Abstract
Background and Objectives: Methotrexate is widely prescribed for the treatment of moderate-to-severe psoriasis. As drug survival encompasses efficacy, safety, and treatment satisfaction, such studies provide insights into successful drug treatments in the real-life scenario. The objective was to define methotrexate drug survival and reasons for discontinuation, along with factors associated with drug survival, in a cohort of adult patients with moderate-to-severe plaque psoriasis. Materials and Methods: Data on methotrexate treatment were extracted from our institutional registry. Drug survival was estimated by Kaplan–Meier analysis, and predictors of drug survival were analyzed by Cox proportional hazards regression. Results: We included 133 patients treated with methotrexate. Due to significant effects of the year of treatment initiation, drug survival analysis was performed for 117 patients who started methotrexate in 2010 or later. Median methotrexate drug survival was 11.0 months. Overall, 89% of patients discontinued treatment, with over half of these (51%) due to lack of efficacy. Significantly longer drug survival was seen for patients who discontinued treatment due to lack of efficacy versus drug safety (p = 0.049); when stratified by sex, this remained significant only for women (p = 0.002). The patient ABCC2 rs717620 genotype was significantly associated with drug survival in both univariate log-rank and multivariate Cox regression analyses, with variant T allele associated with longer drug survival (hazard ratio, 0.606; 95% confidence interval, 0.380–0.967; p = 0.036). Conclusions: We have identified the novel association of patient ABCC2 rs717620 genotype with methotrexate drug survival. This pharmacogenetic marker might thus help in the management of psoriasis patients in daily practice.
1. Introduction
Methotrexate is an immunosuppressive and antiproliferative agent with a well-established place in the treatment of patients with psoriasis [1,2]. It is the most commonly used conventional systemic drug and remains a first-line treatment for patients with moderate-to-severe psoriasis [2,3,4].
The chronic nature of psoriasis implies that patients require long-term treatment. Methotrexate is often used for maintenance therapy, although long-term treatment outcomes are rarely reported [5]. Furthermore, data from short-duration clinical trials performed with pre-selected patients need to be supported by data on methotrexate use in daily practice, e.g., from drug survival studies [6,7]. These studies are considered as real-life measures of treatment success, as they reflect drug efficacy and safety and indicate patient and physician preference [8,9]. Several studies have described drug survival of biologics [9], although data on methotrexate remain limited, even though methotrexate is still prescribed routinely as a first-line systemic agent and often represents a stepping-stone toward the introduction of biologics [7,10]. Therefore, an improved understanding of methotrexate treatment success in daily practice and the factors that impact upon methotrexate drug survival should contribute further to successful patient management.
Few studies have examined methotrexate drug survival in adult patients with psoriasis, with most comparing it to other conventional [10,11,12,13,14] or biological [10,11,12,14,15] agents. Four studies extensively focused on methotrexate [13,16,17,18], three of which looked for predictors of drug survival [13,16,18]. None of these investigated the possible effects of pharmacogenetic factors. Indeed, genetic factors might contribute to the marked variability of methotrexate treatment outcomes in psoriasis [2]. Most pharmacogenetic research of methotrexate in patients with psoriasis has investigated the polymorphisms in genes coding for proteins involved in the pharmacokinetic or pharmacodynamic pathways of methotrexate, with the latter predominantly focusing on enzymes of the folate cycle and the adenosine signaling pathway [2,19,20,21,22,23]. In our previous research, we used a hypothesis-driven approach for the selection of 16 candidate polymorphisms previously shown to affect the response to methotrexate or its pharmacokinetics or to be important for folate–homocysteine metabolism. Of these, five potential biomarkers of methotrexate treatment outcomes were identified: polymorphisms in genes coding for enzymes of the methionine cycle GNMT, DNMT3b, and BHMT (the first two were associated with methotrexate efficacy and the third with hepatotoxicity) and polymorphisms in genes for transporters ABCC2 and SLCO1B1 (both associated with efficacy) [24]. In this study we hypothesized that these pharmacogenetic markers also impact methotrexate drug survival.
The aim of this study was to define methotrexate drug survival and the reasons for discontinuation in a daily practice cohort of adult patients with moderate-to-severe plaque psoriasis. Furthermore, we investigated the factors associated with methotrexate drug survival, with a focus on pharmacogenetic markers.
2. Materials and Methods
2.1. Patients and Data Collection
Patients were recruited within a project for the establishment of an institutional registry of systemic treatment of adults with moderate-to-severe psoriasis, within which a methotrexate pharmacogenetics study was performed [24]. This study was approved by the National Medical Ethics Committee of Slovenia (No. 85/06/15) and performed according to the Declaration of Helsinki. Written informed consent was obtained from all patients who participated.
This retrospective study of methotrexate drug survival included 133 patients with moderate-to-severe plaque psoriasis treated with methotrexate as the only systemic agent (concomitant topical treatment was allowed) and for whom exact data on methotrexate treatment duration were available. Patient clinical characteristics and details of their methotrexate treatment were retrospectively collected from patient records and included age at introduction, start and stop dates, and reasons for treatment discontinuation.
