Effects of Black Garlic Extract and Nanoemulsion on the Deoxy Corticosterone Acetate-Salt Induced Hypertension and Its Associated Mild Cognitive Impairment in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Instrumentation
2.3. Processing of Garlic into Black Garlic
2.4. Extraction and Purification of Organosulfur Compounds from Raw and Black Garlic by QuEChERS
2.5. GC-MS Analysis of Organosulfur Compounds in Raw and Black Garlic
- As = peak area of organosulfur compounds
- Ai = peak area of internal standard
- b = intercept of the regression equation
- a = slope of the regression equation
- Ci = internal standard concentration (μg/mL)
- V = extract volume (mL)
- DF = dilution factor
- R = recovery (%)
- Ws = sample weight (g)
2.6. Method Validation
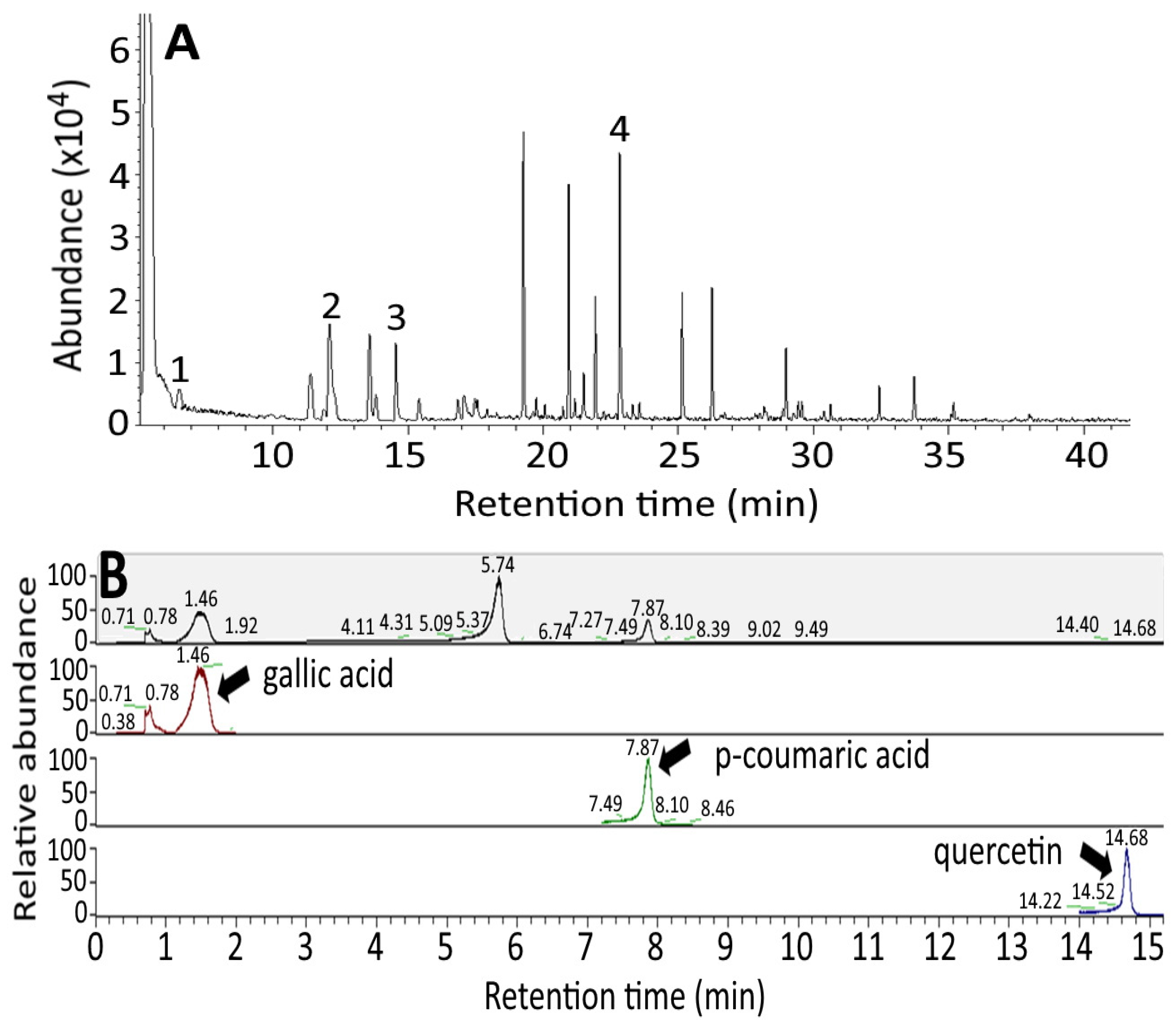
2.7. Determination of Phenolic Acids and Flavonoids in Raw and Black Garlic
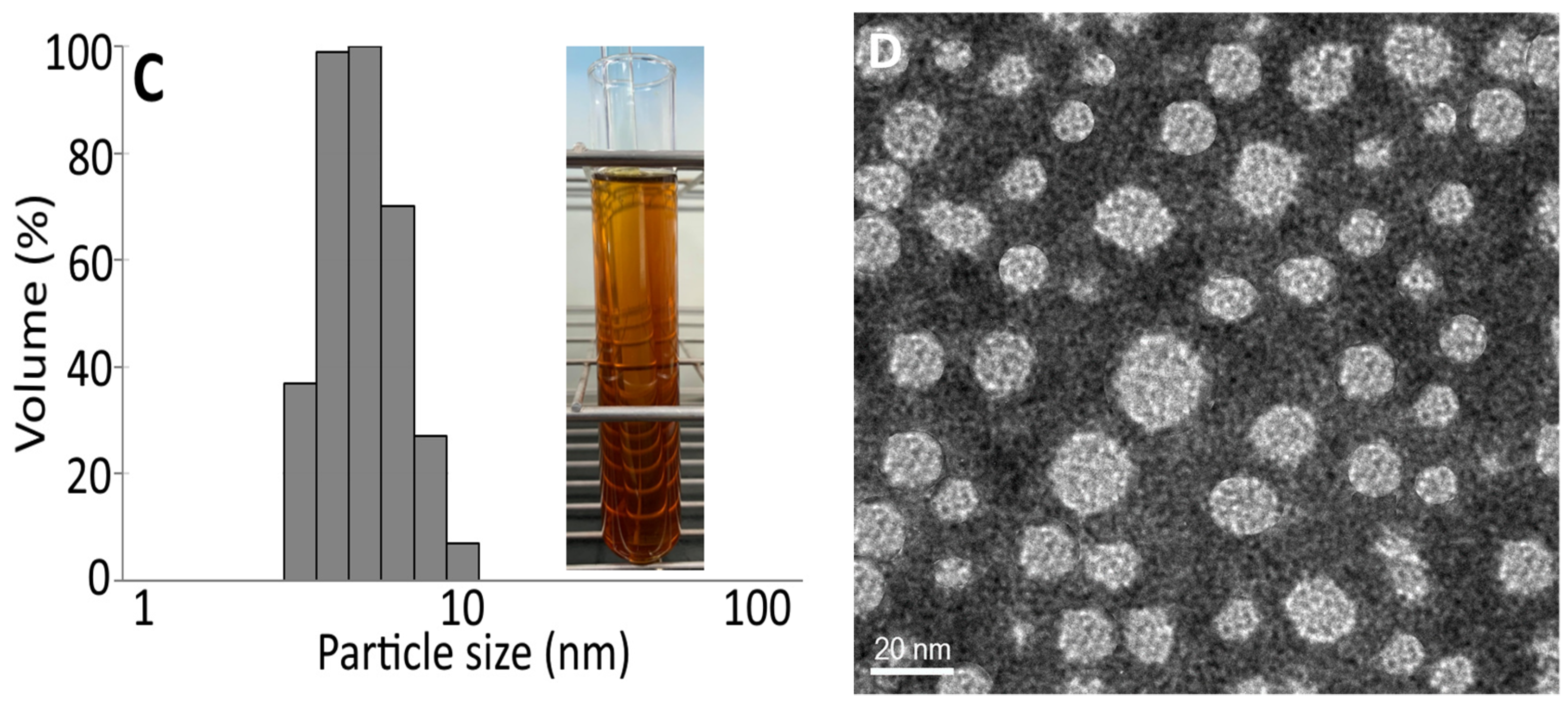
2.8. Preparation of Black Garlic Nanoemulsion and Characteristic Determination
2.9. Determination of Encapsulation Efficiency
2.10. Stability Study
2.11. Animal Study
2.12. Morris Water Maze Test
2.13. Biochemical Analysis of Blood
2.14. Determination of Oxidative Index in Brain Tissue
2.15. Determination of Inflammation Index in Brain Tissue
2.16. Statistical Analysis
3. Results and Discussion
3.1. Analysis of Organosulfur Compounds in Raw and Black Garlic by GC-MS
3.2. Analysis of Phenolic Acids and Flavonoids in Raw and Black Garlic
3.3. Characteristic of Black Garlic Nanoemulsion
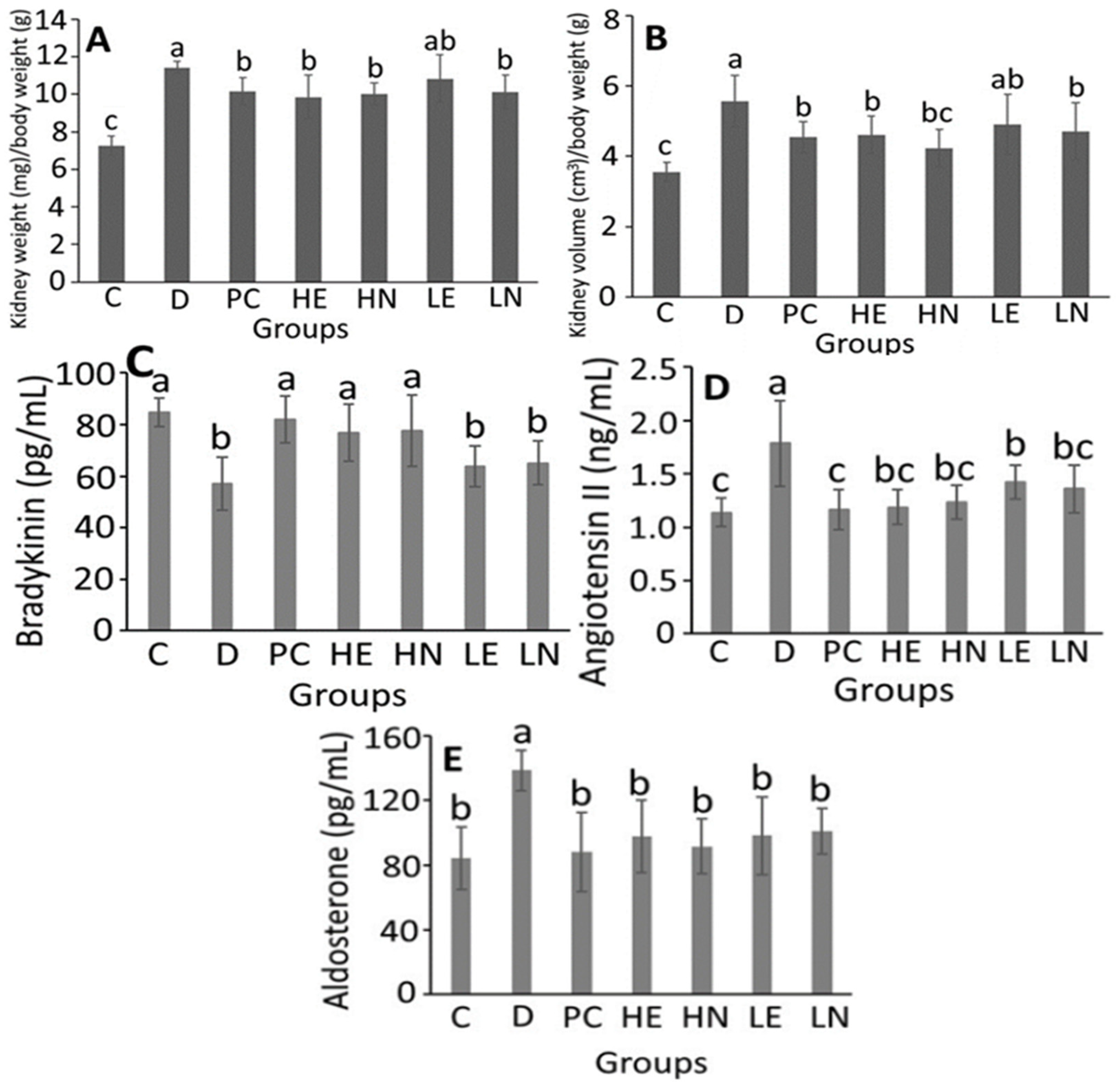
3.4. Animal Study
3.5. Morris Water Maze Test
3.6. Determination of NO Concentration in Plasma, Oxidative and Inflammation Index in Hippocampus in DOCA-Salt Induced Hypertension and Its Associated Mild Cognitive Impairment in Rats
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Qattan, K.; Thomson, M.; Ali, M. Garlic (Allium sativum) and ginger (Zingiber officinale) attenuate structural nephropathy progression in streptozotocin-induced diabetic rats. Eur. e-J. Clin. Nutr. Metab. 2008, 3, e62–e71. [Google Scholar] [CrossRef]
- Queiroz, Y.S.; Ishimoto, E.Y.; Bastos, D.H.M.; Sampaio, G.R.; Torres, E.A.F.S. Garlic (Allium sativum L.) and ready-to-eat garlic products: In vitro antioxidant activity. Food Chem. 2009, 115, 371–374. [Google Scholar] [CrossRef]
- Choi, I.S.; Cha, H.S.; Lee, Y.S. Physicochemical and antioxidant properties of black garlic. Molecules 2014, 19, 16811–16823. [Google Scholar] [CrossRef] [PubMed]
