Overview of Multiple Applications of Basil Species and Cultivars and the Effects of Production Environmental Parameters on Yields and Secondary Metabolites in Hydroponic Systems
Abstract
1. Introduction
- Provide an overview of the multiple uses of basil;
- Collate and present common hydroponic systems available in the market;
- Review effects of key production environmental parameters on basil yields in quantity and quality in hydroponic systems;
- Summarize the effects of the growth environments on basil microgreen yield quantity and quality.
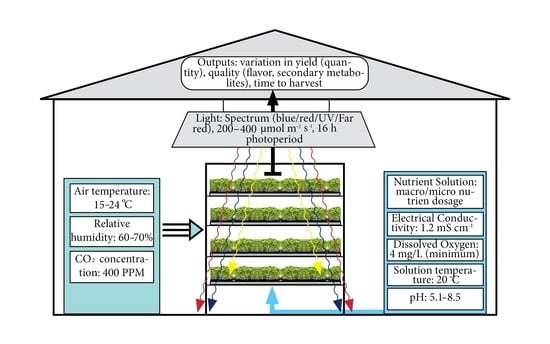
2. Materials and Methods
3. Results
3.1. Multiple Applications of Basil
3.2. Overview of Hydroponics Systems for Basil Microgreen Production
3.3. Production Environment in Hydroponics Systems
3.3.1. Germination
3.3.2. Light: Light Intensity, Color Spectrum, Photoperiodism, Position
- Light intensity, which is a quantitative measure that refers to the number of photons that reach the leaf level during a unit of time (μmol m−2 s−1). This is the most important parameter for the photosynthetic process. Upon the placement of lamps, one should consider their type and the fact that light intensity is reduced by the square of distance [63]. Different types of lamps also produce variable amounts of heat, which necessitates a certain distance from the young vulnerable microgreens;
- Spectral distribution of light is the wavelength of light that the plants are exposed to. For photosynthesis, plants respond best to red and blue light spectra (300–450 nm for blue, 620–750 for red). However, new research is pinpointing that photomorphogenesis occurs when plants, including microgreens, are exposed to wavelengths outside the traditional range: for instance, it has been shown that plants respond to UV light, in the range of 260–400, and to far-red light, from 750 to 780; this photomorphogenesis results in higher accumulations of specific phytochemicals and nutrients that improve the flavor and health benefits of microgreens [64,65,66,67,68];
- Photoperiodism, which refers to the lighting duration, has the most relevance for inciting phenological stages, such as blooming. According to the crop species, the generative phase can be controlled by manipulating photoperiodism [69]. It is, therefore, appropriate to have a long photoperiod for increased photosynthesis as well as ensuring the microgreens do not become cued to a more autumnal day length.
3.3.3. Air and Water Temperature
3.3.4. Relative Humidity
3.3.5. Dissolved Oxygen
3.3.6. The Nutrient Solution
3.3.7. pH
3.3.8. Electrical Conductivity (EC)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giurgiu, G.M. Research on Hydroponic Cultivation of Some Medicinal and Aromatic Plant Species and the Influence of the Technology on the Bioactive Substance. Ph.D. Thesis, USAMV Cluj-Napoca, Cluj-Napoca, Romania, 2016. [Google Scholar]
- De Vries, G. Feeding the world in the 21st century. Trends Plant. Sci. 2000, 5, 190. [Google Scholar] [CrossRef]
- Khazaie, H.R.; Nadjafi, F.; Bannayan, M. Effect of irrigation frequency and planting density on herbage biomass and oil production of thyme (Thymus vulgaris) and hyssop (Hyssopus officinalis). Ind. Crop. Prod. 2008, 27, 315–321. [Google Scholar] [CrossRef]
- Chen, F.; Tang, Y.N.; Shen, M.Y. Coordination control of greenhouse environ-mental factors. Int. J. Autom. Comput. 2011, 8, 147–153. [Google Scholar] [CrossRef]
- Ncube, B.; Finnie, J.F.; Van Staden, J. Quality from the field: The impact of environmental factors as quality determinants in medicinal plants. S. Afr. J. Bot. 2012, 82, 11–20. [Google Scholar] [CrossRef]
- Tawaha, A.A.; Karaki, G.A.; Adnan, M. Antioxidant activity, total phenols and variation of chemical composition from essential oil in sage (Salvia officinalis L.) grown under protected soilless condition and open field conditions. Adv. Environ. Biol. 2013, 7, 894–901. [Google Scholar]
- Lee, J.S.; Pill, W.G.; Cobb, B.B.; Olszewski, M. Seed treatments to advance greenhouse establishment of beet and chard microgreens. J. Hortic. Sci. Biotechnol. 2004, 79, 565–570. [Google Scholar] [CrossRef]
- Xiao, Z.; Lester, G.E.; Luo, Y.; Wang, Q. Assessment of vitamin and carotenoid concentrations of emerging food products: Edible microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651. [Google Scholar] [CrossRef] [PubMed]
- Pannico, A.; El-Nakhel, C.; Graziani, G.; Kyriacou, M.C.; Giordano, M.; Soteriou, G.A.; Zarrelli, A.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Selenium biofortification impacts the nutritive value, polyphenolic content, and bioactive constitution of variable microgreens genotypes. Antioxidants 2020, 9, 272. [Google Scholar] [CrossRef] [PubMed]
- Bulgari, R.; Baldi, A.; Ferrante, A.; Lenzi, A. Yield and quality of basil, Swiss chard, and rocket microgreens grown in a hydroponic system, New Zealand. J. Crop. Hortic. Sci. 2017, 45, 119–129. [Google Scholar] [CrossRef]
- Murphy, C.J.; Llort, K.F.; Pill, W.G. Factors affecting the growth of microgreen table beet. Int. J. Veg. Sci. 2010, 16, 253–266. [Google Scholar] [CrossRef]
- Kou, L.P.; Yang, T.B.; Luo, Y.G.; Liu, X.J.; Huang, L.H.; Codling, E. Pre-harvest calcium application increases biomass and delays senescence of broccoli microgreens. Postharvest Biol. Technol. 2014, 87, 70–78. [Google Scholar] [CrossRef]
