At a Crossroads to Cancer: How p53-Induced Cell Fate Decisions Secure Genome Integrity
Abstract
:1. Introduction
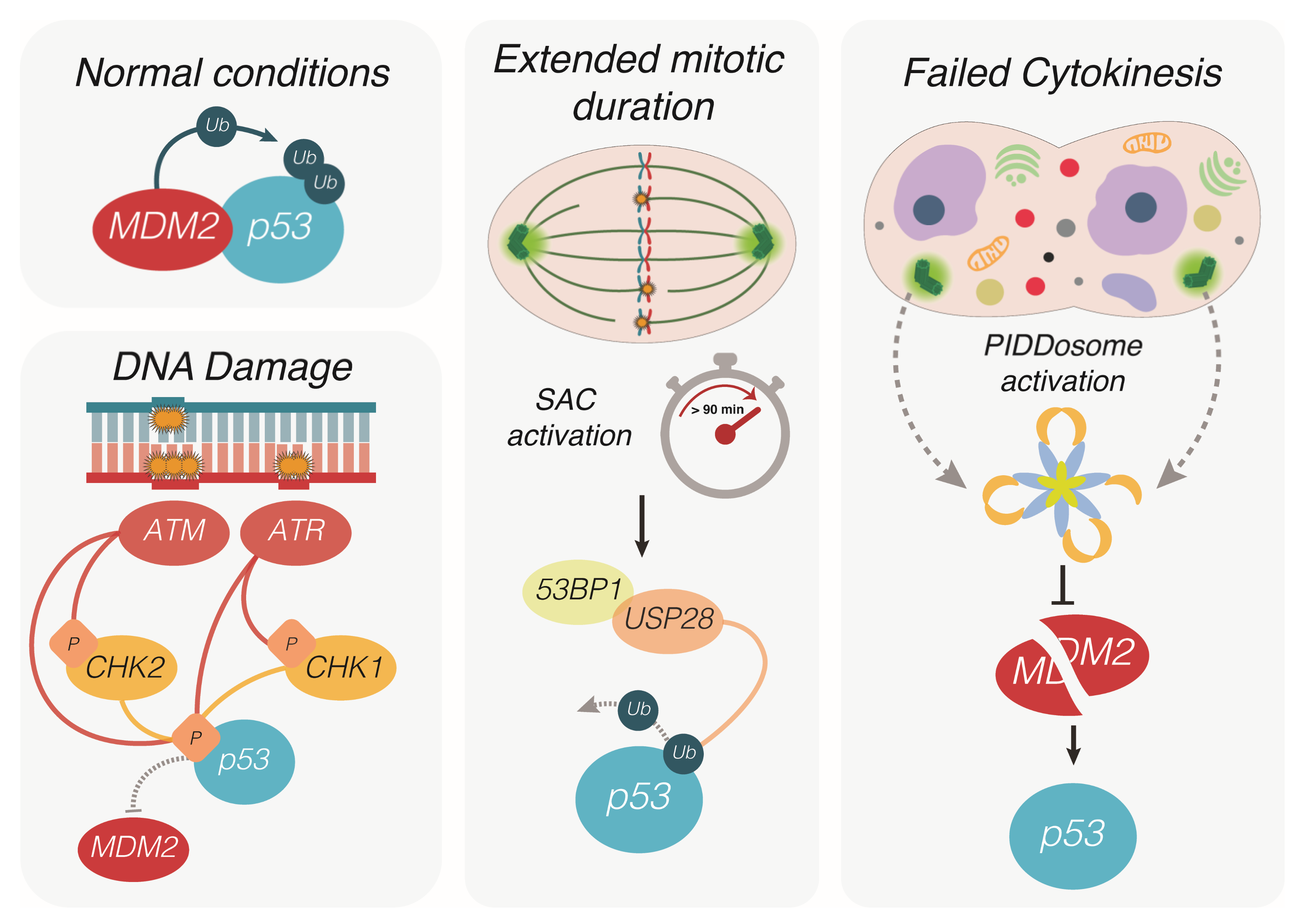
2. How p53 Puts the Break on CIN and Aneuploidy
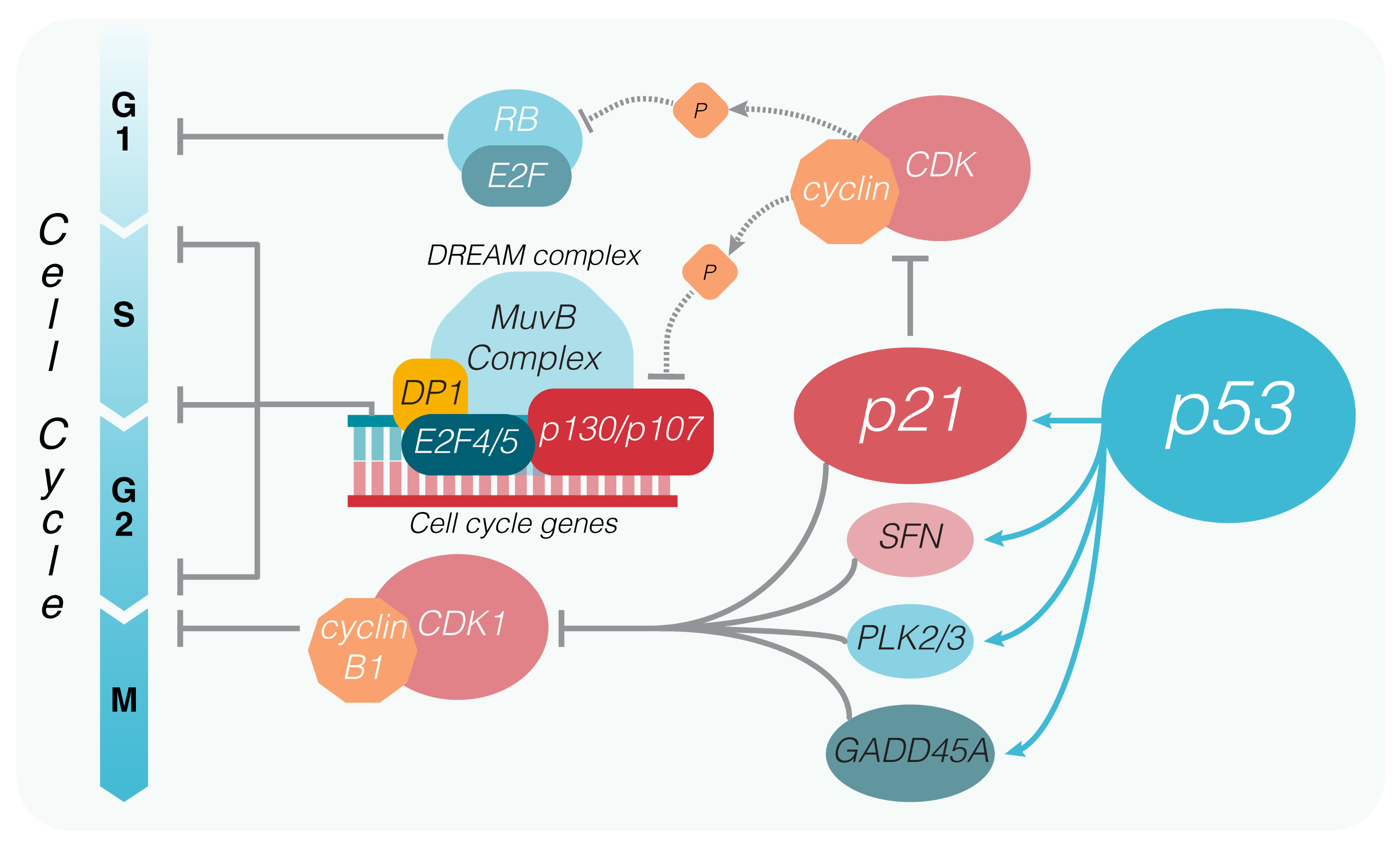
3. P53-Induced Cell Cycle Arrest and Senescence
4. P53 and the DREAM Complex
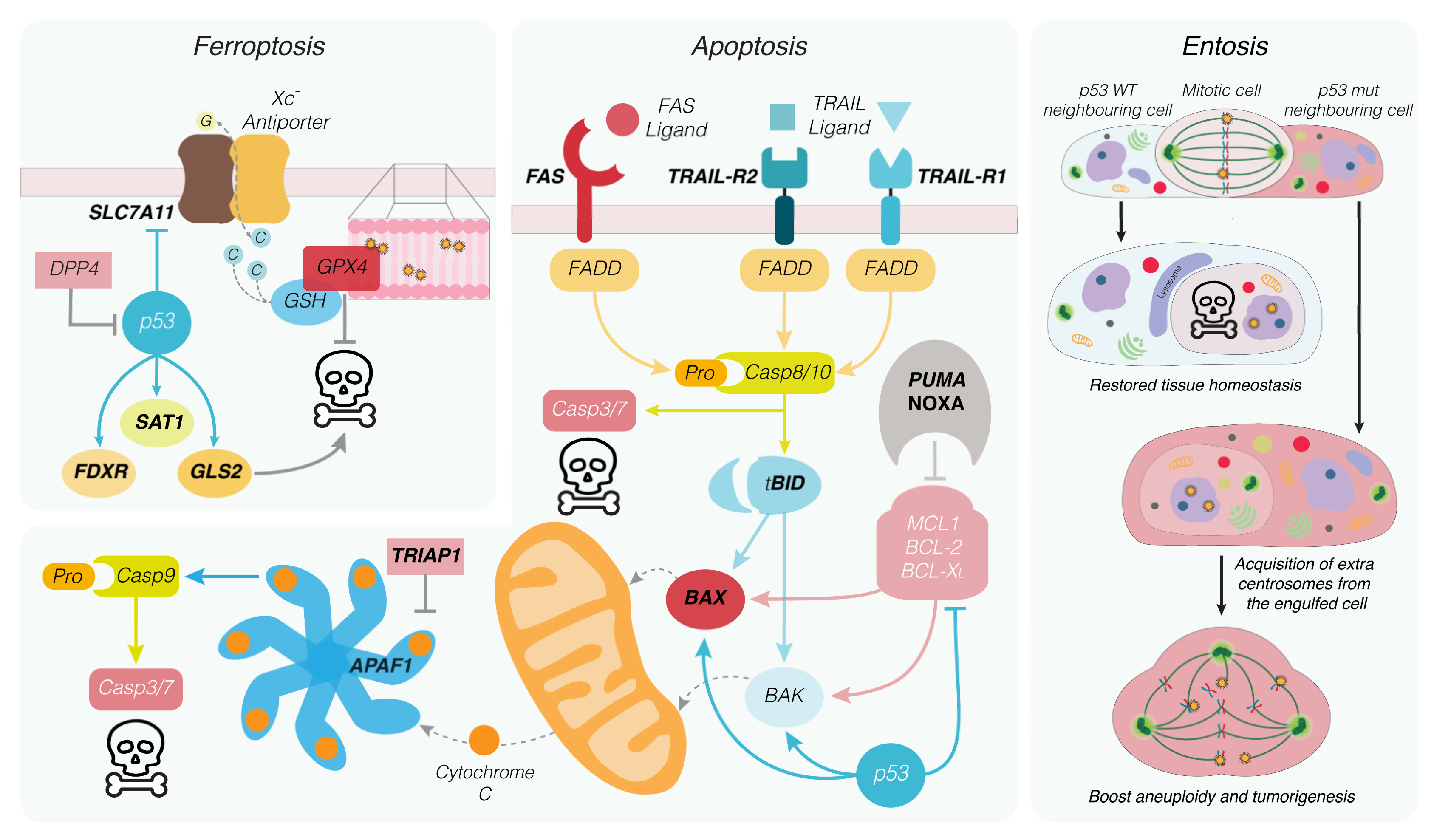
5. P53-Induced Apoptosis
6. Non-Apoptotic Cell Death Forms Regulated by p53
7. Life–Death Decisions by p53—Flicking the Switch
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACD | autophagic cell death |
| BH | BCL2 homology |
| ChIP | chromatin immunoprecipitation |
| CiC | cell-in-cell structure |
| CIN | chromosomal instability |
| DD | death domain |
| DDR | DNA damage response |
| DR | death receptor |
| MEF | mouse embryonic fibroblast |
| PTM | post-translational modifications |
| RBP | RNA binding proteins |
| SAC | spindle assembly checkpoint |
| SNP | single-nucleotide polymorphism |
| TNFR | tumor necrosis factor receptor |
References
- Báez, A. Genetic and Environmental Factors in Head and Neck Cancer Genesis. J. Environ. Sci. Health Part C 2008, 26, 174–200. [Google Scholar] [CrossRef] [PubMed]
- Landi, S. Genetic Predisposition and Environmental Risk Factors to Pancreatic Cancer: A Review of the Literature. Mutat. Res./Rev. Mutat. Res. 2009, 681, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.E.; Wark, J.D.; Hopper, J.L.; Erbas, B.; Garland, S.M.; CeCaGeEn Study Group. The Roles of Genetic and Environmental Factors on Risk of Cervical Cancer: A Review of Classical Twin Studies. Twin Res. Hum. Genet. 2012, 15, 79–86. [Google Scholar] [CrossRef]
- Nickels, S.; Truong, T.; Hein, R.; Stevens, K.; Buck, K.; Behrens, S.; Eilber, U.; Schmidt, M.; Häberle, L.; Vrieling, A.; et al. Evidence of Gene–Environment Interactions between Common Breast Cancer Susceptibility Loci and Established Environmental Risk Factors. PLoS Genet. 2013, 9, e1003284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M.S.; Suresh, S.; Waly, M.I. Risk Factors for Cancer: Genetic and Environment. In Bioactive Components, Diet and Medical Treatment in Cancer Prevention; Waly, M.I., Rahman, M.S., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–23. ISBN 978-3-319-75692-9. [Google Scholar]
- Rudolph, A.; Chang-Claude, J.; Schmidt, M.K. Gene–Environment Interaction and Risk of Breast Cancer. Br. J. Cancer 2016, 114, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, K.D.; Galbraith, M.D.; Andrysik, Z.; Espinosa, J.M. Mechanisms of Transcriptional Regulation by P53. Cell Death Differ. 2018, 25, 133–143. [Google Scholar] [CrossRef] [Green Version]
- Aubrey, B.J.; Kelly, G.L.; Janic, A.; Herold, M.J.; Strasser, A. How Does P53 Induce Apoptosis and How Does This Relate to P53-Mediated Tumour Suppression? Cell Death Differ. 2018, 25, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Chen, J. The Cell-Cycle Arrest and Apoptotic Functions of P53 in Tumor Initiation and Progression. Cold Spring Harb. Perspect. Med. 2016, 6, a026104. [Google Scholar] [CrossRef]
- Hofseth, L.J.; Hussain, S.P.; Harris, C.C. P53: 25 Years after Its Discovery. Trends Pharmacol. Sci. 2004, 25, 177–181. [Google Scholar] [CrossRef]
- Kastenhuber, E.R.; Lowe, S.W. Putting P53 in Context. Cell 2017, 170, 1062–1078. [Google Scholar] [CrossRef] [Green Version]
- El-Deiry, W.S.; Tokino, T.; Velculescu, V.E.; Levy, D.B.; Parsons, R.; Trent, J.M.; Lin, D.; Mercer, W.E.; Kinzler, K.W.; Vogelstein, B. WAF1, a Potential Mediator of P53 Tumor Suppression. Cell 1993, 75, 817–825. [Google Scholar] [CrossRef]
- Amaral, J.D.; Xavier, J.M.; Steer, C.J.; Rodrigues, C.M. The Role of P53 in Apoptosis. Discov. Med. 2010, 9, 145–152. [Google Scholar]
- Bieging, K.T.; Mello, S.S.; Attardi, L.D. Unravelling Mechanisms of P53-Mediated Tumour Suppression. Nat. Rev. Cancer 2014, 14, 359–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humpton, T.; Vousden, K.H. Taking up the Reins of Power: Metabolic Functions of P53. J. Mol. Cell Biol. 2019, 11, 610–614. [Google Scholar] [CrossRef]
- Vousden, K.H.; Prives, C. Blinded by the Light: The Growing Complexity of P53. Cell 2009, 137, 413–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Kon, N.; Jiang, L.; Tan, M.; Ludwig, T.; Zhao, Y.; Baer, R.; Gu, W. Tumor Suppression in the Absence of P53-Mediated Cell-Cycle Arrest, Apoptosis, and Senescence. Cell 2012, 149, 1269–1283. [Google Scholar] [CrossRef] [Green Version]
- Valente, L.J.; Gray, D.H.D.; Michalak, E.M.; Pinon-Hofbauer, J.; Egle, A.; Scott, C.L.; Janic, A.; Strasser, A. P53 Efficiently Suppresses Tumor Development in the Complete Absence of Its Cell-Cycle Inhibitory and Proapoptotic Effectors P21, Puma, and Noxa. Cell Rep. 2013, 3, 1339–1345. [Google Scholar] [CrossRef] [Green Version]
- Aylon, Y.; Oren, M. P53: Guardian of Ploidy. Mol. Oncol. 2011, 5, 315–323. [Google Scholar] [CrossRef]
- Gronroos, E.; López-García, C. Tolerance of Chromosomal Instability in Cancer: Mechanisms and Therapeutic Opportunities. Cancer Res. 2018, 78, 6529–6535. [Google Scholar] [CrossRef] [Green Version]
- Abbas, T.; Dutta, A. P21 in Cancer: Intricate Networks and Multiple Activities. Nat. Rev. Cancer 2009, 9, 400–414. [Google Scholar] [CrossRef]
- Dansen, T.B.; Whitfield, J.; Rostker, F.; Brown-Swigart, L.; Evan, G.I. Specific Requirement for Bax, Not Bak, in Myc-Induced Apoptosis and Tumor Suppression in Vivo. J. Biol. Chem. 2006, 281, 10890–10895. [Google Scholar] [CrossRef] [Green Version]
