Beneficial Effects of High Intensity Interval Training and/or Linseed Oil Supplementation to Limit Obesity-Induced Oxidative Stress in High Fat Diet-Fed Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
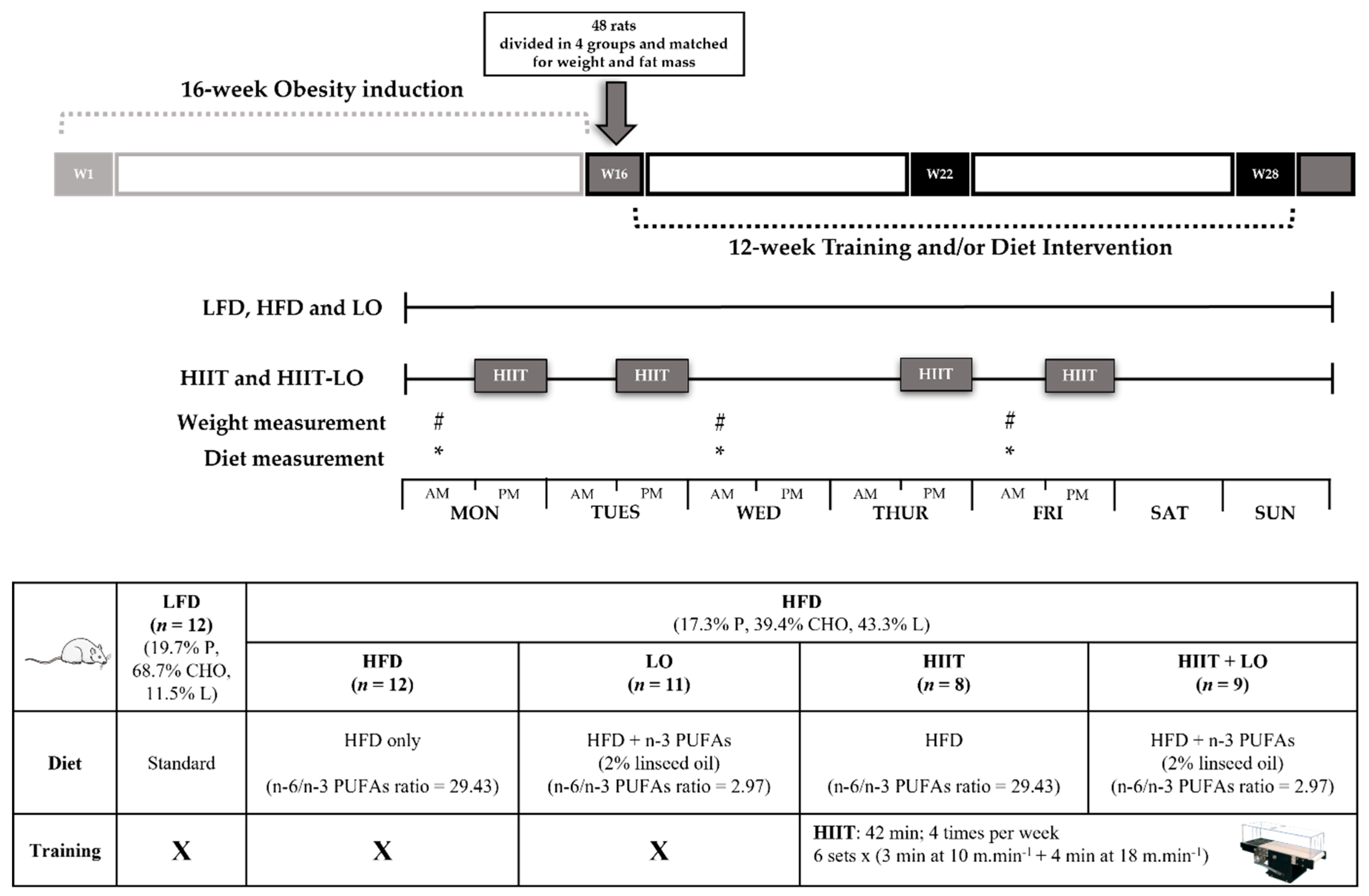
2.2. Animal Model and Experimental Groups
2.3. LO Supplementation
2.4. Training Protocol
2.5. Biochemical Analyses
2.5.1. Oxidative Stress Markers
2.5.2. Antioxidant System Markers
2.5.3. Pro-Oxidant Enzymes
2.6. Statistical Analysis
3. Results
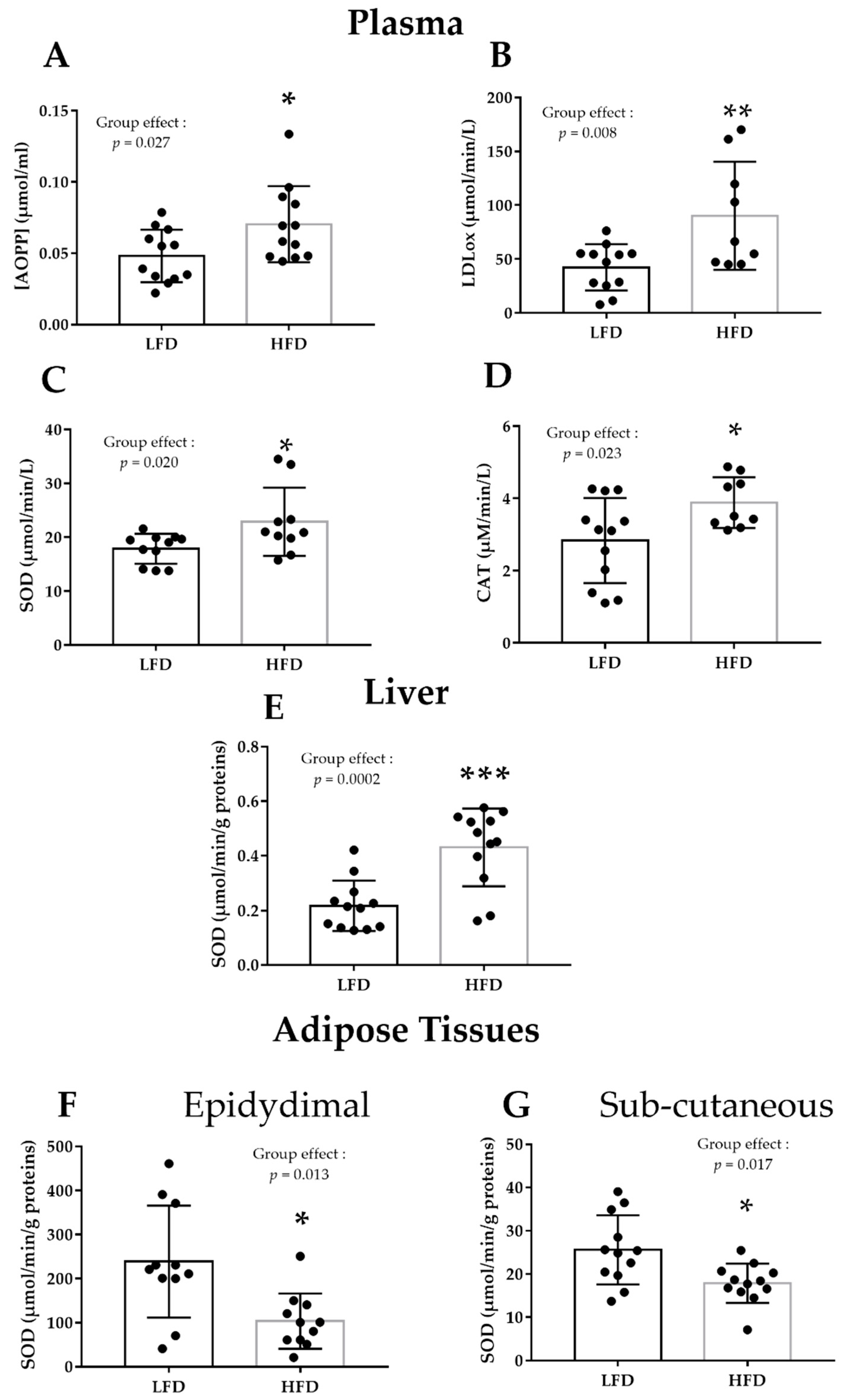
3.1. HFD Alters Plasma and Liver Pro/Antioxidant Status
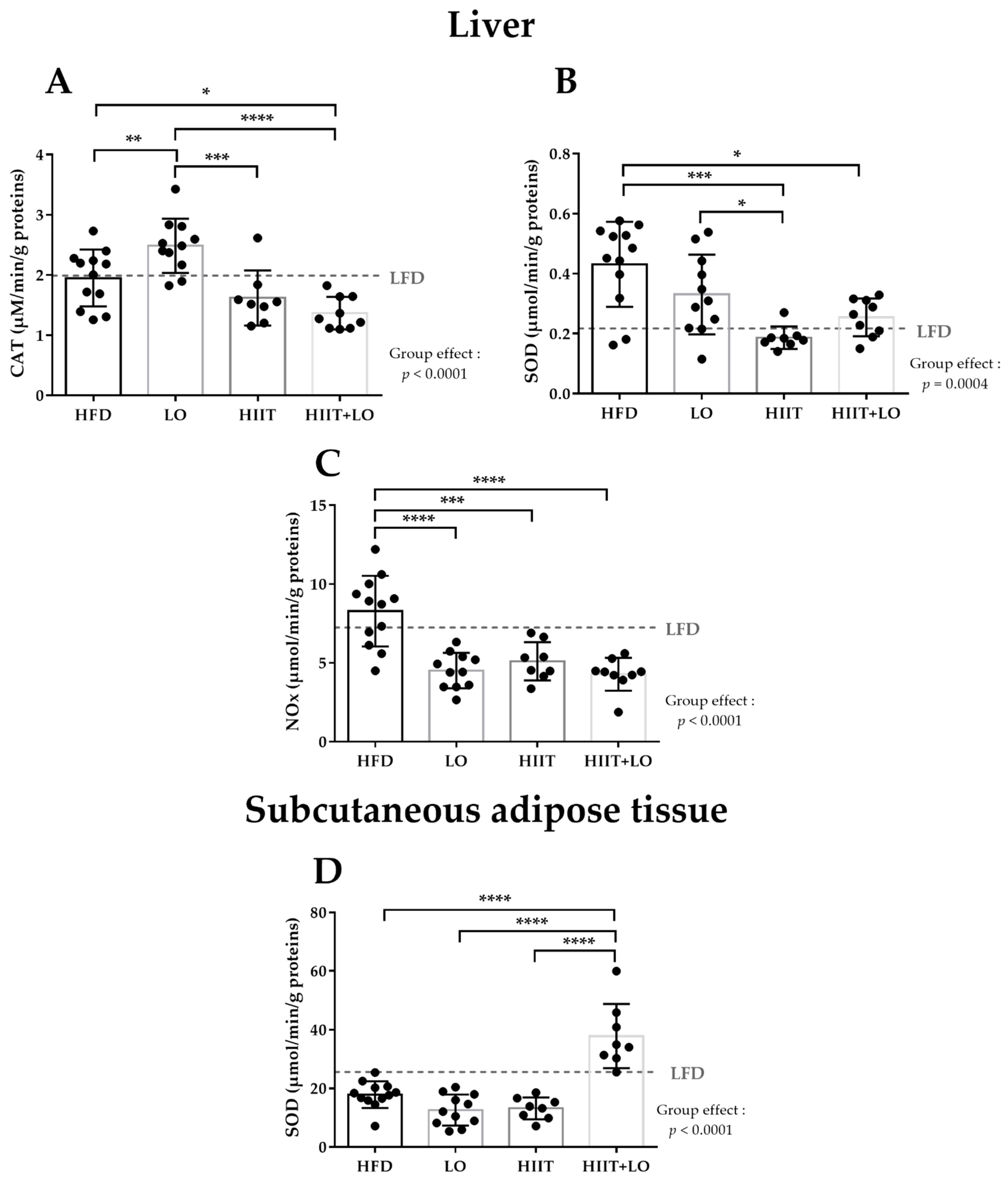
3.2. LO Supplementation Exerts Beneficial Effects in Liver by Upregulating CAT Activity and by Decreasing NOx Activity
3.3. HIIT Alleviates HFD Negative Effects by Reducing SOD and NOx Activities in Liver
3.4. The HIIT and LO Combination Potentiates Their Effect on SOD Activity in Subcutaneous Adipose Tissue
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Abdelaal, M.; le Roux, C.W.; Docherty, N.G. Morbidity and mortality associated with obesity. Ann. Transl. Med. 2017, 5, 161. [Google Scholar] [CrossRef]
- Wright, S.M.; Aronne, L.J. Causes of obesity. Abdom. Imaging 2012, 37, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Perez-Escamilla, R.; Obbagy, J.E.; Altman, J.M.; Essery, E.V.; McGrane, M.M.; Wong, Y.P.; Spahn, J.M.; Williams, C.L. Dietary energy density and body weight in adults and children: A systematic review. J. Acad. Nutr. Diet. 2012, 112, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Jebb, S.A.; Moore, M.S. Contribution of a sedentary lifestyle and inactivity to the etiology of overweight and obesity: Current evidence and research issues. Med. Sci. Sports Exerc. 1999, 31, S534–S541. [Google Scholar] [CrossRef] [PubMed]
- Rakhra, V.; Galappaththy, S.L.; Bulchandani, S.; Cabandugama, P.K. Obesity and the Western Diet: How We Got Here. Mo. Med. 2020, 117, 536–538. [Google Scholar] [PubMed]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef]
- Hu, C.; Yuan, Y.V.; Kitts, D.D. Antioxidant activities of the flaxseed lignan secoisolariciresinol diglucoside, its aglycone secoisolariciresinol and the mammalian lignans enterodiol and enterolactone in vitro. Food Chem. Toxicol. 2007, 45, 2219–2227. [Google Scholar] [CrossRef]
- Maillard, F.; Pereira, B.; Boisseau, N. Effect of High-Intensity Interval Training on Total, Abdominal and Visceral Fat Mass: A Meta-Analysis. Sports Med. 2018, 48, 269–288. [Google Scholar] [CrossRef]
- Racil, G.; Coquart, J.B.; Elmontassar, W.; Haddad, M.; Goebel, R.; Chaouachi, A.; Amri, M.; Chamari, K. Greater effects of high-compared with moderate-intensity interval training on cardio-metabolic variables, blood leptin concentration and ratings of perceived exertion in obese adolescent females. Biol. Sport 2016, 33, 145–152. [Google Scholar] [CrossRef]
- Rezzani, R.; Franco, C. Liver, Oxidative Stress and Metabolic Syndromes. Nutrients 2021, 13, 301. [Google Scholar] [CrossRef]
- Sethi, J.K.; Vidal-Puig, A.J. Targeting fat to prevent diabetes. Cell Metab. 2007, 5, 323–325. [Google Scholar] [CrossRef][Green Version]
