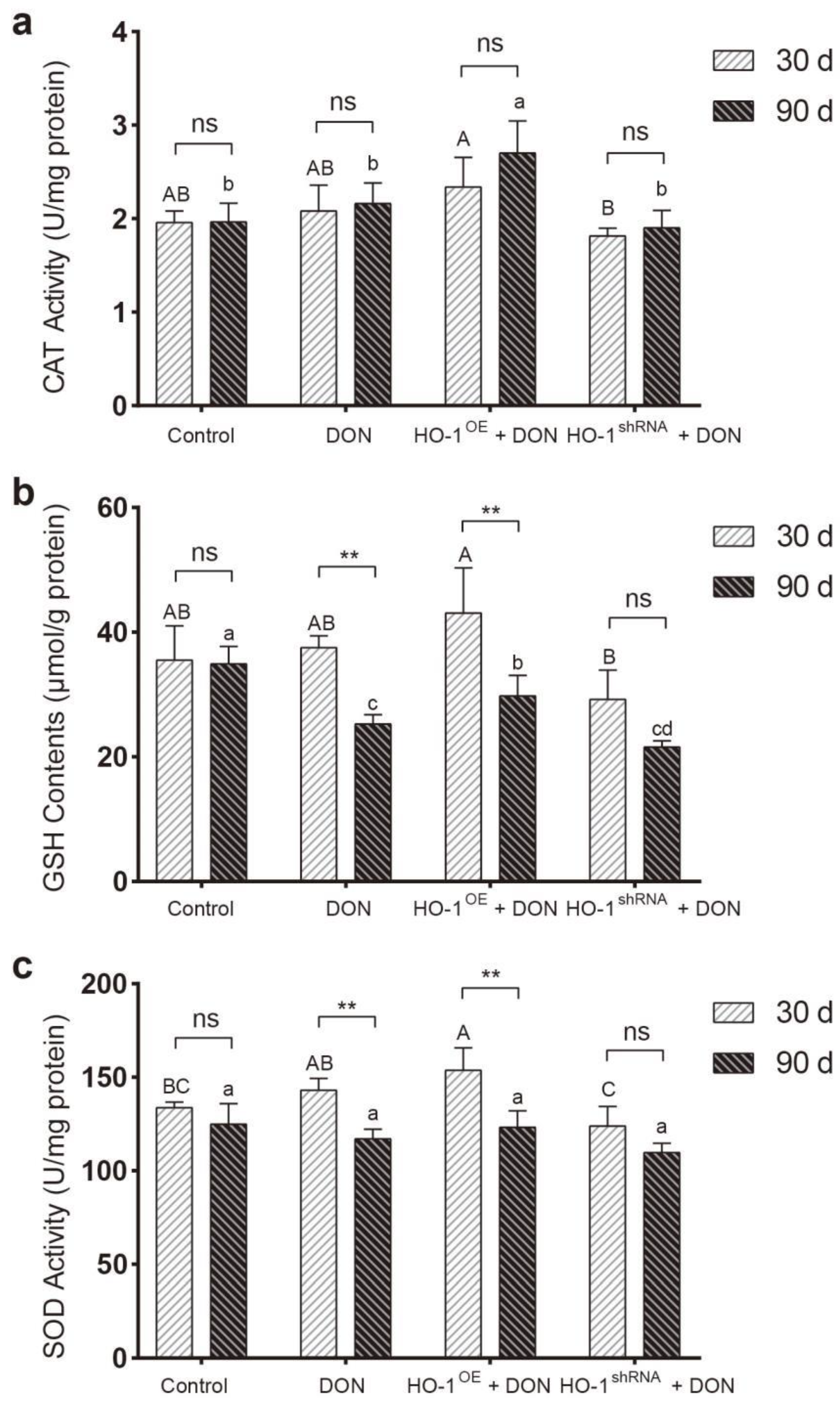
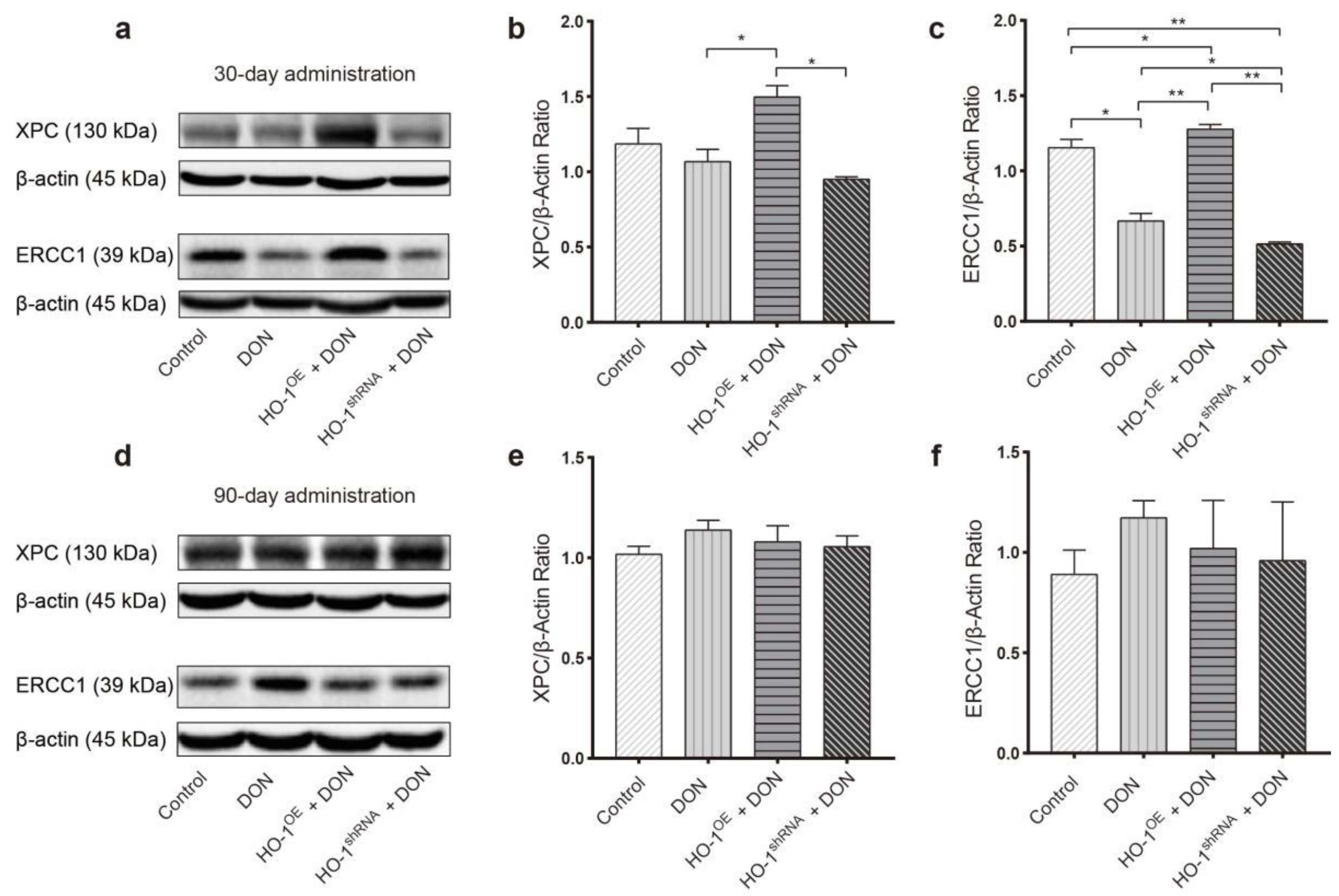
2.2. HO-1 Can Maintain the Antioxidant Enzyme/DNA Repair System in Mouse Liver during Low-Dose DON Exposure
2.2.1. The Protective Effect of HO-1 after 30 Days of Exposure to DON
2.2.2. The Protective Effect of HO-1 after 90 Days of Exposure to DON
2.3. HO-1 Can Also Maintain the Antioxidant Enzyme/DNA Repair/Autophagy of Hepa 1–6 Cells during Low-Dose DON Exposure
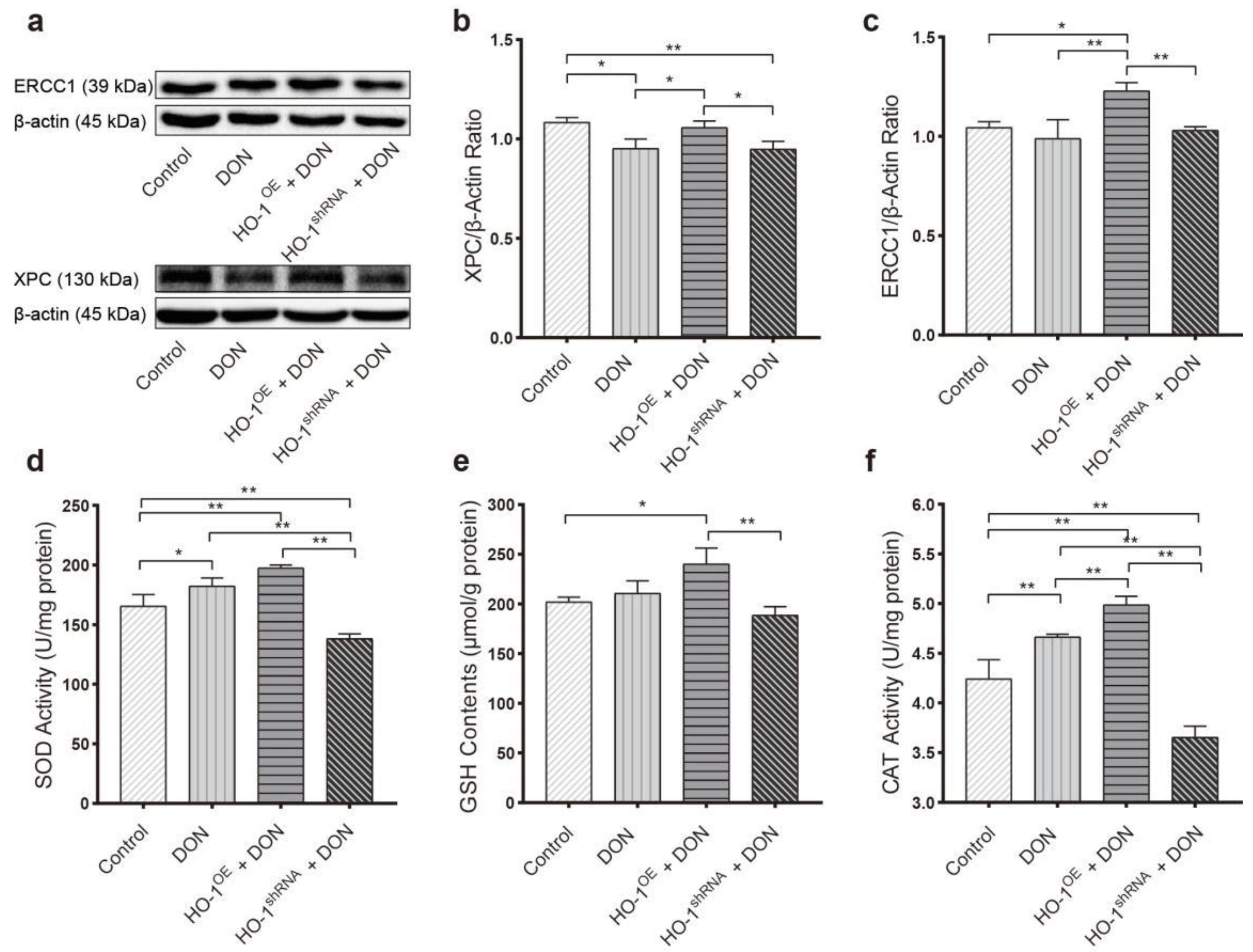
2.3.1. HO-1 Maintains Antioxidant Activity/DNA Repair in Hepa 1–6 Cells, under DON Exposure Conditions