- Eischen, C.M.; Roussel, M.F.; Korsmeyer, S.J.; Cleveland, J.L. Bax Loss Impairs Myc-Induced Apoptosis and Circumvents the Selection of P53 Mutations during Myc-Mediated Lymphomagenesis. Mol. Cell. Biol. 2001, 21, 7653–7662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemann, M.T.; Zilfou, J.T.; Zhao, Z.; Burgess, D.J.; Hannon, G.J.; Lowe, S.W. Suppression of Tumorigenesis by the P53 Target PUMA. Proc. Natl. Acad. Sci. USA 2004, 101, 9333–9338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalak, E.M.; Jansen, E.S.; Happo, L.; Cragg, M.S.; Tai, L.; Smyth, G.K.; Strasser, A.; Adams, J.M.; Scott, C.L. Puma and to a Lesser Extent Noxa Are Suppressors of Myc-Induced Lymphomagenesis. Cell Death Differ. 2009, 16, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Valente, L.J.; Grabow, S.; Vandenberg, C.J.; Strasser, A.; Janic, A. Combined Loss of PUMA and P21 Accelerates C-MYC-Driven Lymphoma Development Considerably Less than Loss of One Allele of P53. Oncogene 2016, 35, 3866–3871. [Google Scholar] [CrossRef]
- Giam, M.; Rancati, G. Aneuploidy and Chromosomal Instability in Cancer: A Jackpot to Chaos. Cell Div. 2015, 10, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, Y.; Wong, C.; Thoma, R.S.; Richman, R.; Wu, Z.; Piwnica-Worms, H.; Elledge, S.J. Conservation of the Chk1 Checkpoint Pathway in Mammals: Linkage of DNA Damage to Cdk Regulation Through Cdc25. Science 1997, 277, 1497–1501. [Google Scholar] [CrossRef]
- Faesen, A.C.; Thanasoula, M.; Maffini, S.; Breit, C.; Müller, F.; van Gerwen, S.; Bange, T.; Musacchio, A. Basis of Catalytic Assembly of the Mitotic Checkpoint Complex. Nature 2017, 542, 498–502. [Google Scholar] [CrossRef] [Green Version]
- Maresca, T.J.; Salmon, E.D. Welcome to a New Kind of Tension: Translating Kinetochore Mechanics into a Wait-Anaphase Signal. J. Cell Sci. 2010, 123, 825–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holland, A.J.; Cleveland, D.W. Boveri Revisited: Chromosomal Instability, Aneuploidy and Tumorigenesis. Nat. Rev. Mol. Cell Biol. 2009, 10, 478–487. [Google Scholar] [CrossRef] [Green Version]
- Levine, M.S.; Holland, A.J. The Impact of Mitotic Errors on Cell Proliferation and Tumorigenesis. Genes Dev. 2018, 32, 620–638. [Google Scholar] [CrossRef] [Green Version]
- Nigg, E.A.; Holland, A.J. Once and Only Once: Mechanisms of Centriole Duplication and Their Deregulation in Disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 297–312. [Google Scholar] [CrossRef]
- Thompson, S.L.; Bakhoum, S.F.; Compton, D.A. Mechanisms of Chromosomal Instability. Curr. Biol. 2010, 20, R285–R295. [Google Scholar] [CrossRef] [Green Version]
- Williams, B.R.; Prabhu, V.R.; Hunter, K.E.; Glazier, C.M.; Whittaker, C.A.; Housman, D.E.; Amon, A. Aneuploidy Affects Proliferation and Spontaneous Immortalization in Mammalian Cells. Science 2008, 322, 703–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-David, U.; Amon, A. Context Is Everything: Aneuploidy in Cancer. Nat. Rev. Genet. 2020, 21, 44–62. [Google Scholar] [CrossRef]
- Sansregret, L.; Vanhaesebroeck, B.; Swanton, C. Determinants and Clinical Implications of Chromosomal Instability in Cancer. Nat. Rev. Clin. Oncol. 2018, 15, 139–150. [Google Scholar] [CrossRef] [Green Version]
- Chunduri, N.K.; Storchová, Z. The Diverse Consequences of Aneuploidy. Nat. Cell Biol. 2019, 21, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, A.; Ohori, M.; Iwai, K.; Nakayama, Y.; Nambu, T.; Morishita, D.; Kawamoto, T.; Miyamoto, M.; Hirayama, T.; Okaniwa, M.; et al. Aneuploidy Generates Proteotoxic Stress and DNA Damage Concurrently with P53-Mediated Post-Mitotic Apoptosis in SAC-Impaired Cells. Nat. Commun. 2015, 6, 7668. [Google Scholar] [CrossRef] [Green Version]
- Pfau, S.J.; Silberman, R.E.; Knouse, K.A.; Amon, A. Aneuploidy Impairs Hematopoietic Stem Cell Fitness and Is Selected against in Regenerating Tissues in Vivo. Genes Dev. 2016, 30, 1395–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrero, A.B.; Rojas, E.A.; Misiewicz-Krzeminska, I.; Krzeminski, P.; Gutiérrez, N.C. Molecular Mechanisms of P53 Deregulation in Cancer: An Overview in Multiple Myeloma. Int. J. Mol. Sci. 2016, 17, 2003. [Google Scholar] [CrossRef]
- Soussi, T.; Lozano, G. P53 Mutation Heterogeneity in Cancer. Biochem. Biophys. Res. Commun. 2005, 331, 834–842. [Google Scholar] [CrossRef]
- Joerger, A.C.; Fersht, A.R. The P53 Pathway: Origins, Inactivation in Cancer, and Emerging Therapeutic Approaches. Annu. Rev. Biochem. 2016, 85, 375–404. [Google Scholar] [CrossRef] [PubMed]
- Guha, T.; Malkin, D. Inherited TP53 Mutations and the Li–Fraumeni Syndrome. Cold Spring Harb. Perspect. Med. 2017, 7, a026187. [Google Scholar] [CrossRef] [Green Version]
- McBride, K.A.; Ballinger, M.L.; Killick, E.; Kirk, J.; Tattersall, M.H.N.; Eeles, R.A.; Thomas, D.M.; Mitchell, G. Li-Fraumeni Syndrome: Cancer Risk Assessment and Clinical Management. Nat. Rev. Clin. Oncol. 2014, 11, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Whibley, C.; Pharoah, P.D.P.; Hollstein, M. P53 Polymorphisms: Cancer Implications. Nat. Rev. Cancer 2009, 9, 95–107. [Google Scholar] [CrossRef]
- Barnoud, T.; Parris, J.L.D.; Murphy, M.E. Common Genetic Variants in the TP53 Pathway and Their Impact on Cancer. J. Mol. Cell Biol. 2019, 11, 578–585. [Google Scholar] [CrossRef]
- Grochola, L.F.; Zeron-Medina, J.; Mériaux, S.; Bond, G.L. Single-Nucleotide Polymorphisms in the P53 Signaling Pathway. Cold Spring Harb. Perspect. Biol. 2010, 2, a001032. [Google Scholar] [CrossRef] [Green Version]