- Sun, J.; Kou, L.; Geng, P.; Huang, H.; Yang, T.; Luo, Y.; Chen, P. Metabolomic assessment reveals an elevated level of glucosinolate content in CaCl2 treated broccoli microgreens. J. Agric. Food Chem. 2015, 63, 1863–1868. [Google Scholar] [CrossRef]
- Murphy, C.J.; Pill, W.G. Cultural practices to speed the growth of microgreen arugula (roquette; Eruca vesicaria subsp. sativa). J. Hortic. Sci. Biotechnol. 2010, 85, 171–176. [Google Scholar] [CrossRef]
- Biró-Janka, B. Studies Regarding the Biology and Cultivation Technology of Basil (Ocimum basilicum L.) in the Eastern Part of the Transylvanian Plain. Ph.D. Thesis, USAMV Cluj-Napoca, Cluj-Napoca, Romania, 2020. [Google Scholar]
- Al Murad, M.; Razi, K.; Jeong, B.R.; Samy, P.M.A.; Muneer, S. Light emitting diodes (LEDs) as agricultural lighting: Impact and its potential on improving physiology, flowering, and secondary metabolites of crops. Sustainability 2021, 13, 1985. [Google Scholar] [CrossRef]
- El-Tantawy, W.H.; Temraz, A. Natural products for controlling hyperlipidemia: Review. Arch. Physiol. Biochem. 2018, 125, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.A.; Abourashed, E.A. Leung’s Encyclopedia of Common Natural Ingredients: Used in Food, Drugs, and Cosmetics, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 74–75. [Google Scholar]
- Grayer, R.J.; Kite, G.C.; Goldstone, F.J.; Bryan, S.E.; Paton, A.; Putievsky, E. Infraspecific taxonomy and essential oil chemotypes in sweet basil, Ocimum basilicum. Phytochemistry 1996, 43, 1033–1039. [Google Scholar] [CrossRef]
- Carović-Stanko, K.; Orlić, S.; Politeo, O.; Strikić, F.; Kolak, I.; Milos, M.; Satovic, Z. Composition and antibacterial activities of essential oils of seven Ocimum taxa. Food Chem. 2010, 119, 196–201. [Google Scholar] [CrossRef]
- Taie, H.A.A.; Abd-El, Z.; Salama, R.; Radwan, S. Potenial activity of basyl plants as a source of antioxidants and anticancer agents as affected by organic and bio-organic fertilization. Not. Bot. Horti Agrobot. Cluj-Napoca 2010, 38, 119–127. [Google Scholar] [CrossRef]
- Oxenham, S.K.; Svoboda, K.P.; Walters, D.R. Antifungal activity of the essential oil of basil (Ocimum basilicum). J. Phytopathol. 2005, 153, 174–180. [Google Scholar] [CrossRef]
- Lee, S.J.; Umano, K.; Shibamoto, T.; Lee, K.G. Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food Chem. 2005, 91, 131–137. [Google Scholar] [CrossRef]
- Politeo, O.; Jukic, M.; Milos, M. Chemical composition and antioxidant capacity of free volatile aglycones from basil (Ocimum basilicum L.) compared with its essential oil. Food Chem. 2007, 101, 379–385. [Google Scholar] [CrossRef]
- Erler, F.; Ulug, I.; Yalcinkaya, B. Repellent activity of five essential oils against culex pipiens. Fitoterapia 2006, 77, 491–494. [Google Scholar] [CrossRef]
- Chiriac, I.P.; Ulea, E. Study Regarding effectiveness of some plant extracts and different pesticides against an Erwinia amylovora (Burrill.) Winslow et al. strain isolated from quince. Cercet. Agron. Mold. 2012, 45, 69–74. [Google Scholar] [CrossRef]
- Gonceariuc, M. Plante Medicinale și Aromatice Cultivate, 1st ed.; Academia de Științe a Moldovei: Chișinău, Moldova, 2008; pp. 18–25. [Google Scholar]
- Zhang, J.W.; Li, S.K.; Wu, W.J. The main chemical composition and in vitro antifungal activity of the essential oils of Ocimum basilicum Linn. var. Pilosum (Willd.) Benth. Molecules 2009, 14, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.L.; Cho, I.K.; Li, Q.X. Insecticidal activity of basil oil, trans-anethole, estragole, and linalool to adult fruit flies Ceratitis capitata, Bactrocera dorsalis, and Bactrocera cucurbitae. J. Econ. Entomol. 2009, 102, 203–209. [Google Scholar] [CrossRef]
- Ciocan, V. Plante Etnobotanice din România: Între Adevăr şi Pericol; Editura Ceres: Bucureşti, Romania, 2011; pp. 129–134. [Google Scholar]
- Oshaghi, E.; Tavilani, H.; Khodadadi, I.; Goodarzi, M. Dill tablet: A potential anti-oxidant and anti-diabetic medicine. Asian Pac. J. Trop. Biomed. 2015, 5, 720–727. [Google Scholar] [CrossRef]
- Zahra, A.A.; Al Fadhil, A.O. Antibacterial activity of Ocimum basilicum (Rehan) leaf extract against bacterial pathogens in Sudan. Am. J. Res. Commun. 2015, 3, 94–99. [Google Scholar]
- Milică, C.I.; Roman, C.N.; Troia, D. Flora Medicinală a României; Editura Doxologia: Iaşi, Romania, 2012; p. 57. [Google Scholar]
- Muntean, L.S.; Tămaş, M.; Muntean, S. Tratat de Plante Medicinale Cultivate şi Spontane, 2nd ed.; Edit. Risoprint: Cluj-Napoca, Romania, 2016; pp. 485–495. [Google Scholar]
- Araújo, S.V.; Sousa, J.P.D.; Pessôa, H.D.L.F.; Freitas, A.F.R.D.; Coutinho, H.D.M.; Alves, L.B.N.; Lima, E.O. Ocimum basilicum: Antibacterial activity and association study with antibiotics against bacteria of clinical importance. Pharm. Biol. 2016, 54, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Takwa, S.; Caleja, C.; Barreira, J.C.M.; Soković, M.; Achour, L.; Barros, L.; Ferreira, I.C.F.R. Arbutus unedo L. and Ocimum basilicum L. as sources of natural preservatives for food industry: A case study using loaf bread. LWT 2018, 88, 47–55. [Google Scholar] [CrossRef]