Treatment discontinuation was defined as the cessation of treatment that resulted in a change of therapy (e.g., methotrexate with another conventional systemic drug, switch to a different systemic agent). By definition, drug survival is the time for which a patient continues a drug treatment [8], which was here calculated as the time from methotrexate start to when the event (methotrexate discontinuation) occurred. The reasons for discontinuation were classified into three categories: lack of efficacy; adverse events (AEs); and other reasons (as documented in patient records by treating physicians). Lack of methotrexate efficacy was defined as either inadequate response (i.e., Psoriasis Area and Severity Index (PASI) 75 not reached within 6 months) or when the patient records contained another explicit record of unsuccessful treatment or loss of efficacy beyond 6 months of treatment; both of these had to lead to methotrexate discontinuation and a change in therapy. The patients were stratified according to the year of methotrexate introduction, to examine changes in prescribing practice. As 2010 was the first full year of ustekinumab availability, it was considered the most appropriate cut-off for these patients.
2.2. Determinants of Drug Survival
Patient characteristics in terms of their temporal relationship to methotrexate treatment were defined as possible determinants of drug survival. Furthermore, previously identified pharmacogenetic markers of methotrexate treatment outcomes [24] were also considered. Six single nucleotide polymorphisms (SNPs) in five genes encoding enzymes of the methionine cycle or methotrexate transporters were included in the analysis; they are listed in Section 2.3.
2.3. DNA Extraction and Genotyping
DNA was extracted from patient venous blood samples using the MasterPure Complete DNA and RNA purification kits (Epicentre, Illumina, Madison, WI, USA) or QIAamp DNA Mini kits (Qiagen, Hilden, Germany) as per the manufacturers’ instructions. Genotyping was performed by means of TaqMan genotyping assays (Applied Biosystems, Foster City, CA, USA) using the Roche LightCycler 480 system, in line with manufacturer instructions. The following assays were used for analysis of selected SNPs: TaqMan assay ID C__11646606_20 (rs3733890, betaine-homocysteine methyltransferase; BHMT); assay ID C__11425842_10 (rs10948059, glycine N-methyltransferase; GNMT); assay ID C__25620192_20 (rs2424913, DNA [cytosine-5-]-methyltransferase 3β; DNMT3b); assay ID C___2814642_10 (rs717620, ATP-binding cassette transporter C2; ABCC2); assay IDs C___1901697_20 and C__30633906_10 (rs2306283 and rs4149056, respectively, solute carrier organic anion transporter family member 1B1; SLCO1B1).
2.4. Statistical Analysis
Descriptive statistics were used for the characteristics of the study cohort. Continuous variables are reported as means ± standard deviation (SD) if parametric and medians ± ranges if non-parametric. Counts and percentages were calculated for categorical data.
For the analysis of genotype associations, patients were divided into two groups using the dominant model (wild-type vs. heterozygous/homozygous genotype). The exception was SLCO1B1, where two groups were formed according to reported transporter activity associated with established haplotypes: (i) high-activity group comprising of haplotypes SLCO1B1*1a (wild-type) and SLCO1B1*1b (variant rs2306238) and (ii) low-activity group including patients with haplotypes SLCO1B1*5 (variant rs4149056) and SLCO1B1*15 (variant for both rs2306238 and rs4149056).
Drug survival was investigated using Kaplan–Meier survival analysis. Observations were censored either at the time of data-lock if no discontinuation was seen or for patients lost to follow-up. Log-rank tests examined the differences in drug survival for the reasons for discontinuation and selected factors, including sex, age at methotrexate introduction, age at psoriasis onset, family history, and the six genotypes previously identified as significant for methotrexate treatment outcomes. The effects of changing prescription practices were investigated using log-rank tests for the year of methotrexate introduction (pre-2010/2010 and later).
Multivariate Cox proportional hazards regression with forward/backward selection was performed to identify the predictors of drug discontinuation. All variables tested by log-rank tests were entered into the model, noting that the number of variables included in multivariate Cox regression models should not exceed 10% of the observed events [8]. Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated to describe the risks associated with the analyzed factors. The level of significance was set at α = 0.05. All analyses were performed using IBM SPSS version 25.0 (IMB Corp., Armonk, NY, USA).
3. Results
3.1. Patient and Treatment Characteristics
Out of 133 included patients, 81 (60.9%) were male. The mean age at psoriasis onset was 29.9 ± 14.4 years, and 102 patients (76.7%) showed early onset psoriasis (diagnosis before 40 years [20]). Information on family history of psoriasis was available for 131 patients and was positive in 64 (48.9%) individuals. Genotypes for six candidate polymorphisms in five genes were determined for the included patients. Variant allele frequencies are presented in Table 1.

Table 1.
Genotype data and variant allele frequencies of the analyzed polymorphisms in included patients.