- Proestling, K.; Hebar, A.; Pruckner, N.; Marton, E.; Vinatzer, U.; Schreiber, M. The Pro Allele of the P53 Codon 72 Polymorphism Is Associated with Decreased Intratumoral Expression of BAX and P21, and Increased Breast Cancer Risk. PLoS ONE 2012, 7, e47325. [Google Scholar] [CrossRef] [Green Version]
- Gunaratna, R.T.; Santos, A.; Luo, L.; Nagi, C.; Lambertz, I.; Spier, M.; Conti, C.J.; Fuchs-Young, R.S. Dynamic Role of the Codon 72 P53 Single-Nucleotide Polymorphism in Mammary Tumorigenesis in a Humanized Mouse Model. Oncogene 2019, 38, 3535–3550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storey, A.; Thomas, M.; Kalita, A.; Harwood, C.; Gardiol, D.; Mantovani, F.; Breuer, J.; Leigh, I.M.; Matlashewski, G.; Banks, L. Role of a P53 Polymorphism in the Development of Human Papillomavirus-Associated Cancer. Nature 1998, 393, 229–234. [Google Scholar] [CrossRef]
- Rosenthal, A.N.; Ryan, A.; Al-Jehani, R.M.; Storey, A.; Harwood, C.A.; Jacobs, I.J. P53 Codon 72 Polymorphism and Risk of Cervical Cancer in UK. Lancet 1998, 352, 871–872. [Google Scholar] [CrossRef]
- Papadakis, E.N.; Dokianakis, D.N.; Spandidos, D.A. P53 Codon 72 Polymorphism as a Risk Factor in the Development of Breast Cancer. Mol. Cell Biol. Res. Commun. 2000, 3, 389–392. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, M.; Wu, D.; Wang, M.; Tong, N.; Tian, Y.; Zhang, Z. P53 Codon 72 Polymorphism Contributes to Breast Cancer Risk: A Meta-Analysis Based on 39 Case–Control Studies. Breast Cancer Res. Treat. 2010, 120, 509–517. [Google Scholar] [CrossRef]
- Stracquadanio, G.; Wang, X.; Wallace, M.D.; Grawenda, A.M.; Zhang, P.; Hewitt, J.; Zeron-Medina, J.; Castro-Giner, F.; Tomlinson, I.P.; Goding, C.R.; et al. The Importance of P53 Pathway Genetics in Inherited and Somatic Cancer Genomes. Nat. Rev. Cancer 2016, 16, 251–265. [Google Scholar] [CrossRef]
- Green, R.A.; Paluch, E.; Oegema, K. Cytokinesis in Animal Cells. Annu. Rev. Cell Dev. Biol. 2012, 28, 29–58. [Google Scholar] [CrossRef] [Green Version]
- Lens, S.M.A.; Medema, R.H. Cytokinesis Defects and Cancer. Nat. Rev. Cancer 2019, 19, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Zack, T.I.; Schumacher, S.E.; Carter, S.L.; Cherniack, A.D.; Saksena, G.; Tabak, B.; Lawrence, M.S.; Zhang, C.-Z.; Wala, J.; Mermel, C.H.; et al. Pan-Cancer Patterns of Somatic Copy Number Alteration. Nat. Genet. 2013, 45, 1134–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganem, N.J.; Godinho, S.A.; Pellman, D. A Mechanism Linking Extra Centrosomes to Chromosomal Instability. Nature 2009, 460, 278–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, A.D.; Feldman, J.L. Microtubule-Organizing Centers: From the Centrosome to Non-Centrosomal Sites. Curr. Opin. Cell Biol. 2017, 44, 93–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, J.Y. A Clinical Overview of Centrosome Amplification in Human Cancers. Int. J. Biol. Sci. 2011, 7, 1122–1144. [Google Scholar] [CrossRef]
- Godinho, S.A.; Picone, R.; Burute, M.; Dagher, R.; Su, Y.; Leung, C.T.; Polyak, K.; Brugge, J.S.; Théry, M.; Pellman, D. Oncogene-like Induction of Cellular Invasion from Centrosome Amplification. Nature 2014, 510, 167–171. [Google Scholar] [CrossRef] [Green Version]
- Thompson, S.L.; Compton, D.A. Proliferation of Aneuploid Human Cells Is Limited by a P53-Dependent Mechanism. J. Cell Biol. 2010, 188, 369–381. [Google Scholar] [CrossRef] [Green Version]
- Fong, C.S.; Mazo, G.; Das, T.; Goodman, J.; Kim, M.; O’Rourke, B.P.; Izquierdo, D.; Tsou, M.-F.B. 53BP1 and USP28 Mediate P53-Dependent Cell Cycle Arrest in Response to Centrosome Loss and Prolonged Mitosis. eLife 2016, 5, e16270. [Google Scholar] [CrossRef] [PubMed]
- Lambrus, B.G.; Daggubati, V.; Uetake, Y.; Scott, P.M.; Clutario, K.M.; Sluder, G.; Holland, A.J. A USP28–53BP1–P53–P21 Signaling Axis Arrests Growth after Centrosome Loss or Prolonged Mitosis. J. Cell Biol. 2016, 214, 143–153. [Google Scholar] [CrossRef]
- Meitinger, F.; Anzola, J.V.; Kaulich, M.; Richardson, A.; Stender, J.D.; Benner, C.; Glass, C.K.; Dowdy, S.F.; Desai, A.; Shiau, A.K.; et al. 53BP1 and USP28 Mediate P53 Activation and G1 Arrest after Centrosome Loss or Extended Mitotic Duration. J. Cell Biol. 2016, 214, 155–166. [Google Scholar] [CrossRef]
- Panier, S.; Boulton, S.J. Double-Strand Break Repair: 53BP1 Comes into Focus. Nat. Rev. Mol. Cell Biol. 2014, 15, 7–18. [Google Scholar] [CrossRef]
- Lakin, N.D.; Jackson, S.P. Regulation of P53 in Response to DNA Damage. Oncogene 1999, 18, 7644–7655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsson, A.; Manzl, C.; Strasser, A.; Villunger, A. How Important Are Post-Translational Modifications in P53 for Selectivity in Target-Gene Transcription and Tumour Suppression? Cell Death Differ. 2007, 14, 1561–1575. [Google Scholar] [CrossRef]
- Zimmermann, M. 53BP1: Pro Choice in DNA Repair. Trends Cell Biol. 2014, 24, 108–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuella-Martin, R.; Oliveira, C.; Lockstone, H.E.; Snellenberg, S.; Grolmusova, N.; Chapman, J.R. 53BP1 Integrates DNA Repair and P53-Dependent Cell Fate Decisions via Distinct Mechanisms. Mol. Cell 2016, 64, 51–64. [Google Scholar] [CrossRef] [Green Version]
- Fava, L.L.; Schuler, F.; Sladky, V.; Haschka, M.D.; Soratroi, C.; Eiterer, L.; Demetz, E.; Weiss, G.; Geley, S.; Nigg, E.A.; et al. The PIDDosome Activates P53 in Response to Supernumerary Centrosomes. Genes Dev. 2017, 31, 34–45. [Google Scholar] [CrossRef]
