Optimizing Temperature and Photoperiod in a Home Cultivation System to Program Normal, Delayed, and Hastened Growth and Development Modes for Leafy Oak-Leaf and Romaine Lettuces
Abstract
:1. Introduction
2. Materials and Methods
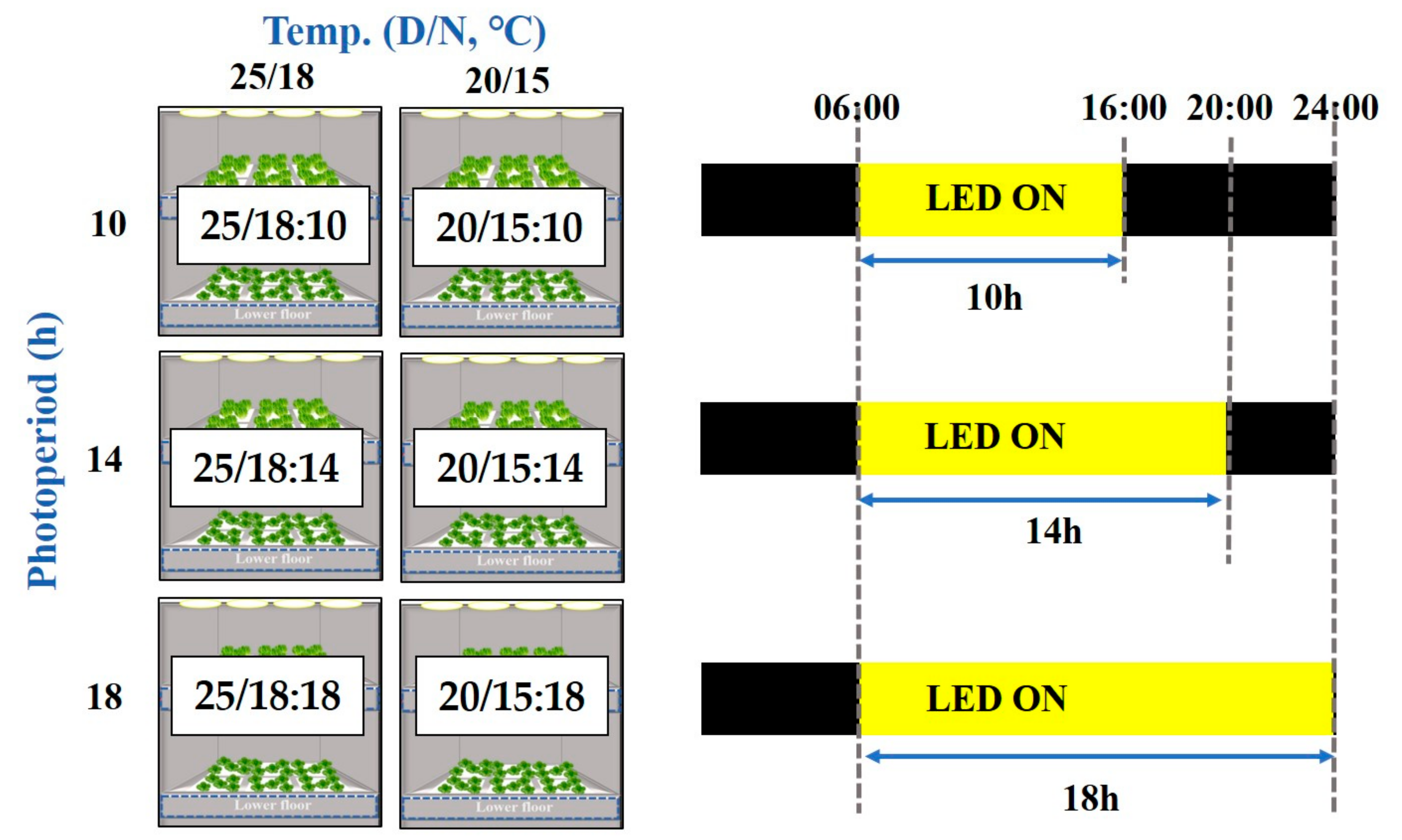
2.1. Cultivation System and Environment
2.2. Nutrient Solution
2.3. Plant Materials and Mesurements
2.4. Statistical Analysis
3. Results
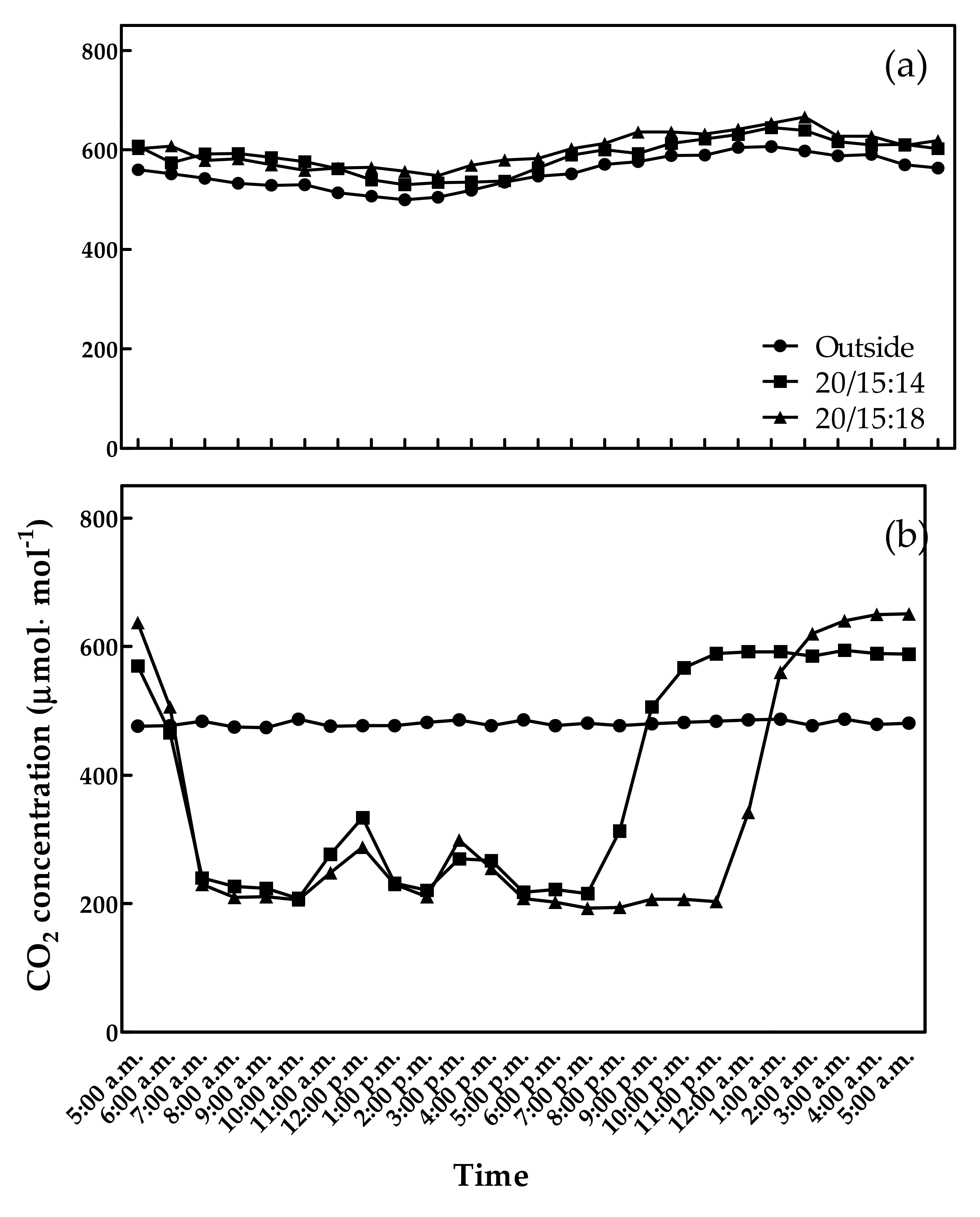
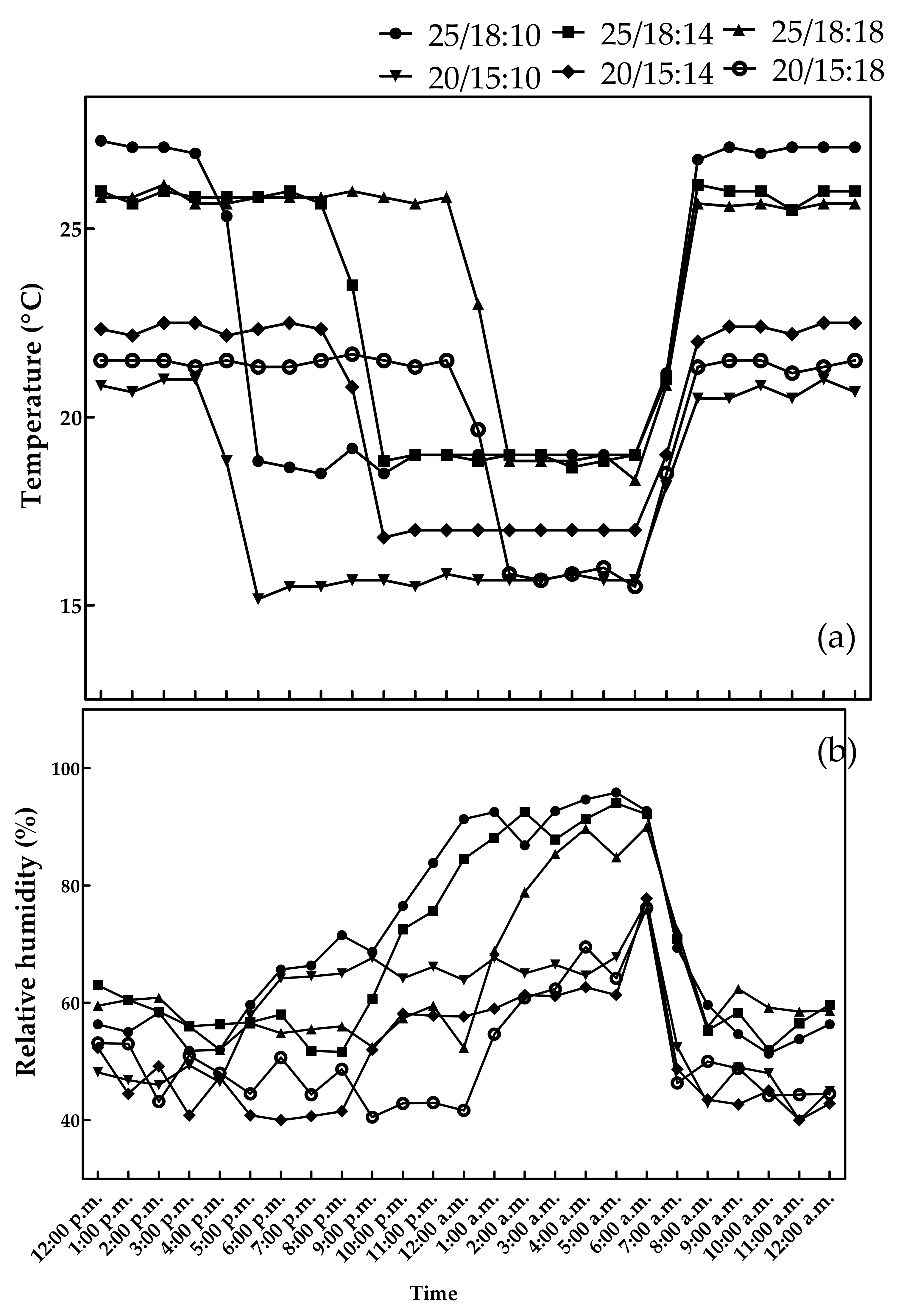
3.1. CO2 Concentrations, Temperature, and Relative Humidity
3.2. Nutrient Solution
3.3. Germination and Emergence Rate
3.4. Comparison of the Early Growth Stages
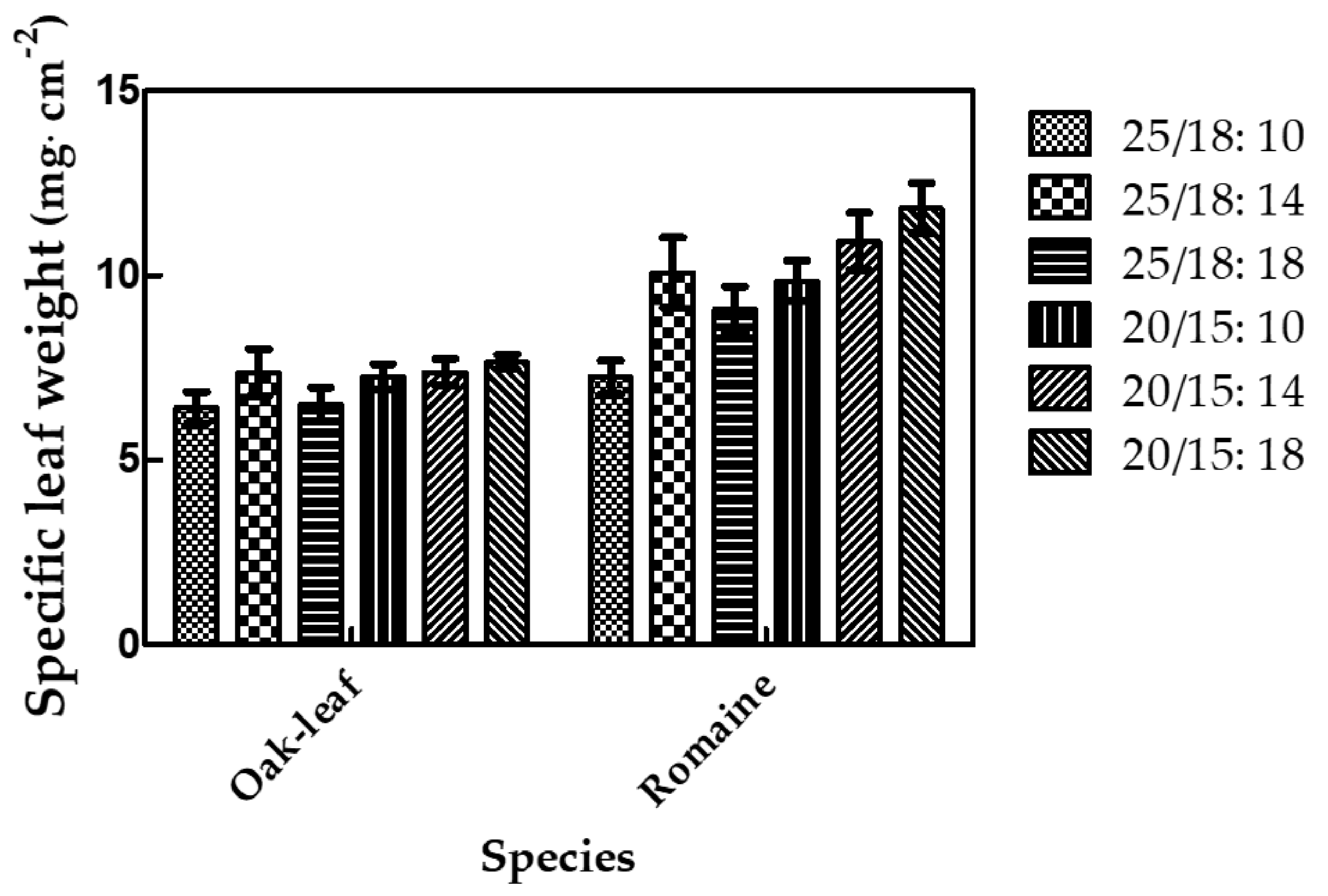
3.5. Yield and Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Plaut, T.R. Urban expansion and the loss of farmland in the United States: Implications for the future. Am. J. Agric. Econ. 1980, 62, 537–542. [Google Scholar] [CrossRef]
- Hande, M.J. Indoor Farming Hydroponic Plant Grow Chamber. Int. J. Sci. Res. Eng. Trends 2021, 7, 2050–2052. [Google Scholar]
- Eigenbrod, C.; Gruda, N. Urban vegetable for food security in cities. A review. Agron. Sustain. Dev. 2015, 35, 483–498. [Google Scholar] [CrossRef] [Green Version]
- Van Tuijl, E.; Hospers, G.J.; Van Den Berg, L. Opportunities and challenges of urban agriculture for sustainable city development. Eur. Spat. Res. Policy 2018, 25, 5–22. [Google Scholar] [CrossRef]
- Jensen, M.H. In Controlled Environment agriculture in deserts, tropics and temperate regions-A World Review. Int. Symp. Des. Environ. Control Trop. Subtrop. Greenh. 2001, 578, 19–25. [Google Scholar] [CrossRef]