All patients were initially treated with 15 mg methotrexate weekly, with concomitant folic acid (5 mg weekly, 24 h post-methotrexate). At methotrexate introduction, the mean age was 49.9 ± 12.7 years, with median disease duration 17.0 years (range 0.00–66.0 years). The median methotrexate treatment duration was 11.1 months (range 0.92–202.1 months).
Most patients discontinued methotrexate treatment during the period of analysis. Only 14 patients (10.5%) still received methotrexate at data-lock. Most of the 119 patients who discontinued treatment (n = 62; 52.1%) did so for lack of efficacy, while 41 patients (34.5%) experienced AEs that required treatment cessation. The remaining 16 patients (8.04%) discontinued treatment for other reasons.
3.2. Change in Prescription Practice: Influence of Time of Methotrexate Introduction
Patient inclusion was not restricted by year of therapy initiation. Methotrexate introduction ranged from May 1999 to September 2016, during which time the psoriasis treatment landscape changed drastically. As the availability of treatment alternatives might influence drug survival [8,25], patients were classified according to the year of treatment introduction, with the first full year of ustekinumab availability (i.e., 2010) as the cut-off. Of the 133 patients, 16 (12.0%) started treatment before 2010.
To test our assumption of an influence of the introduction year on methotrexate drug survival, the two groups (pre-2010 vs. 2010 and later) were compared using log-rank tests. A significant difference (p = 0.005) was identified, whereby patients who started methotrexate therapy before 2010 continued treatment for longer (median drug survival, 36.0 months; 95% CI, 0.00–110.4 months) compared to patients who started methotrexate treatment in 2010 or later (11.0 months; 95% CI, 7.97–14.0 months).
3.3. Methotrexate Drug Survival in Patients Starting Therapy in 2010 or Later
Due to the identified influence of the year of treatment introduction, the analysis of methotrexate drug survival using Kaplan–Meier survival analysis was conducted only for the subpopulation of patients who started methotrexate treatment in 2010 or later (n = 117). Their patient and treatment characteristics are shown in Table 2.

Table 2.
Patient and treatment characteristics of the subpopulation of patients with methotrexate introduction in 2010 or later.
The overall median methotrexate drug survival was 11.0 months (95% CI, 7.97–14.0 months). After 1 year of methotrexate treatment, 46.2% of patients were still on the therapy; the drug survival rate then dropped to 28.2% at 2 years of methotrexate treatment. Only 6.8% of patients reached 5 years of methotrexate treatment. Patients who discontinued methotrexate treatment due to lack of efficacy showed longer drug survival than those who experienced AEs: median drug survival, 8.02 months (95% CI, 6.37–9.67 months) versus 5.98 months (95% CI, 3.12–8.84 months), respectively. Figure 1 shows Kaplan–Meier survival curves for overall drug survival and according to reasons for discontinuation.
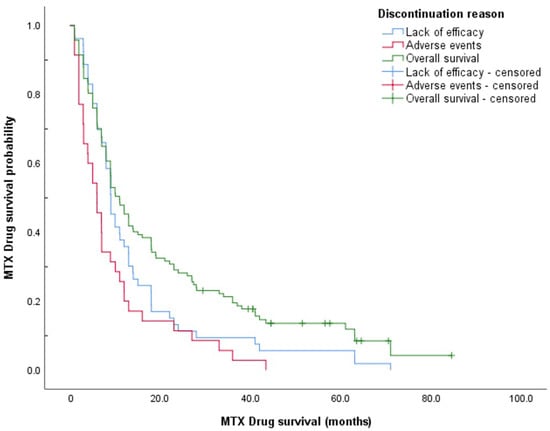
Figure 1.
Methotrexate drug survival curves according to reason for discontinuation.
Survival curves of the two discontinuation groups (i.e., lack of efficacy vs. AEs) were compared using log-rank tests, with a significant difference seen (p = 0.049). To gain further insight into this finding, the groups were stratified by sex. This difference in drug survival according to the reason for discontinuation appears to be solely driven by the significant difference in survival curves for females (log-rank test, p = 0.002), as this was lost for males (p > 0.05). Median drug survival for women discontinuing methotrexate treatment for lack of efficacy was 9.04 months (95% CI, 6.02–12.1 months) compared to 3.02 months (95% CI, 0.96–5.08 months) for AEs.
Further analyses using log-rank tests determined whether selected categorical determinants led to differences in methotrexate drug survival. A significant difference in survival curves was seen when patients were split according to the ABCC2 rs717620 genotype (p = 0.030) (Figure 2). At least one copy of variant allele T (-24CT, -24TT genotypes) was associated with longer median drug survival compared to the wild-type (-24CC) genotype: 19.1 months (95% CI, 7.02–31.1 months) versus 9.04 months (95% CI, 7.47–10.6 months), respectively. No significant differences were identified for patient sex, family history, BHMT rs3733890, GNMT rs10948059, and DNMT3b rs2424913 genotypes, and SLCO1B1 haplotype (rs2306238/rs4149056).