- Amor, G.; Sabbah, M.; Caputo, L.; Idbella, M.; De Feo, V.; Porta, R.; Fechtali, T.; Mauriello, G. Basil essential oil: Composition, antimicrobial properties, and microencapsulation to produce active chitosan films for food packaging. Foods 2021, 10, 121. [Google Scholar] [CrossRef]
- Tacchini, M.; Guevara, M.P.E.; Grandini, A.; Maresca, I.; Radice, M.; Angiolella, L.; Guerrini, A. Ocimum campechianum Mill. from Amazonian Ecuador: Chemical composition and biological activities of extracts and their main constituents (eugenol and rosmarinic acid). Molecules 2021, 26, 84. [Google Scholar] [CrossRef]
- Bernáth, J.; Székely, J. Gyógy—És Aromanövények; Editura Mezőgazda: Budapesta, Hungary, 2006; pp. 367–370. [Google Scholar]
- Purkayastha, J.; Nath, S.C. Composition of the camphor-rich essential oil of Ocimum basilicum L. native to Northeast India. J. Essent. Oil Res. 2006, 18, 332–334. [Google Scholar] [CrossRef]
- Kutta, G. A Lamiaceae Családra Jellemző Illó És Nem Illó Terpén, Illetve Fenolos Komponensek Kivonása Szuperkritikus Fluid Extrakcióval; Universitatea Corvinus din Budapesta: Budapesta, Hungary, 2010; pp. 17–19. [Google Scholar]
- Lawrence, B.M. A further examination of the variation of Ocimum basilicum L. Dev. Food Sci. 1988, 18, 161–170. [Google Scholar]
- Koutsos, T.V.; Chatzopoulou, P.S.; Katsiotis, S.T. Effects of individual selection on agronomical and morphological traits and essential oil of a “Greek Basil” population. Euphytica 2009, 170, 365–370. [Google Scholar] [CrossRef]
- Sestili, P.; Ismail, T.; Calcabrini, C.; Guescini, M.; Catanzaro, E.; Turrini, E.; Layla, A.; Akhtar, S.; Fimognari, C. The potential effects of Ocimum basilicum on health: A review of pharmacological and toxicological studies. Expert Opin. Drug Metab. Toxicol. 2018, 14, 679–692. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Lee, J.; Scagel, C.F. Chicoric acid found in basil (Ocimum basilicum L.) Leaves. Food Chem. 2009, 115, 650–656. [Google Scholar] [CrossRef]
- Surveswaran, S.; Cai, Y.; Corke, H.; Sun, M. Systematic evaluation of natural phenolic antioxidants from 133 indian medicinal plants. Food Chem. 2007, 102, 938–953. [Google Scholar] [CrossRef]
- Kwee, E.M.; Niemeyer, E.D. Variations in phenolic composition and antioxidant properties among 15 basil (Ocimum basilicum L.) cultivars. Food Chem. 2011, 128, 1044–1050. [Google Scholar] [CrossRef]
- Asghari, G.; Gholamali, H.; Mahmoudi, Z.; Asghari, M. Diurnal variation of essential oil components of Pycnocycla spinosa Decne. ex Boiss. Junidishapur J. Nat. Pharm. Maceutical Prod. 2014, 9, 35–38. [Google Scholar] [CrossRef]
- Dzida, K. Nutrients contents in sweet basil (Ocimum basilicum L.) Herb depending on calcium carbonate dose and cultivar. Acta scientiarum Polonorum. Hortorum Cultus 2010, 9, 143–151. [Google Scholar]
- Snežana, F. Basil (Ocimum basilicum L.) a source of valuable phytonutrients. Int. J. Clin. Nutr. Diet. 2017, 3, 118. [Google Scholar] [CrossRef]
- Bîlteanu, G. Fitotehnie, 2nd ed.; Editura Ceres: București, Romania, 2001; p. 544. [Google Scholar]
- Fallahi, H.R.; Mohammadi, M.; Aghhavani-Shajari, M.; Ranjbar, F. Determination of germination cardinal temperatures in two basil (Ocimum basilicum L.) cultivars using non-linear regression models. J. Appl. Res. Med. Aromat. Plants 2015, 2, 140–145. [Google Scholar] [CrossRef]
- Nadjafi, E.; Tabrizi, L.; Shabahang, J.; Mahdavi-Damghani, A.M. Cardinal germination temperatures of some medicinal plant species. Seed Technol. 2009, 31, 156–163. [Google Scholar]
- Kumar, B. Prediction of germination potential in seeds of Indian basil (Ocimum basilicum L.). J. Crop. Improv. 2012, 26, 532–539. [Google Scholar] [CrossRef]
- Zhou, D.; Jacob, B.; Ponder, M.A.; Welbaum, G.E. Germination response of six sweet basil (Ocimum basilicum) cultivars to temperature. Seed Technol. J. 2016, 37, 43–51. [Google Scholar]
- Lariguet, P.; Ranocha, P.; De Meyer, M.; Barbier, O.; Penel, C.; Dunand, C. Identification of a hydrogen peroxide signaling pathway in the control of light-dependent germination in Arabidopsis. Planta 2013, 238, 381–395. [Google Scholar] [CrossRef]
- Rakshit, A.; Singh, H.B. Advances in Seed Priming; Springer: Singapore, 2018; pp. 147–183. [Google Scholar] [CrossRef]
- Noorhosseini, S.A.; Jokar, N.K.; Damalas, C.A. Improving seed germination and early growth of garden cress (Lepidium sativum) and basil (Ocimum basilicum) with hydro-priming. J. Plant Growth Regul. 2018, 37, 323–334. [Google Scholar] [CrossRef]
- Delavari, P.; Baghizadeh, A.; Enteshari, S.; Kalantari, K.; Yazdanpanah, A.; Mousavi, E.A. The effects of salicylic acid on some of biochemical and morphological characteristic of Ocimum basilicucm under salinity stress. Aust. J. Basic Appl. Sci. 2010, 4, 4832–4845. [Google Scholar]
- Elhindi, K.M.; Dewir, Y.H.; Asrar, A.W.; Abdel-Salam, E.; Sharaf El-Din, A.; Ali, M. Improvement of seed germination in three medicinal plant species by plant growth regulators. HortScience 2016, 51, 887–891. [Google Scholar] [CrossRef]
- Rusu, T. Agrotehnica, 1st ed.; Editura Risoprint: Cluj-Napoca, Romania, 2005; p. 68. [Google Scholar]
- Sabzalian, M.R.; Heydarizadeh, P.; Zahedi, M.; Boroomand, A.; Agharockh, M.; Sahba, M.R.; Schoefs, B. High performance of vegetables, flowers, and medicinal plants in a red-blue LED incubator for indoor plant production. Agron. Sustain. Dev. 2014, 34, 879–886. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Morrow, R.C.; Sams, C.R. Blue wavelengths from LED lighting increase nutritionally important metabolites in specialty crops. HortScience 2015, 50, 1285–1288. [Google Scholar] [CrossRef]