- Campiotti, C.; Balducchi, R.; Incrocci, L.; Pardossi, A.; Popovski, K.; Popovska, S. In Hydroponics technology as a tool for plant fresh food support in Antarctica. World Congr. Soil. Cult. Agric. Coming Millenn. 2000, 554, 279–284. [Google Scholar] [CrossRef]
- Bamsey, M.T.; Zabel, P.; Zeidler, C.; Gyimesi, D.; Schubert, D.; Kohlberg, E.; Mengedoht, D.; Rae, J.; Graham, T. In Review of Antarctic greenhouses and plant production facilities: A historical account of food plants on the ice. In Proceedings of the 45th International Conference on Environmental Systems, Bellevue, DC, USA, 12–16 July 2015. [Google Scholar]
- Oyama, N.; Nair, J.; Ho, G. Recycling of treated domestic effluent from an on-site wastewater treatment system for hydroponics. Water Sci. Technol. 2005, 51, 211–219. [Google Scholar] [CrossRef]
- Oyama, N.; Nair, J.; Ho, G. Wastewater hydroponics for horticulture. In Proceedings of the International Water Association 4th World Water Congress, Marrakesh, Morocco, 21–22 September 2004. [Google Scholar]
- Kumar, R.R.; Cho, J.Y. Reuse of hydroponic waste solution. Environ. Sci. Pollut. Res. 2014, 21, 9569–9577. [Google Scholar] [CrossRef]
- Ikeda, A.; Tanimura, Y.; Ezaki, K.; Kawai, Y.; Nakayama, S.; Iwao, K.; Kageyama, H. In Environmental control and operation monitoring in a plant factory using artificial light. In Proceedings of the International Workshop on Sensors in Horticulture, Noordwijkerhout, The Netherlands, 29–31 January 1991; Volume 304, pp. 151–158. [Google Scholar]
- Vidhya, R.; Valarmathi, K. In Survey on automatic monitoring of hydroponics farms using IoT. In Proceedings of the 2018 3rd International Conference on Communication and Electronics Systems (ICCES), Coimbatore, India, 15–16 October 2018; pp. 125–128. [Google Scholar]