- Sato, E.; Kohno, M.; Hamano, H.; Niwano, Y. Increased anti-oxidative potency of garlic by spontaneous short-term fermentation. Plant Foods Hum. Nutr. 2006, 61, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Nam, S.H.; Rico, C.W.; Kang, M.Y. A comparative study on the antioxidative and anti-allergic activities of fresh and aged black garlic extracts. Int. J. Food Sci. Technol. 2012, 47, 1176–1182. [Google Scholar] [CrossRef]
- Martínez-Casas, L.; Lage-Yusty, M.; López-Hernández, J. Changes in the aromatic profile, sugars, and bioactive compounds when purple garlic is transformed into black garlic. J. Agric. Food Chem. 2017, 65, 10804–10811. [Google Scholar] [CrossRef]
- Xu, G.; Ye, X.; Chen, J.; Liu, D. Effect of heat treatment on the phenolic compounds and antioxidant capacity of citrus peel extract. J. Agric. Food Chem. 2007, 55, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Kang, O.-J.; Gweon, O.-C. Changes in the content of fat- and water-soluble vitamins in black garlic at the different thermal processing steps. Food Sci. Biotechnol. 2013, 22, 283–287. [Google Scholar] [CrossRef]
- Tsai, J.-C.; Chen, Y.-A.; Wu, J.-T.; Cheng, K.-C.; Lai, P.-S.; Liu, K.-F.; Lin, Y.-K.; Huang, Y.-T.; Hsieh, C.-W. Extracts from fermented black garlic exhibit a hepatoprotective effect on acute hepatic injury. Molecules 2019, 24, 1112. [Google Scholar] [CrossRef]
- Kim, M.J.; Yoo, Y.C.; Kim, H.J.; Shin, S.K.; Sohn, E.J.; Min, A.Y.; Sung, N.Y.; Kim, M.R. Aged black garlic exerts anti-inflammatory effects by decreasing no and proinflammatory cytokine production with less cytoxicity in LPS-stimulated raw 264.7 macrophages and LPS-induced septicemia mice. J. Med. Food 2014, 17, 1057–1063. [Google Scholar] [CrossRef]
- Dong, M.; Yang, G.; Liu, H.; Liu, X.; Lin, S.; Sun, D.; Wang, Y. Aged black garlic extract inhibits HT29 colon cancer cell growth via the PI3K/Akt signaling pathway. Biomed. Rep. 2014, 2, 250–254. [Google Scholar] [CrossRef] [PubMed]
- ADI. Alzheimer’s Disease International. Available online: https://www.alzint.org/ (accessed on 4 April 2021).
- Walker, K.A.; Power, M.C.; Gottesman, R.F. Defining the Relationship Between Hypertension, Cognitive Decline, and Dementia: A Review. Curr. Hypertens. Rep. 2017, 19, 24. [Google Scholar] [CrossRef]
- Zhang, X.; Li, N.; Lu, X.; Liu, P.; Qiao, X. Effects of temperature on the quality of black garlic. J. Sci. Food Agric. 2016, 96, 2366–2372. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.-W.; Kao, T.-H.; Chen, B.-H. Improved analytical method for determination of cholesterol-oxidation products in meat and animal fat by QuEChERS coupled with gas chromatography–mass spectrometry. J. Agric. Food Chem. 2018, 66, 3561–3571. [Google Scholar] [CrossRef] [PubMed]