- Ouzounis, T.; Heuvelink, E.; Ji, Y.; Schouten, H.J.; Visser, R.G.F.; Marcelis, L.F.M. Blue and red LED lighting effects on plant biomass, stomatal conductance, and metabolite content in nine tomato genotypes. Acta Hortic. 2016, 1134, 251–258. [Google Scholar] [CrossRef]
- Lobiuc, A.; Vasilache, V.; Oroian, M.; Stoleru, T.; Burducea, M.; Pintilie, O.; Zamfirache, M.M. Blue and red LED illumination improves growth and bioactive compounds contents in acyanic and cyanic Ocimum basilicum L. microgreens. Molecules 2017, 22, 2111. [Google Scholar] [CrossRef] [PubMed]
- Ying, Q.L.; Kong, Y.; Zheng, Y.B. Growth and appearance quality of four microgreen species under light-emitting diode lights with different spectral combinations. Hortscience 2020, 55, 1399–1405. [Google Scholar] [CrossRef]
- Singh, D.; Basu, C.; Meinhardt-Wollweber, M.; Roth, B. LEDs for energy efficient greenhouse lighting. Renew. Sustain. Energy Rev. 2015, 49, 139–147. [Google Scholar] [CrossRef]
- Samuolienė, G.; Brazaitytė, A.; Sirtautas, R.; Sakalauskienė, S.; Jankauskiene, J.; Duchovskis, P.; Novickovas, A. The impact of supplementary short-term red LED lighting on the antioxidant properties of microgreens. Acta Hortic. 2012, 956, 649–655. [Google Scholar] [CrossRef]
- Leroy, C.; Petitclerc, F.; Orivel, J.; Corbara, B.; Carrias, J.F.; Dejean, A.; Céréghino, R. The influence of light, substrate and seed origin on the germination and establishment of ant-garden bromeliad. Plant Biol. 2017, 19, 70–78. [Google Scholar] [CrossRef]
- Jacobsen, J.V.; Barrero, J.M.; Hughes, T.; Julkowska, M.; Taylor, J.M.; Xu, Q.; Gubler, F. Roles for blue light, jasmonate and nitric oxide in the regulation of dormancy and germination in wheat grain (Triticum aestivum L.). Planta 2013, 238, 121–138. [Google Scholar] [CrossRef]
- Wang, Y.; Folta, K.M. Contributions of green light to the plant growth and development. Am. J. Bot. 2013, 100, 70–78. [Google Scholar] [CrossRef]
- Flores, A.M.; Schutte, B.J.; Shukla, M.K.; Picchioni, G.A.; Ulery, A.L. Time-integrated measurements of seeds germination for salt tolerant plant species. Seed Sci. Technol. 2015, 43, 541–547. [Google Scholar] [CrossRef]
- Footitt, S.; Huang, Z.; Clay, H.A.; Mead, A.; Finch-Savage, W.E. Temperature, light and nitrate sensing coordinate Arabidopsis seed dormancy cycling, resulting in winter and summer annual phenotypes. Plant. J. 2013, 74, 1003–1015. [Google Scholar] [CrossRef]
- Renade, S.S.; Gil, M.R.G. Application of monochromatic blue light during germination and hypocotyl development improves outplanted Scot spine (Pinus sylvestris L.) trees performance. For. Ecol. Manag. 2016, 361, 368–374. [Google Scholar] [CrossRef]
- Rahman, M.M.; Vasiliev, M.; Alameh, K. LED illumination spectrum manipulation for increasing the yield of sweet basil (Ocimum basilicum L.). Plants 2021, 10, 344. [Google Scholar] [CrossRef] [PubMed]
- Samuolienė, G.; Brazaitytė, A.; Viršilė, A.; Jankauskienė, J.; Sakalauskienė, S.; Duchovskis, P. Red light-dose or wavelength-dependent photoresponse of antioxidants in herb microgreens. PLoS ONE 2016, 11, e0163405. [Google Scholar] [CrossRef] [PubMed]
- Brazaitytë, A.; Virsilë, A.; Samuolienë, G.; Jankauskienë, J.; Sakalauskienë, S.; Sirtautas, R.; Novièkovas, A.; Dabašinskas, L.; Vaštakaitë, V.; Miliauskienë, J.; et al. Light quality: Growth and nutritional value of microgreens under indoor and greenhouse conditions. Acta Hortic. 2016, 1134, 277–284. [Google Scholar] [CrossRef]
- Vaštakaitė, V.; Viršilė, A.; Brazaitytė, A.; Samuolienė, G.; Jankauskienė, J.; Sirtautas, R.; Duchovskis, P. The effect of UV-a supplemental lighting on antioxidant properties of Ocimum basilicum L. microgreens in greenhouse. In Proceedings of the 7th International Scientific Conference Rural Development, Lithuania, 19–20 November 2015. [Google Scholar] [CrossRef]
- Sakamoto, M.; Uenishi, M.; Miyamoto, K.; Suzuki, T. Effect of root-zone temperature of the growth and fruit quality of hydroponically grown strawberry plants. J. Agric. Sci. 2016, 8, 122–131. [Google Scholar] [CrossRef]
- Waller, P.; Yitayew, M. Hydroponic irrigation systems. Environ. Sci. 2016, 369–386. [Google Scholar] [CrossRef]
- Bita, C.E.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant. Sci. 2013, 4, 273. [Google Scholar] [CrossRef]
- Chun, C.; Takakura, T. Rate of root respiration of lettuce under various dissolved oxygen concentrations in hydroponics. Environ. Control. Biol. 1993, 32, 125–135. [Google Scholar] [CrossRef]
- Cornejo, H.A.C.; Rodriguez, L.M.; Vergara, A.G.; Bucio, J.L. Trichoderma modulates stomatal aperture and leaf transpiration through an abscisic acid-dependent mechanism in arabidopsis. J. Plant. Growth Regul. 2015, 34, 425–434. [Google Scholar] [CrossRef]
- Roosta, H.R.; Bagheri, M.H.; Hamidpour, M.; Roozban, M.R. Interactive effects of nitrogen form and oxygen concentration on growth and nutritional status of eggplant in hydroponics. J. Agric. Sci. Technol. 2016, 18, 731–739. [Google Scholar]