- Dinpanah11, G.; Jamshidi, A.; Lashgarara, F. Influencing factors on attitude toward hydroponics cultivation viewpoint of experts. J. Biodivers. Environ. Sci. 2014, 5, 141–147. [Google Scholar]
- Barbosa, G.L.; Gadelha, F.D.A.; Kublik, N.; Proctor, A.; Reichelm, L.; Weissinger, E.; Wohlleb, G.M.; Halden, R.U. Comparison of land, water, and energy requirements of lettuce grown using hydroponic vs. conventional agricultural methods. Int. J. Environ. Res. Public Health 2015, 12, 6879–6891. [Google Scholar] [CrossRef] [Green Version]
- Noh, K.; Jeong, B.R. Particulate matter in the cultivation area may contaminate leafy vegetables with heavy metals above safe levels in Korea. Environ. Sci. Pollut. Res. 2019, 26, 25762–25774. [Google Scholar] [CrossRef] [Green Version]
- Sugano, M. In Elemental technologies for realizing a fully-controlled artificial light-type plant factory. In Proceedings of the 2015 12th International Conference & Expo on Emerging Technologies for a Smarter World (CEWIT), Melville, NY, USA, 19–20 October 2015; pp. 1–5. [Google Scholar]
- Venter, G.D. Hydroponics today. SABI Mag.-Tydskr. 2015, 7, 8–10. [Google Scholar]
- Quagrainie, K.K.; Flores, R.M.V.; Kim, H.-J.; McClain, V. Economic analysis of aquaponics and hydroponics production in the US Midwest. J. Appl. Aquac. 2018, 30, 1–14. [Google Scholar] [CrossRef]
- Walters, K.J.; Behe, B.K.; Currey, C.J.; Lopez, R.G. Historical, current, and future perspectives for controlled environment hydroponic food crop production in the United States. HortScience 2020, 55, 758–767. [Google Scholar] [CrossRef]