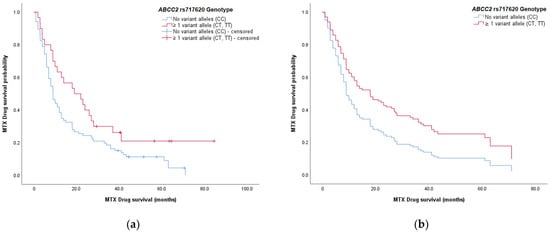
Figure 2.
Methotrexate drug survival curves stratified according to the ABCC2 rs717620 genotype: (a) drug survival curves obtained by Kaplan–Meier survival analysis; (b) theoretically predicted survival curves obtained by multivariate Cox regression analysis. Both of the survival plots show a higher probability of longer drug survival for patients who carry at least one variant allele T in ABCC2 (red), compared to the wild-type genotype (blue).
3.4. Predictors of Drug Survival
To search for the determinants of drug survival, a multivariate analysis was performed using Cox proportional hazards regression with forward and backward variable selection. The selection model included all variables tested in the Kaplan–Meier drug survival analysis with log-rank tests (i.e., sex; BHMT, GNMT, DNMT3b, and ABCC2 genotypes; SLCO1B1 haplotype) and numerical variables (age at methotrexate introduction, age at disease onset). As family history correlates with age at disease onset, this was left out of the model.
The ABCC2 genotype was significantly associated with methotrexate drug survival (Table 3). Patients carrying at least one variant allele T (-24CT, -24TT genotypes) had a lower hazard ratio for treatment discontinuation (HR, 0.606; 95% CI, 0.380–0.967; p = 0.036). Therefore, they had a greater chance of longer methotrexate use compared to patients with the wild-type-24CC ABCC2 rs717620 genotype; this was in line with the univariate log-rank tests (Figure 2).

Table 3.
Determinants of methotrexate drug survival from the results of the univariate tests and multivariate analyses.
None of the other factors in the multivariate model were significantly associated with drug survival. Results from the univariate and multivariate analyses are given in Table 3.
4. Discussion
Methotrexate is widely used for treatment of moderate-to-severe psoriasis. It is the most frequently prescribed conventional systemic agent, and its use commonly precedes biologic therapy [7,10]. Treatment outcomes for methotrexate vary widely among patients, and many discontinue methotrexate due to a lack of efficacy or the occurrence of AEs [2]. Despite the frequent use of methotrexate, real-life data on its efficacy, safety, and duration of therapy remain lacking [10,16]. Drug survival studies provide such data and can offer insight into the therapeutic success of methotrexate in daily practice.
In the present study, we described methotrexate drug survival in a cohort of 133 patients with moderate-to-severe plaque psoriasis. We recorded a median methotrexate drug survival of 11.1 months. Almost 90% of patients discontinued treatment during the observation time, predominantly due to a lack of efficacy. We identified a significant impact of the year of methotrexate introduction on drug survival, and although we divided the patients into only two groups according to the entry of ustekinumab onto the market, a pronounced reduction in the median methotrexate drug survival was observed. This is in line with the suggested impact of changing prescription practices and the reported effects of increased biologics availability, which resulted in a shorter treatment duration of both conventional and biologic agents [8,13,25].
To prevent confounding by the changes in the psoriasis management landscape, we performed drug survival analyses on the group of 117 patients who started methotrexate treatment in 2010 or later. Almost the same proportion of patients discontinued treatment in this group, with a similar median methotrexate drug survival of 11.0 months. Other studies that have investigated methotrexate drug survival in adult patients with psoriasis have reported median drug survivals of 9.15 months [26], 12.1 months [11], 15 months [18], 18 months [13], 21.6 months [16], and 29.3 months [15]. Additionally, mean drug survivals have been reported as 7.7 months [14], 17.2 months [27], 18.8 months [17], and 22.3 months [10]. The median methotrexate drug survival in the present study thus lies on the low end of this spectrum, as half of the patients included had discontinued treatment after 11 months. However, differences in the studies performed make comparisons difficult to interpret, such as the inclusion of patients with different psoriasis types [10,11,12,15,26] versus plaque psoriasis only [14,16,18] and uncertainty for the combination therapy or concomitant folic acid [10,11,12,13,14,15,16,26]. For these aspects, the present cohort was similar to that of Otero and colleagues [16], where patients with plaque psoriasis were prescribed methotrexate monotherapy and folic acid supplementation. Despite this, the median methotrexate drug survival in the present study was almost half that of Otero and colleagues [16]. This might have arisen from a difference in prescription guidelines and also, importantly, in reimbursement practices [7,10,28].
Almost 90% of patients in the present study discontinued methotrexate treatment during the observation period. A lack of efficacy was the main reason given, with a little over half of the treatment cessations (51.0% of discontinuations in the subgroup of patients starting methotrexate in 2010 or later). This was a surprise finding, as AEs were expected to be the main driver of therapy cessation, in line with existing data [10,11,16,17,18]. Furthermore, at 51%, the rate of discontinuation due to a lack of efficacy in the present study was significantly greater than that for other methotrexate drug survival studies, which ranged from 16% to 33% [10,11,12,16,17,18,29]. In contrast, the proportion of patients who stopped methotrexate therapy due to AEs was relatively consistent with previously reported data (present study: 34%; other studies: 12–47% [10,11,12,16,17,18,29]).