- Higuchi, M.; Dusting, G.J.; Peshavariya, H.; Jiang, F.; Hsiao, S.T.; Chan, E.C.; Liu, G.S. Differentiation of human adipose-derived stem cells into fat involves reactive oxygen species and Forkhead box O1 mediated upregulation of antioxidant enzymes. Stem Cells Dev. 2013, 22, 878–888. [Google Scholar] [CrossRef]
- Tobore, T.O. Towards a comprehensive theory of obesity and a healthy diet: The causal role of oxidative stress in food addiction and obesity. Behav. Brain Res. 2020, 384, 112560. [Google Scholar] [CrossRef]
- Huang, C.J.; McAllister, M.J.; Slusher, A.L.; Webb, H.E.; Mock, J.T.; Acevedo, E.O. Obesity-Related Oxidative Stress: The Impact of Physical Activity and Diet Manipulation. Sports Med. Open 2015, 1, 32. [Google Scholar] [CrossRef]
- Vincent, H.K.; Innes, K.E.; Vincent, K.R. Oxidative stress and potential interventions to reduce oxidative stress in overweight and obesity. Diabetes Obes. Metab. 2007, 9, 813–839. [Google Scholar] [CrossRef]
- Keating, S.E.; Johnson, N.A.; Mielke, G.I.; Coombes, J.S. A systematic review and meta-analysis of interval training versus moderate-intensity continuous training on body adiposity. Obes. Rev. 2017, 18, 943–964. [Google Scholar] [CrossRef] [PubMed]
- Burgomaster, K.A.; Howarth, K.R.; Phillips, S.M.; Rakobowchuk, M.; Macdonald, M.J.; McGee, S.L.; Gibala, M.J. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J. Physiol. 2008, 586, 151–160. [Google Scholar] [CrossRef]
- Gibala, M.J.; Little, J.P.; Macdonald, M.J.; Hawley, J.A. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J. Physiol. 2012, 590, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Gibala, M.J.; Little, J.P.; van Essen, M.; Wilkin, G.P.; Burgomaster, K.A.; Safdar, A.; Raha, S.; Tarnopolsky, M.A. Short-term sprint interval versus traditional endurance training: Similar initial adaptations in human skeletal muscle and exercise performance. J. Physiol. 2006, 575, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Groussard, C.; Maillard, F.; Vazeille, E.; Barnich, N.; Sirvent, P.; Otero, Y.F.; Combaret, L.; Madeuf, E.; Sourdrille, A.; Delcros, G.; et al. Tissue-Specific Oxidative Stress Modulation by Exercise: A Comparison between MICT and HIIT in an Obese Rat Model. Oxidative Med. Cell Longev. 2019, 2019, 1965364. [Google Scholar] [CrossRef]
- Tamtaji, O.R.; Milajerdi, A.; Reiner, Z.; Dadgostar, E.; Amirani, E.; Asemi, Z.; Mirsafaei, L.; Mansournia, M.A.; Dana, P.M.; Sadoughi, F.; et al. Effects of flaxseed oil supplementation on biomarkers of inflammation and oxidative stress in patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. ESPEN 2020, 40, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Zhang, W.; Li, J.; Liang, H.; Zhou, H.; Duan, W.; Xu, X.; Yu, S.; Zhang, H.; Yi, D. alpha-Linolenic acid intake attenuates myocardial ischemia/reperfusion injury through anti-inflammatory and anti-oxidative stress effects in diabetic but not normal rats. Arch. Med. Res. 2011, 42, 171–181. [Google Scholar] [CrossRef]