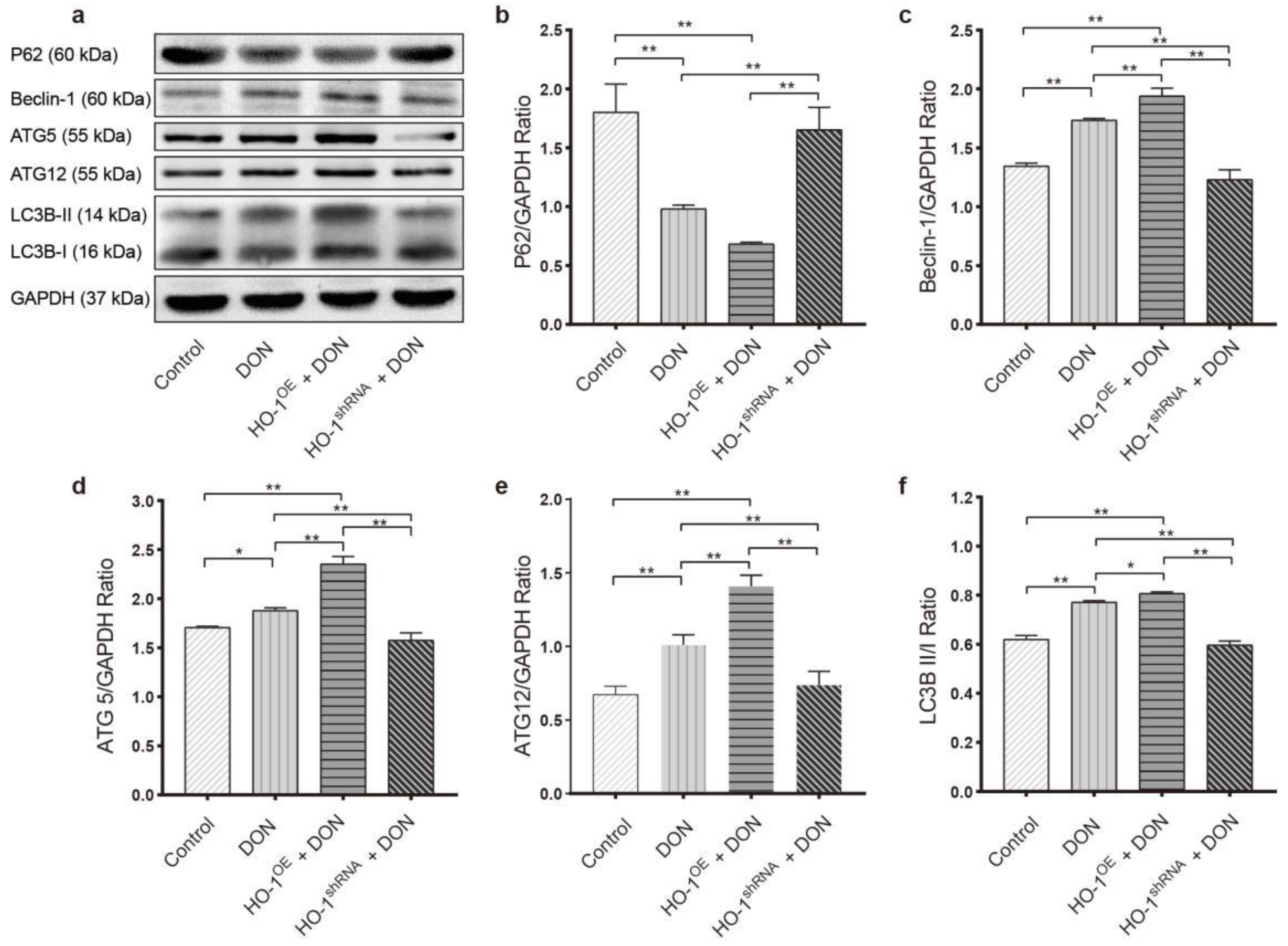
2.3.2. HO-1 Could Protect the Ability of Autophagy in Hepa 1–6 Cells under DON Exposure Conditions
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Reagents
5.2. Animals
5.3. Cell Lines
5.4. Determination of Antioxidant Enzyme Contents in Tissues and Cells
5.5. Determination of 8-OHdG Levels
5.6. Western Blot Analysis
5.7. Cellular ROS Measurement
5.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DON | Deoxynivalenol; |
| HO-1 | Heme oxygenase-1; |
| CAT | Catalase; |
| GSH | Glutathione; |
| 8-OHdG | 8-hydroxy-2 deoxyguanosine; |
| ROS | Reactive oxygen species; |
| SOD | Superoxide dismutase; |
| ERCC1 | Excision repair cross-complementation group 1; |
| XPC | Xeroderma pigmentosum complementation group C; |
| OE | Overexpression; |
| shRNA | Short hairpin Ribonucleic Acid. |
References
- Bryla, M.; Ksieniewicz-Wozniak, E.; Waskiewicz, A.; Szymczyk, K.; Jedrzejczak, R. Natural Occurrence of Nivalenol, Deoxynivalenol, and Deoxynivalenol-3-Glucoside in Polish Winter Wheat. Toxins 2018, 10, 81. [Google Scholar] [CrossRef] [Green Version]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef]
- Sirot, V.; Fremy, J.M.; Leblanc, J.C. Dietary exposure to mycotoxins and health risk assessment in the second French total diet study. Food Chem. Toxicol. 2013, 52, 1–11. [Google Scholar] [CrossRef]
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef]
- World Health Organization. Safety Evaluation of Certain Contaminants in Food; Food and Drug Administration: College Park, MD, USA, 2006; Volume 82, pp. 1–778.