- Tocmo, R.; Wang, C.; Liang, D.; Huang, D. Organosulphide profile and hydrogen sulphide-releasing capacity of garlic (Allium sativum L.) scape oil: Effects of pH and cooking. J. Funct. Foods 2015, 17, 410–421. [Google Scholar] [CrossRef]
- Kao, T.H.; Huang, C.W.; Chen, B.H. Functional components in Luffa cylindrica and their effects on anti-inflammation of macrophage cells. Food Chem. 2012, 135, 386–395. [Google Scholar] [CrossRef] [PubMed]
- American Psychological Association. Guidelines for Ethical Conduct in the Care and Use of Nonhuman Animals in Research; American Psychological Association: Washington, DC, USA, 2012. [Google Scholar]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Sun, Z.K.; Ma, X.R.; Jia, Y.J.; Liu, Y.R.; Zhang, J.W.; Zhang, B.A. Effects of resveratrol on apoptosis in a rat model of vascular dementia. Exp. Ther. Med. 2014, 7, 843–848. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Statistical Analysis System. SAS Procedures and SAS/Graph User’s Guide, Version 6; Statistical Analysis System Institute: Cary, NC, USA, 2014. [Google Scholar]
- Taiwan Food and Drug Administration. Method Validation of Food Analysis; Taiwan Food and Drug Administration: Taipei, Taiwan, 2013. (In Chinese) [Google Scholar]
- Locatelli, D.A.; Altamirano, J.C.; González, R.E.; Camargo, A.B. Home-cooked garlic remains a healthy food. J. Funct. Foods 2015, 16, 1–8. [Google Scholar] [CrossRef]
- Tocmo, R.; Wu, Y.; Liang, D.; Fogliano, V.; Huang, D. Boiling enriches the linear polysulfides and the hydrogen sulfide-releasing activity of garlic. Food Chem. 2017, 221, 1867–1873. [Google Scholar] [CrossRef]
- Zhang, Z.; Lei, M.; Liu, R.; Gao, Y.; Xu, M.; Zhang, M. Evaluation of alliin, saccharide contents and antioxidant activities of black garlic during thermal processing. J. Food Biochem. 2015, 39, 39–47. [Google Scholar] [CrossRef]
- Purev, U.; Chung, M.J.; Oh, D.-H. Individual differences on immunostimulatory activity of raw and black garlic extract in human primary immune cells. Immunopharmacol. Immunotoxicol. 2012, 34, 651–660. [Google Scholar] [CrossRef]
- Thach, N.A.; Thuy, N.M. Effect of extraction conditions on polyphenols, flavonoids, s-allyl cysteine content and antioxidant activity of black garlic extracts. Vietnam J. Sci. Technol. 2017, 55, 18. [Google Scholar] [CrossRef]
- Lakshmi, P.; Kumar, G.A. Nanosuspension technology: A review. Int. J. Pharm. Sci. 2010, 2, 35–40. [Google Scholar]