- Sakamoto, M.; Suzuki, T. Elevated root-zone temperature modulates growth and quality of hydroponically grown carrots. Agric. Sci. 2015, 6, 749–757. [Google Scholar] [CrossRef]
- Msayleb, N. Soil Ozonation as a Sustainable Alternative to Methyl Bromide Fumigation and Synthetic Pesticides. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 2014. [Google Scholar] [CrossRef]
- Oliveira, L.L.; Macedo, A.F. The effect of light quality, temperature and substrate on seed germination and epicotyl development of Carapa guianensis, a multi-use neotropical tree. J. Med. Plants Res. 2015, 9, 582–593. [Google Scholar] [CrossRef]
- Baenas, N.; Garcia-Viguera, C.; Moreno, D.A. Elicitation: A tool for enriching the bioactive composition of foods. Molecules 2014, 19, 13541–13563. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.C.; Xue, X.Z.; Li, Y.K.; Li, F.; Zhang, F.; Guo, W.Z. Effect of pH upper control limit on nutrient solution component and water spinach growth under hydroponics. Adv. J. Food Sci. Technol. 2015, 9, 717–721. [Google Scholar] [CrossRef]
- Chitarra, W.; Pugliese, M.; Gilardi, G.; Gullino, M.L.; Garibaldi, A. Effect of silicates and electrical conductivity on Fusarium wilt of hydroponically grown lettuce. Commun. Agric. Appl. Biol. Sci. 2013, 78, 555–557. [Google Scholar] [PubMed]
- Bulgari, R.; Negri, M.; Santoro, P.; Ferrante, A. Quality evaluation of indoor-grown microgreens cultivated on three different substrates. Horticulturae 2021, 7, 96. [Google Scholar] [CrossRef]
- Manawasinghe, N.K.G.K.R.; Weerasekara, S.H.; Karunaratne, C.S.L.M.; Weerakkody, W.A.P.; Kulapala, B. Influence of substrate and supplementary LED lighting on vertical farming of basil (Ocimum basilicum L.) and pak choi (Brassica rapa var. chinensis). Asian J. Agric. Hortic. Res. 2021, 7, 42–52. [Google Scholar] [CrossRef]
- Puccinelli, M.; Malorgio, F.; Rosellini, I.; Pezzarossa, B. Production of selenium-biofortified microgreens from selenium-enriched seeds of basil. J. Sci. Food Agric. 2019, 99, 5601–5605. [Google Scholar] [CrossRef]
- Scagel, F.C.; Lee, J.; Mitchell, J.N. Salinity from NaCl changes the nutrient and polyphenolic composition of basil leaves. Ind. Crop. Prod. 2019, 127, 119–128. [Google Scholar] [CrossRef]
- Saha, S.; Monroe, A.; Day, M.R. Growth, yield, plant quality and nutrition of basil (Ocimum basilicum L.) under soilless agricultural systems. Ann. Agric. Sci. 2016, 61, 181–186. [Google Scholar] [CrossRef]
- Walters, K.J.; Currey, C.J. Hydroponic greenhouse basil production: Comparing systems and cultivars. HortTechnology 2015, 25, 645–650. [Google Scholar] [CrossRef]
- Kiferle, C.; Maggini, R.; Pardossi, A. Influence of nitrogen nutrition on growth and accumulation of rosmarinic acid in sweet basil (Ocimum basilicum L.) grown in hydroponic culture. Aust. J. Crop. Sci. 2013, 7, 321–327. [Google Scholar]
| No. | References | Use and Advantages of Basil |
|---|---|---|
| 1 | [19,20,21] | Used in traditional medicinal tea for the treatment of bronchial asthma, pertussis, etc., and pharmaceutical products are used for the treatment of inflammation of the airways, as a tonic of the nervous system, or as an antispasmodic remedy |
| 2 | [22] | Substances extracted have a fungicidal effect, and grain plants treated with it remained healthy |
| 3 | [23,24] | Has antibacterial, antioxidant, and antitumor effects |
| 4 | [25,26] | Volatile oil can also be used as a repellent against Culex pipiens, a mosquito that spreads diseases such as meningitis, or against fire blight (Erwinia amylovora) |
| 5 | [27] | Fresh juice can be used in otitis (inflammation or infection of the ear) |
| 6 | [28] | Volatile oil has been shown to prevent the development of Fulvia fulva, Glomerella cingulata, Alternaria alternate, and Fusarium solani var. coeruleum phytopathogens in lab conditions |
| 7 | [29] | Volatile oil and extracted estragole, linalool, methyl-eugenol, and trans-anethole compounds have shown insecticidal effects on Ceratitis capitata, Bactrocera dorsalis, and Bactrocera cucurbitae fruit flies |
| 8 | [18] | Often been used in traditional medicine, especially against intestinal worms, flu, or kidney disease, for the carminative effect (that relieves abdominal pain and promotes the evacuation of intestinal gas) and even for the treatment of insect or snake bites |
| 9 | [30] | Has shown positive impacts on diseases such as: depression, neurosis, dysfunctions of the sweat, adrenal, mammary, and sexual glands, diseases of the respiratory system (asthma, bronchitis, cold and flu, allergy), nausea, liver and gallbladder disease, kidney disease, rheumatisme, parasitic disease, insect bites and different types of skin cancer |
| 10 | [31] | Has been widely cultivated globally and used to reduce the serum lipid content |
| 11 | [32] | Extraction of ethyl alcohol has shown an antibacterial effect in the case of Escherichia coli, Pseudomonas auruginosa, Proteus mirabilis, Klebsiella pneumoniae, Staphylococcus aureus, and Enterococcus faecalis pathogens, in certain concentrations equaling the effectiveness of synthetic antibacterial substances |
| 12 | [33,34] | Known to have stimulant-digestive, bactericidal, antifungal, spasmolytic, carminative, galactogoge, expectorant, antipyretic, febrifuge, diuretic, sedative, and anti-inflammatory properties. Cultivated for use in the perfume industry to prepare different cosmetic products such as perfumes, soaps, shampoos, and toothpaste, for example |