- Wang, L.L.; Liao, Y.X.; Peng, Z.; Chen, L.K.; Zhang, W.H.; Nussler, A.K.; Shi, S.J.; Liu, L.G.; Yang, W. Food raw materials and food production occurrences of deoxynivalenol in different regions. Trends Food Sci. Technol. 2019, 83, 41–52. [Google Scholar] [CrossRef]
- Wu, F.; Groopman, J.D.; Pestka, J.J. Public health impacts of foodborne mycotoxins. Annu. Rev. Food Sci. Technol. 2014, 5, 351–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzo, A.F.; Atroshi, F.; Ahotupa, M.; Sankari, S.; Elovaara, E. Protective effect of antioxidants against free radical-mediated lipid peroxidation induced by DON or T-2 toxin. Zent. Vet. A 1994, 41, 81–90. [Google Scholar] [CrossRef]
- Yu, M.; Peng, Z.; Liao, Y.; Wang, L.; Li, D.; Qin, C.; Hu, J.; Wang, Z.; Cai, M.; Cai, Q.; et al. Deoxynivalenol-induced oxidative stress and Nrf2 translocation in maternal liver on gestation day 12.5d and 18.5d. Toxicon 2019, 161, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jin, Y.; Wu, S.; Yu, H.; Zhao, Y.; Fang, H.; Shen, J.; Zhou, C.; Fu, Y.; Li, R.; et al. Deoxynivalenol induces oxidative stress, inflammatory response and apoptosis in bovine mammary epithelial cells. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1663–1674. [Google Scholar] [CrossRef]
- Hou, Y.J.; Zhao, Y.Y.; Xiong, B.; Cui, X.S.; Kim, N.H.; Xu, Y.X.; Sun, S.C. Mycotoxin-containing diet causes oxidative stress in the mouse. PLoS ONE 2013, 8, e60374. [Google Scholar] [CrossRef]
- Hallaj Salahipour, M.; Hasanzadeh, S.; Malekinejad, H.; Razi, M.; Farrokhi-Ardebili, F. Deoxynivalenol reduces quality parameters and increases DNA damage in mice spermatozoa. Andrologia 2019, 51, e13238. [Google Scholar] [CrossRef]
- Kouadio, J.H.; Dano, S.D.; Moukha, S.; Mobio, T.A.; Creppy, E.E. Effects of combinations of Fusarium mycotoxins on the inhibition of macromolecular synthesis, malondialdehyde levels, DNA methylation and fragmentation, and viability in Caco-2 cells. Toxicon 2007, 49, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Guo, W.; Zhao, Y.; Liu, G.; Wu, J.; Chang, C. Deoxynivalenol-Induced Cytotoxicity and Apoptosis in IPEC-J2 Cells Through the Activation of Autophagy by Inhibiting PI3K-AKT-mTOR Signaling Pathway. ACS Omega 2019, 4, 18478–18486. [Google Scholar] [CrossRef]
- Han, J.; Wang, Q.C.; Zhu, C.C.; Liu, J.; Zhang, Y.; Cui, X.S.; Kim, N.H.; Sun, S.C. Deoxynivalenol exposure induces autophagy/apoptosis and epigenetic modification changes during porcine oocyte maturation. Toxicol. Appl. Pharmacol. 2016, 300, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Gozzelino, R.; Jeney, V.; Soares, M.P. Mechanisms of cell protection by heme oxygenase-1. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 323–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, H.; Amersi, F.; Buelow, R.; Melinek, J.; Coito, A.J.; Ke, B.; Busuttil, R.W.; Kupiec-Weglinski, J.W. Heme oxygenase-1 overexpression protects rat livers from ischemia/reperfusion injury with extended cold preservation. Am. J. Transplant. 2001, 1, 121–128. [Google Scholar] [CrossRef]
- Barikbin, R.; Neureiter, D.; Wirth, J.; Erhardt, A.; Schwinge, D.; Kluwe, J.; Schramm, C.; Tiegs, G.; Sass, G. Induction of heme oxygenase 1 prevents progression of liver fibrosis in Mdr2 knockout mice. Hepatology 2012, 55, 553–562. [Google Scholar] [CrossRef]