- Alessandri, C.; Pignatelli, P.; Loffredo, L.; Lenti, L.; Del Ben, M.; Carnevale, R.; Perrone, A.; Ferro, D.; Angelico, F.; Violi, F. Alpha-linolenic acid-rich wheat germ oil decreases oxidative stress and CD40 ligand in patients with mild hypercholesterolemia. Arter. Thromb. Vasc. Biol. 2006, 26, 2577–2578. [Google Scholar] [CrossRef]
- Grajzer, M.; Szmalcel, K.; Kuzminski, L.; Witkowski, M.; Kulma, A.; Prescha, A. Characteristics and Antioxidant Potential of Cold-Pressed Oils-Possible Strategies to Improve Oil Stability. Foods 2020, 9, 1630. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Kowalczyk, A.; Sarritzu, E.; Cabras, P. Determination of antioxidant compounds and antioxidant activity in commercial oilseeds for food use. Food Chem. 2007, 103, 1494–1501. [Google Scholar] [CrossRef]
- Simopoulos, A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.X.S.; Palma, A.S.V.; Reis, B.R.; Franco, C.S.R.; Marconi, A.P.S.; Shiozaki, F.A.; Reis, L.G.; Salles, M.S.V.; Netto, A.S. Inclusion of soybean and linseed oils in the diet of lactating dairy cows makes the milk fatty acid profile nutritionally healthier for the human diet. PLoS ONE 2021, 16, e0246357. [Google Scholar] [CrossRef] [PubMed]
- Menzies, K.J.; Singh, K.; Saleem, A.; Hood, D.A. Sirtuin 1-mediated effects of exercise and resveratrol on mitochondrial biogenesis. J. Biol. Chem. 2013, 288, 6968–6979. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, L.; Ramirez-Sanchez, I.; Perkins, G.A.; Murphy, A.; Taub, P.R.; Ceballos, G.; Villarreal, F.J.; Hogan, M.C.; Malek, M.H. (-)-Epicatechin enhances fatigue resistance and oxidative capacity in mouse muscle. J. Physiol. 2011, 589, 4615–4631. [Google Scholar] [CrossRef] [PubMed]
- Plissonneau, C.; Capel, F.; Chassaing, B.; Dupuit, M.; Maillard, F.; Wawrzyniak, I.; Combaret, L.; Dutheil, F.; Etienne, M.; Mairesse, G.; et al. High-Intensity Interval Training and alpha-Linolenic Acid Supplementation Improve DHA Conversion and Increase the Abundance of Gut Mucosa-Associated Oscillospira Bacteria. Nutrients 2021, 13, 788. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Friedlander, M.; Capeillere-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Oberley, L.W.; Spitz, D.R. Assay of superoxide dismutase activity in tumor tissue. Methods Enzymol. 1984, 105, 457–464. [Google Scholar]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Johansson, L.H.; Borg, L.A. A spectrophotometric method for determination of catalase activity in small tissue samples. Anal. Biochem. 1988, 174, 331–336. [Google Scholar] [CrossRef]
- Laouafa, S.; Ribon-Demars, A.; Marcouiller, F.; Roussel, D.; Bairam, A.; Pialoux, V.; Joseph, V. Estradiol Protects Against Cardiorespiratory Dysfunctions and Oxidative Stress in Intermittent Hypoxia. Sleep 2017, 40. [Google Scholar] [CrossRef] [PubMed]
- Rios, J.L.; Bomhof, M.R.; Reimer, R.A.; Hart, D.A.; Collins, K.H.; Herzog, W. Protective effect of prebiotic and exercise intervention on knee health in a rat model of diet-induced obesity. Sci. Rep. 2019, 9, 3893. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Willett, W.C.; Hu, F.B. Global obesity: Trends, risk factors and policy implications. Nat. Rev. Endocrinol. 2013, 9, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Meng, R.; Zhu, D.L.; Bi, Y.; Yang, D.H.; Wang, Y.P. Anti-oxidative effect of apocynin on insulin resistance in high-fat diet mice. Ann. Clin. Lab. Sci. 2011, 41, 236–243. [Google Scholar] [PubMed]