Interestingly, patients who discontinued methotrexate due to a lack of efficacy did so with a longer drug survival than their counterparts who experienced AEs, with significantly different drug survival curves between these two groups. Moreover, when we stratified the data according to sex, there was a pronounced difference for women: the median methotrexate drug survival for women who discontinued due to a lack of efficacy was three-fold that for AEs, with this relationship lost for men. The women in this cohort did not experience more AEs or report them more often; however, these data suggested that when women did experience AEs, methotrexate therapy was more readily discontinued. As far as we know, differences in the discontinuation of methotrexate according to sex for patients with psoriasis have not been reported previously; further, studies of methotrexate in rheumatoid and psoriatic arthritis have not identified sex as a determinant for drug survival [30,31]. In addition, we note that our overall drug survival analysis using both Kaplan–Meier analysis and Cox regression analysis did not identify sex as a factor that influenced methotrexate drug survival. Separate analyses according to reasons for discontinuation would have been needed to confirm this influence of sex, but these were not performed due to the limited sample size and the ensuing concerns of statistical power. However, these findings warrant further exploration.
To the best of our knowledge, this is the first study to date to investigate the effects of genetic polymorphisms on methotrexate drug survival in patients with psoriasis. Here, we identified the ABCC2 rs717620 genotype as a determinant of methotrexate drug survival in plaque psoriasis in both the univariate analysis using log-rank tests and the multivariate Cox proportional hazards regression. The variant allele conveyed a significant protective effect (HR, 0.606), as patients carrying at least one variant allele T (i.e., -24CT, -24TT) achieved longer drug survival compared to patients with wild-type -24CC genotype. ABCC2 is a transporter that is important for the efflux of natural folates and antifolate drugs from cells [32]. The common polymorphism rs717620 has been associated with reduced transporter activity, which leads to decreased methotrexate elimination and hence higher methotrexate exposure [33,34,35,36]. Our previous study showed an important association between ABCC2 rs717620 genotype and methotrexate efficacy in psoriasis, whereby variant allele carriers were more likely to achieve adequate disease control with methotrexate [24], possibly due to this increased methotrexate exposure. The impact of ABCC2 rs717620 polymorphism on methotrexate drug survival reported here is in line with these previous data [24], whereby patients carrying variant allele T were more likely to continue treatment for longer, implying longer duration of disease control. Treatment response itself was associated with rs717620 polymorphism in the same way, i.e., variant allele T increased the odds of adequate treatment response [24]. Interestingly, considering the polymorphisms that we previously identified as significant for methotrexate efficacy and safety, the polymorphism in the ABCC2 transporter gene was the only one to also have effects here on methotrexate drug survival. Polymorphisms in genes for the transporter SLCO1B1 and the methionine cycle enzymes GNMT, DNMT3b, and BHMT did not have any effects on methotrexate drug survival in the present study, even though some of these had shown strong associations with methotrexate treatment outcomes in our previous study [24].
Only a handful of studies have investigated predictors of methotrexate drug survival to date. These have identified only isolated factors, without consistency among studies. Age at methotrexate introduction was identified as significant in two studies [13,18], but not in a third [16], and now not in the present study. Age at psoriasis onset was also not identified as a significant factor here, in line with previous results [16]. Concomitant metabolic syndrome, oral administration, and folic acid supplementation were identified as protective factors by Shalom et al. [13] which was also the only study to examine these factors [13]. As all patients in our cohort received methotrexate orally and were supplemented with folic acid, such an analysis was not feasible here. A positive association of ≥15 mg methotrexate weekly and drug survival has also been reported [18], in line with the known dose–response effects of methotrexate [2]. Otero et al. [16] reported no association of baseline disease severity and overall survival in multivariate analyses; however, severity perception by patients predicted short drug survival when methotrexate was discontinued due to AEs [16]. We did not include severity variables in the present analysis; although we assessed severity by PASI, Body Surface Area, and/or the Dermatology Life Quality Index, none of these were used for all or the majority of patients. Interestingly, neither the present study nor other studies have identified sex as an important factor for drug survival in multivariate Cox analyses [13,16,18], which contrasts with the results for biologic treatments [9]. Overall, at this stage, the determinants of methotrexate drug survival identified to date need further replication before they can lead to any meaningful applications in clinical practice.
An interesting finding from the present study was the identification of a novel genetic factor associated with methotrexate drug survival—a result that supplements a previously reported association with methotrexate treatment outcome. It therefore appears that ABCC2 rs717620 has additional potential as a biomarker of methotrexate treatment in adults with moderate-to-severe psoriasis.