- Li, D.; Zhao, D.; Du, J.; Dong, S.; Aldhamin, Z.; Yuan, X.; Li, W.; Du, H.; Zhao, W.; Cui, L.; et al. Heme oxygenase-1 alleviated non-alcoholic fatty liver disease via suppressing ROS-dependent endoplasmic reticulum stress. Life Sci. 2020, 253, 117678. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Mazumder, S.; Siddiqui, A.A.; Iqbal, M.S.; Banerjee, C.; Sarkar, S.; De, R.; Goyal, M.; Bindu, S.; Bandyopadhyay, U. Association of heme oxygenase 1 with the restoration of liver function after damage in murine malaria by Plasmodium yoelii. Infect. Immun. 2014, 82, 3113–3126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Z.; Liao, Y.X.; Wang, X.Q.; Chen, L.K.; Wang, L.L.; Qin, C.Y.; Wang, Z.T.; Cai, M.Y.; Hu, J.W.; Li, D.; et al. Heme oxygenase-1 regulates autophagy through carbon-oxygen to alleviate deoxynivalenol-induced hepatic damage. Arch. Toxicol. 2020, 94, 573–588. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J. Deoxynivalenol-induced proinflammatory gene expression: Mechanisms and pathological sequelae. Toxins 2010, 2, 1300–1317. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Yu, M.; Fu, J.; Bao, W.; Wang, D.; Hao, L.; Yao, P.; Nussler, A.K.; Yan, H.; Liu, L. Deoxynivalenol induced oxidative stress and genotoxicity in human peripheral blood lymphocytes. Food Chem. Toxicol. 2014, 64, 383–396. [Google Scholar] [CrossRef]
- Iverson, F.; Armstrong, C.; Nera, E.; Truelove, J.; Fernie, S.; Scott, P.; Stapley, R.; Hayward, S.; Gunner, S. Chronic feeding study of deoxynivalenol in B6C3F1 male and female mice. Teratog. Carcinog. Mutagen. 1995, 15, 283–306. [Google Scholar] [CrossRef]
- Tardivel, C.; Airault, C.; Djelloul, M.; Guillebaud, F.; Barbouche, R.; Troadec, J.D.; Gaige, S.; Dallaporta, M. The food born mycotoxin deoxynivalenol induces low-grade inflammation in mice in the absence of observed-adverse effects. Toxicol. Lett. 2015, 232, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Tiemann, U.; Brussow, K.P.; Kuchenmeister, U.; Jonas, L.; Kohlschein, P.; Pohland, R.; Danicke, S. Influence of diets with cereal grains contaminated by graded levels of two Fusarium toxins on selected enzymatic and histological parameters of liver in gilts. Food Chem. Toxicol. 2006, 44, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Tiemann, U.; Brussow, K.P.; Kuchenmeister, U.; Jonas, L.; Pohland, R.; Reischauer, A.; Jager, K.; Danicke, S. Changes in the spleen and liver of pregnant sows and full-term piglets after feeding diets naturally contaminated with deoxynivalenol and zearalenone. Vet. J. 2008, 176, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Gouze, M.E.; Laffitte, J.; Dedieu, G.; Galinier, A.; Thouvenot, J.P.; Oswald, I.P.; Galtier, P. Individual and combined effects of low oral doses of deoxynivalenol and nivalenol in mice. Cell. Mol. Biol. (Noisy-Le-Grand Fr.) 2005, 51, OL809–OL817. [Google Scholar]
- Pollmann, D.S.; Koch, B.A.; Seitz, L.M.; Mohr, H.E.; Kennedy, G.A. Deoxynivalenol-contaminated wheat in swine diets. J. Anim. Sci. 1985, 60, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Bouaziz, C.; Abid-Essefi, S.; Bouslimi, A.; El Golli, E.; Bacha, H. Cytotoxicity and related effects of T-2 toxin on cultured Vero cells. Toxicon 2006, 48, 343–352. [Google Scholar] [CrossRef]
- Sahu, S.C.; Garthoff, L.H.; Robl, M.G.; Chirtel, S.J.; Ruggles, D.I.; Flynn, T.J.; Sobotka, T.J. Rat liver clone-9 cells in culture as a model for screening hepatotoxic potential of food-related products: Hepatotoxicity of deoxynivalenol. J. Appl. Toxicol. 2008, 28, 765–772. [Google Scholar] [CrossRef]