- Mahdi, E.S.; Noor, A.M.; Sakeena, M.H.; Abdullah, G.Z.; Abdulkarim, M.F.; Sattar, M.A. Formulation and in vitro release evaluation of newly synthesized palm kernel oil esters-based nanoemulsion delivery system for 30% ethanolic dried extract derived from local Phyllanthus urinaria for skin antiaging. Int. J. Nanomed. 2011, 6, 2499. [Google Scholar] [CrossRef]
- Baccarin, T.; Lemos-Senna, E. Potential application of nanoemulsions for skin delivery of pomegranate peel polyphenols. AAPS PharmSciTech 2017, 18, 3307–3314. [Google Scholar] [CrossRef]
- Bazana, M.T.; da Silva, S.S.; Codevilla, C.F.; de Deus, C.; Lucas, B.N.; Ugalde, G.A.; Mazutti, M.A.; Moraes Flores, E.M.; Barin, J.S.; de Bona da Silva, C.; et al. Development of nanoemulsions containing Physalis peruviana calyx extract: A study on stability and antioxidant capacity. Food Res. Int. 2019, 125, 108645. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V.; Huang, D.Y.; Grahammer, F.; Wyatt, A.W.; Osswald, H.; Wulff, P.; Kuhl, D.; Lang, F. SGK1 as a determinant of kidney function and salt intake in response to mineralocorticoid excess. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R395–R401. [Google Scholar] [CrossRef] [PubMed]
- Thunhorst, R.L.; Beltz, T.G.; Johnson, A.K. Glucocorticoids increase salt appetite by promoting water and sodium excretion. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R1444–R1451. [Google Scholar] [CrossRef] [PubMed]
- Asdaq, S.M.B.; Inamdar, M.N. The potential benefits of a garlic and hydrochlorothiazide combination as antihypertensive and cardioprotective in rats. J. Nat. Med. 2011, 65, 81–88. [Google Scholar] [CrossRef]
- Sharifi, A.M.; Darabi, R.; Akbarloo, N. Investigation of antihypertensive mechanism of garlic in 2K1C hypertensive rat. J. Ethnopharmacol. 2003, 86, 219–224. [Google Scholar] [CrossRef]
- Harauma, A.; Moriguchi, T. Aged garlic extract improves blood pressure in spontaneously hypertensive rats more safely than raw garlic. J. Nutr. 2006, 136, 769S–773S. [Google Scholar] [CrossRef]
- Jadhav, A.; Torlakovic, E.; Ndisang, J.F. Hemin therapy attenuates kidney injury in deoxycorticosterone acetate-salt hypertensive rats. Am. J. Physiol. Ren. Physiol. 2009, 296, F521–F534. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Takaoka, M.; Kobayashi, Y.; Yuba, M.; Ohkita, M.; Matsumura, Y. Effects of α-lipoic acid on deoxycorticosterone acetate–salt-induced hypertension in rats. Eur. J. Pharmacol. 2001, 424, 121–129. [Google Scholar] [CrossRef]
- O’Brien, D.; Chunduri, P.; Iyer, A.; Brown, L. l-Carnitine attenuates cardiac remodelling rather than vascular remodelling in deoxycorticosterone acetate-salt hypertensive rats. Basic Clin. Pharmacol. Toxicol. 2010, 106, 296–301. [Google Scholar] [CrossRef]