Some limitations of the present study should be acknowledged when interpreting these findings. First, we gathered data retrospectively, which precluded the collection of some patient or disease characteristics (e.g., smoking at the time of treatment, disease severity using the same standardized criteria in all patients). Second, the observational nature of the study carries a risk of bias inherent to this type of study; confounding and selection bias cannot be fully excluded as sources of error. Furthermore, we included a limited number of patients, and thus it was not feasible to perform separate multivariate analyses according to reasons for discontinuation. Of note, approximately one-third of Slovenian patients with moderate-to-severe psoriasis are treated at our institution (and virtually all consented to inclusion in the patient registry); hence our cohort can be considered as representative for the country. Despite this, we should also acknowledge that the influence of the pharmacogenetic marker identified in this study should be considered as preliminary, as no definite conclusions can be made due to the small sample size. Our findings hence need further validation in larger patient cohorts, if possible, in prospective studies.
5. Conclusions
This study describes methotrexate treatment in a daily practice cohort of patients with moderate-to-severe psoriasis. To the best of our knowledge, this is the first study that has investigated the effects of genetic predisposition on methotrexate drug survival. These data show that genetic variability in the gene coding for the ABCC2 transporter influences methotrexate drug survival, whereby a patient carrying at least one copy of variant ABCC2 rs717620 allele is likely to remain on treatment for longer compared to patients with wild-type ABCC2 rs717620. Our findings suggest pharmacogenetic markers might have further applications in the management of moderate-to-severe psoriasis and might represent an interesting addition to studies of methotrexate use in daily practice.
Author Contributions
Conceptualization, J.G., M.M., I.M.-R., P.B.M., T.G. and A.Š.; Methodology, J.G. and A.Š.; Formal analysis, J.G. and A.Š.; Investigation, J.G., M.M., P.B.M., T.G. and A.Š.; Resources: I.M.-R.; Writing—original draft preparation, J.G., M.M. and A.Š.; Writing—review and editing, J.G., M.M., I.M.-R., P.B.M., T.G. and A.Š.; Supervision, I.M.-R.; Funding acquisition, I.M.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Slovenian Research Agency, grant number P1-0208, and the European Regional Development Fund, grant number C333-19-952061 EATRIS-TRI.SI.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the National Medical Ethics Committee of Slovenia (No. 85/06/15, 23 June 2015).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Due to patient privacy concerns, data used and/or analyzed during this study are not available for public access. Data are available from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to thank the patients for taking part in this study and Christopher P. Berrie for proofreading the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kaushik, S.B.; Lebwohl, M.G. Review of safety and efficacy of approved systemic psoriasis therapies. Int. J. Dermatol. 2019, 58, 649–658. [Google Scholar] [CrossRef]
- Yélamos, O.; Puig, L. Systemic methotrexate for the treatment of psoriasis. Expert Rev. Clin. Immunol. 2015, 11, 553–563. [Google Scholar] [CrossRef]
- Menter, A.; Gelfand, J.M.; Connor, C.; Armstrong, A.W.; Cordoro, K.M.; Davis, D.M.; Elewski, B.E.; Gordon, K.B.; Gottlieb, A.B.; Kaplan, D.H.; et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J. Am. Acad. Dermatol. 2020, 82, 1445–1486. [Google Scholar] [CrossRef] [PubMed]
- Nast, A.; Smith, C.; Spuls, P.I.; Avila Valle, G.; Bata-Csörgö, Z.; Boonen, H.; Boonen, E.; De Jong, I.; Garcia-Doval, P.; Gisondi, D.; et al. EuroGuiDerm Guideline on the systemic treatment of Psoriasis vulgaris—Part 1: Treatment and monitoring recommendations. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2461–2498. [Google Scholar] [CrossRef] [PubMed]
- Zweegers, J.; Otero, M.E.; van ven Reek, J.M.P.A.; van Lümig, P.P.; Driessen, R.J.; Kievit, W.; Seyger, M.M.B.; van de Kerkhof, P.C.M.; de Jong, E.M.G. Effectiveness of biologic and conventional systemic therapies in adults with chronic plaque psoriasis in daily practice: A systematic review. Acta Derm. Venereol. 2016, 96, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Doval, I.; Carretero, G.; Vanaclocha, F.; Ferrandiz, C.; Daudén, E.; Sánchez-Carazo, J.L.; Alsina, M.; Herrera-Ceballos, E.; Gómez-García, F.-J.; Ferrán, M.; et al. Risk of serious adverse events associated with biologic and nonbiologic psoriasis systemic therapy: Patients ineligible vs eligible for randomized controlled trials. Arch. Dermatol. 2012, 148, 463–470. [Google Scholar] [CrossRef] [PubMed]