- Zhang, X.O.; Jiang, L.P.; Geng, C.Y.; Cao, J.; Zhong, L.F. The role of oxidative stress in deoxynivalenol-induced DNA damage in HepG2 cells. Toxicon 2009, 54, 513–518. [Google Scholar] [CrossRef]
- Braicu, C.; Berindan-Neagoe, I.; Tudoran, O.; Balacescu, O.; Rugina, D.; Gherman, C.; Socaciu, C.; Irimie, A. In vitro evaluation of the chemoprotective action of flavan-3-ols against deoxynivalenol related toxicity. Arch. Zootech. 2009, 12, 45–55. [Google Scholar]
- Johnson, S.A.S.; Pardini, R.S. Antioxidant enzyme response to hypericin in EMT6 mouse mammary carcinoma cells. Free Radic. Biol. Med. 1998, 24, 817–826. [Google Scholar] [CrossRef]
- Li, R.; Zhou, X.Q.; Liu, D.; Feng, W. Enhancing the activity and stability of Mn-superoxide dismutase by one-by-one ligation to catalase. Free. Radic. Bio. Med. 2018, 129, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Branicky, R.; Noe, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Dogar, I.; Dixon, S.; Gill, R.; Young, A.; Mallay, S.; Oldford, C.; Mailloux, R.J. C57BL/6J mice upregulate catalase to maintain the hydrogen peroxide buffering capacity of liver mitochondria. Free Radic. Bio. Med. 2020, 146, 59–69. [Google Scholar] [CrossRef]
- Mondola, P.; Damiano, S.; Sasso, A.; Santillo, M. The Cu, Zn Superoxide Dismutase: Not Only a Dismutase Enzyme. Front. Physiol. 2016, 7, 594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeLeve, L.D.; Kaplowitz, N. Glutathione metabolism and its role in hepatotoxicity. Pharmacol. Ther. 1991, 52, 287–305. [Google Scholar] [CrossRef]
- Braca, A.; Sortino, C.; Politi, M.; Morelli, I.; Mendez, J. Antioxidant activity of flavonoids from Licania licaniaeflora. J. Ethnopharmacol. 2002, 79, 379–381. [Google Scholar] [CrossRef]
- De Bont, R.; van Larebeke, N. Endogenous DNA damage in humans: A review of quantitative data. Mutagenesis 2004, 19, 169–185. [Google Scholar] [CrossRef] [Green Version]
- Le Drean, G.; Auffret, M.; Batina, P.; Arnold, F.; Sibiril, Y.; Arzur, D.; Parent-Massin, D. Myelotoxicity of trichothecenes and apoptosis: An in vitro study on human cord blood CD34(+) hematopoietic progenitor. Toxicol. Vitr. 2005, 19, 1015–1024. [Google Scholar] [CrossRef]
- Minervini, F.; Fornelli, F.; Flynn, K.M. Toxicity and apoptosis induced by the mycotoxins nivalenol, deoxynivalenol and fumonisin B1 in a human erythroleukemia cell line. Toxicol. Vitr. 2004, 18, 21–28. [Google Scholar] [CrossRef]
- Islam, Z.; Moon, Y.S.; Zhou, H.R.; King, L.E.; Fraker, P.J.; Pestka, J.J. Endotoxin potentiation of trichothecene-induced lymphocyte apoptosis is mediated by up-regulation of glucocorticoids. Toxicol. Appl. Pharmacol. 2002, 180, 43–55. [Google Scholar] [CrossRef]
- Islam, Z.; Pestka, J.J. Role of IL-1 beta in endotoxin potentiation of deoxynivalenol-induced corticosterone response and leukocyte apoptosis in mice. Toxicol. Sci. 2003, 74, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W.; Choi, A.M.K. Heme oxygenase-1: Molecular mechanisms of gene expression in oxygen-related stress. Antioxid. Redox Signal. 2002, 4, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Melchiorri, D.; Sewerynek, E.; Poeggeler, B.; Barlow-Walden, L.; Chuang, J.; Ortiz, G.G.; Acuña-Castroviejo, D. A review of the evidence supporting melatonin’s role as an antioxidant. J. Pineal Res. 1995, 18, 1–11. [Google Scholar] [CrossRef]