- Loh, S. Living walls–a way to green the built environment. Environ. Des. Guide 2008, 26, 1–7. [Google Scholar]
- Brown-Paul, C. Diversifying for success. Pract. Hydroponics Greenh. 2015, 155, 32–39. [Google Scholar]
- Sadeghianfar, P.; Nazari, M.; Backes, G. Exposure to ultraviolet (UV-C) radiation increases germination rate of maize (Zea maize L.) and sugar beet (Beta vulgaris) seeds. Plants 2019, 8, 49. [Google Scholar] [CrossRef] [Green Version]
- Afsheen, S.; Naseer, H.; Iqbal, T.; Abrar, M.; Bashir, A.; Ijaz, M. Synthesis and characterization of metal sulphide nanoparticles to investigate the effect of nanoparticles on germination of soybean and wheat seeds. Mater. Chem. Phys. 2020, 252, 123216. [Google Scholar] [CrossRef]
- Zhang, T.; Fan, S.; Xiang, Y.; Zhang, S.; Wang, J.; Sun, Q. Non-destructive analysis of germination percentage, germination energy and simple vigour index on wheat seeds during storage by Vis/NIR and SWIR hyperspectral imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 239, 118488. [Google Scholar] [CrossRef]
- Wang, M.; Xiao, J.; Wei, H.; Jeong, B.R. Supplementary light source affects growth and development of carnation ‘Dreambyul’cuttings. Agronomy 2020, 10, 1217. [Google Scholar] [CrossRef]
- Rubatzky, V.E.; Yamaguchi, M. Spinach, table beets, and other vegetable chenopods. World Veg. 1997, 457–473. [Google Scholar] [CrossRef]
- Kozai, T. Plant Factories with Artificial Lighting (PFALs): Benefits, Problems, and Challenges. Smart Plant Fact. 2018, 1227, 15–29. [Google Scholar]
- Lee, S.-Y.; Kim, H.-J.; Bae, J.-H. Growth, vitamin C, and mineral contents of Sedum sarmentosum in soil and hydroponic cultivation. Hortic. Sci. Technol. 2011, 29, 195–200. [Google Scholar]