- Zaman, M.A.; Oparil, S.; Calhoun, D.A. Drugs targeting the renin–angiotensin–aldosterone system. Nat. Rev. Drug Discov. 2002, 1, 621–636. [Google Scholar] [CrossRef]
- Carey, R.M.; Siragy, H.M. Newly recognized components of the renin-angiotensin system: Potential roles in cardiovascular and renal regulation. Endocr. Rev. 2003, 24, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.-C.; Pan, T.-M. Prevention of hypertension-induced vascular dementia by Lactobacillus paracasei subsp. paracasei NTU 101-fermented products. Pharm. Biol. 2017, 55, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Akcılar, R.; Turgut, S.; Caner, V.; Akcılar, A.; Ayada, C.; Elmas, L.; Özcan, T.O. Apelin effects on blood pressure and RAS in DOCA-salt-induced hypertensive rats. Clin. Exp. Hypertens. 2013, 35, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Hermawati, E.; Sari, D.C.R.; Partadiredja, G. The effects of black garlic ethanol extract on the spatial memory and estimated total number of pyramidal cells of the hippocampus of monosodium glutamate-exposed adolescent male Wistar rats. Anat. Sci. Int. 2015, 90, 275–286. [Google Scholar] [CrossRef]
- Javed, H.; Khan, M.M.; Khan, A.; Vaibhav, K.; Ahmad, A.; Khuwaja, G.; Ahmed, M.E.; Raza, S.S.; Ashafaq, M.; Tabassum, R.; et al. S-allyl cysteine attenuates oxidative stress associated cognitive impairment and neurodegeneration in mouse model of streptozotocin-induced experimental dementia of Alzheimer’s type. Brain Res. 2011, 1389, 133–142. [Google Scholar] [CrossRef]
- Hiranya, P.; Jirapas, S.; Luerat, S.; Nattayaporn, A.; Nipon, C.; Siriporn, C. Garlic extract attenuates brain mitochondrial dysfunction and cognitive deficit in obese-insulin resistant rats. Appl. Physiol. Nutr. Metab. 2014, 39, 1373–1379. [Google Scholar] [CrossRef]
- Ghasemi, S.; Hosseini, M.; Feizpour, A.; Alipour, F.; Sadeghi, A.; Vafaee, F.; Mohammadpour, T.; Soukhtanloo, M.; Ebrahimzadeh Bideskan, A.; Beheshti, F. Beneficial effects of garlic on learning and memory deficits and brain tissue damages induced by lead exposure during juvenile rat growth is comparable to the effect of ascorbic acid. Drug Chem. Toxicol. 2017, 40, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, T.; Farooqui, A.A. Neuroprotective Effects of garlic in model systems of neurodegenerative diseases. In Role of the Mediterranean Diet in the Brain and Neurodegenerative Diseases; Farooqui, T., Farooqui, A.A., Eds.; Academic Press: Cambridge, MA, USA, 2018; Chapter 16; pp. 253–269. [Google Scholar]
