Abstract
Botulinum toxins or neurotoxins (BoNTs) are the most potent neurotoxins known, and are currently extensively studied, not only for their potential lethality, but also for their possible therapeutic and cosmetic uses. Currently, seven types of antigenically distinct toxins are known and characterized, produced by a rod-shaped bacterium, Clostridium botulinum. Human poisoning by botulism (presenting with severe neuromuscular paralytic disease) is usually caused by toxins A, B, E, and F type. Poisoning from contaminated food preparations is the most common cause of noniatrogenic botulism. The spores are highly resistant to heat but are easily destroyed at 80 °C for thirty minutes. Type A and B toxins are resistant to digestion by the enzymes of the gastrointestinal system. After their entry, BoNTs irreversibly bind to cholinergic nerve endings and block the release of acetylcholine from the synapses. In contrast, in wound botulism, the neurotoxin is instead product by the growth of C. botulium in infected tissues. The contamination by BoNT inhalation does not occur by a natural route but it is certainly the most dangerous. It can be caused by the dispersion of the botulinum toxin in the atmosphere in the form of an aerosol and therefore can be deliberately used for bioterrorist purposes (e.g., during CBRN (chemical, biological, radiological, and nuclear) unconventional events). In addition, BoNTs are currently used to treat a variety of diseases or alleviate their symptoms, such as the onabotulinumtoxinA for migraine attacks and for cosmetic use. Indeed, this paper aims to report on updated knowledge of BoNTs, both their toxicological mechanisms and their pharmacological action.
1. Introduction
The Clostridium genus, a part of the phylum Firmicutes, belongs to the family Clostridiaceae, class Clostridiales, prokaryotic microorganisms and is sporogenic, Gram (+) bacteria, and anaerobic (even if some species can live in the microaerophilic environment such as C. tertium and C. histolyticum). Some species are primarily pathogenic to humans, many are potentially pathogenic, and many other species are saprophytic. It is the largest and most heterogeneous genus of bacteria and can be divided into several phylogenetic groups (clusters). Currently, there are 228 species and subspecies classified in the genus. The basic G + C ratio has a very wide range (22–55 mol%) [1,2]. Clostridia are large bacteria (3–8 μm long and 0.4–1.2 μm wide) that have the ability to form spores. Morphologically, the germinal forms and their seeds can be distinguished. The germinal form in most Clostridia has the shape of a straight or curved bacterium [1]. Bacteria have parallel sides, and their edge can be gradually thinned, rounded, or rectangular. In some species, different bacteria attach themselves to solid helical formations. Almost all species are motile because of the presence of peritrichous flagella. Immobile species isolated from clinical specimens are C. perfringens, C. ramnosum, and C. innocuum [3,4]. The spores of the Clostridia are spherical to oval in shape and appear as a bulge in the body of the bacterium. They grow in different parts of the microbial cell, depending on the species, but usually they can be found at a pole of the bacterial body or a short distance from it, or can be central, giving different shapes to the Clostridia. Characteristic of the Clostridia is the ability to produce powerful proteic exotoxins [5] that are largely responsible for the onset of symptoms of infections (Table 1). The infections by Clostridia are divided into endogenous (presence in the patient microbiota) and exogenous (origin from the external environment). Clostridium spp. are usually fermentative (saccharolytic), such as C. novyi, C. perfringens, and C. septicum, or proteolytic, such as C. botulinum, C. histolyticum and C. The proteolytic C. sporogenes exhibits partial saccharolytic activity, while the saccharolytic C. perfringens is also slightly proteolytic. Finally, there are fermentative and nonproteolytic species [1,6,7].

Table 1.
Classification of pathogenic Clostridium spp. according to the toxins’ target.
The C. botulinum is mobile and arranged in pairs or small chains. The bacteria and especially their spores are ubiquitous, mainly found in soil and water, also found in the gut microbiota of many animals, and live in the natural environment for a few years (they can germinate in an anaerobic environment). They are destroyed at acid pH in the presence of NaCl [8]. C. botulinum spores are ubiquitous in nature with a pH ranging 4.6–7 and produce the toxins. During their ingestion they do not cause damage to the organism, because they do not metabolize and do not germinate at the gastroenteric level. Their presence in environments suitable for development is dangerous because the vegetative cells of C. botulinum produce several types of proteins toxins (BoNTs or BTXs), resistant to the action of gastric juice and intestinal proteolytic enzymes. They are water soluble, degraded by alcohols and bacterial growth, and toxins are inhibited at pH values < 4.6, and are relatively thermostable (inactive at 100 °C for 10 min or 80 °C for 30 min) [9]. Moreover, adding nitrites to meats and canned food inhibits the growth of Clostridium. The toxins do not pass the blood–brain barrier [10].
2. Timeline of Historical Events about BoNTs Discovery
The term botulism, which derives from the Latin word “botulus” (meaning sausage), was used in Europe in the 18th century to describe a highly lethal disease with muscle paralysis leading to respiratory arrest after eating raw sausages. In the period 1817–1822, the German Dr. Justinus Kerner (1786–1862) first described botulism, a food poisoning after consumption of meats, performing confirmative studies. It is noteworthy that he was also the first physician to propose the therapeutic use of the botulinum toxin. In 1897, after an epidemic of botulism in the city of Elezel (Belgium) due to the consumption of ham of homemade pork, the microbiologist Emile van Ermengem, discovered the bacterium and concluded that the disease was caused by the anaerobic growth of the microorganism and that the endospores produced a toxin [11]. In the 1920s, scientists were able to isolate one of the seven toxins produced by Clostridium, such as the toxin BoNT-A, which is now the active form of the commercial drug Botox™. During the Second World War, the research on the manufacture of biological weapons that could infect and affect the nervous system had a strong impulse, so two military laboratories, one in the United States and one in England, studied the effects of toxin B [11,12].
After the end of the II World war, in the 1950s and 1960s, research revealed the mechanism of action of the toxin, but the medical use of botulinum toxin began in 1977. In fact, in 1978, Alan Scott, an American ophthalmologist, decided to try the toxin to treat strabismus that caused the rolling of the eyes, thus becoming the first to use the botulinum neurotoxin drug in humans [12]. The results of his studies were published in medical journals in the 1980s and then many doctors applied his technique to treat eyelid twitching and other muscle spasms. The drug was originally called Oculinum, from the Latin oculi, which means eyes [13]. Since the 1970s the BoNT-A, has been used not only for aesthetic purposes but also as a medicine in a wide variety of diseases and its indications have increased over time. Its action appears a few days after administration and usually lasts between 7 and 10 days. In fact, BoNT-A was first used extensively for the neurological disorder as treatment of focal dystonia and is now used in other diseases such as in the field of dermatology, urology, gastroenterology, and others. In 1982 discovered that the drug had a side effect of eliminating skin wrinkles at the injection site. Later, the same drug was recalled Botox, and was licensed by the American FDA for wrinkles. Botox has already caused a small revolution in cosmetic plastic surgery in an easy, simple, and safe way [11,14]. In England, in 1993, Khalaf Bushara and David Park were the first to use BTX-A for nonmuscular problems demonstrating that injections of toxins inhibit sweating and can therefore be useful in the treatment of hyperhidrosis [15]. In 1996, other aesthetic applications began and in 2002, he obtained FDA approval for these. In 2010, the FDA approved intramuscular injections for the prophylactic treatment of migraines and headaches and in 2014, the UK’s Medicines and Healthcare Products Regulatory Agency (MHRA) approved BoNTA for the treatment of limited ankle movement (due to stroke-related spasticity of the lower limbs) [16]. Subsequently, in 2016, the United States Food and Drug Administration (FDA) approved abobotulinumtoxinA for injection for the treatment of lower extremity spasticity in pediatric patients two years of age and older [17].