- Saiyasit, N.; Chunchai, T.; Apaijai, N.; Pratchayasakul, W.; Sripetchwandee, J.; Chattipakorn, N.; Chattipakorn, S.C. Chronic high-fat diet consumption induces an alteration in plasma/brain neurotensin signaling, metabolic disturbance, systemic inflammation/oxidative stress, brain apoptosis, and dendritic spine loss. Neuropeptides 2020, 82, 102047. [Google Scholar] [CrossRef]
- Labban, R.S.M.; Alfawaz, H.A.; Almnaizel, A.T.; Al-Muammar, M.N.; Bhat, R.S.; El-Ansary, A. Garcinia mangostana extract and curcumin ameliorate oxidative stress, dyslipidemia, and hyperglycemia in high fat diet-induced obese Wistar albino rats. Sci. Rep. 2021, 11, 7278. [Google Scholar] [CrossRef]
- Valenzuela, R.; Espinosa, A.; Gonzalez-Manan, D.; D’Espessailles, A.; Fernandez, V.; Videla, L.A.; Tapia, G. N-3 long-chain polyunsaturated fatty acid supplementation significantly reduces liver oxidative stress in high fat induced steatosis. PLoS ONE 2012, 7, e46400. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.M.; de Freitas, A.F.S.; Costa, M.D.S.; Torres, M.; Castro, Y.A.A.; Almeida, A.M.R.; Paiva, P.M.G.; Carvalho, B.M.; Napoleao, T.H. Pilosocereus gounellei (Cactaceae) stem extract decreases insulin resistance, inflammation, oxidative stress, and cardio-metabolic risk in diet-induced obese mice. J. Ethnopharmacol. 2021, 265, 113327. [Google Scholar] [CrossRef]
- Kaithwas, G.; Singh, P.; Bhatia, D. Evaluation of in vitro and in vivo antioxidant potential of polysaccharides from Aloe vera (Aloe barbadensis Miller) gel. Drug Chem. Toxicol. 2014, 37, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Kaithwas, G.; Majumdar, D.K. Effect of L. usitatissimum (Flaxseed/Linseed) Fixed Oil against Distinct Phases of Inflammation. ISRN Inflamm. 2013, 2013, 735158. [Google Scholar] [CrossRef] [PubMed]
- Derbali, A.; Mnafgui, K.; Affes, M.; Derbali, F.; Hajji, R.; Gharsallah, N.; Allouche, N.; El Feki, A. Cardioprotective effect of linseed oil against isoproterenol-induced myocardial infarction in Wistar rats: A biochemical and electrocardiographic study. J. Physiol. Biochem 2015, 71, 281–288. [Google Scholar] [CrossRef]
- Roy, S.; Rawat, A.K.; Sammi, S.R.; Devi, U.; Singh, M.; Gautam, S.; Yadav, R.K.; Rawat, J.K.; Singh, L.; Ansari, M.N.; et al. Alpha-linolenic acid stabilizes HIF-1 alpha and downregulates FASN to promote mitochondrial apoptosis for mammary gland chemoprevention. Oncotarget 2017, 8, 70049–70071. [Google Scholar] [CrossRef]
- Hosseinian, F.S.; Muir, A.D.; Westcott, N.D.; Krol, E.S. Antioxidant capacity of flaxseed lignans in two model systems. J. Am. Oil Chem. Soc. 2006, 83, 835. [Google Scholar] [CrossRef]
- An, W.S.; Kim, H.J.; Cho, K.H.; Vaziri, N.D. Omega-3 fatty acid supplementation attenuates oxidative stress, inflammation, and tubulointerstitial fibrosis in the remnant kidney. Am. J. Physiol. Renal Physiol. 2009, 297, F895–F903. [Google Scholar] [CrossRef]