| 13 | [1] | Volatile oils (methylchavicol, linalool) reduced the development of the Botrytis fabae phytopathogen in grain (Vicia faba) in vitro conditions |
| 14 | [35] | Volatile oils have proven effective against Staphylococcus aureus and Pseudomonas aeruginosa pathogens, acting even synergistically with certain antibacterial substances |
| 15 | [36] | Can be used in the food industry as a preservative and antioxidant for different food products |
| 16 | [17] | The World Health Organization (WHO) has estimated that medicines based on plants, especially extracts of plants, are used by almost 80% of the world population for their first treatment |
| 17 | [15] | Can be used for the treatment of a wide range of diseases and ailments: diarrhea, dysentery, nausea, vomiting, gastric ulcer, biliary colics, bloating, flatulence, aerophagia, fermentation colitis, abdominal cramps, anorexia, migraines, neuroses, depression, insomnia, headaches, gonorrhea, acne, thrush, stomatitis, etc. |
| 18 | [37] | Essential oils have been used in a new type of packing system to extend the validity period of food: microcapsules and packing systems that inhibit the bacterial activity and increase the pH of packed food |
| 19 | [38] | Essential oils have shown effectiveness when used in cosmetic formulations and food supplements as antioxidant agents |
| No. | Systems | Characteristics | Website |
|---|---|---|---|
| 1 | Wick System | The plants are placed in a container on an absorbent growth medium, and the connection to the tank with nutrient solution is made with absorbant wicks, through which the nutrient solution diffuses to the level of the plant roots. | www.hydroponics.eu (accessed on 1 July 2021) |
| 2 | Drip System | The plants are installed in a medium, and the nutrient solution is transported from the solution tank through drip tubes; the excess solution reaches the tank again and recirculates through the system. | www.trees.com (accessed on 1 July 2021) |
| 3 | Ebb and Flow System | It works by flooding and then draining the growing environment in which the roots of plants with nutrient solution are located. A pump pushes the nutrient solution out of the tank, and then the excess drains back slowly, allowing the plants to receive nutrients regularly. | www.nosoilsolutions.com (accessed on 1 July 2021) |
| 4 | Deep Water Culture (DWC) System | The plants have their roots immersed directly in the nutrient solution and float above it. As support, one can use expanded polystyrene plates with perforations in which the plants are inserted. Oxygenation of the solution is necessary. | www.epicgardening.com (accessed on 1 July 2021) |
| 5 | Nutrient Film Technology (NFT) | This system ensures a constant flow of nutrient solution directly to the plant roots. The plants are grown in perforated polyethylene tubes and PVC pipes. | www.thespruce.com (accessed on 1 July 2021) |
| 6 | Aeroponic System | This system suspends the plants on top of a water pump that directly sprays the roots with nutrient solution every few minutes. The advantages of this system are the use of a much smaller amount of water, the roots receive oxygen in large quantities, and the plants grow faster. However, the roots of the plants being suspended in the air are more prone to drying faster than in any other hydroponic system. | https://aeroponicsdiy.com (accessed on 1 July 2021) |
| 7 | Aquaponic Systems | Aquaponics integrates aquaculture and hydroponics into a single culture system. The water used in these crops normally comes from the fish farming system. The fish secrete nitrogen compounds that are captured and used by plants in their growth, prolonging water use and reducing the adjustment of the nutrient solution for plants. | www.futurefarming.group (accessed on 1 July 2021) |
| No. | Parameter | Unit of Measurement | Average Value of Parameters (Parameter Variation) |
|---|---|---|---|
| 1 | Light | W | 400 |
| 1.1 | Photoperiodicity | h | 06:30–21:30 (15 h) (10–20 h) |
| 1.2 | Light intensity | μmol m−2 s−1 | 300 (200–400) |
| 1.3 | Color spectrum | nm | 440–460 (260–780) |
| 1.4 | Position | cm | 150—Lamps HPS (high-pressure sodium) 40—Lamps LED |
| 2 | Ambient temperature | °C | 21 ± 2 day; 17 night |
| 3 | Humidity | % | 65 ± 5 (50–60) |
| 4 | Nutrient | N-P-K: 3-2-3 (%) | Changed every 10 days |
| 5 | pH | pH units | 6.8 ± 0.4 |
| 6 | Electrical conductivity | mS | 1.2 ± 0.2 |
| 7 | Dissolved oxygen | mg L−1 | 6.5 |
| 8 | Solution temperature | °C | 20 ± 2 |
| Basil Cultivar | Germinated Seeds at Different Temperatures (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 10 °C | 15 °C | 20 °C | |||||||
| Control | Gibberellic Acid | Ascorbic Acid | Control | Gibberellic Acid | Ascorbic Acid | Control | Gibberellic Acid | Ascorbic Acid | |
| Aromat de Buzău | - | - | - | 8 | 6 | 7.75 | 3 | 3 | 3.5 |
| Serafim | - | 14 | - | 4.5 | 3.75 | 5 | 2.75 | 2 | 2 |
| Busuioc Dulce | - | - | - | 5.5 | 3 | 5.25 | 3 | 2 | 2 |
| Italiano Classico | - | - | - | 4.5 | 3 | 5 | 3 | 2 | 1.75 |
| Dark Opal | - | 11 | - | 4.5 | 3.5 | 4 | 2.75 | 2 | 1.5 |
| Genovese | - | 14 | - | 3.25 | 3 | 5 | 2 | 2 | 2 |
| Grand Verte | - | 13 | - | 3.5 | 3.25 | 4 | 3 | 2 | 2 |