- Zhang, H.; Li, Y.; Zhu, J.-K. Developing naturally stress-resistant crops for a sustainable agriculture. Nat. Plants 2018, 4, 989–996. [Google Scholar] [CrossRef]
- Anpo, M.; Fukuda, H.; Wada, T. Plant Factory Using Artificial Light: Adapting to Environmental Disruption and Clues to Agricultural Innovation; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Choi, K.Y.; Paek, K.Y.; Lee, Y.B. Effect of air temperature on tipburn incidence of butterhead and leaf lettuce in a plant factory. In Transplant Production in the 21st Century; Springer: Dordrecht, The Netherlands, 2000; pp. 166–171. [Google Scholar]
- Challa, H.; Heuvelink, E.; Van Meeteren, U. Crop growth and development. In Greenhouse Climate Control: An Integrated Approach; Wageningen Academic Publishers: Wageningen, The Netherlands, 1995; pp. 62–84. [Google Scholar]
- Kang, J.H.; KrishnaKumar, S.; Atulba, S.L.S.; Jeong, B.R.; Hwang, S.J. Light intensity and photoperiod influence the growth and development of hydroponically grown leaf lettuce in a closed-type plant factory system. Hortic. Environ. Biotechnol. 2013, 54, 501–509. [Google Scholar] [CrossRef]
- Mou, B. Lettuce. In Vegetables I; Springer: New York, NY, USA, 2008; pp. 75–116. [Google Scholar]
- Lee, R.J.; Bhandari, S.R.; Lee, G.; Lee, J.G. Optimization of temperature and light, and cultivar selection for the production of high-quality head lettuce in a closed-type plant factory. Hortic. Environ. Biotechnol. 2019, 60, 207–216. [Google Scholar] [CrossRef]
- Collier, G.; Tibbitts, T. Effects of relative humidity and root temperature on calcium concentration and tipburn development in lettuce. J. Am. Soc. Hortic. Science. Am. Soc. Hortic. Sci. 1984, 109, 128–131. [Google Scholar]