3. Materials and Methods
Data Sources
This article is based on a detailed analysis of studies on the historical, pharmacological, and toxicological significance of C. botulinum, using international scientific databases, medical books, statistical data from government reports, and texts available on Medline, PubMed, Web of Knowledge, Web of Science, Google Scholar, Scopus, and Elsevier (EMBASE.com, accessed 30 May 2021). Key articles have been selected that could provide scientific insight into toxicity, biomolecular action, antidotes, medical applications purposes, and their use in CBRN (chemical, biological, radiological, and nuclear) terrorist events. Ancient books from public libraries and private collections were very useful to collect historical data about botulism and its treatment.
4. Results and Discussion
The Biomolecular Toxicity and Activity of Botulinum Neurotoxins (BoNTs)
- (a)
- The physical–chemical features
The BoNT [Chemical Abstracts Service Registry Numbers (CASRNs): 107231-15-2 (botulin b > f), 93384-43-1 (botulin b > a; botulin b > um toxin a), 93384-45-3 (toxins botulin b > c)] exotoxins are produced and secreted under anaerobic conditions by the C. botulinum (categorized based on what BoNTs produce and not on their phylogenetic affinity) [1,18]. BoNTs are the most potent biotoxins found in nature (in mice LD50 = 1 ng/kg for type A) and therefore are 100 billion times more toxic than cyanide. BoNTs belong to the zinc endopeptidases that are proteins with a molecular weight of about 150 kDa. The toxin is released during bacterial cell lysis and each strain of C. botulinum usually produces only one type. Its structure consists of two fractions, M and L (larger), linked by a disulfide bond. An additional hemoglutin is also attached in the L chain [19]. Thus, BoNT-A and BoNT-B contain two polypeptide chains, the A and B chains, that play different roles and are linked together by a disulfide bond and a nontoxic hemagglutinin. The light chain A has a molecular weight of about 50 kDa and the heavy chain B has a molecular weight of about 100 kDa [20]. It is the heavy polypeptide chain that binds to the acetylcholine synapses of peripheral nerve cells. Light chain is a zinc-containing protease that acts by binding to proteins that play a role in the mechanism of acetylcholine release (SNAP-25, syntaxine synaptobevin-VAMP). In cultures, BoNTs bind to other proteins to form protein complexes weighing 300–900 kDa [21,22]. Some BoNTs are not inactivated by enzymes in the intestine but are absorbed in the upper part of the neuromuscular junction and inhibit the release of acetylcholine. There are seven types of C. botulinum toxins in terms of their antigenicity, denoted by the Latin letters A, B, C, D, E, F, and G and a number of subtypes (e.g., for type A there are five subtypes A1, A2, A3, A4, A5) [1,22]. The neurotoxins of each type differ up to 70% in amino acid level, while the subtypes differ between 2.6 and 32%. The most common types that affect humans are A, B, E, and rarely F, and the types C, D, and E can cause diseases in other mammals, birds, and fish [10,23]. Thus, there are four C. botulinum groups I, II, III, and IV. Group I proteolytic C. botulinum produces toxins of types A, B, or F and the nonproteolytic group II C. botulinum produces toxins of types B, E, or F. These two strains differ significantly genetically and are responsible for most cases of botulism [24]. These bacteria are present in soil and in aquatic sediments worldwide, growing and producing botulinum toxins under anaerobic conditions. The genetic information that encodes their production can be chromosomal or plasmid [25].
- (b)
- Modality of the toxic action
BoNTs are the most potent toxins found in nature (e.g., in mice the LD50 = 1 ng/Kg for type A) and are 100 billion times more toxic than hydrogen cyanide and Sarin (an organophosphorus nerve gas compound) [26]. Since even a small amount of BoNTs, entered through ingestion, inhalation or absorption through the eye or a continuous solution of the skin can cause serious illness, all materials suspected of containing the contaminating toxin require particular attention in handling. BoNTs are active by either ingestion or inhalation (they do not occur naturally) and the clinical effect on the patient is similar in both ways of entering the body. Samples should be placed in unbreakable sterile sealed refrigerated containers and examined as soon as possible. Samples taken from wounds are an exception and should not be refrigerated [1,9].
As mentioned, the action of BoNTs is the effect they have on specific proteins associated with nerve activation, blocking the peripheral release of acetylcholine at presynaptic cholinergic nerve endings through the breakdown of SNAP-25, a protein that is essential for and release of acetylcholine from vesicles found inside nerve endings (Table 2) [27].

Table 2.
The action of BoNTs in the Synaptic Fusion Snare Proteins Complex.
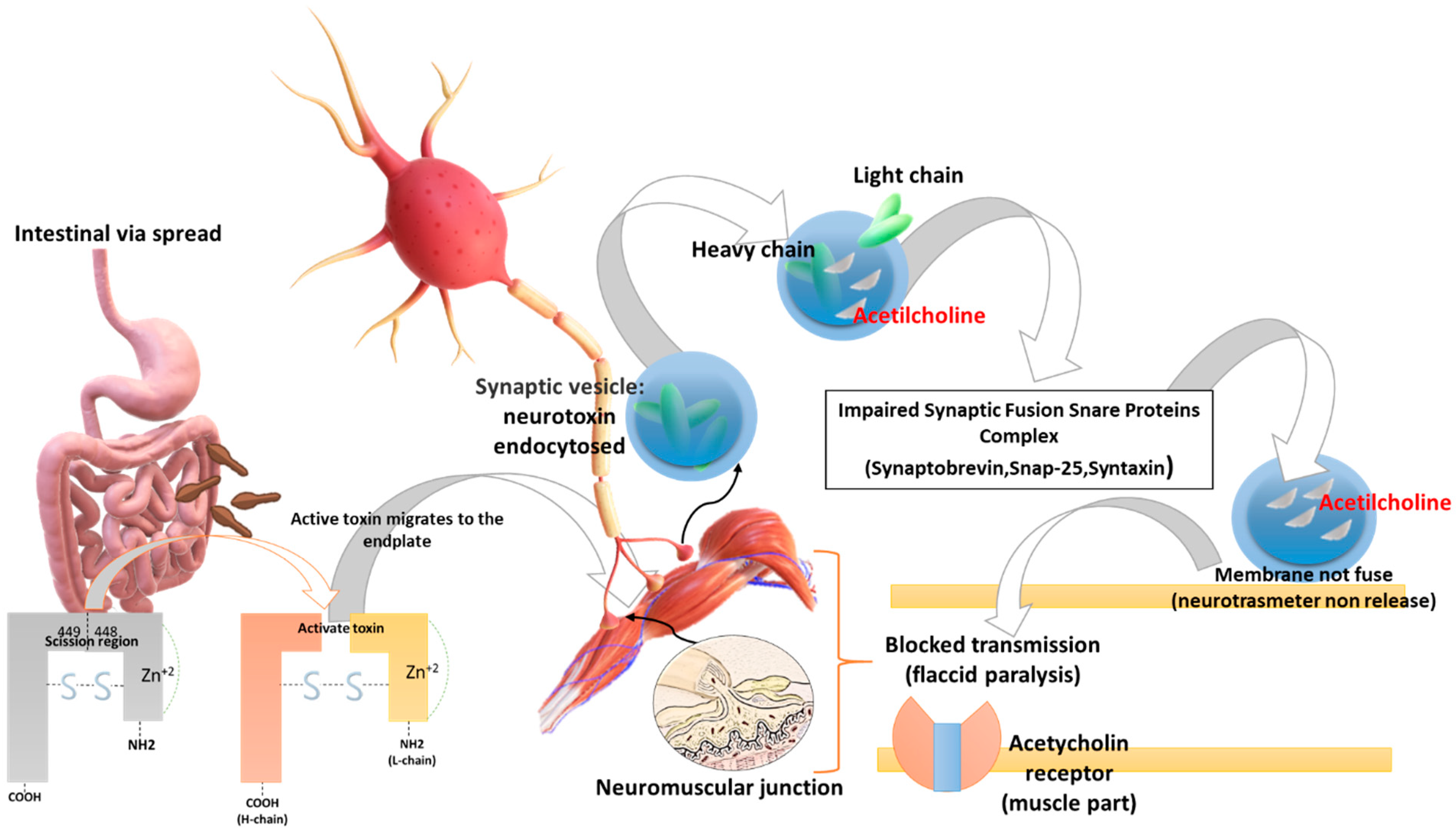
Initially the BoNT heavy chain is specifically attached to nerves that use the neurotransmitter acetylcholine. Then the neuron encloses the toxin in a vesicle, which is oxidized as it moves inside the neuron. So, after entering the cytoplasm of the neuron, the light chain zinc-containing protease acts by binding to proteins that play a role in the mechanism of acetylcholine release (SNAP-25, syntaxine, and synaptobrevin-VAMP). Thus, the light chain binds to SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) proteins preventing the neuron from releasing the acetylcholine neurotransmitter. The result is that the communication of the nerve cells is blocked, leading to paralysis (Figure 1) [28].