| No. | Crop | Treatments | Effects | Reference |
|---|---|---|---|---|
| 1 | Basil, Ocimum basilicum L., sweet basil | Three treatments: only white LED (W); combination of blue and red LED (BR) (84% R, 16% B); combination of red (R), blue (B) and far-red (F) LED (BRF) (79% R, 11% B, and 10% F); PPFD was set at 155 µmol m−2 s−1; photoperiod: 20 h | Combination of blue (B), red (R), and far-red (F) LED illumination led to a one-fold increase in the yield in comparison with only white LED illumination (W); on the other hand, the use of blue (B) and red (R) LED illumination resulted in a half-fold increase in plant yield; the results of this study demonstrated the commercial viability of both BRF-, and BR- illuminated grow tents compared to the commonly used W-illuminated counterparts | [77] |
| 2 | Basil, Ocimum basilicum L., Sweet Genovese, and Red Rubin | Different ratios of LED blue and red illumination; 4 light treatments were 100% white (White) and various red (R) to blue (B) ratios, as follows: 2R:1B, 1R:1B, and 1R:2B, intensities | Growth was enhanced with predominantly blue illumination, leading to larger cotyledon area and higher fresh mass. The same treatment elevated chlorophyll a and anthocyanin pigments contents. Stimulation of phenolic synthesis and free radical scavenging activity was improved by predominantly red light in the green cultivar and by predominantly blue light in the red cultivar | [68] |
| 3 | Basil, Ocimum basilicum L., Sweet Genovese | LED’s blue 447 nm, red 638– 665 nm, and far-red 731 nm from increased or supplemental red light. PPFD was set to 231 during growth, up to 300 μmol m−2 s−1 during 3-day treatment changing R638 or R665 PPFD level; 16 h photoperiod | The treatments significantly increased contents of phenolics, beta-tocopherol, ascorbic acid, and DPPH (1,1-diphenyl-2-picrylhydrazyl) center dot, an indicator of antioxidant capacity, but suppressed accumulation of lutein and beta-carotene; under supplemental or increased red 638 nm light, amounts of tested antioxidants were greater | [78] |
| 4 | Basil, Ocimum basilicum L., Sweet Genovese | Supplemental 520 and 622 nm lighting; supplemental 366- and 390 nm UV-A radiation; lighting with high PPFD level of red (638 nm) LEDs; 16 h photoperiod | Supplemental 520 and 622 nm lighting was more efficient for nitrate reduction, while the antioxidative system indices were enhanced by 595 nm diodes; supplemental 366 and 390 nm UV-A radiation were more favorable for antioxidant accumulation; short- term (3-days before harvesting) lighting with high PPFD level of red (638 nm) LEDs increased the amounts of the secondary metabolites of microgreens | [79] |
| 5 | Purple-leaf, Dark opal, and green- leaf Sweet Genovese forms of Ocimum basilicum L. | The main lighting system (HPS lamps and natural daylight) was supplemented with ~13.0 μmol m−2 s−1 flux of UV-A 390 nm blue light, and total PPFD was ~125 μmol m−2 s−1 (16 h photoperiod) for 1 or 7 days before harvest, or entire growth period, 14 days | UV-A enhanced antioxidant properties in green-leaf. Generally, UV-A irradiation for 7 days significantly inhibited growth and hypocotyl elongation of green- leaf, and for 14 days of both varieties. The total phenols and anthocyanin contents significantly decreased after 1 day UV-A irradiation in purple-leaf basils, with continuous decrease following UV-A irradiation for 7 or 14 days being shown. No significant differences in leaf chlorophyll index were determined | [80] |
| No. | Reference | Investigation Context | Treatment | Results (Yield, Quality, and Quantity, Chlorophyll, etc.) |
|---|---|---|---|---|
| 1 | [93] | Influence of three growing media (vermiculite, coconut fiber, and jute fabric) on yield and quality parameters of two varieties (green and red) | Microgreens were grown in a floating micro experimental growing system equipped with LED lamps, with modulation of both quantity and spectra of the light | Results showed high yield, comprised from 2 to 3 kg m−2, and nutritional quality varied among species, and higher antioxidant compounds were found in red basil on vermiculite and jute; coconut fiber allowed the differentiation of crop performance in terms of sucrose and above all nitrate; the choice of the substrate significantly affected the yield, the dry matter percentage, and the nitrate concentration |
| 2 | [94] | Influence of substrate: nutrient film technique (NFT) culture compared with conventional soil culture and compost mixed coco-peat substrate | The treatments were: top soil (control; T1) as compost and coir dust mixture at the rate of 1:1 (T2) and NFT (T3); the pH and EC of the supply solution were 5.9 (at 27.9 °C) and 1.5 mS cm−1, respectively | A significantly high vegetative growth and total yield was found in the NFT; the nitrate accumulation was well below the maximum permissible limit (MPL), set forth by the recommendations of the European Health Commission |