- Tinel, A.; Tschopp, J. The PIDDosome, a Protein Complex Implicated in Activation of Caspase-2 in Response to Genotoxic Stress. Science 2004, 304, 843–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Resnick-Silverman, L.; Clair, S.S.; Maurer, M.; Zhao, K.; Manfredi, J.J. Identification of a Novel Class of Genomic DNA-Binding Sites Suggests a Mechanism for Selectivity in Target Gene Activation by the Tumor Suppressor Protein P53. Genes Dev. 1998, 12, 2102–2107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Bitar, S.; Gali-Muhtasib, H. The Role of the Cyclin Dependent Kinase Inhibitor P21cip1/Waf1 in Targeting Cancer: Molecular Mechanisms and Novel Therapeutics. Cancers 2019, 11, 1475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giacinti, C.; Giordano, A. RB and Cell Cycle Progression. Oncogene 2006, 25, 5220–5227. [Google Scholar] [CrossRef] [Green Version]
- Andrysik, Z.; Galbraith, M.D.; Guarnieri, A.L.; Zaccara, S.; Sullivan, K.D.; Pandey, A.; MacBeth, M.; Inga, A.; Espinosa, J.M. Identification of a Core TP53 Transcriptional Program with Highly Distributed Tumor Suppressive Activity. Genome Res. 2017, 27, 1645–1657. [Google Scholar] [CrossRef]
- Taylor, W.R.; Stark, G.R. Regulation of the G2/M Transition by P53. Oncogene 2001, 20, 1803–1815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, Q. Gadd45a, a P53- and BRCA1-Regulated Stress Protein, in Cellular Response to DNA Damage. Mutat. Res./Fundam. Mol. Mech. Mutagenesis 2005, 569, 133–143. [Google Scholar] [CrossRef]
- Eckerdt, F.; Yuan, J.; Strebhardt, K. Polo-like Kinases and Oncogenesis. Oncogene 2005, 24, 267–276. [Google Scholar] [CrossRef] [Green Version]
- Fischer, M.; Steiner, L.; Engeland, K. The Transcription Factor P53: Not a Repressor, Solely an Activator. Cell Cycle 2014, 13, 3037–3058. [Google Scholar] [CrossRef] [Green Version]
- Fischer, M.; Quaas, M.; Steiner, L.; Engeland, K. The P53-P21-DREAM-CDE/CHR Pathway Regulates G2/M Cell Cycle Genes. Nucleic Acids Res. 2016, 44, 164–174. [Google Scholar] [CrossRef] [Green Version]
- Fischer, M.; Grossmann, P.; Padi, M.; DeCaprio, J.A. Integration of TP53, DREAM, MMB-FOXM1 and RB-E2F Target Gene Analyses Identifies Cell Cycle Gene Regulatory Networks. Nucleic Acids Res. 2016, 44, 6070–6086. [Google Scholar] [CrossRef] [PubMed]
- Sadasivam, S.; DeCaprio, J.A. The DREAM Complex: Master Coordinator of Cell Cycle-Dependent Gene Expression. Nat. Rev. Cancer 2013, 13, 585–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engeland, K. Cell Cycle Arrest through Indirect Transcriptional Repression by P53: I Have a DREAM. Cell Death Differ. 2018, 25, 114–132. [Google Scholar] [CrossRef] [Green Version]
- Graña, X.; Garriga, J.; Mayol, X. Role of the Retinoblastoma Protein Family, PRB, P107 and P130 in the Negative Control of Cell Growth. Oncogene 1998, 17, 3365–3383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stengel, K.R.; Thangavel, C.; Solomon, D.A.; Angus, S.P.; Zheng, Y.; Knudsen, E.S. Retinoblastoma/P107/P130 Pocket Proteins: Protein dynamics and interactions with target gene promoters. J. Biol. Chem. 2009, 284, 19265–19271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quaas, M.; Müller, G.A.; Engeland, K. P53 Can Repress Transcription of Cell Cycle Genes through a P21WAF1/CIP1-Dependent Switch from MMB to DREAM Protein Complex Binding at CHR Promoter Elements. Cell Cycle 2012, 11, 4661–4672. [Google Scholar] [CrossRef] [Green Version]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 485. [Google Scholar] [CrossRef]
- Sage, J.; Miller, A.L.; Pérez-Mancera, P.A.; Wysocki, J.M.; Jacks, T. Acute Mutation of Retinoblastoma Gene Function Is Sufficient for Cell Cycle Re-Entry. Nature 2003, 424, 223–228. [Google Scholar] [CrossRef]
- Beausejour, C.M. Reversal of Human Cellular Senescence: Roles of the P53 and P16 Pathways. EMBO J. 2003, 22, 4212–4222. [Google Scholar] [CrossRef]
- Gire, V.; Wynford-Thomas, D. Reinitiation of DNA Synthesis and Cell Division in Senescent Human Fibroblasts by Microinjection of Anti-P53 Antibodies. Mol. Cell. Biol. 1998, 18, 1611–1621. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Schmitt, C.A. The Dynamic Nature of Senescence in Cancer. Nat. Cell Biol. 2019, 21, 94–101. [Google Scholar] [CrossRef]
- Litovchick, L.; Florens, L.A.; Swanson, S.K.; Washburn, M.P.; DeCaprio, J.A. DYRK1A Protein Kinase Promotes Quiescence and Senescence through DREAM Complex Assembly. Genes Dev. 2011, 25, 801–813. [Google Scholar] [CrossRef] [Green Version]
- Hauser, S.; Ulrich, T.; Wurster, S.; Schmitt, K.; Reichert, N.; Gaubatz, S. Loss of LIN9, a Member of the DREAM Complex, Cooperates with SV40 Large T Antigen to Induce Genomic Instability and Anchorage-Independent Growth. Oncogene 2012, 31, 1859–1868. [Google Scholar] [CrossRef] [Green Version]
- Reichert, N.; Wurster, S.; Ulrich, T.; Schmitt, K.; Hauser, S.; Probst, L.; Götz, R.; Ceteci, F.; Moll, R.; Rapp, U.; et al. Lin9, a Subunit of the Mammalian DREAM Complex, Is Essential for Embryonic Development, for Survival of Adult Mice, and for Tumor Suppression. Mol. Cell. Biol. 2010, 30, 2896–2908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.; Ma, W.; Benchimol, S. Pidd, a New Death-Domain–Containing Protein, Is Induced by P53 and Promotes Apoptosis. Nat. Genet 2000, 26, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Sladky, V.C.; Knapp, K.; Soratroi, C.; Heppke, J.; Eichin, F.; Rocamora-Reverte, L.; Szabo, T.G.; Bongiovanni, L.; Westendorp, B.; Moreno, E.; et al. E2F-Family Members Engage the PIDDosome to Limit Hepatocyte Ploidy in Liver Development and Regeneration. Dev. Cell 2020, 52, 335–349.e7. [Google Scholar] [CrossRef]