- Bhardwaj, K.; Verma, N.; Trivedi, R.K.; Bhardwaj, S.; Shukla, N. Significance of Ratio of Omega-3 and Omega-6 in Human Health with Special Reference to Flaxseed Oil. Int. J. Biol. Chem. 2016, 10, 1–6. [Google Scholar] [CrossRef]
- Han, H.; Qiu, F.; Zhao, H.; Tang, H.; Li, X.; Shi, D. Dietary Flaxseed Oil Prevents Western-Type Diet-Induced Nonalcoholic Fatty Liver Disease in Apolipoprotein-E Knockout Mice. Oxidative Med. Cell Longev. 2017, 2017, 3256241. [Google Scholar] [CrossRef]
- Pilar, B.; Gullich, A.; Oliveira, P.; Stroher, D.; Piccoli, J.; Manfredini, V. Protective Role of Flaxseed Oil and Flaxseed Lignan Secoisolariciresinol Diglucoside Against Oxidative Stress in Rats with Metabolic Syndrome. J. Food Sci. 2017, 82, 3029–3036. [Google Scholar] [CrossRef] [PubMed]
- Jangale, N.M.; Devarshi, P.P.; Dubal, A.A.; Ghule, A.E.; Koppikar, S.J.; Bodhankar, S.L.; Chougale, A.D.; Kulkarni, M.J.; Harsulkar, A.M. Dietary flaxseed oil and fish oil modulates expression of antioxidant and inflammatory genes with alleviation of protein glycation status and inflammation in liver of streptozotocin-nicotinamide induced diabetic rats. Food Chem. 2013, 141, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Jangale, N.M.; Devarshi, P.P.; Bansode, S.B.; Kulkarni, M.J.; Harsulkar, A.M. Dietary flaxseed oil and fish oil ameliorates renal oxidative stress, protein glycation, and inflammation in streptozotocin-nicotinamide-induced diabetic rats. J. Physiol. Biochem. 2016, 72, 327–336. [Google Scholar] [CrossRef] [PubMed]
- El Midaoui, A.; Haddad, Y.; Couture, R. Beneficial effects of argan oil on blood pressure, insulin resistance, and oxidative stress in rat. Nutrition 2016, 32, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Rastogi, R.; Guan, L.; Li, F.; Du, H.; Geng, X.; Ding, Y. Omega-3 fatty acid supplement reduces activation of NADPH oxidase in intracranial atherosclerosis stenosis. Neurol. Res. 2018, 40, 499–507. [Google Scholar] [CrossRef]
- Richard, D.; Wolf, C.; Barbe, U.; Kefi, K.; Bausero, P.; Visioli, F. Docosahexaenoic acid down-regulates endothelial Nox 4 through a sPLA2 signalling pathway. Biochem. Biophys. Res. Commun. 2009, 389, 516–522. [Google Scholar] [CrossRef]
- Dupuit, M.; Chavanelle, V.; Chassaing, B.; Perriere, F.; Etienne, M.; Plissonneau, C.; Boscaro, A.; Barnich, N.; Pialoux, V.; Maugard, T.; et al. The TOTUM-63 Supplement and High-Intensity Interval Training Combination Limits Weight Gain, Improves Glycemic Control, and Influences the Composition of Gut Mucosa-Associated Bacteria in Rats on a High Fat Diet. Nutrients 2021, 13, 1569. [Google Scholar] [CrossRef]
- Maillard, F.; Vazeille, E.; Sauvanet, P.; Sirvent, P.; Combaret, L.; Sourdrille, A.; Chavanelle, V.; Bonnet, R.; Otero, Y.F.; Delcros, G.; et al. High intensity interval training promotes total and visceral fat mass loss in obese Zucker rats without modulating gut microbiota. PLoS ONE 2019, 14, e0214660. [Google Scholar] [CrossRef]