- West, J.; Ogston, S.; Foerster, J. Safety and efficacy of methotrexate in psoriasis: A meta-analysis of published trials. PLoS ONE 2016, 11, e0153740. [Google Scholar] [CrossRef]
- Van den Reek, J.M.P.A.; Kievit, W.; Gniadecki, R.; Goeman, J.J.; Zweegers, J.; van de Kerkhof, P.C.M.; Seyger, M.M.B.; de Jong, E.M.G.J. Drug survival studies in dermatology: Principles, purposes, and pitfalls. J. Investig. Dermatol. 2015, 135, 1–5. [Google Scholar] [CrossRef]
- Mourad, A.; Straube, S.; Armijo-Olivo, S.; Gniadecki, R. Factors predicting persistence of biologic drugs in psoriasis: A systematic review and meta-analysis. Br. J. Dermatol. 2019, 181, 450–458. [Google Scholar] [CrossRef]
- Arnold, T.; Schaarschmidt, M.L.; Herr, R.; Fischer, J.E.; Goerdt, S.; Peitsch, W.K. Drug survival rates and reasons for drug discontinuation in psoriasis. J. Dtsch. Dermatol. Ges. 2016, 14, 1089–1099. [Google Scholar] [CrossRef]
- Dávila-Seijo, P.; Dauden, E.; Carretero, G.; Ferrandiz, C.; Vanaclocha, F.; Gómez-García, F.J.; Herrera-Ceballos, E.; De la Cueva-Dobao, P.; Belinchón, I.; Sánchez-Carazo, J.-L.; et al. Survival of classic and biological systemic drugs in psoriasis: Results of the BIOBADADERM registry and critical analysis. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1942–1950. [Google Scholar] [CrossRef]
- Puig, L.; Carrascosa, J.M.; Daudén, E.; Sulleiro, S.; Guisado, C. Drug survival of conventional systemic and biologic therapies for moderate-to-severe psoriasis in clinical practice in Spain: Prospective results from the SAHARA study. J. Dermatolog. Treat. 2020, 31, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Shalom, G.; Zisman, D.; Harman-Boehm, I.; Biterman, H.; Greenberg-Dotan, S.; Polishchuk, I.; Moser, H.; Freud, T.; Feldhamer, I.; Cohen, A.D. Factors associated with drug survival of methotrexate and acitretin in patients with psoriasis. Acta Derm. Venereol. 2015, 95, 973–977. [Google Scholar] [CrossRef][Green Version]
- Maul, J.-T.; Djamei, V.; Kolios, A.G.A.; Meier, B.; Czernielewski, J.; Jungo, P.; Yawalkar, N.; Mainetti, C.; Laffitte, E.; Spehr, C.; et al. Efficacy and survival of systemic psoriasis treatments: An analysis of the Swiss registry SDNTT. Dermatology 2016, 232, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Svedbom, A.; Ståhle, M. Real-world comparative effectiveness of adalimumab, etanercept and methotrexate: A Swedish register analysis. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Otero, M.E.; van den Reek, J.M.; Seyger, M.M.; van de Kerkhof, P.C.; Kievit, W.; de Jong, E.M. Determinants for drug survival of methotrexate in patients with psoriasis, split according to different reasons for discontinuation: Results of the prospective MTX-CAPTURE. Br. J. Dermatol. 2017, 177, 497–504. [Google Scholar] [CrossRef]
- Busger op Vollenbroek, F.T.M.; Doggen, C.J.M.; Janssens, R.W.A.; Bernelot Moens, H.J. Dermatological guidelines for monitoring methotrexate treatment reduce drug-survival compared to rheumatological guidelines. PLoS ONE 2018, 13, e0194401. [Google Scholar] [CrossRef]
- Akbulut Ozkok, T.; Topaloglu Demir, F.; Oguz Topal, I.; Kara Polat, A.; Karadag, A.S.; Aslan Kayiran, M.; Ozkur, E.; Altunay, I.K. Drug survival and predictor factors for discontinuation of methotrexate in psoriasis: A real-life multicenter study. Int. J. Dermatol. 2021, 60, 1140–1147. [Google Scholar] [CrossRef]
- Campalani, E.; Arenas, M.; Marinaki, A.M.; Lewis, C.M.; Barker, J.N.W.N.; Smith, C.H. Polymorphisms in folate, pyrimidine, and purine metabolism are associated with efficacy and toxicity of methotrexate in psoriasis. J. Investig. Dermatol. 2007, 127, 1860–1867. [Google Scholar] [CrossRef]
- Warren, R.B.; Smith, R.L.; Campalani, E.; Eyre, S.; Smith, C.H.; Barker, J.N.W.N.; Worthington, J.; Griffiths, C.E.M. Outcomes of methotrexate therapy for psoriasis and relationship to genetic polymorphisms. Br. J. Dermatol. 2009, 160, 438–441. [Google Scholar] [CrossRef]
- Warren, R.B.; Smith, R.L.L.; Campalani, E.; Eyre, S.; Smith, C.H.; Barker, J.N.W.N.; Worthington, J.; Griffiths, C.E.M. Genetic variation in efflux transporters influences outcome to methotrexate therapy in patients with psoriasis. J. Investig. Dermatol. 2008, 128, 1925–1929. [Google Scholar] [CrossRef]