- Ito, T. More intensive production of lettuce under artificially controlled conditions. Int. Symp. Growth Yield Control Veg. Prod. 1989, 260, 381–390. [Google Scholar] [CrossRef]


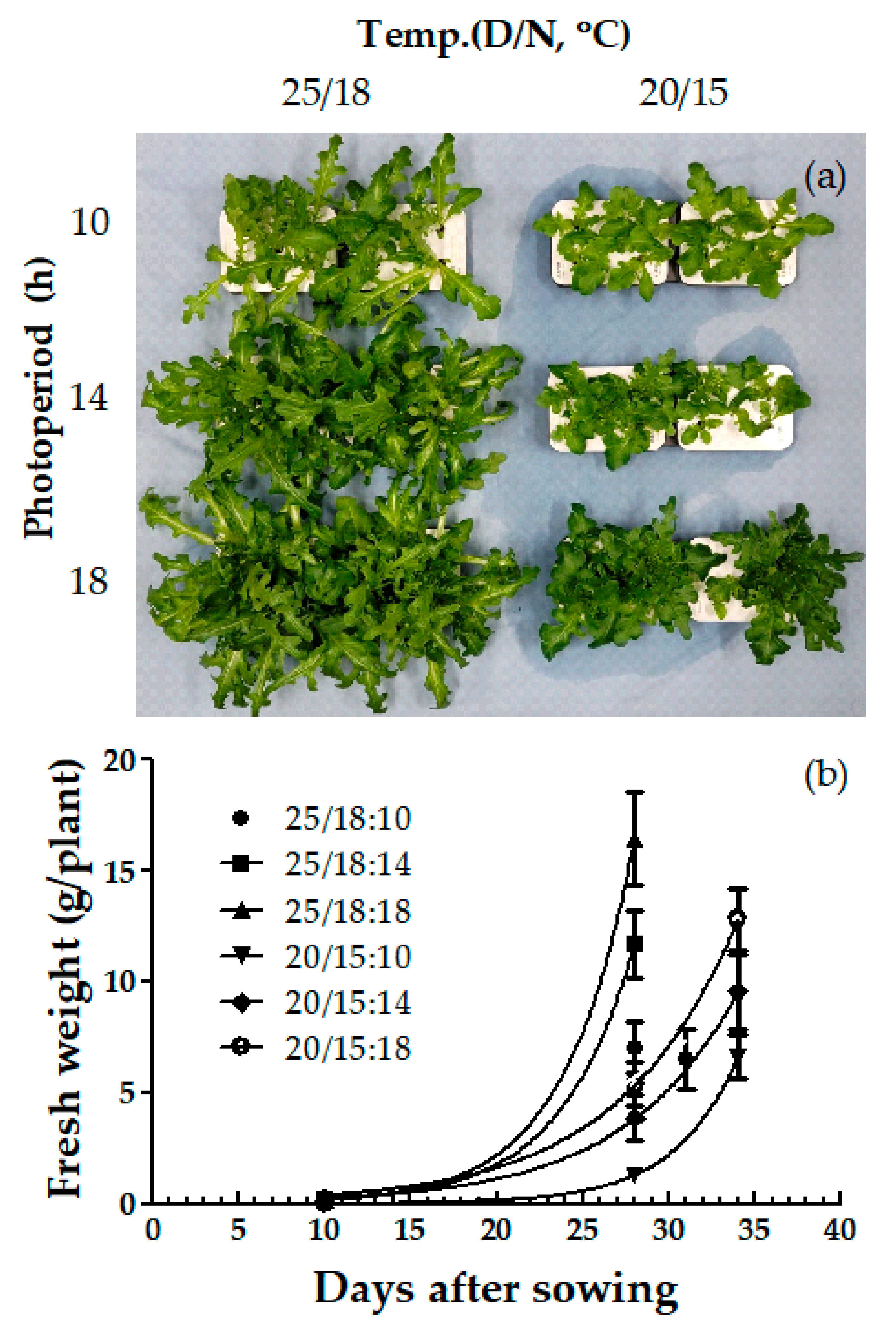
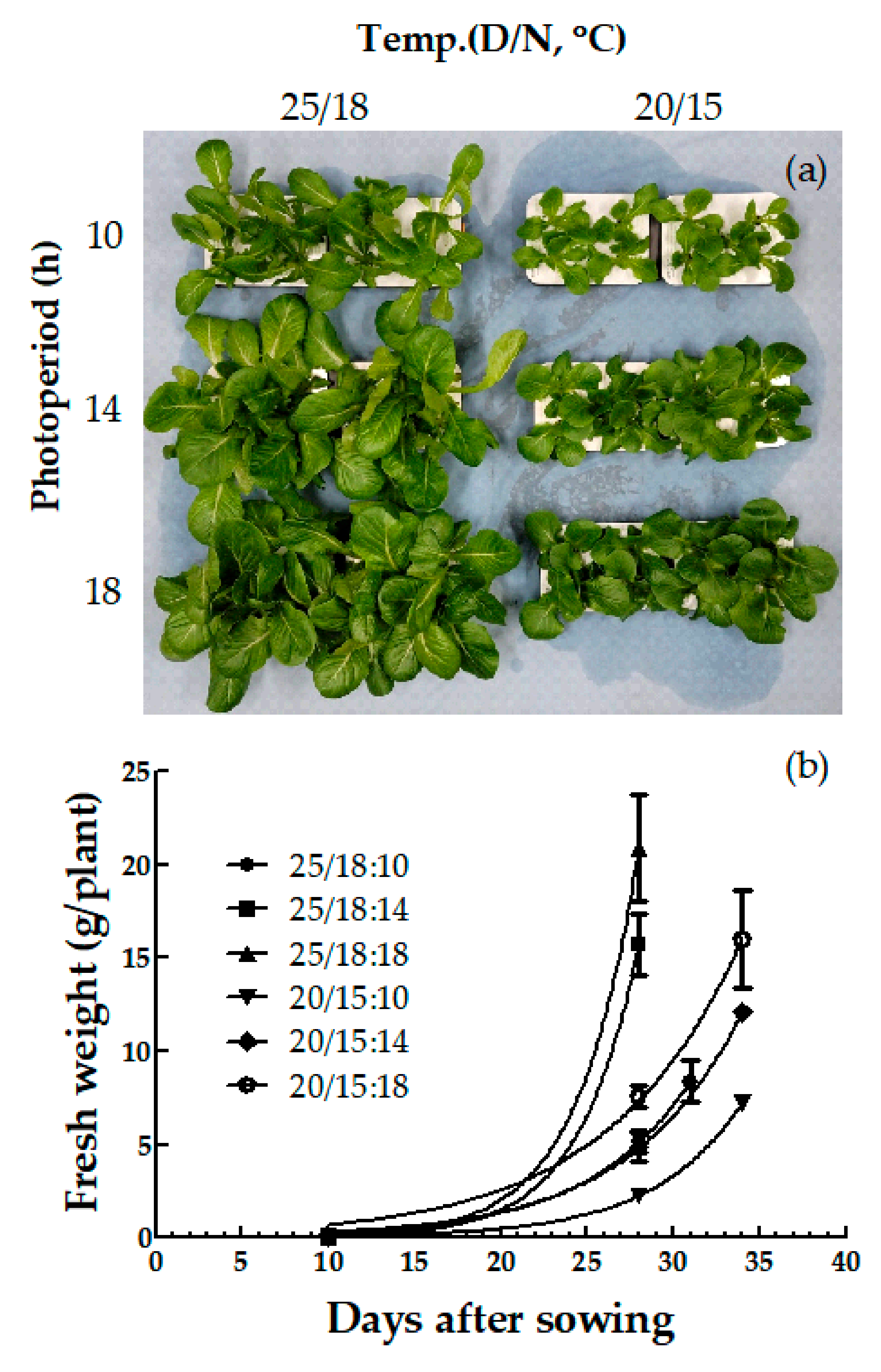
| Species | Temp. (D/N, °C) (A) | Photoperiod, (h) (B) | Germination Rate (GR) | Mean Germination Time (MGT, day) | Germination Energy (GE) |
|---|---|---|---|---|---|
| Oak-leaf | 25/18 | 10 | 5.6 | 1.9 | 83.3 |
| 14 | 6.7 | 2.0 | 93.3 | ||
| 18 | 5.9 | 1.6 | 80.0 | ||
| 20/15 | 10 | 5.4 | 2.5 | 93.3 | |
| 14 | 6.5 | 2.3 | 96.7 | ||
| 18 | 4.3 | 2.2 | 76.7 | ||
| F-test | A | z NS | NS | NS | |
| B | NS | NS | NS | ||
| A* B | NS | NS | NS | ||
| Romaine | 25/18 | 10 | 6.9 ab y | 2.2 ab | 96.7 |
| 14 | 7.4 a | 2.0 b | 100.0 | ||
| 18 | 7.3 a | 2.1 b | 100.0 | ||
| 20/15 | 10 | 6.2 ab | 2.5 ab | 100.0 | |
| 14 | 6.9 ab | 2.3 ab | 100.0 | ||
| 18 | 5.7 b | 2.7 a | 100.0 | ||
| F-test | A | * | * | NS | |
| B | NS | NS | NS | ||
| A* B | NS | NS | NS |
| Species | Temp. (D/N, °C) (A) | Photoperiod (h) (B) | Emergence Rate (ER) | Mean Emergence Time (MET, day) | Emergence Energy (EE) | ||
|---|---|---|---|---|---|---|---|
| 5th | 6th | 7th | |||||
| Oak-leaf | 25/18 | 10 | 7.3 ab | 3.3 c | 85.0 a | 100.0 a | 100.0 |
| 14 | 8.5 a | 3.4 bc | 85.0 a | 100.0 a | 100.0 | ||
| 18 | 7.0 ab | 3.2 c | 85.0 a | 100.0 a | 100.0 | ||
| 20/15 | 10 | 4.6 c | 4.0 a | 10.0 b | 55.0 b | 95.0 | |
| 14 | 5.5 bc | 4.3 a | 25.0 b | 50.0 b | 95.0 | ||
| 18 | 4.3 c | 3.9 ab | 5.0 b | 60.0 b | 80.0 | ||
| F-test | A | z * | * | ** | *** | NS | |