- Delwing-de Lima, D.; Ulbricht, A.; Werlang-Coelho, C.; Delwing-Dal Magro, D.; Joaquim, V.H.A.; Salamaia, E.M.; de Quevedo, S.R.; Desordi, L. Effects of two aerobic exercise training protocols on parameters of oxidative stress in the blood and liver of obese rats. J. Physiol. Sci. 2018, 68, 699–706. [Google Scholar] [CrossRef]
- Pimenta, M.; Bringhenti, I.; Souza-Mello, V.; Dos Santos Mendes, I.K.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. High-intensity interval training beneficial effects on body mass, blood pressure, and oxidative stress in diet-induced obesity in ovariectomized mice. Life Sci. 2015, 139, 75–82. [Google Scholar] [CrossRef]
- Gomez-Cabrera, M.C.; Domenech, E.; Vina, J. Moderate exercise is an antioxidant: Upregulation of antioxidant genes by training. Free. Radic. Biol. Med. 2008, 44, 126–131. [Google Scholar] [CrossRef]
- Ostrom, E.L.; Valencia, A.P.; Marcinek, D.J.; Traustadottir, T. High intensity muscle stimulation activates a systemic Nrf2-mediated redox stress response. Free. Radic. Biol. Med. 2021, 172, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Done, A.J.; Newell, M.J.; Traustadottir, T. Effect of exercise intensity on Nrf2 signalling in young men. Free. Radic. Res. 2017, 51, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Nakao, C.; Ookawara, T.; Sato, Y.; Kizaki, T.; Imazeki, N.; Matsubara, O.; Haga, S.; Suzuki, K.; Taniguchi, N.; Ohno, H. Extracellular superoxide dismutase in tissues from obese (ob/ob) mice. Free. Radic. Res. 2000, 33, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Vincent, H.K.; Taylor, A.G. Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int. J. Obes. (Lond.) 2006, 30, 400–418. [Google Scholar] [CrossRef]
- Alves, R.; Suehiro, C.L.; Oliveira, F.G.; Frantz, E.D.C.; Medeiros, R.F.; Vieira, R.P.; Martins, M.A.; Lin, C.J.; Nobrega, A.; Toledo-Arruda, A.C. Aerobic exercise modulates cardiac NAD(P)H oxidase and the NRF2/KEAP1 pathway in a mouse model of chronic fructose consumption. J. Appl. Physiol. 2020, 128, 59–69. [Google Scholar] [CrossRef]
- Veras, A.S.C.; Gomes, R.L.; Almeida Tavares, M.E.; Giometti, I.C.; Cardoso, A.; da Costa Aguiar Alves, B.; Lenquiste, S.A.; Vanderlei, L.C.M.; Teixeira, G.R. Supplementation of polyunsaturated fatty acids (PUFAs) and aerobic exercise improve functioning, morphology, and redox balance in prostate obese rats. Sci. Rep. 2021, 11, 6282. [Google Scholar] [CrossRef]
- Napoli, C.; Williams-Ignarro, S.; De Nigris, F.; Lerman, L.O.; Rossi, L.; Guarino, C.; Mansueto, G.; Di Tuoro, F.; Pignalosa, O.; De Rosa, G.; et al. Long-term combined beneficial effects of physical training and metabolic treatment on atherosclerosis in hypercholesterolemic mice. Proc. Natl. Acad. Sci. USA 2004, 101, 8797–8802. [Google Scholar] [CrossRef]
- Gueritat, J.; Lefeuvre-Orfila, L.; Vincent, S.; Cretual, A.; Ravanat, J.L.; Gratas-Delamarche, A.; Rannou-Bekono, F.; Rebillard, A. Exercise training combined with antioxidant supplementation prevents the antiproliferative activity of their single treatment in prostate cancer through inhibition of redox adaptation. Free. Radic. Biol. Med. 2014, 77, 95–105. [Google Scholar] [CrossRef]