- Fischer, M. Census and Evaluation of P53 Target Genes. Oncogene 2017, 36, 3943–3956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakano, K.; Vousden, K.H. PUMA, a Novel Proapoptotic Gene, Is Induced by P53. Mol. Cell 2001, 7, 683–694. [Google Scholar] [CrossRef]
- Villunger, A.; Michalak, E.M.; Coultas, L.; Müllauer, F.; Böck, G.; Ausserlechner, M.J.; Adams, J.M.; Strasser, A. P53- and Drug-Induced Apoptotic Responses Mediated by BH3-Only Proteins Puma and Noxa. Science 2003, 302, 1036–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naik, E.; Michalak, E.M.; Villunger, A.; Adams, J.M.; Strasser, A. Ultraviolet Radiation Triggers Apoptosis of Fibroblasts and Skin Keratinocytes Mainly via the BH3-Only Protein Noxa. J. Cell Biol. 2007, 176, 415–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oda, E.; Ohki, R.; Murasawa, H.; Nemoto, J.; Shibue, T.; Yamashita, T.; Tokino, T.; Taniguchi, T.; Tanaka, N. Noxa, a BH3-Only Member of the Bcl-2 Family and Candidate Mediator of P53-Induced Apoptosis. Science 2000, 288, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of Apoptosis by the BCL-2 Protein Family: Implications for Physiology and Therapy. Nat. Rev. Mol. Cell Biol. 2014, 15, 49–63. [Google Scholar] [CrossRef]
- Letai, A.; Bassik, M.C.; Walensky, L.D.; Sorcinelli, M.D.; Weiler, S.; Korsmeyer, S.J. Distinct BH3 Domains Either Sensitize or Activate Mitochondrial Apoptosis, Serving as Prototype Cancer Therapeutics. Cancer Cell 2002, 2, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Sax, J.K.; Fei, P.; Murphy, M.E.; Bernhard, E.; Korsmeyer, S.J.; El-Deiry, W.S. BID Regulation by P53 Contributes to Chemosensitivity. Nat. Cell Biol. 2002, 4, 842–849. [Google Scholar] [CrossRef]
- Selvakumaran, M.; Lin, H.K.; Miyashita, T.; Wang, H.G.; Krajewski, S.; Reed, J.C.; Hoffman, B.; Liebermann, D. Immediate Early Up-Regulation of Bax Expression by P53 but Not TGF Beta 1: A Paradigm for Distinct Apoptotic Pathways. Oncogene 1994, 9, 1791–1798. [Google Scholar]
- Toshiyuki, M.; Reed, J.C. Tumor Suppressor P53 Is a Direct Transcriptional Activator of the Human Bax Gene. Cell 1995, 80, 293–299. [Google Scholar] [CrossRef] [Green Version]
- Marchenko, N.D.; Zaika, A.; Moll, U.M. Death Signal-Induced Localization of P53 Protein to Mitochondria: A potential role in apoptotic signaling. J. Biol. Chem. 2000, 275, 16202–16212. [Google Scholar] [CrossRef] [Green Version]
- Mihara, M.; Erster, S.; Zaika, A.; Petrenko, O.; Chittenden, T.; Pancoska, P.; Moll, U.M. P53 Has a Direct Apoptogenic Role at the Mitochondria. Mol. Cell 2003, 11, 577–590. [Google Scholar] [CrossRef]
- Chipuk, J.E.; Kuwana, T.; Bouchier-Hayes, L.; Droin, N.M.; Newmeyer, D.D.; Schuler, M.; Green, D.R. Direct Activation of Bax by P53 Mediates Mitochondrial Membrane Permeabilization and Apoptosis. Science 2004, 303, 1010–1014. [Google Scholar] [CrossRef] [Green Version]
- Leu, J.I.-J.; Dumont, P.; Hafey, M.; Murphy, M.E.; George, D.L. Mitochondrial P53 Activates Bak and Causes Disruption of a Bak–Mcl1 Complex. Nat. Cell Biol. 2004, 6, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Follis, A.V.; Chipuk, J.E.; Fisher, J.C.; Yun, M.-K.; Grace, C.R.; Nourse, A.; Baran, K.; Ou, L.; Min, L.; White, S.W.; et al. PUMA Binding Induces Partial Unfolding within BCL-XL to Disrupt P53 Binding and Promote Apoptosis. Nat. Chem. Biol. 2013, 9, 163–168. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Qu, L.; Dai, S.; Li, Y.; Wang, H.; Feng, Y.; Chen, X.; Jiang, L.; Guo, M.; Li, J.; et al. Structural Insight into the Molecular Mechanism of P53-Mediated Mitochondrial Apoptosis. Nat. Commun. 2021, 12, 2280. [Google Scholar] [CrossRef]
- Mai, W.X.; Gosa, L.; Daniels, V.W.; Ta, L.; Tsang, J.E.; Higgins, B.; Gilmore, W.B.; Bayley, N.A.; Harati, M.D.; Lee, J.T.; et al. Cytoplasmic P53 Couples Oncogene-Driven Glucose Metabolism to Apoptosis and Is a Therapeutic Target in Glioblastoma. Nat. Med. 2017, 23, 1342–1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kannan, K.; Kaminski, N.; Rechavi, G.; Jakob-Hirsch, J.; Amariglio, N.; Givol, D. DNA Microarray Analysis of Genes Involved in P53 Mediated Apoptosis: Activation of Apaf-1. Oncogene 2001, 20, 3449–3455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robles, A.I.; Bemmels, N.A.; Foraker, A.B.; Harris, C.C. APAF-1 Is a Transcriptional Target of P53 in DNA Damage-Induced Apoptosis. Cancer Res. 2001, 61, 6660–6664. [Google Scholar]
- Potting, C.; Tatsuta, T.; König, T.; Haag, M.; Wai, T.; Aaltonen, M.J.; Langer, T. TRIAP1/PRELI Complexes Prevent Apoptosis by Mediating Intramitochondrial Transport of Phosphatidic Acid. Cell Metab. 2013, 18, 287–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, W.-R.; Nakamura, Y. P53CSV, a Novel P53-Inducible Gene Involved in the P53-Dependent Cell-Survival Pathway. Cancer Res. 2005, 65, 1197–1206. [Google Scholar] [CrossRef] [Green Version]
- Carneiro, B.A.; El-Deiry, W.S. Targeting Apoptosis in Cancer Therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef]