- Chen, M.; Chen, W.; Liu, P.; Yan, K.; Lv, C.; Zhang, M.; Lu, Y.; Qin, Q.; Kuang, Y.; Zhu, W.; et al. The impacts of gene polymorphisms on methotrexate in Chinese psoriatic patients. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2059–2065. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.H.; Lu, Y.; Yan, K.X.; Liu, P.P.; Chen, W.Q.; Shen, M.X.; He, Y.J.; Wu, L.S.; Qin, Q.S.; Zhou, X.C.; et al. Genetic polymorphism predicting Methotrexate efficacy in Chinese patients with psoriasis vulgaris. J. Dermatol. Sci. 2019, 93, 8–13. [Google Scholar] [CrossRef]
- Grželj, J.; Mlinarič-Raščan, I.; Marko, P.B.; Marovt, M.; Gmeiner, T.; Šmid, A. Polymorphisms in GNMT and DNMT3b are associated with methotrexate treatment outcome in plaque psoriasis. Biomed. Pharmacother. 2021, 138, 111456. [Google Scholar] [CrossRef] [PubMed]
- Shalom, G.; Cohen, A.D.; Feldhamer, I.; Comaneshter, D.; Freud, T.; Pavlovsky, L. Drug survival in patients with psoriasis is associated with the availability of biologic medications. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1524–1528. [Google Scholar] [CrossRef] [PubMed]
- Sbidian, E.; Billionnet, C.; Weill, A.; Maura, G.; Mezzarobba, M. Persistence of apremilast in moderate-to-severe psoriasis: A real-world analysis of 14 147 apremilast- and methotrexate-naive patients in the French National Health Insurance database. Br. J. Dermatol. 2020, 182, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Cabello Zurita, C.; Grau Pérez, M.; Hernández Fernández, C.P.; González Quesada, A.; Valerón Almazán, P.; Vilar Alejo, J.; Carretero Hernández, G. Effectiveness and safety of Methotrexate in psoriasis: An eight-year experience with 218 patients. J. Dermatolog. Treat. 2017, 28, 401–405. [Google Scholar] [CrossRef]
- Dávila-Seijo, P.; García-Doval, I. Drug survival analysis is not a good method for assessing the safety or effectiveness of systemic therapies in psoriasis. Actas Dermo-Sifiliogr. 2017, 108, 3–5. [Google Scholar] [CrossRef]
- Pongparit, K.; Chularojanamontri, L.; Limphoka, P.; Silpa-Archa, N.; Wongpraparat, C. Effectiveness of and factors associated with clinical response to methotrexate under daily life conditions in Asian patients with psoriasis: A retrospective cohort study. J. Dermatol. 2018, 45, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Lie, E.; van der Heijde DDer Uhlig, T.; Heiberg, M.S.; Koldingsnes, W.; Rødevand, E.; Kaufmann, C.; Mikkelsen, K.; Kvien, T.K. Effectiveness and retention rates of methotrexate in psoriatic arthritis in comparison with methotrexate-treated patients with rheumatoid arthritis. Ann. Rheum. Dis. 2010, 69, 671–676. [Google Scholar] [CrossRef]
- Jacobs, M.E.; Pouw, J.N.; Welsing, P.; Radstake, T.R.D.J.; Leijten, E.F.A. First-line csDMARD monotherapy drug retention in psoriatic arthritis: Methotrexate outperforms sulfasalazine. Rheumatology 2021, 60, 780–784. [Google Scholar] [CrossRef]
- Bruhn, O.; Cascorbi, I. Polymorphisms of the drug transporters ABCB1, ABCG2, ABCC2 and ABCC3 and their impact on drug bioavailability and clinical relevance. Expert Opin. Drug Metab. Toxicol. 2014, 10, 1337–1354. [Google Scholar] [CrossRef]
- Zgheib, N.K.; Akra-Ismail, M.; Aridi, C.; Mahfouz, R.; Abboud, M.R.; Solh, H.; Muwakkit, S.A. Genetic polymorphisms in candidate genes predict increased toxicity with methotrexate therapy in Lebanese children with acute lymphoblastic leukemia. Pharm. Genom. 2014, 24, 381–396. [Google Scholar] [CrossRef]
- Razali, R.H.; Noorizhab, M.N.F.; Jamari, H.; James, R.J.; Teh, K.H.; Ibrahim, H.M.; Teh, L.K.; Salleh, M. Association of ABCC2 with levels and toxicity of methotrexate in Malaysian Childhood Acute Lymphoblastic Leukemia (ALL). Pediatr. Hematol. Oncol. 2020, 37, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Rau, T.; Eerney, B.; Göres, R.; Eschenhagen, T.; Beck, J.; Langer, T. High-dose methotrexate in pediatric acute lymphoblastic leukemia: Impact of ABCC2 polymorphisms on plasma concentrations. Clin. Pharmacol. Ther. 2006, 80, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Haenisch, S.; Zimmermann, U.; Dazert, E.; Wruck, C.J.; Dazert, P.; Siegmund, W.; Kroemer, H.K.; Warzok, R.W.; Cascorbi, I. Influence of polymorphisms of ABCB1 and ABCC2 on mRNA and protein expression in normal and cancerous kidney cortex. Pharm. J. 2007, 7, 56–65. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).