- Jiang, F.; Lim, H.K.; Morris, M.J.; Prior, L.; Velkoska, E.; Wu, X.; Dusting, G.J. Systemic upregulation of NADPH oxidase in diet-induced obesity in rats. Redox Rep. 2011, 16, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Bondia-Pons, I.; Ryan, L.; Martinez, J.A. Oxidative stress and inflammation interactions in human obesity. J. Physiol. Biochem. 2012, 68, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Vogiatzi, G.; Tousoulis, D.; Stefanadis, C. The role of oxidative stress in atherosclerosis. Hell. J. Cardiol. 2009, 50, 402–409. [Google Scholar]
| LFD | HFD | p | |
|---|---|---|---|
| Plasma | |||
| GPx (μmol·min−1·g−1) | 0.22 ± 0.02 | 0.21 ± 0.02 | 0.08 |
| Liver | |||
| AOPP (μmol·g−1) | 3.40 ± 1.23 | 3.24 ± 1.06 | 0.73 |
| MDA (μmol·g−1) | 0.21 ± 0.16 | 0.29 ± 0.21 | 0.31 |
| XO (μmol·min−1·g−1) | 0.14 ± 0.05 | 0.18 ± 0.05 | 0.07 |
| NOx (μmol·min−1·g−1) | 0.72 ± 0.39 | 0.83 ± 0.24 | 0.44 |
| CAT (μmol·min−1·g−1) | 0.20 ± 0.03 | 0.19 ± 0.01 | 0.89 |
| GPx (μmol·min−1·g−1) | 3.58 ± 1.68 | 2.94 ± 1.34 | 0.31 |
| Muscle | |||
| AOPP (μmol·g−1) | 9.51 ± 3.64 | 10.76 ± 8.01 | 0.96 |
| MDA (μmol·g−1) | 0.92 ± 0.22 | 0.9 ± 0.23 | 0.80 |
| XO (μmol·min−1·g−1) | 11.46 ± 2.13 | 11.85 ± 3.02 | 0.73 |
| NOx (μmol·min−1·g−1) | 10.99 ± 3.03 | 9.38 ± 2.64 | 0.19 |
| SOD (μmol·min−1·g−1) | 3.85 ± 1.61 | 3.18 ± 1.90 | 0.38 |
| CAT (μmol·min−1·g−1) | 1.96 ± 0.43 | 2.10 ± 0.95 | 0.65 |
| GPx (μmol·min−1·g−1) | 109.57 ± 24.96 | 118.96 ± 43.00 | 0.54 |
| Subcutaneous adipose tissue | |||
| AOPP (μmol·mg−1) | 2.13 ± 1.30 | 1.83 ± 0.87 | 0.68 |
| MDA (μmol·g−1) | 4.42 ± 1.73 | 3.74 ± 1.79 | 0.35 |
| XO (μmol·min−1·g−1) | 2.27 ± 0.70 | 1.99 ± 0.40 | 0.24 |
| NOx (μmol·min−1·g−1) | 3.07 ± 0.70 | 2.67 ± 0.55 | 0.13 |
| CAT (μmol·min−1·g−1) | 1.83 ± 0.60 | 1.70 ± 0.39 | 0.55 |
| GPx (μmol·min−1·g−1) | 60.51 ± 22.90 | 49.29 ± 8.0 | 0.36 |
| Epididymal adipose tissue | |||
| AOPP (μmol·g−1) | 25.11 ± 38.97 | 15.53 ± 11.84 | 0.77 |
| MDA (μmol·g−1) | 64.21 ± 67.60 | 26.47 ± 12.65 | 0.07 |
| XO (μmol·min−1·g−1) | 22.24 ± 9.56 | 23.57 ± 8.80 | 0.73 |
| NOx (μmol·min−1·g−1) | 22.54 ± 8.77 | 22.30 ± 7.52 | 0.85 |
| CAT (μmol·min−1·g−1) | 22.40 ± 16.38 | 12.95 ± 5.45 | 0.08 |
| GPx (μmol·min−1·g−1) | 119.56 ± 60.54 | 115.93 ± 57.36 | 0.88 |
| HFD (n = 12) | LO (n = 11) | HIIT (n = 8) | LO + HIIT (n = 9) | p | |
|---|---|---|---|---|---|
| Liver | |||||
| AOPP (μmol·g−1) | 3.24 ± 1.06 | 3. 40 ± 1.02 | 3.25 ± 1.40 | 2.98 ± 0.53 | 0.89 |
| MDA (μmol·g−1) | 0.29 ± 0.21 | 0.25 ± 0.20 | 0.21 ± 0.17 | 0.23 ± 0.19 | 0.23 |
| XO (μmol·min−1·g−1) | 0.17 ± 0.05 ab | 0.21 ± 0.05 b | 0.14 ± 0.03 a | 0.15 ± 0.04 a | 0.007 |
| GPx (μmol·min−1·g−1) | 2.95 ± 1.34 | 3.17 ± 0.87 | 2.36 ± 0.79 | 2.41 ± 0.78 | 0.15 |
| Plasma | |||||
| AOPP (μmol·g−1) | 0.07 ± 0.03 | 0.08 ± 0.04 | 0.08 ± 0.03 | 0.07 ± 0.02 | 0.82 |
| CAT (μmol·min−1·g−1) | 3.89 ± 0.70 | 4.07 ± 0.98 | 3.94 ± 0.68 | 3.56 ± 0.59 | 0.53 |
| GPx (μmol·min−1·g−1) | 0.21 ± 0.02 | 0.22 ± 0.02 | 0.22 ± 0.02 | 0.21 ± 0.02 | 0.74 |
| Muscle | |||||
| AOPP (μmol·g−1) | 10.76 ± 8.01 | 9.10 ± 3.34 | 11.48 ± 7.31 | 10.48 ± 5.71 | 0.94 |