| 3 | [9] | Identification and quantification of polyphenols, major carotenoids, and macro/micro- minerals; twenty-seven phenolic compounds were quantified, of which the most abundant were: cichoric acid and rosmarinic acid | Sodium selenate applications at three concentrations (0, 8, and 16 μM Se) on green and purple basil; Hoagland nutrient solution was used, and they used a pH: 6 and EC: 0.35 dS cm−1 | In green and purple varieties, the 8 μM Se application enhanced the lutein concentration by 7% and 19%, respectively; the same application rate also increased the overall macroelement content by 35% and total polyphenols concentration by 32% but only in the green; the latter had a tripled chicoric acid content compared to the untreated control |
| 4 | [95] | Selenium biofortified microgreens from selenium- enriched seeds; substrate: perlite and vermiculite with a pH: 5.6 and EC: 2.04 dS m−1 | Grown in a nutrient solution containing 0 (control), 4 or 8 mg Se L−1 as sodium selenate | Seeds from plants treated with Se showed a significantly higher germination index than seeds from control plants, and the microgreens retained the Se; the antioxidant capacity of Se-fortified microgreens was higher compared to the control |
| 5 | [96] | Effect of salinity on biomass yield; for every 10 mM increase in NaCl, treatment solution EC increased 1.1 dS m−1; pH: 5.1– 5.2; hydroponic solution pH decreased slightly during the experiment (7.5 to 6.8), but all treatments had similar pH | Two cultivars were grown hydroponically for 71 d with four different concentrations of NaCl (no NaCl, low, moderate, and high (20 dS m−1)) | In both cultivars, salinity increased leaf concentrations of certain caffeic acid derivatives, caftaric acid, cinnamyl malic acid, and feruloyl tartaric acid and decreased concentrations of chicoric acid; salinity increased leaf concentrations of the two of the major polyphenolics, quercetin-rutinoside, and rosmarinic acid; salinity decreased concentrations of rosmarinic acid in leaves |
| 6 | [10] | Yield, mineral uptake, and quality were measured, among other outputs | Hoagland’s nutrient solution; pH: 5.56; EC: 1.12 dS cm−1; minimum and maximum temperatures: 9.7–43.1 °C; microgreens were harvested at the first true leaf stage, with green and swollen cotyledons | Results showed high concentrations of some minerals, but their nutrient uptake was limited due to low yield; nitrates content was lower if compared with that usually measured in baby leaf or adult vegetables of the same species, as well as the concentration of chlorophylls, carotenoids, phenols, and sugars |
| 7 | [97] | Nutritional dynamics | Compared between aquaponic and hydroponic systems using crayfish (Procambarus spp.) as the aquatic species | Aquaponic (AqB) showed 14%, 56%, and 65% more height, fresh weight, and dry weight, respectively, compared to hydroponic (HyB) |
| 8 | [98] | Quantify productivity and characterize growth of 35 cultivars grown in two hydroponic production systems | In this study, two hydroponic systems were compared: nutrient film technique (NFT) and deep flow technique (DFT) systems, grown for 3 weeks | Fresh weight of plants grown in DFT systems was 2.6 g greater compared with plants grown in NFT systems. Cultivars differed greatly in fresh weight; however, the yield seems to be affected more by cultivar selection than hydroponic production system |
| 9 | [99] | The nutrient solutions contained different NO3− concentrations (0.5, 5.0 and 10.0 mol m−3) or NO3−/NH4+ molar ratios (1:0, 1:1 and 0:1; total N concentration was 10.0 mol m−3); concentration of other nutrients were as follows: 1.0 mol m−3 P- H2PO4, 10.0 mol m−3 K+; 3.0 mol m−3 Ca2+; 1.5 mol m−3 Mg2+ plus trace elements | Influence of nitrogen nutrition on growth and accumulation of rosmarinic acid | The use of a total NO3− concentration of 5 mol m−3 resulted in optimal plant growth and rosmarinic acid production; this suggests that the standard N concentration used in hydroponic culture (10 mol m−3 or higher) could be reduced considerably, with important implications from the environmental point of view; in contrast, the addition of NH4+ to the nutrient solution was detrimental to both growth and rosmarinic acid production |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusu, T.; Cowden, R.J.; Moraru, P.I.; Maxim, M.A.; Ghaley, B.B. Overview of Multiple Applications of Basil Species and Cultivars and the Effects of Production Environmental Parameters on Yields and Secondary Metabolites in Hydroponic Systems. Sustainability 2021, 13, 11332. https://doi.org/10.3390/su132011332
Rusu T, Cowden RJ, Moraru PI, Maxim MA, Ghaley BB. Overview of Multiple Applications of Basil Species and Cultivars and the Effects of Production Environmental Parameters on Yields and Secondary Metabolites in Hydroponic Systems. Sustainability. 2021; 13(20):11332. https://doi.org/10.3390/su132011332
Chicago/Turabian StyleRusu, Teodor, Reed John Cowden, Paula Ioana Moraru, Mihai Avram Maxim, and Bhim Bahadur Ghaley. 2021. "Overview of Multiple Applications of Basil Species and Cultivars and the Effects of Production Environmental Parameters on Yields and Secondary Metabolites in Hydroponic Systems" Sustainability 13, no. 20: 11332. https://doi.org/10.3390/su132011332
APA StyleRusu, T., Cowden, R. J., Moraru, P. I., Maxim, M. A., & Ghaley, B. B. (2021). Overview of Multiple Applications of Basil Species and Cultivars and the Effects of Production Environmental Parameters on Yields and Secondary Metabolites in Hydroponic Systems. Sustainability, 13(20), 11332. https://doi.org/10.3390/su132011332