- Ghosh, M.; Wang, H.D.; McNeill, J.R. Role of oxidative stress and nitric oxide in regulation of spontaneous tone in aorta of DOCA-salt hypertensive rats. Br. J. Pharmacol. 2004, 141, 562–573. [Google Scholar] [CrossRef]
- Schulz, E.; Jansen, T.; Wenzel, P.; Daiber, A.; Münzel, T. Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid. Redox Signal. 2008, 10, 1115–1126. [Google Scholar] [CrossRef]
- Soreq, H.; Seidman, S. Acetylcholinesterase—New roles for an old actor. Nat. Rev. Neurosci. 2001, 2, 294–302. [Google Scholar] [CrossRef]
- Tian, Q.; Lin, Z.Q.; Wang, X.C.; Chen, J.; Wang, Q.; Gong, C.X.; Wang, J.Z. Injection of okadaic acid into the meynert nucleus basalis of rat brain induces decreased acetylcholine level and spatial memory deficit. Neuroscience 2004, 126, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Kim, M.R. Effect of aged garlic ethyl acetate extract on oxidative stress and cholinergic function of scopolamine-Induced cognitive impairment in mice. Prev. Nutr. Food Sci. 2019, 24, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. Dual inhibition of acetylcholinesterase and butyrylcholineesterase enzymes by allicin. Indian J. Pharmacol. 2015, 47, 444–446. [Google Scholar] [CrossRef] [PubMed]
| (A) | ||||||
|---|---|---|---|---|---|---|
| Diallyl Sulfide | Diallyl Disulfide | Diallyl Trisulfide | ||||
| Raw garlic | 0.93 c | 2.51 b | 13.49 a | |||
| Black garlic | 87.8 c | 203.9 b | 282.6 a | |||
| (B) | ||||||
| 50% EtOH | 70% EtOH | 95% EtOH | ||||
| Raw Garlic | Black Garlic | Raw Garlic | Black Garlic | Raw Garlic | Black Garlic | |
| Total phenolic acids A | 0.590 ± 0.049 e | 5.49 ± 0.35 b | 0.641 ± 0.023 d | 6.75 ± 0.46 a | 0.534 ± 0.024 f | 1.40 ± 0.05 c |
| Total flavonoids B | 0.044 ± 0.006 e | 0.75 ± 0.05 b | 0.120 ± 0.010 d | 1.28 ± 0.12 a | 0.025 ± 0.006 f | 0.15 ± 0.01 c |
| Groups | Systolic Blood Pressure (mmHg) | ||||||
|---|---|---|---|---|---|---|---|
| Week 0 | Week 2 | Week 4 | Week 6 | Week 8 | Week 10 | Week 12 | |
| C | 117.2 ± 3.5 Ba | 121.4 ± 3.6 ABb | 120.3 ± 6.4 ABb | 123.2 ± 3.9 Ab | 117.0 ± 4.9 Bc | 119.2 ± 4.2 ABc | 120.2 ± 4.9 ABd |
| D | 118.5 ± 2.9 Ea | 126.0 ± 4.0 Dab | 137.5 ± 6.3 Ca | 160.0 ± 6.3 Ba | 166.9 ± 5.1 Aa | 169.5 ± 7.7 Aa | 173.4 ± 4.4 Aa |
| PC | 120.5 ± 4.8 Da | 127.7 ± 6.6 Cab | 138.4 ± 8.2 Ba | 158.3 ± 5.7 Aa | 158.0 ± 4.6 Ab | 152.6 ± 6.9 Ab | 144.4 ± 7.3 Bc |
| HE | 119.4 ± 4.5 Da | 125.6 ± 6.5 Dab | 136.8 ± 9.5 Ca | 157.2 ± 9.0 ABa | 161.5 ± 7.7 Aab | 156.7 ± 6.6 ABb | 150.0 ± 7.6 Bbc |
| HN | 118.6 ± 3.6 Ea | 129.0 ± 5.8 Da | 141.3 ± 4.9 Ca | 158.7 ± 7.0 Aa | 159.8 ± 8.7 Aab | 155.2 ± 6.8 Ab | 149.4 ± 8.1 Bbc |