| B | NS | NS | NS | NS | NS | ||
| A* B | NS | NS | NS | NS | NS | ||
| Romaine | 25/18 | 10 | 5.8 | 3.0 b y | 100.0 a | 100.0 | 100.0 |
| 14 | 7.5 | 3.1 b | 100.0 a | 100.0 | 100.0 | ||
| 18 | 5.8 | 3.1 b | 90.0 ab | 100.0 | 100.0 | ||
| 20/15 | 10 | 6.0 | 3.9 a | 25.0 c | 85.0 | 100.0 | |
| 14 | 8.2 | 3.6 a | 65.0 b | 95.0 | 100.0 | ||
| 18 | 6.5 | 3.8 a | 30.0 c | 100.0 | 100.0 | ||
| F-test | A | NS | ** | ** | NS | NS | |
| B | NS | NS | NS | NS | NS | ||
| A* B | NS | NS | NS | NS | NS | ||
| Species | Temperature (Day/Night, °C) (A) | Photoperiod (h) (B) | FW (mg) | Shoot Length (cm) | Leaf Length (cm) | Leaf Width (cm) | No. of Leaves |
|---|---|---|---|---|---|---|---|
| Oak-leaf | 25/18 | 10 | 153.5 b | 6.6 a | 5.3 a | 3.9 a | 1.8 a |
| 14 | 169.4 b | 6.0 b | 5.1 a | 3.3 b | 1.5 b | ||
| 18 | 221.9 a | 5.4 b | 5.0 a | 3.4 ab | 1.5 ab | ||
| 20/15 | 10 | 30.2 c | 3.2 d | 3.5 d | 1.3 d | 0.7 d | |
| 14 | 51.0 c | 3.7 cd | 4.1 c | 1.8 d | 1.0 c | ||
| 18 | 72.4 c | 3.9 c | 4.6 b | 2.6 c | 1.2 c | ||
| F-test | A | z *** | *** | *** | *** | *** | |
| B | ** | NS | * | * | NS | ||
| A* B | NS | *** | *** | *** | *** | ||
| Romaine | 25/18 | 10 | 66.8 b y | 4.7 a | 1.9 b | 1.0 b | 3.9 b |
| 14 | 70.4 b | 4.3 b | 2.1 b | 1.1 b | 3.9 b | ||
| 18 | 96.9 a | 4.3 b | 2.6 a | 1.3 a | 4.4 a | ||
| 20/15 | 10 | 28.6 d | 3.3 c | 0.4 d | 0.3 c | 2.9 d | |
| 14 | 32.2 cd | 2.9 c | 0.7 d | 0.5 c | 3.4 c | ||
| 18 | 44.8 c | 3.2 c | 1.0 c | 0.6 c | 3.9 b | ||
| F-test | A | *** | *** | *** | *** | *** | |
| B | *** | * | *** | *** | *** | ||
| A* B | NS | NS | NS | NS | ** |
| Temperature (Day/Night, °C) (A) | Photoperiod (h) (B) | Dry Weight/Fresh Weight Ratio (%) | |