- Müller, M.; Wilder, S.; Bannasch, D.; Israeli, D.; Lehlbach, K.; Li-Weber, M.; Friedman, S.L.; Galle, P.R.; Stremmel, W.; Oren, M.; et al. P53 Activates the CD95 (APO-1/Fas) Gene in Response to DNA Damage by Anticancer Drugs. J. Exp. Med. 1998, 188, 2033–2045. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Yue, P.; Khuri, F.R.; Sun, S.-Y. P53 Upregulates Death Receptor 4 Expression through an Intronic P53 Binding Site. Cancer Res. 2004, 64, 5078–5083. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.S.; Burns, T.F.; McDonald, E.R.; Jiang, W.; Meng, R.; Krantz, I.D.; Kao, G.; Gan, D.-D.; Zhou, J.-Y.; Muschel, R.; et al. KILLER/DR5 Is a DNA Damage–Inducible P53–Regulated Death Receptor Gene. Nat. Genet. 1997, 17, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Sprick, M.R.; Weigand, M.A.; Rieser, E.; Rauch, C.T.; Juo, P.; Blenis, J.; Krammer, P.H.; Walczak, H. FADD/MORT1 and Caspase-8 Are Recruited to TRAIL Receptors 1 and 2 and Are Essential for Apoptosis Mediated by TRAIL Receptor 2. Immunity 2000, 12, 599–609. [Google Scholar] [CrossRef] [Green Version]
- Kischkel, F.C.; Lawrence, D.A.; Chuntharapai, A.; Schow, P.; Kim, K.J.; Ashkenazi, A. Apo2L/TRAIL-Dependent Recruitment of Endogenous FADD and Caspase-8 to Death Receptors 4 and 5. Immunity 2000, 12, 611–620. [Google Scholar] [CrossRef] [Green Version]
- Bodmer, J.-L.; Holler, N.; Reynard, S.; Vinciguerra, P.; Schneider, P.; Juo, P.; Blenis, J.; Tschopp, J. TRAIL Receptor-2 Signals Apoptosis through FADD and Caspase-8. Nat. Cell Biol. 2000, 2, 241–243. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, H.; Xu, C.; Yuan, J. Cleavage of BID by Caspase 8 Mediates the Mitochondrial Damage in the Fas Pathway of Apoptosis. Cell 1998, 94, 491–501. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Budihardjo, I.; Zou, H.; Slaughter, C.; Wang, X. Bid, a Bcl2 Interacting Protein, Mediates Cytochrome c Release from Mitochondria in Response to Activation of Cell Surface Death Receptors. Cell 1998, 94, 481–490. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Yue, P.; Khuri, F.R.; Sun, S.-Y. Decoy Receptor 2 (DcR2) Is a P53 Target Gene and Regulates Chemosensitivity. Cancer Res. 2005, 65, 9169–9175. [Google Scholar] [CrossRef] [Green Version]
- Sheikh, M.S.; Huang, Y.; Fernandez-Salas, E.A.; El-Deiry, W.S.; Friess, H.; Amundson, S.; Yin, J.; Meltzer, S.J.; Holbrook, N.J.; Fornace, A.J. The Antiapoptotic Decoy Receptor TRID/TRAIL-R3 Is a P53-Regulated DNA Damage-Inducible Gene That Is Overexpressed in Primary Tumors of the Gastrointestinal Tract. Oncogene 1999, 18, 4153–4159. [Google Scholar] [CrossRef] [Green Version]
- Fais, S.; Overholtzer, M. Cell-in-Cell Phenomena in Cancer. Nat. Rev. Cancer 2018, 18, 758–766. [Google Scholar] [CrossRef]
- Overholtzer, M.; Brugge, J.S. The Cell Biology of Cell-in-Cell Structures. Nat. Rev. Mol. Cell Biol. 2008, 9, 796–809. [Google Scholar] [CrossRef]
- Overholtzer, M.; Mailleux, A.A.; Mouneimne, G.; Normand, G.; Schnitt, S.J.; King, R.W.; Cibas, E.S.; Brugge, J.S. A Nonapoptotic Cell Death Process, Entosis, That Occurs by Cell-in-Cell Invasion. Cell 2007, 131, 966–979. [Google Scholar] [CrossRef] [Green Version]
- Krajcovic, M.; Johnson, N.B.; Sun, Q.; Normand, G.; Hoover, N.; Yao, E.; Richardson, A.L.; King, R.W.; Cibas, E.S.; Schnitt, S.J.; et al. A Non-Genetic Route to Aneuploidy in Human Cancers. Nat. Cell Biol. 2011, 13, 324–330. [Google Scholar] [CrossRef] [Green Version]
- Mackay, H.L.; Moore, D.; Hall, C.; Birkbak, N.J.; Jamal-Hanjani, M.; Karim, S.A.; Phatak, V.M.; Piñon, L.; Morton, J.P.; Swanton, C.; et al. Genomic Instability in Mutant P53 Cancer Cells upon Entotic Engulfment. Nat. Commun. 2018, 9, 3070. [Google Scholar] [CrossRef] [Green Version]
- Durgan, J.; Tseng, Y.-Y.; Hamann, J.C.; Domart, M.-C.; Collinson, L.; Hall, A.; Overholtzer, M.; Florey, O. Mitosis Can Drive Cell Cannibalism through Entosis. eLife 2017, 6, e27134. [Google Scholar] [CrossRef]
- Liang, J.; Niu, Z.; Zhang, B.; Yu, X.; Zheng, Y.; Wang, C.; Ren, H.; Wang, M.; Ruan, B.; Qin, H.; et al. P53-Dependent Elimination of Aneuploid Mitotic Offspring by Entosis. Cell Death Differ. 2021, 28, 799–813. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, R.; Kroemer, G.; Tang, D. The Tumor Suppressor Protein P53 and the Ferroptosis Network. Free Radic. Biol. Med. 2019, 133, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of Ferroptotic Cancer Cell Death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, L.; Kon, N.; Li, T.; Wang, S.-J.; Su, T.; Hibshoosh, H.; Baer, R.; Gu, W. Ferroptosis as a P53-Mediated Activity during Tumour Suppression. Nature 2015, 520, 57–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Liu, X.; Jiang, L.; Manfredi, J.; Zha, S.; Gu, W. Loss of P53-Mediated Cell-Cycle Arrest, Senescence and Apoptosis Promotes Genomic Instability and Premature Aging. Oncotarget 2016, 7, 11838–11849. [Google Scholar] [CrossRef]
- Ou, Y.; Wang, S.-J.; Li, D.; Chu, B.; Gu, W. Activation of SAT1 Engages Polyamine Metabolism with P53-Mediated Ferroptotic Responses. PNAS 2016, 113, E6806–E6812. [Google Scholar] [CrossRef] [Green Version]