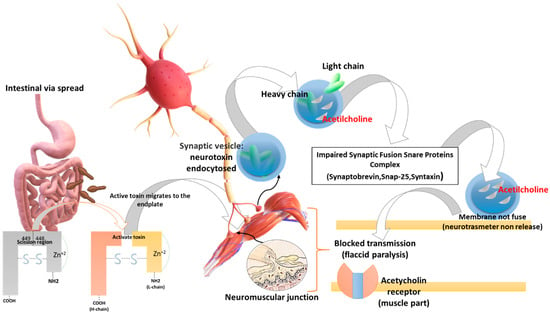
Figure 1.
Biomolecular mechanism of toxin action: BoNTs (a) have tropism for peripheral motor neurons, (b) cut the SNAP 25 protein, (c) inhibit synaptic vesicle fusion with the presynaptic membrane, (d) inhibit the release of the neurotransmitter acetylcholine at the level of the neuromuscular plate causing flaccid paralysis and death from respiratory arrest. BoNTs (e.g., BoNT-A) initially are activated in the digestive system by the action of the trypsin (a serine protease) in the scission region (449–448) and are therefore absorbed by the gut. BoNTs spread in the blood until they reach the neuromuscular and cholinergic junctions, penetrate the synaptic cholinergic endings, and block the release of acetylcholine, causing flaccid paralysis. The heavy chain of the toxin is important for its penetration into the axonic extremities, a condition to which the onset of paralysis is linked. Following the binding of the heavy chain to terminal axon proteins, the toxin can enter neurons via endocytosis. The binding of the heavy chain occurs with the protein receptor SV2, whose expression is increased when the synapse is most active. The light chain is able to leave the endocytic vesicles and reach the cytoplasm. The light chain of the toxin possesses protease activity. Toxin A proteolytically degrades SNAP-25 protein, a type of SNARE protein. The SNAP-25 protein is necessary for the release of neurotransmitters from terminal axons (in particular, botulinum toxin degrades SNAREs, preventing the release of neurotransmitters at the synapse level). Credits: Original figure by I.A. Charitos.
- (c)
- The clinical picture and diagnosis of botulism
It exists in six main intoxication clinical forms: (a) alimentary, (b) inhaled, (c) wound (incubation period 4–14 days), (d) infant (age 6 > months), (e) adult intestinal, and (f) iatrogenic. Several improperly made food preparations can provide botulism such as: preserves and semipreserves in oil, water, industrial or domestic brine, the products called RE.P.F.E.D. (Refrigerated Processed Food with Extended Durability), creams and sauces, some macrobiotic products (seitan or tofu), spices in oil, and flavored oils. Other causes are preserved products, vacuum-packed with equipment available at home, honey, and exposure to water. Symptoms of BoNTs poisoning by inhalation or ingestion usually occur with an incubation period of 12 to 36 h and in exceptional cases up to eight days (maximum time range four hours to eight days) and the onset time is dose-dependent. Thus, a higher dose means a shorter onset of symptoms [29].
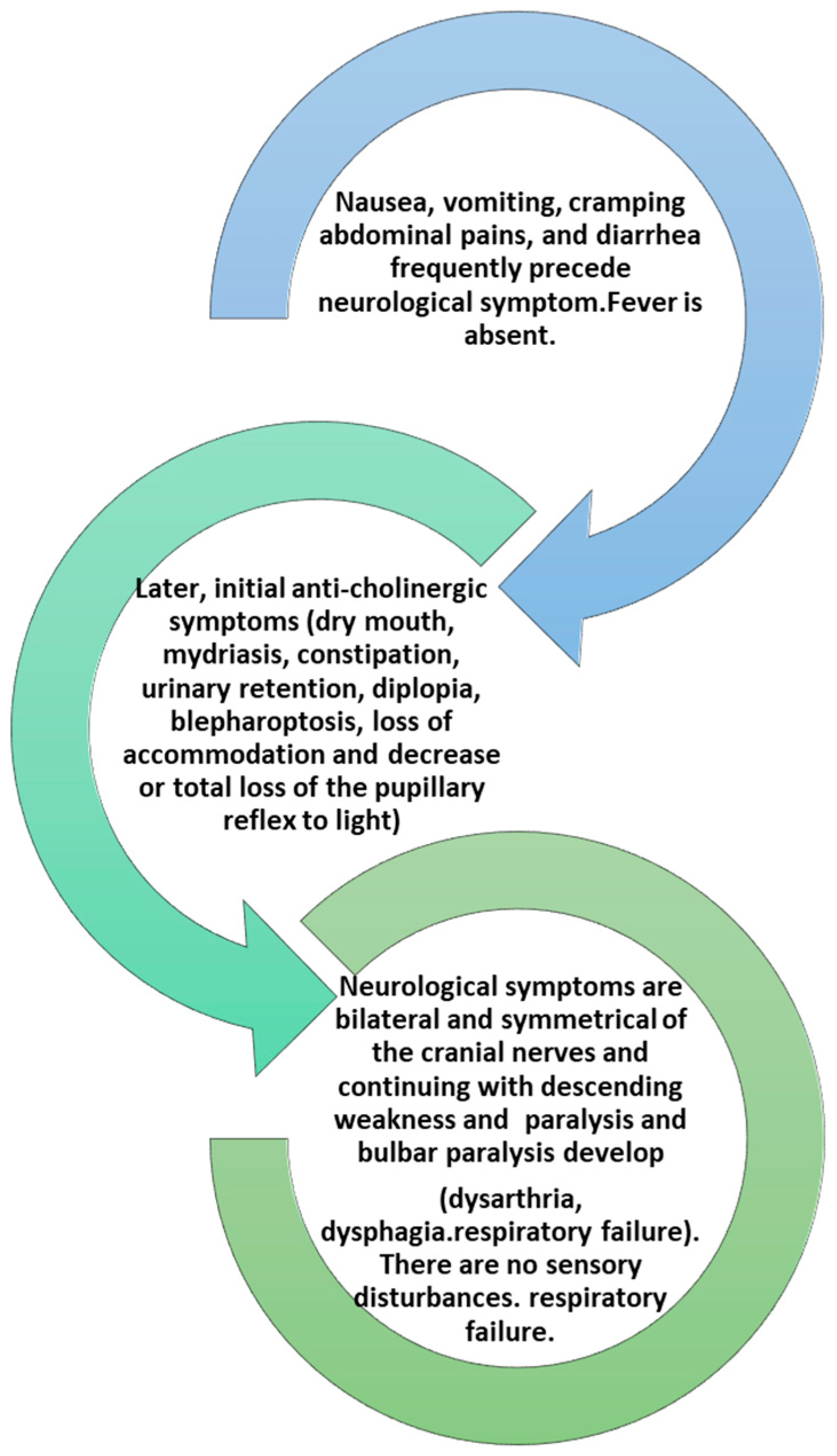
Initial symptoms include fatigue, weakness, and dizziness followed by obvious cranial nerve dysfunction IX, X, XI, and XII with blurred vision due to dilation of the pupil, diplopia, and photophobia, drooping eyelids with symptoms of difficulty in articulation and speech. Symptoms may include vomiting, diarrhea or constipation, and bloating [30]. This is followed by paralysis of skeletal muscles such as those of the arms and legs with symmetrical progressive weakness. The completion of the symptomatology is sudden respiratory failure (Figure 2) [30,31].