| LE | 116.8 ± 5.1 Da | 124.8 ± 8.5 Cab | 144.5 ± 6.7 Ba | 162.7 ± 6.5 Aa | 161.5 ± 6.7 Aab | 158.2 ± 8.0 Ab | 155.0 ± 8.0 Ab |
| LN | 119.1 ± 6.0 Da | 129.5 ± 5.6 Ca | 142.1 ± 6.3 Ba | 160.2 ± 7.1 Aa | 159.7 ± 7.3 Aab | 156.3 ± 8.2 Ab | 152.3 ± 5.3 Abc |
| Group | Total Swimming Distance (cm) | Escape Latency (s) | ||||
|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 1 | Day 2 | Day 3 | |
| Reference memory task | ||||||
| C | 767.73 ± 103.73 Aa | 441.75 ± 112.66 Bb | 224.02 ± 97.25 Cb | 31.87 ± 10.15 Aa | 18.92 ± 5.85 Bb | 9.92 ± 4.29 Bb |
| D | 995.84 ± 188.73 Aa | 713.38 ± 209.50 ABa | 541.26 ± 205.86 Ba | 50.24 ± 11.48 Aa | 39.19 ± 8.04 ABa | 28.44 ± 9.08 Ba |
| PC | 842.06 ± 313.78Aa | 371.96 ± 173.97 Bb | 281.97 ± 163.49 Bb | 34.33 ± 13.37 Aa | 16.67 ± 7.05 Bb | 13.61 ± 7.11 Bb |
| HE | 791.77 ± 182.28 Aa | 439.65 ± 182.25 Bb | 307.71 ± 75.56 Bb | 37.13 ± 12.21 Aa | 19.89 ± 7.01 Bb | 14.57 ± 5.38 Bb |
| HN | 746.88 ± 269.54 Aa | 359.68 ± 129.05 Bb | 250.27 ± 125.73 Bb | 36.25 ± 13.51 Aa | 16.28 ± 6.31 Bb | 12.21 ± 5.65 Bb |
| LE | 907.87 ± 294.82 Aa | 444.94 ± 78.91 Bb | 301.83 ± 108.25 Bb | 43.02 ± 12.34 Aa | 21.01 ± 2.67 Bb | 15.44 ± 4.57 Bb |
| LN | 890.78 ± 343.01 Aa | 428.43 ± 137.43 Bb | 375.43 ± 185.71 Bab | 41.31 ± 20.36 Aa | 20.11 ± 6.31 Bb | 18.64 ± 5.92 Bb |
| Day 5 | Day 6 | Day 7 | Day 5 | Day 6 | Day 7 | |
| Working memory task | ||||||
| C | 193.56 ± 114.26 A | 156.76 ± 43.02 B | 142.78 ± 34.08 B | 8.93 ± 6.14 A | 6.73 ± 2.06 B | 6.94 ± 1.93 B |
| D | 783.07 ± 123.26 A | 552.35 ± 220.46 B | 439.66 ± 101.02 B | 35.91 ± 6.61 A | 28.12 ± 7.57 A | 21.52 ± 3.12 B |
| PC | 247.31 ± 137.00 A | 231.64 ± 89.02 A | 166.59 ± 73.52 B | 10.49 ± 7.11 A | 8.93 ± 3.09 B | 7.31 ± 2.53 B |
| HE | 306.80 ± 92.97 A | 281.27 ± 81.48 A | 164.28 ± 91.38 B | 14.43 ± 5.22 A | 13.48 ± 4.54 A | 6.93 ± 3.21 B |
| HN | 323.90 ± 107.48 A | 243.63 ± 85.15 B | 142.37 ± 98.54 C | 14.87 ± 6.05 A | 11.33 ± 4.21 A | 6.70 ± 5.61 B |
| LE | 426.64 ± 126.97 A | 298.02 ± 78.58 B | 189.55 ± 66.06 C | 18.23 ± 5.67 A | 12.92 ± 1.30 B | 8.46 ± 3.01 C |
| LN | 447.39 ± 119.98 A | 283.72 ± 94.82 BC | 185.92 ± 105.31 C | 9.43 ± 6.26 A | 7.58 ± 3.13 B | 8.32 ± 4.12 B |
| Groups | Nitrate + Nitrite (μM) | TNF-α (pg/g Tissue) | IL-6 (pg/g Tissue) | IL-1β (pg/g Tissue) | ||||
|---|---|---|---|---|---|---|---|---|
| C | 23.28 ± 5.95 A | 310.53 ± 79.33 C | 1890.75 ± 84.36 D | 1465.38 ± 118.46 D | ||||
| D | 12.94 ± 3.54 C | 593.75 ± 121.77 A | 2684.75 ± 281.72 A | 2833.01 ± 208.09 A | ||||