- Chu, B.; Kon, N.; Chen, D.; Li, T.; Liu, T.; Jiang, L.; Song, S.; Tavana, O.; Gu, W. ALOX12 Is Required for P53-Mediated Tumour Suppression through a Distinct Ferroptosis Pathway. Nat. Cell Biol. 2019, 21, 579–591. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, Y.; Zhang, J.; Yan, W.; Jung, Y.-S.; Chen, M.; Huang, E.; Lloyd, K.; Duan, Y.; Wang, J.; et al. Ferredoxin Reductase Is Critical for P53-Dependent Tumor Suppression via Iron Regulatory Protein 2. Genes Dev. 2017, 31, 1243–1256. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.; Monian, P.; Quadri, N.; Ramasamy, R.; Jiang, X. Glutaminolysis and Transferrin Regulate Ferroptosis. Mol. Cell 2015, 59, 298–308. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.-J.; Li, D.; Ou, Y.; Jiang, L.; Chen, Y.; Zhao, Y.; Gu, W. Acetylation Is Crucial for P53-Mediated Ferroptosis and Tumor Suppression. Cell Rep. 2016, 17, 366–373. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Zhu, S.; Song, X.; Sun, X.; Fan, Y.; Liu, J.; Zhong, M.; Yuan, H.; Zhang, L.; Billiar, T.R.; et al. The Tumor Suppressor P53 Limits Ferroptosis by Blocking DPP4 Activity. Cell Rep. 2017, 20, 1692–1704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarangelo, A.; Magtanong, L.; Bieging-Rolett, K.T.; Li, Y.; Ye, J.; Attardi, L.D.; Dixon, S.J. P53 Suppresses Metabolic Stress-Induced Ferroptosis in Cancer Cells. Cell Rep. 2018, 22, 569–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitale, I.; Galluzzi, L.; Castedo, M.; Kroemer, G. Mitotic Catastrophe: A Mechanism for Avoiding Genomic Instability. Nat. Rev. Mol. Cell Biol. 2011, 12, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Vitale, I.; Manic, G.; Castedo, M.; Kroemer, G. Caspase 2 in Mitotic Catastrophe: The Terminator of Aneuploid and Tetraploid Cells. Mol. Cell. Oncol. 2017, 4, e1299274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown-Suedel, A.N.; Bouchier-Hayes, L. Caspase-2 Substrates: To Apoptosis, Cell Cycle Control, and Beyond. Front. Cell Dev. Biol. 2020, 8, 1662. [Google Scholar] [CrossRef] [PubMed]
- Manzl, C.; Krumschnabel, G.; Bock, F.; Sohm, B.; Labi, V.; Baumgartner, F.; Logette, E.; Tschopp, J.; Villunger, A. Caspase-2 Activation in the Absence of PIDDosome Formation. J. Cell Biol. 2009, 185, 291–303. [Google Scholar] [CrossRef] [Green Version]
- Manzl, C.; Peintner, L.; Krumschnabel, G.; Bock, F.; Labi, V.; Drach, M.; Newbold, A.; Johnstone, R.; Villunger, A. PIDDosome-Independent Tumor Suppression by Caspase-2. Cell Death Differ. 2012, 19, 1722–1732. [Google Scholar] [CrossRef]
- Oliver, T.G.; Meylan, E.; Chang, G.P.; Xue, W.; Burke, J.R.; Humpton, T.J.; Hubbard, D.; Bhutkar, A.; Jacks, T. Caspase-2-Mediated Cleavage of Mdm2 Creates a P53-Induced Positive Feedback Loop. Mol. Cell 2011, 43, 57–71. [Google Scholar] [CrossRef] [Green Version]
- Tsabar, M.; Mock, C.S.; Venkatachalam, V.; Reyes, J.; Karhohs, K.W.; Oliver, T.G.; Regev, A.; Jambhekar, A.; Lahav, G. A Switch in P53 Dynamics Marks Cells That Escape from DSB-Induced Cell Cycle Arrest. Cell Rep. 2020, 32, 107995. [Google Scholar] [CrossRef]
- Ho, L.; Taylor, R.; Dorstyn, L.; Cakouros, D.; Bouillet, P.; Kumar, S. A Tumor Suppressor Function for Caspase-2. Proc. Natl. Acad. Sci. USA 2009, 106, 5336–5341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawar, S.; Lim, Y.; Puccini, J.; White, M.; Thomas, P.; Bouchier-Hayes, L.; Green, D.R.; Dorstyn, L.; Kumar, S. Caspase-2-Mediated Cell Death Is Required for Deleting Aneuploid Cells. Oncogene 2017, 36, 2704–2714. [Google Scholar] [CrossRef]
- Dorstyn, L.; Puccini, J.; Wilson, C.H.; Shalini, S.; Nicola, M.; Moore, S.; Kumar, S. Caspase-2 Deficiency Promotes Aberrant DNA-Damage Response and Genetic Instability. Cell Death Differ. 2012, 19, 1288–1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranjan, A.; Iwakuma, T. Non-Canonical Cell Death Induced by P53. Int. J. Mol. Sci. 2016, 17, 2068. [Google Scholar] [CrossRef] [Green Version]
- Kroemer, G.; Levine, B. Autophagic Cell Death: The Story of a Misnomer. Nat. Rev. Mol. Cell Biol. 2008, 9, 1004–1010. [Google Scholar] [CrossRef]
- Crighton, D.; Wilkinson, S.; O’Prey, J.; Syed, N.; Smith, P.; Harrison, P.R.; Gasco, M.; Garrone, O.; Crook, T.; Ryan, K.M. DRAM, a P53-Induced Modulator of Autophagy, Is Critical for Apoptosis. Cell 2006, 126, 121–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crighton, D.; Wilkinson, S.; Ryan, K.M. DRAM Links Autophagy to P53 and Programmed Cell Death. Autophagy 2007, 3, 72–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, L.; Song, Z.; Liang, B.; Jia, L.; Ma, S.; Liu, X. Radiation Induces Autophagic Cell Death via the P53/DRAM Signaling Pathway in Breast Cancer Cells. Oncol. Rep. 2016, 35, 3639–3647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K.-J.; Lee, S.-H.; Lee, C.-H.; Jang, J.-Y.; Chung, J.; Kwon, M.-H.; Kim, Y.-S. Upregulation of Beclin-1 Expression and Phosphorylation of Bcl-2 and P53 Are Involved in the JNK-Mediated Autophagic Cell Death. Biochem. Biophys. Res. Commun. 2009, 382, 726–729. [Google Scholar] [CrossRef]
- Cheng, Y.; Qiu, F.; Tashiro, S.; Onodera, S.; Ikejima, T. ERK and JNK Mediate TNFα-Induced P53 Activation in Apoptotic and Autophagic L929 Cell Death. Biochem. Biophys. Res. Commun. 2008, 376, 483–488. [Google Scholar] [CrossRef]
- Kim, S.M.; Ha, S.E.; Lee, H.J.; Rampogu, S.; Vetrivel, P.; Kim, H.H.; Venkatarame Gowda Saralamma, V.; Lee, K.W.; Kim, G.S. Sinensetin Induces Autophagic Cell Death through P53-Related AMPK/MTOR Signaling in Hepatocellular Carcinoma HepG2 Cells. Nutrients 2020, 12, 2462. [Google Scholar] [CrossRef]
- Liu, J.; Xia, H.; Kim, M.; Xu, L.; Li, Y.; Zhang, L.; Cai, Y.; Norberg, H.V.; Zhang, T.; Furuya, T.; et al. Beclin1 Controls the Levels of P53 by Regulating the Deubiquitination Activity of USP10 and USP13. Cell 2011, 147, 223–234. [Google Scholar] [CrossRef] [Green Version]