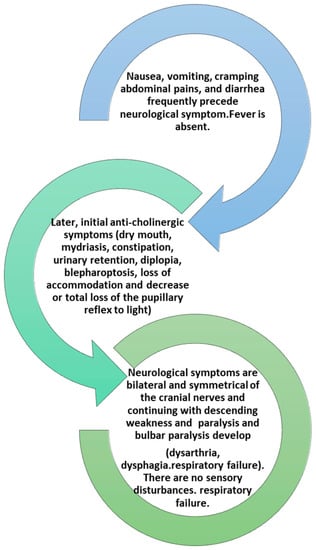
Figure 2.
The development of clinical symptoms in an intoxication from BoNTs.
Food botulism case control measures provide treatments that prevent germination and toxin genesis or, secondly, the inactivation of the already produced toxins (heat treatment at a core temperature of at least 85 °C for 10 min). The treatments consist of (a) acidification by acid fermentation or the addition of acidifiers in order to obtain a final pH < 4.5; (b) addition of solutes such as salt or sugar until reaching a value of free water (Aw) <0.935 (10% NaCl brine), (c) additives and preservatives (nitrites, polyphosphates, sorbic acid, nisin, etc.), and (d) industrial sterilization (121 °C × 15 min) for all those foods that cannot be subjected to the indicated treatments. To obtain the same effect as industrial sterilization, the product must be boiled for 7–10 h [32,33].
The second clinical form concerns botulism from a wound. This case is particularly rare and occurs when endospores of C. botulinum enter from an open wound that causes discontinuity of the skin. The active strains are converted and under anaerobic conditions secrete botulinum toxins. Here, the symptoms appears slowly and can start even after two weeks. Wound botulism arises with the same symptoms of neurological involvement present in food, with progressive paralysis starting from the injection site, but there are no symptoms affecting the gastrointestinal system. Such cases have occurred in drug addicts and especially in people who injected acetylated heroin (black tar) [34,35].
Infant botulism is a syndrome caused by neurotoxic Clostridia such as C. botulinum and more rarely C. butyricum or C. baratii. They receive C. botulinum endospores with food, which germinate and then turn into active strains, which form colonies in the gastrointestinal tract and thus release the toxin [36]. Thanks to the immaturity of the intestinal microbiota and therefore of competition that leads to bacterial dysbiosis, the spores can germinate, multiply, temporarily colonize the intestinal lumen in the colon, and produce the neurotoxin in situ. This cannot occur in adults and children under six months of age as the body’s natural defenses prevent the endospores from turning into active bacteria. Since spores of neurotoxic Clostridia are normally present in all types of environments, both terrestrial and aquatic, soil and dust play have a critical role as a source of Clostridia for newborns [36,37]. Such incidents have occurred also in cases of honey contaminated with endospores C. botulinum, which is why there is a clear warning to parents and caregivers not to feed honey to infants under one year. The study of cases of infant botulism has made it possible to correlate the onset of the infection with the inhalation of spores present in the environment surrounding the newborn. Clinical presentation is the same as for adults and the intestinal mucosa is not affected by the infection. The clinical diagnosis for infant botulism depends on a careful examination of symptoms such as neurological ones (lethargy and muscular flaccidity, mydriasis) and on characteristic elements such as the absence of fever and integrity of the sensory functions. During the illness, the child’s state of consciousness remains intact, and the central nervous system is not affected, while the possible appearance of fever is a consequence of concomitant infections. The typical sign of the clinical picture of infantile botulism is instead generalized paralysis involving the cranial nerves [30,36,38,39].
Adult intestinal toxemia is a very rare kind of botulism. Typically, patients that have some anatomical or functional abnormalities of the intestine or are subjected to prolonged antimicrobial therapies, can allow bacterial colonization. Thus, the adult intestinal colonization botulism is the result of the production of the toxin in the intestinal lumen of adults that leads to gut microbiota dysbiosis [40,41,42].
The differential diagnosis of botulism includes dehydration and electrolyte imbalance, sepsis, polyneuropathies such as Guillain-Barré syndrome, myasthenia gravis, spinal muscular atrophy, stroke, encephalitis, and others (Table 3) [43,44,45,46].

Table 3.
The table shows the main pathologies for which the differential diagnosis must be made in comparison with C. botulinum intoxication. (Adapted from https://www.cdc.gov/botulism/health-professional.html, 2021, accessed date, accessed date 30 June 2021).