| MDA (μmol·g−1) | 0.90 ± 0.23 | 0.90 ± 0.27 | 0.82 ± 0.33 | 1.00 ± 0.52 | 0.74 |
| XO (μmol·min−1·g−1) | 11.85 ± 3.02 | 11.85 ± 3.02 | 11.64 ± 4.22 | 10.92 ± 2.07 | 0.91 |
| NOx (μmol·min−1·g−1) | 9.38 ± 2.64 | 10.52 ± 3.46 | 11.21 ± 2.52 | 10.98 ± 2.09 | 0.48 |
| SOD (μmol·min−1·g−1) | 3.18 ± 1.90 ab | 2.02 ± 0.92 b | 4.14 ± 1.20 a | 3.52 ± 0.875 ab | 0.01 |
| CAT (μmol·min−1·g−1) | 2.10 ± 0.95 | 2.22 ± 0.86 | 2.63 ± 0.89 | 2.11 ± 0.71 | 0.55 |
| GPx (μmol·min−1·g−1) | 118.96 ± 43.00 | 115.81 ± 31.60 | 117.83 ± 40.40 | 100.28 ± 33.51 | 0.71 |
| Subcutaneous adipose tissue | |||||
| AOPP (μmol·mg−1) | 1.83 ± 0.87 | 1.64 ± 0.55 | 1.91 ± 1.03 | 1.52 ± 0.35 | 0.98 |
| MDA (μmol·g−1) | 3.74 ± 1.79 a | 3.35 ± 2.16 a | 6.46 ± 3.69 a | 5.95 ± 3.15 a | 0.03 |
| XO (μmol·min−1·g−1) | 1.99 ± 0.40 | 1.79 ± 0.78 | 1.95 ± 0.49 | 1.83 ± 0.79 | 0.87 |
| NOx (μmol·min−1·g−1) | 2.67 ± 0.55 | 2.33 ± 0.91 | 2.82 ± 0.64 | 2.46 ± 0.90 | 0.52 |
| CAT (μmol·min−1·g−1) | 1.70 ± 0.39 | 1.52 ± 0.51 | 1.47 ± 0.28 | 1.52 ± 0.26 | 0.55 |
| Epididymal adipose tissue | |||||
| AOPP (μmol·g−1) | 15.53 ± 11.84 | 15.64 ± 8.59 | 20.80 ± 9.40 | 16.41 ± 8.19 | 0.58 |
| MDA (μmol·g−1) | 26.47 ± 12.65 | 29.07 ± 20.02 | 29.75 ± 14.10 | 30.58 ± 15.44 | 0.94 |
| XO (μmol·min−1·g−1) | 23.57 ± 8.80 | 25.75 ± 8.69 | 28.16 ± 7.48 | 28.63 ± 15.73 | 0.69 |
| NOx (μmol·min−1·g−1) | 22.30 ± 7.52 | 23.74 ± 8.16 | 25.39 ± 6.48 | 29.74 ± 14.44 | 0.44 |
| CAT (μmol·min−1·g−1) | 12.95 ± 5.45 | 15.71 ± 10.98 | 16.36 ± 8.10 | 19.00 ± 14.09 | 0.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Groussard, C.; Plissonneau, C.; Josset, L.; Capel, F.; Mura, M.; Gouraud, E.; Mairesse, G.; Chesneau, G.; Barnich, N.; Pialoux, V.; et al. Beneficial Effects of High Intensity Interval Training and/or Linseed Oil Supplementation to Limit Obesity-Induced Oxidative Stress in High Fat Diet-Fed Rats. Nutrients 2021, 13, 3531. https://doi.org/10.3390/nu13103531
Groussard C, Plissonneau C, Josset L, Capel F, Mura M, Gouraud E, Mairesse G, Chesneau G, Barnich N, Pialoux V, et al. Beneficial Effects of High Intensity Interval Training and/or Linseed Oil Supplementation to Limit Obesity-Induced Oxidative Stress in High Fat Diet-Fed Rats. Nutrients. 2021; 13(10):3531. https://doi.org/10.3390/nu13103531
Chicago/Turabian StyleGroussard, Carole, Claire Plissonneau, Laurie Josset, Fréderic Capel, Mathilde Mura, Etienne Gouraud, Guillaume Mairesse, Guillaume Chesneau, Nicolas Barnich, Vincent Pialoux, and et al. 2021. "Beneficial Effects of High Intensity Interval Training and/or Linseed Oil Supplementation to Limit Obesity-Induced Oxidative Stress in High Fat Diet-Fed Rats" Nutrients 13, no. 10: 3531. https://doi.org/10.3390/nu13103531
APA StyleGroussard, C., Plissonneau, C., Josset, L., Capel, F., Mura, M., Gouraud, E., Mairesse, G., Chesneau, G., Barnich, N., Pialoux, V., & Boisseau, N. (2021). Beneficial Effects of High Intensity Interval Training and/or Linseed Oil Supplementation to Limit Obesity-Induced Oxidative Stress in High Fat Diet-Fed Rats. Nutrients, 13(10), 3531. https://doi.org/10.3390/nu13103531