- Sperandio, S.; de Belle, I.; Bredesen, D.E. An Alternative, Nonapoptotic Form of Programmed Cell Death. PNAS 2000, 97, 14376–14381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Zhao, J.; Wang, C.-Z.; Searle, J.; He, T.-C.; Yuan, C.-S.; Du, W. Ginsenoside Rh2 Induces Apoptosis and Paraptosis-like Cell Death in Colorectal Cancer Cells through Activation of P53. Cancer Lett. 2011, 301, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Pehar, M.; O’Riordan, K.J.; Burns-Cusato, M.; Andrzejewski, M.E.; del Alcazar, C.G.; Burger, C.; Scrable, H.; Puglielli, L. Altered Longevity-Assurance Activity of P53:P44 in the Mouse Causes Memory Loss, Neurodegeneration and Premature Death. Aging Cell 2010, 9, 174–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.M.; Kim, I.Y.; Seo, M.J.; Kwon, M.R.; Choi, K.S. Nutlin-3 Enhances the Bortezomib Sensitivity of P53-Defective Cancer Cells by Inducing Paraptosis. Exp. Mol. Med. 2017, 49, e365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bensaad, K.; Vousden, K.H. P53: New Roles in Metabolism. Trends Cell Biol. 2007, 17, 286–291. [Google Scholar] [CrossRef]
- Stewart-Ornstein, J.; Iwamoto, Y.; Miller, M.A.; Prytyskach, M.A.; Ferretti, S.; Holzer, P.; Kallen, J.; Furet, P.; Jambhekar, A.; Forrester, W.C.; et al. P53 Dynamics Vary between Tissues and Are Linked with Radiation Sensitivity. Nat. Commun. 2021, 12, 898. [Google Scholar] [CrossRef]
- Tovar, C.; Rosinski, J.; Filipovic, Z.; Higgins, B.; Kolinsky, K.; Hilton, H.; Zhao, X.; Vu, B.T.; Qing, W.; Packman, K.; et al. Small-Molecule MDM2 Antagonists Reveal Aberrant P53 Signaling in Cancer: Implications for Therapy. PNAS 2006, 103, 1888–1893. [Google Scholar] [CrossRef] [Green Version]
- DeHart, C.J.; Chahal, J.S.; Flint, S.J.; Perlman, D.H. Extensive Post-Translational Modification of Active and Inactivated Forms of Endogenous P53. Mol. Cell. Proteom. 2014, 13, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Tavana, O.; Gu, W. P53 Modifications: Exquisite Decorations of the Powerful Guardian. J. Mol. Cell Biol. 2019, 11, 564–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, J.; Wang, D. Deciphering the PTM Codes of the Tumor Suppressor P53. J. Mol. Cell Biol. 2021. [Google Scholar] [CrossRef]
- Liebl, M.C.; Hofmann, T.G. Cell Fate Regulation upon DNA Damage: P53 Serine 46 Kinases Pave the Cell Death Road. BioEssays 2019, 41, 1900127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smeenk, L.; van Heeringen, S.J.; Koeppel, M.; Gilbert, B.; Janssen-Megens, E.; Stunnenberg, H.G.; Lohrum, M. Role of P53 Serine 46 in P53 Target Gene Regulation. PLoS ONE 2011, 6, e17574. [Google Scholar] [CrossRef] [Green Version]
- Ichwan, S.J.A.; Yamada, S.; Sumrejkanchanakij, P.; Ibrahim-Auerkari, E.; Eto, K.; Ikeda, M.-A. Defect in Serine 46 Phosphorylation of P53 Contributes to Acquisition of P53 Resistance in Oral Squamous Cell Carcinoma Cells. Oncogene 2006, 25, 1216–1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahav, G.; Rosenfeld, N.; Sigal, A.; Geva-Zatorsky, N.; Levine, A.J.; Elowitz, M.B.; Alon, U. Dynamics of the P53-Mdm2 Feedback Loop in Individual Cells. Nat Genet 2004, 36, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Purvis, J.E.; Karhohs, K.W.; Mock, C.; Batchelor, E.; Loewer, A.; Lahav, G. P53 Dynamics Control Cell Fate. Science 2012, 336, 1440–1444. [Google Scholar] [CrossRef] [Green Version]
- Kracikova, M.; Akiri, G.; George, A.; Sachidanandam, R.; Aaronson, S.A. A Threshold Mechanism Mediates P53 Cell Fate Decision between Growth Arrest and Apoptosis. Cell Death Differ. 2013, 20, 576–588. [Google Scholar] [CrossRef] [Green Version]
- Paek, A.L.; Liu, J.C.; Loewer, A.; Forrester, W.C.; Lahav, G. Cell-to-Cell Variation in P53 Dynamics Leads to Fractional Killing. Cell 2016, 165, 631–642. [Google Scholar] [CrossRef] [Green Version]
- Hafner, A.; Stewart-Ornstein, J.; Purvis, J.E.; Forrester, W.C.; Bulyk, M.L.; Lahav, G. P53 Pulses Lead to Distinct Patterns of Gene Expression Albeit Similar DNA-Binding Dynamics. Nat. Struct. Mol. Biol. 2017, 24, 840–847. [Google Scholar] [CrossRef]
- Rizzotto, D.; Zaccara, S.; Rossi, A.; Galbraith, M.D.; Andrysik, Z.; Pandey, A.; Sullivan, K.D.; Quattrone, A.; Espinosa, J.M.; Dassi, E.; et al. Nutlin-Induced Apoptosis Is Specified by a Translation Program Regulated by PCBP2 and DHX30. Cell Rep. 2020, 30, 4355–4369.e6. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.A.; Espinosa, J.M. The Impact of Post-Transcriptional Regulation in the P53 Network. Brief. Funct. Genom. 2013, 12, 46–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaccara, S.; Tebaldi, T.; Pederiva, C.; Ciribilli, Y.; Bisio, A.; Inga, A. P53-Directed Translational Control Can Shape and Expand the Universe of P53 Target Genes. Cell Death Differ 2014, 21, 1522–1534. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzotto, D.; Englmaier, L.; Villunger, A. At a Crossroads to Cancer: How p53-Induced Cell Fate Decisions Secure Genome Integrity. Int. J. Mol. Sci. 2021, 22, 10883. https://doi.org/10.3390/ijms221910883
Rizzotto D, Englmaier L, Villunger A. At a Crossroads to Cancer: How p53-Induced Cell Fate Decisions Secure Genome Integrity. International Journal of Molecular Sciences. 2021; 22(19):10883. https://doi.org/10.3390/ijms221910883
Chicago/Turabian StyleRizzotto, Dario, Lukas Englmaier, and Andreas Villunger. 2021. "At a Crossroads to Cancer: How p53-Induced Cell Fate Decisions Secure Genome Integrity" International Journal of Molecular Sciences 22, no. 19: 10883. https://doi.org/10.3390/ijms221910883
APA StyleRizzotto, D., Englmaier, L., & Villunger, A. (2021). At a Crossroads to Cancer: How p53-Induced Cell Fate Decisions Secure Genome Integrity. International Journal of Molecular Sciences, 22(19), 10883. https://doi.org/10.3390/ijms221910883