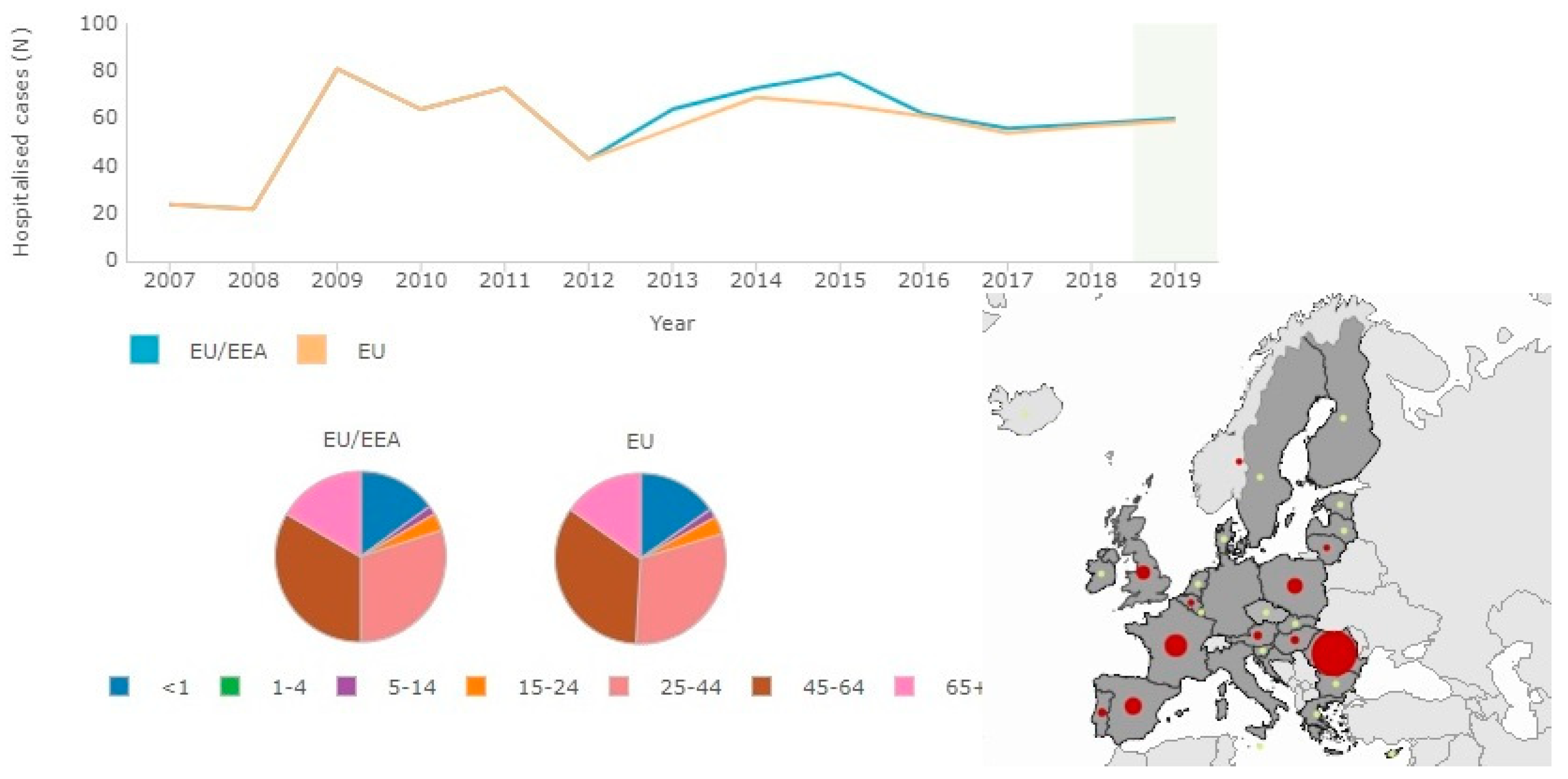
The criteria for laboratory conferment of botulism for all the clinical forms are based on the detection of BoNTs in serum, feces, and/or food samples and on the identification of Clostridi, which produces BoNTs in feces, wound sites, and in food samples (Figure 3) [38,45,47].
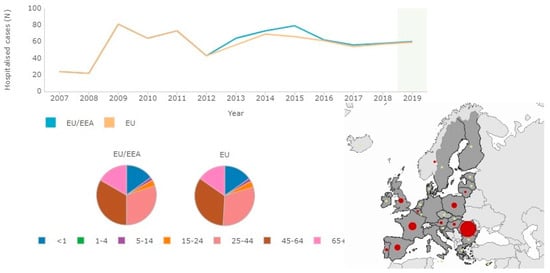
Figure 3.
Sixty confirmed hospitalized cases for botulism in the EU/EEA in comparison with age (highest incidence aged 45–64) for 2019 (source: http://atlas.ecdc.europa.eu/public/index.aspx?Dataset=27&FixDataset=1, accessed date 5 July 2021).
The electromyogram shows the typical results of exposure to botulism including normal nerve conduction velocity, normal sensory nerve function, a pattern of short small-amplitude motor potentials, and a clear incremental response (facilitation) to repetitive stimulation often seen only at 50 Hz [40]. The CT/CT–Angio imaging of the brain, spine, and chest are normal in botulism and the CSF is unchanged in botulism and autoantibodies are not present in botulism [38,41,45].
Botulism infection is fatal in 5–10% of cases and requires rapid diagnosis and immediate hospitalization for rapid treatment with early antidotic (antitoxin) administration. If the neurological conditions are severe the patient should be managed in intensive care for mechanical respiratory support, symptomatic therapy, and antidotism [47,48]. An important role in the initial management of the patient is played by the Poison Control Center, both in an out-of-hospital and in-hospital health care in several countries. The only treatment available is the antitoxin antidotes. The CDC (Centers for Disease Control and Prevention, USA) recommends the use of Botulism Antitoxin Heptavalent-(Equine) or (HBAT) and BabyBIG. The polyvalent antitoxin (ABCDEF) is more efficient (even for specific epidemics such as those related to botulism C or F) and the Botulism Immune Globulin Intravenous (Human) is an antitoxin antibotulism of human origin and is administered for botulism infantile type A and B [47,49,50]. The antitoxin must be administered as soon as possible after diagnosis, because it stops the progression of the paralysis and so there will be recovery through the regeneration of new neuromuscular connections [47]. Currently animal experimental studies continue for botulinum intoxication therapy. In fact, in a study a protein was created by fusing a single-domain antibody (nanobody) against BoNT/A demonstrated (into cultured neurons) that neutralized the BoNT/A in neurons in the mouse. Even in lethal doses of BoNT/A this protein caused a shorter duration of local muscle paralysis and the recovery of muscle strength within hours. Another study showed that nontoxic BoNT derivatives can carry an antibody that blocks BoNT serotype A (BoNT/A1) so they can be used as therapeutic protein portions. Hence, it could allow for the administration of antibodies to intraneuronal targets [51,52]. Finally, animal studies are currently determining the efficacy of 3,4-diaminopyridine (3,4-DAP) as a potential treatment againts paralysis from botulism, which in cases of bioterrorism would be useful in mass intoxication (due to lack of stocks of the antitoxin antidotes). Furthermore, in an older pilot study after the oral administration of a single dose of 3,4-DAP a delay in the evolution of paralysis to fatal respiratory failure was noted among intoxicated patients [53,54].
5. Pharmacological and Therapeutics Aspects
In the medical field, new techniques of treatments (such as those of stem cells) and already known pharmacological treatments are being tested, such as the BoNTs. In fact, they are used to treat several disorders characterized by muscle hyperactivity such as poststroke spasticity, spasticity due to spinal cord injury, blepharospasm, hemifacial spasm spasmodic dysphonia, and bruxism. They are used to relax muscle contraction, including achalasia overactive bladder and anal fissures. Another indication would be that of strabismus. It can sometimes be relieved by the weakening that causes the administration of the toxin so that the muscles adapt to the length corrected over time [54,55,56,57,58,59,60]. In fact, if a paralyzed muscle is stretched by its competitor, it increases more, while the competitor decreases and therefore over time, both eyes align, and this leads to a correct alignment between the two eyes. BONTs can be used offlabel for a variety of pediatric conditions, including pediatric esotropia [61,62,63]. BTX-A is used to treat severe primary axillary hyperhidrosis (with some doubts because of adverse effects), which cannot be treated with topical agents [58]. Intramuscular injections of BONTs also can be used for prophylactic treatment of chronic migraine headache. Finally, as we have mentioned, one of the cosmetic applications is to reduce facial wrinkles (especially in the upper third of the face) because it causes the relaxation of these muscles, which is visible 7–10 days after treatment and lasts around three to six months (Table 4) [64,65].

Table 4.