| PC | 23.69 ± 5.07 A | 377.44 ± 54.96 BC | 1994.86 ± 215.67 CD | 1599.86 ± 166.94 D | ||||
| HE | 20.15 ± 3.81 AB | 401.88 ± 94.12 BC | 2227.14 ± 241.24 BC | 1922.57 ± 130.81 C | ||||
| HN | 21.23 ± 5.08 AB | 390.57 ± 78.31 BC | 2046.83 ± 123.61 CD | 2012.33 ± 267.47 C | ||||
| LE | 15.98 ± 3.87 BC | 490.35 ± 107.76 AB | 2305.17 ± 194.02 B | 2564.34 ± 208.08 B | ||||
| LN | 15.65 ± 3.94 BC | 477.63 ± 97.51 B | 2416.67 ± 175.18 B | 2360.83 ± 200.97 B | ||||
| Groups | AChE (mU/g Tissue) | SOD (U/g Tissue) | CAT (mU/g Tissue) | GSH (nmol/g Tissue) | GSH-Px (nmol/min/g Tissue) | MDA (nmol/g Tissue) | ||
| C | 56.73 ± 14.27 B | 44.80 ± 3.15 A | 514.93 ± 76.89 A | 631.83 ± 64.16 A | 3.91 ± 1.06 A | 113.84 ± 10.39 B | ||
| D | 80.78 ± 16.41 A | 33.77 ± 7.89 B | 280.71 ± 70.29 D | 417.23 ± 69.74 B | 1.92 ± 0.57 D | 141.61 ± 14.77 A | ||
| PC | 51.23 ± 8.06 B | 45.81 ± 6.02 A | 457.38 ± 114.79 AB | 595.44 ± 68.85 A | 3.58 ± 0.71 A | 116.18 ± 8.89 B | ||
| HE | 54.37 ± 9.97 B | 45.00 ± 5.95 A | 369.23 ± 90.53 C | 625.28 ± 96.64 A | 3.14 ± 0.70 AB | 121.97 ± 8.30 B | ||
| HN | 52.38 ± 24.09 B | 43.51 ± 4.74 A | 398.63 ± 73.87 C | 651.88 ± 65.66 A | 3.28 ± 0.67 AB | 117.90 ± 4.88 B | ||
| LE | 64.68 ± 12.40 B | 37.92 ± 7.31 AB | 320.65 ± 61.41 CD | 427.19 ± 82.71 B | 2.25 ± 0.78 C | 137.24 ± 7.00 A | ||
| LN | 58.87 ± 11.14 B | 40.45 ± 9.29 AB | 353.03 ± 99.85 C | 463.07 ± 47.70 B | 2.55 ± 0.76 C | 133.86 ± 11.45 A | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-Y.; Tsai, T.-Y.; Chen, B.-H. Effects of Black Garlic Extract and Nanoemulsion on the Deoxy Corticosterone Acetate-Salt Induced Hypertension and Its Associated Mild Cognitive Impairment in Rats. Antioxidants 2021, 10, 1611. https://doi.org/10.3390/antiox10101611
Chen C-Y, Tsai T-Y, Chen B-H. Effects of Black Garlic Extract and Nanoemulsion on the Deoxy Corticosterone Acetate-Salt Induced Hypertension and Its Associated Mild Cognitive Impairment in Rats. Antioxidants. 2021; 10(10):1611. https://doi.org/10.3390/antiox10101611
Chicago/Turabian StyleChen, Chun-Yu, Tsung-Yu Tsai, and Bing-Huei Chen. 2021. "Effects of Black Garlic Extract and Nanoemulsion on the Deoxy Corticosterone Acetate-Salt Induced Hypertension and Its Associated Mild Cognitive Impairment in Rats" Antioxidants 10, no. 10: 1611. https://doi.org/10.3390/antiox10101611
APA StyleChen, C.-Y., Tsai, T.-Y., & Chen, B.-H. (2021). Effects of Black Garlic Extract and Nanoemulsion on the Deoxy Corticosterone Acetate-Salt Induced Hypertension and Its Associated Mild Cognitive Impairment in Rats. Antioxidants, 10(10), 1611. https://doi.org/10.3390/antiox10101611