- Sies, H. Strategies of antioxidant defense. Eur. J. Biochem. 1993, 215, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Bump, E.A.; Brown, J.M. Role of glutathione in the radiation response of mammalian cells in vitro and in vivo. Pharmacol. Ther. 1990, 47, 117–136. [Google Scholar] [CrossRef]
- Chu, G. Cellular responses to cisplatin. The roles of DNA-binding proteins and DNA repair. J. Biol. Chem. 1994, 269, 787–790. [Google Scholar] [CrossRef]
- Lautier, D.; Luscher, P.; Tyrrell, R.M. Endogenous glutathione levels modulate both constitutive and uva radiation/hydrogen peroxide inducible expression of the human heme oxygenase gene. Carcinogenesis 1992, 13, 227–232. [Google Scholar] [CrossRef]
- Parzych, K.R.; Klionsky, D.J. An overview of autophagy: Morphology, mechanism, and regulation. Antioxid. Redox Signal. 2014, 20, 460–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizunoe, Y.; Kobayashi, M.; Sudo, Y.; Watanabe, S.; Yasukawa, H.; Natori, D.; Hoshino, A.; Negishi, A.; Okita, N.; Komatsu, M.; et al. Trehalose protects against oxidative stress by regulating the Keap1-Nrf2 and autophagy pathways. Redox Biol. 2018, 15, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Tomasández, S.; Blanco, J.; Rojas, C.; Roca-Martinez, J.; Ojeda-Montes, M.J.; Beltran-Debon, R.; Garcia-Vallve, S.; Pujadas, G.; Arola, L.; Mulero, M. Resveratrol Potently Counteracts Quercetin Starvation-Induced Autophagy and Sensitizes HepG2 Cancer Cells to Apoptosis. Mol. Nutr. Food Res. 2018, 62. [Google Scholar] [CrossRef]
- Xu, D.W.; Chen, L.L.; Chen, X.S.; Wen, Y.K.; Yu, C.; Yao, J.F.; Wu, H.L.; Wang, X.; Xia, Q.; Kong, X.N. The triterpenoid CDDO-imidazolide ameliorates mouse liver ischemia-reperfusion injury through activating the Nrf2/HO-1 pathway enhanced autophagy. Cell Death Dis. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Yao, P.; Nussler, A.; Liu, L.; Hao, L.; Song, F.; Schirmeier, A.; Nussler, N. Quercetin protects human hepatocytes from ethanol-derived oxidative stress by inducing heme oxygenase-1 via the MAPK/Nrf2 pathways. J. Hepatol. 2007, 47, 253–261. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Z.; Wang, L.; Liao, Y.; Peng, Z.; Li, D.; Zhou, X.; Liu, S.; Li, Y.; Nüssler, A.K.; Liu, L.; et al. The Protective Effect of Heme Oxygenase-1 on Liver Injury Caused by DON-Induced Oxidative Stress and Cytotoxicity. Toxins 2021, 13, 732. https://doi.org/10.3390/toxins13100732
Meng Z, Wang L, Liao Y, Peng Z, Li D, Zhou X, Liu S, Li Y, Nüssler AK, Liu L, et al. The Protective Effect of Heme Oxygenase-1 on Liver Injury Caused by DON-Induced Oxidative Stress and Cytotoxicity. Toxins. 2021; 13(10):732. https://doi.org/10.3390/toxins13100732
Chicago/Turabian StyleMeng, Zitong, Liangliang Wang, Yuxiao Liao, Zhao Peng, Dan Li, Xiaolei Zhou, Shuang Liu, Yanmei Li, Andreas K. Nüssler, Liegang Liu, and et al. 2021. "The Protective Effect of Heme Oxygenase-1 on Liver Injury Caused by DON-Induced Oxidative Stress and Cytotoxicity" Toxins 13, no. 10: 732. https://doi.org/10.3390/toxins13100732
APA StyleMeng, Z., Wang, L., Liao, Y., Peng, Z., Li, D., Zhou, X., Liu, S., Li, Y., Nüssler, A. K., Liu, L., Hao, L., & Yang, W. (2021). The Protective Effect of Heme Oxygenase-1 on Liver Injury Caused by DON-Induced Oxidative Stress and Cytotoxicity. Toxins, 13(10), 732. https://doi.org/10.3390/toxins13100732