The BoNTs types currently available: Botox® (onabotulinumtoxinA), Dysport® (abobotulinumtoxinA), and Xeomin® (incobotulinumtoxinA) are the trade names for therapeutic use of botulinum toxin A. The FDA has approved the use of Botox® for glabellar lines and “crow’s feet” and Dysport® and Xeomin® for glabella only, other uses are outside formal approval. Their dosage units are not equivalent, have different pharmacokinetics, and refer to different indications and uses. Less known is botulinum toxin type B (RimabotulinumtoxinB), also with medical applications, mainly in cervical dystonia, neck pain, and chronic sialorrhea, while there are five other serotypes C, D, E, F, and G.
Side effects can occur immediately, within hours, within days, or even months after use. Furthermore, some of them have a long duration or last as long as the action of the toxin, two to four months. The key factors for correct application are that the operator must have knowledge of the safety of the drug and of the adverse symptoms, as well as having an experience of injecting it. The most common side effect is muscle weakness near the injection site, e.g., drooping eyelid, dry eye, and double vision after an injection near the eye. Other unwanted local side effects may include pain, bruising, and bleeding at the injection site, difficulty moving and pursing lips or sucking with a straw, dysarthria, neck flexion weakness, local muscle dysfunction, or muscle wasting in long-term application. Very rarely it can lead to generalized weakness and allergic reactions [66]. About 0.3–6% of patients develop antibodies that neutralize the effectiveness of the drug, especially after frequent and continuous use. Although with changes in formulation the frequency of neurtralizing antibodies has diminished significantly [67]. Contraindications to the application of botulinum toxin are subjects with botulinum toxin allergy, urinary tract infections (for bladder application), myasthenia gravis, Lambert-Eaton syndrome, pregnancy, and breastfeeding. Caution is advised in patients with a history of allergy, asthma, emphysema, predisposition to bleeding, neuromuscolar disorders, Parkinson’s disease, muscle discontinuity due to bruise, and skin diseases [68,69].
6. BoNTs Association with Bioterrorism CBRN (Chemical, Biological, Radiological, Nuclear) Events
There are poisons (biotoxins) produced by living organisms, some of which have chemical–physical characteristics that make them suitable for possible uses as offensive weapons (bioterrorism). They are produced by bacteria, fungi, algae, and other plant and animal organisms, some of which have already been known by the ancients both as poisons for hunting and for their therapeutic properties [70,71]. Some biotoxins have even greater toxicity than that of some synthetic ones (such as nerve gases), but not all have stability characteristics, particularly at temperature and sunlight, so they do not always have the chemical–physical characteristics to be used without risk for whoever intends to use them. A first attempt to overcome these considerable drawbacks was to microencapsulate them (stable to temperature and sunlight, obtaining higher levels of danger). In 1972, the international convention on biological weapons and toxins, signed by 140 countries, decreed a ban on their use for military purposes [72,73,74]. However, it must be emphasized that currently, thanks to genetic engineering techniques, it is possible to produce toxins in large quantities, as well as modify their original structure, both to enhance their aggressive characteristics and improve stability, and to try to obtain a programmed release through a progressive degradation of the envelopes with which they are covered and conveyed [74].
BoNT-A and BoNT-B are among the best known biotoxins that have a high risk of unconventional events CBRN (chemical, biological, radiological, and nuclear) and are bioterrorism agents due to their high lethal toxicity (they are the most toxic known toxins). Their toxicity is about 100,000 times more toxic than organophosphorus nerve gas (Sarin). The mean lethal dose of BoNT-A in humans is 0.09–0.15 μg for 70 kg when administered intravenously or intramuscularly, 0.7–0.9 μg when inhaled, and 70 μg when administered orally. Before the Gulf War, Iraq produced 20,000 L of BoNTs, with the production of warheads bombs containing toxins. Several terrorist groups have experimented with BoNTs, including members of the religious sect of Aum Shinrikyō, known for the 1995 Sarin gas attack on the Tokyo subway [75,76].
The main ways of causing this disease are by inhaling toxins or by administering it through food. In case of botulism due to inhalation, since it cannot occur naturally, there is an association with an accident or a terrorist attack with air pollution with botulinum toxins in aerosol form. One gram of pure crystalline BoNT theoretically with smooth distribution after inhalation could kill more than one million people. For this reason, it belongs to the A category of the CDC (USA) and is also a banned substance of the Biological Weapons Convention [70,72,75].
The factors that would make the use of the BoNTs by terrorists possible are the following: (a) extremely high toxicity, (b) resistance to changing environmental conditions, (c) ease and low cost of production as the bacteria that produce them are widely available, but also the fact that there are commercially available formulations containing botulinum toxins for medical and cosmetic purposes (Botox™) from which they can be isolated, (d) the possibility of adding them to aerosols for spraying as they can be stored in powder form, and (e) the possibility of adding them to foods and beverages that will not be heated before consumption [75,76,77].
7. Conclusions
The findings from this study can be summarized as follows:
- (a)
- The BoNTs produced by C. botulinum are of a protein nature targeting the nervous system (Neurotoxic).
- (b)
- C. botulinum has seven types of toxins in terms of antigenicity; A, B, C, D, E, F, G, and subtypes.
- (c)
- When food is contaminated with toxins, it can cause botulism disease, which can lead to craniocaudal paralysis with respiratory failure.
- (d)
- Differential diagnosis is important because to counter its lethal effects antidote (antitoxin, antibodies or fragments that bind the antibody’s antigen that can block BoNTs), must be administered in the shortest possible time.
- (e)
- Researchers have been successfully converting the BONTs into a safe and effective drug that can be used to treat several diseases and for aesthetic purposes.
- (f)
- Two types of BoNTs are currently available in patient treatment and cosmetic use: Type A (onabotulinumtoxinA, abobotulinumtoxinA, and incobotulinumtoxinA) and Type B (rimabotulinumtoxinB).
- (g)
- BONT injections are effective and expected to provide 50–90% improvement for at least two to three months (if the injections do not seem effective, then the possibility of changing it dose or injection site should be evaluated).
- (h)
- BoNTs have been recognized as potential risk agents for use in bioterrorism (CBRN events).
Author Contributions
Conceptualization: I.A.C., M.A.P., M.M., L.S., F.I. and G.D.; methodology: G.D., F.I., A.D.I., F.L., A.E.W. and M.C.; validation: F.I., L.S., A.E.W., F.L., M.C., I.A.C. and A.D.I.; formal analysis: A.S.; M.M., F.L. and F.I.; investigation: G.D., F.L., A.D.I., L.S., F.I., M.M., M.A.P., G.D., M.C. and I.A.C.; data curation, L.S., M.M. and F.L.; writing—original draft preparation: M.C., F.L., I.A.C., L.S. and F.I.; writing—review and editing: F.I., F.L., A.D.I., G.D., I.A.C., A.E.W., M.A.P. and M.M.; All authors have read and agreed to the published version of the manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding
The authors declared no external funding for the present research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cruz-Morales, P.; Orellana, C.; Moutafis, G.; Moonen, G.; Rincon, G.; Nielsen, L.; Marcellin, E. Revisiting the Evolution and Taxonomy of Clostridia, a Phylogenomic Update. Genome Biol. Evol. 2019, 11, 2035–2044. [Google Scholar] [CrossRef] [Green Version]
- Liberato, V.; Benevenuti, C.; Coelho, F.; Botelho, A.; Amaral, P.; Pereira, J.N.; Ferreira, T. Clostridium sp. as Bio-Catalyst for Fuels and Chemicals Production in a Biorefinery Context. Catalysts 2019, 9, 962. [Google Scholar] [CrossRef] [Green Version]
- Popoff, M.R.; Bouvet, P. Genetic characteristics of toxigenic Clostridia and toxin gene evolution. Toxicon 2013, 75, 63–89. [Google Scholar] [CrossRef]
- Nigam, P.K.; Nigam, A. Botulinum toxin. Indian J. Dermatol. 2010, 55, 8–14. [Google Scholar] [CrossRef]
- Kobayashi, H.; Tanizawa, Y.; Sakamoto, M.; Ohkuma, M.; Tohno, M. Taxonomic status of the species Clostridium methoxyben-zovorans Mechichi et al. 1999. Int. J. Syst. Evol. Microbiol. 2021, 71, 004951. [Google Scholar] [CrossRef]
- Orrell, K.E.; Melnyk, R.A. Large Clostridial Toxins: Mechanisms and Roles in Disease. Microbiol. Mol. Biol. Rev. 2021, 85, e0006421. [Google Scholar] [CrossRef] [PubMed]
- UK Standards for Microbiology Investigations. Identification of Clostridium Species. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/504183/ID_8i4.1.pdf (accessed on 9 July 2021).