|---|---|---|---|
| Oak-Leaf | Romaine | ||
| 25/18 | 10 | 5.1 bc y | 4.6 ab |
| 14 | 4.3 c | 3.9 bc | |
| 18 | 5.1 bc | 3.5 c | |
| 20/15 | 10 | 5.4 b | 5.3 a |
| 14 | 6.7 a | 4.2 bc | |
| 18 | 5.3 bc | 5.1 a | |
| F-test | A | z ** | *** |
| B | NS | ** | |
| A* B | ** | NS | |
| Temperature (D/N °C) | Photoperiod (h) | Tip Burn (%) |
|---|---|---|
| 25/18 | 10 | 0 |
| 14 | 10 | |
| 18 | 80 | |
| 20/15 | 10 | 0 |
| 14 | 50 | |
| 18 | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noh, K.; Jeong, B.R. Optimizing Temperature and Photoperiod in a Home Cultivation System to Program Normal, Delayed, and Hastened Growth and Development Modes for Leafy Oak-Leaf and Romaine Lettuces. Sustainability 2021, 13, 10879. https://doi.org/10.3390/su131910879
Noh K, Jeong BR. Optimizing Temperature and Photoperiod in a Home Cultivation System to Program Normal, Delayed, and Hastened Growth and Development Modes for Leafy Oak-Leaf and Romaine Lettuces. Sustainability. 2021; 13(19):10879. https://doi.org/10.3390/su131910879
Chicago/Turabian StyleNoh, Kyungdeok, and Byoung Ryong Jeong. 2021. "Optimizing Temperature and Photoperiod in a Home Cultivation System to Program Normal, Delayed, and Hastened Growth and Development Modes for Leafy Oak-Leaf and Romaine Lettuces" Sustainability 13, no. 19: 10879. https://doi.org/10.3390/su131910879
APA StyleNoh, K., & Jeong, B. R. (2021). Optimizing Temperature and Photoperiod in a Home Cultivation System to Program Normal, Delayed, and Hastened Growth and Development Modes for Leafy Oak-Leaf and Romaine Lettuces. Sustainability, 13(19), 10879. https://doi.org/10.3390/su131910879