- Carter, A.T.; Peck, M. Genomes, neurotoxins and biology of Clostridium botulinum Group I and Group II. Res. Microbiol. 2015, 166, 303–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Clostridium Botulinum. Available online: https://www.who.int/csr/delibepidemics/clostridiumbotulism.pdf?ua=1 (accessed on 30 June 2021).
- Poulain, B.; Popoff, M.R. Why Are Botulinum Neurotoxin-Producing Bacteria So Diverse and Botulinum Neurotoxins So Toxic? Toxins 2019, 11, 34. [Google Scholar] [CrossRef] [Green Version]
- Lingwood, C. Therapeutic Uses of Bacterial Subunit Toxins. Toxins 2021, 13, 378. [Google Scholar] [CrossRef] [PubMed]
- Pellett, S. Learning from the past: Historical aspects of bacterial toxins as pharmaceuticals. Curr. Opin. Microbiol. 2012, 15, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.J.; AlShaker, S.; Hunter, D.G. Use of Botulinum Toxin in Ophthalmology. Botulinum Toxin Ther. 2019, 263, 147–160. [Google Scholar] [CrossRef]
- Satriyasa, B.K. Botulinum toxin (Botox) A for reducing the appearance of facial wrinkles: A literature review of clinical use and pharmacological aspect. Clin. Cosmet. Investig. Dermatol. 2019, 12, 223–228. [Google Scholar] [CrossRef] [Green Version]
- Nawrocki, S.; Cha, J. Botulinum toxin: Pharmacology and injectable administration for the treatment of primary hyperhi-drosis. J. Am. Acad. Dermatol. 2020, 82, 969–979. [Google Scholar] [CrossRef]
- Escher, C.M.; Paracka, L.; Dressler, D.; Kollewe, K. Botulinum toxin in the management of chronic migraine: Clinical evidence and experience. Ther. Adv. Neurol. Disord. 2017, 10, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Wein, T.; Esquenazi, A.; Jost, W.H.; Ward, A.B.; Pan, G.; Dimitrova, R. OnabotulinumtoxinA for the Treatment of Poststroke Distal Lower Limb Spasticity: A Randomized Trial. PM&R 2018, 10, 693–703. [Google Scholar] [CrossRef]
- Lunn, G.; Sansone, E.B. CAS Registry Number Index. Destruction of Hazardous Chemicals in the Laboratory; Wiley: Hoboken, NJ, USA, 2012; pp. 681–691. Available online: https://onlinelibrary.wiley.com/doi/pdf/10.1002/9781118146606.bindcas (accessed on 2 July 2021).
- Pirazzini, M.; Rossetto, O.; Eleopra, R.; Montecucco, C. Botulinum Neurotoxins: Biology, Pharmacology, and Toxicology. Pharmacol. Rev. 2017, 69, 200–235. [Google Scholar] [CrossRef]
- David, A.B.; Diamant, E.; Barnea, A.; Rosen, O.; Torgeman, A.; Zichel, R. The Receptor Binding Domain of Botulinum Neurotoxin Serotype A (BoNT/A) Inhibits BoNT/A and BoNT/E Intoxications In Vivo. Clin. Vaccine Immunol. 2013, 20, 1266–1273. [Google Scholar] [CrossRef] [Green Version]
- Rossetto, O.; Pirazzini, M.; Fabris, F.; Montecucco, C. Botulinum Neurotoxins: Mechanism of Action. Handb. Exp. Pharmacol. 2021, 263, 35–47. [Google Scholar] [CrossRef]
- Peck, M.W.; Smith, T.J.; Anniballi, F.; Austin, J.W.; Bano, L.; Bradshaw, M.; Cuervo, P.; Cheng, L.W.; Derman, Y.; Dorner, B.G.; et al. Historical Perspectives and Guidelines for Botulinum Neurotoxin Subtype Nomenclature. Toxins 2017, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Bintsis, T. Foodborne pathogens. AIMS Microbiol. 2017, 29, 529–563. [Google Scholar] [CrossRef]
- Sebaihia, M.; Peck, M.; Minton, N.; Thomson, N.; Holden, M.; Mitchell, W.J.; Carter, A.T.; Bentley, S.D.; Mason, D.R.; Crossman, L.; et al. Genome sequence of a proteolytic (Group I) Clostridium botulinum strain Hall A and comparative analysis of the clostridial genomes. Genome Res. 2007, 17, 1082–1092. [Google Scholar] [CrossRef] [Green Version]
- Nawrocki, E.M.; Bradshaw, M.; Johnson, E.A. Botulinum neurotoxin–encoding plasmids can be conjugatively transferred to diverse clostridial strains. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Raza, S.K.; Vijayaraghavan, R. Chemical warfare agents. J. Pharm. Bioallied Sci. 2010, 2, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.Y.; Burns, M.R.; Malaty, I.A. An Update on Botulinum Toxin in Neurology. Neurol Clin. 2021, 39, 209–229. [Google Scholar] [CrossRef]
- Ayyar, B.V.; Aoki, K.R.; Atassi, M.Z. The C-Terminal Heavy-Chain Domain of Botulinum Neurotoxin A Is Not the Only Site That Binds Neurons, as the N-Terminal Heavy-Chain Domain Also Plays a Very Active Role in Toxin-Cell Binding and Interactions. Infect. Immun. 2015, 83, 1465–1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasetti-Escargueil, C.; Lemichez, E.; Popoff, M.R. Toxemia in Human Naturally Acquired Botulism. Toxins 2020, 12, 716. [Google Scholar] [CrossRef]
- Rao, A.K.; Sobel, J.; Chatham-Stephens, K.; Luquez, C. Clinical Guidelines for Diagnosis and Treatment of Botulism, 2021. MMWR. Recomm. Rep. 2021, 70, 1–30. [Google Scholar] [CrossRef]
- Khakshoor, H.; Moghaddam, A.A.; Vejdani, A.H.; Armstrong, B.K.; Moshirfar, M. Diplopia as the primary presentation of foodborne botulism. Oman J. Ophthalmol. 2012, 5, 109–111. [Google Scholar] [CrossRef]
- Sobel, J. Botulism. Clin. Infect. Dis. 2005, 41, 1167–1173. [Google Scholar] [CrossRef]
- Food and Drugs Administration (FAD), USA. Chapter 13: Clostridium Botulinum Toxin Formation. Available online: https://www.fda.gov/files/food/published/Fish-and-Fishery-Products-Hazards-and-Controls-Guidance-Chapter-13-Download.pdf (accessed on 1 June 2021).
- Fernández, P.S.; Peck, M.W. A predictive model that describes the effect of prolonged heating at 70 to 90 degrees C and subsequent incubation at refrigeration temperatures on growth from spores and toxigenesis by nonproteolytic Clostridium botulinum in the presence of lysozyme. Appl. Environ. Microbiol. 1999, 65, 3449–3457. [Google Scholar] [CrossRef] [Green Version]
- Basta, M.; Annamaraju, P. Bacterial Spores; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK556071/ (accessed on 10 June 2021).
- Cagan, E.; Peker, E.; Dogan, M.; Caksen, H. Infant botulism. Eurasian J. Med. 2010, 42, 92–94. [Google Scholar] [CrossRef]
- Santacroce, L.; Man, A.; Charitos, I.A.; Haxhirexha, K.; Topi, S. Current knowledge about the connection between health status and gut microbiota from birth to elderly. A narrative review. Front. Biosci. 2021, 26, 135–148. [Google Scholar] [CrossRef]
- Harris, R.; Tchao, C.; Prystajecky, N.; Cutler, J.; Austin, J.W. A summary of surveillance, morbidity and microbiology of la-boratory-confirmed cases of infant botulism in Canada, 1979–2019. Can. Commun. Dis. Rep. 2021, 47, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Horn, N.L.V.; Street, M. Infantile Botulism; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Harris, R.A.; Anniballi, F.; Austin, J.W. Adult Intestinal Toxemia Botulism. Toxins 2020, 12, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeffery, I.A.; Karim, S. Botulism; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459273/ (accessed on 25 June 2021).
- Cenciarelli, O.; Riley, P.W.; Baka, A. Biosecurity Threat Posed by Botulinum Toxin. Toxins 2019, 11, 681. [Google Scholar] [CrossRef] [Green Version]
- Charitos, I.A.; Topi, S.; Castellaneta, F.; D’Agostino, D. Current Issues and Perspectives in Patients with Possible Sepsis at Emergency Departments. Antibiotics 2019, 8, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polimeno, L.; Lisanti, M.T.; Rossini, M.; Giacovazzo, E.; Polimeno, L.; Debellis, L.; Ballini, A.; Topi, S.; Santacroce, L. Anisakis Allergy: Is Aquacultured Fish a Safe and Alternative Food to Wild-Capture Fisheries for Anisakis sim-plex-Sensitized Patients? Biology 2021, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- Lindström, M.; Korkeala, H. Laboratory Diagnostics of Botulism. Clin. Microbiol. Rev. 2006, 19, 298–314. [Google Scholar] [CrossRef] [Green Version]
- Surveillance Atlas of Infectious Diseases. European Centre for Disease Prevention and Control (ECDC). Available online: http://atlas.ecdc.europa.eu/public/index.aspx?Dataset=27&FixDataset=1 (accessed on 20 June 2021).
- Centres for Disease Control and Prevention (CDC) USA. Botulism. Available online: https://www.cdc.gov/botulism/health-professional.html (accessed on 22 June 2021).
- California Poison Control System. Antidote Chart. Available online: https://calpoison.org/sites/pharm.ucsf.edu/files/calpoison/media-browser/antidote_chart.pdf (accessed on 1 June 2021).
- Brown, J.; Sutter, M.E.; Algren, D.A.; Thomas, J.D.; Ragone, S.; Schier, J.G.; Geller, R.J. The Role of a Poison Control Center in Identifying and Limiting an Outbreak of Foodborne Botulism. Am. J. Prev. Med. 2010, 38, 675–678. [Google Scholar] [CrossRef]
- Yu, P.A.; Lin, N.H.; Mahon, B.E.; Sobel, J.; Yu, Y.; Mody, R.K.; Gu, W.; Clements, J.; Kim, H.-J.; Rao, A.K. Safety and Improved Clinical Outcomes in Patients Treated With New Equine-Derived Heptavalent Botulinum Antitoxin. Clin. Infect. Dis. 2017, 66 (Suppl. S1), S57–S64. [Google Scholar] [CrossRef] [Green Version]
- McNutt, P.M.; Vazquez-Cintron, E.J.; Tenezaca, L.; Ondeck, C.A.; Kelly, K.E.; Mangkhalakhili, M.; Machamer, J.B.; Angeles, C.A.; Glotfelty, E.J.; Cika, J.; et al. Neuronal delivery of antibodies has therapeutic effects in animal models of botulism. Sci. Transl. Med. 2021, 13, eabd7789. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, S.-I.; Zhang, J.; Zhang, S.; Shoemaker, C.B.; Dong, M. Delivery of single-domain antibodies into neurons using a chimeric toxin–based platform is therapeutic in mouse models of botulism. Sci. Transl. Med. 2021, 13, eaaz4197. [Google Scholar] [CrossRef]
- Vazquez-Cintron, E.; Machamer, J.; Ondeck, C.; Pagarigan, K.; Winner, B.; Bodner, P.; Kelly, K.; Pennington, M.R.; McNutt, P. Symptomatic treatment of botulism with a clinically approved small molecule. JCI Insight 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Friggeri, A.; Marçon, F.; Marciniak, S.; Lemaire-Hurtel, A.-S.; Seydi, A.A.; Ammenouche, N.; Levrard, M.; Mahjoub, Y.; Airapetian, N.; Tinturier, F.; et al. 3,4-Diaminopyridine may improve neuromuscular block during botulism. Crit. Care 2013, 17, 449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Francisco, G.E. The Use of Botulinum Toxin for Treatment of Spasticity. Handb. Exp. Pharmacol. 2021, 263, 127–146. [Google Scholar] [CrossRef] [PubMed]
- Moga, M.A.; Dimienescu, O.G.; Bălan, A.; Scârneciu, I.; Barabaș, B.; Pleș, L. Therapeutic Approaches of Botulinum Toxin in Gynecology. Toxins 2018, 10, 169. [Google Scholar] [CrossRef] [Green Version]
- Moore, D.C.; Cohn, J.A.; Dmochowski, R.R. Use of Botulinum Toxin A in the Treatment of Lower Urinary Tract Disorders: A Review of the Literature. Toxins 2016, 8, 88. [Google Scholar] [CrossRef] [Green Version]
- Porter, R.F.; Gyawali, C.P. Botulinum toxin injection in dysphagia syndromes with preserved esophageal peristalsis and incomplete lower esophageal sphincter relaxation. Neurogastroenterol. Motil. 2011, 23, 139-e28. [Google Scholar] [CrossRef]
- Morrissey, D.; El-Khawand, D.; Ginzburg, N.; Wehbe, S.; O’Hare, P., III; Whitmore, K. Botulinum Toxin A Injections Into Pelvic Floor Muscles Under Electromyographic Guidance for Women With Refractory High-Tone Pelvic Floor Dysfunction: A 6-Month Prospective Pilot Study. Female Pelvic Med. Reconstr. Surg. 2015, 21, 277–282. [Google Scholar] [CrossRef]
- Rowe, F.J.; Noonan, C.P. Botulinum toxin for the treatment of strabismus. Cochrane Database Syst. Rev. 2017, 3, CD006499. [Google Scholar] [CrossRef] [PubMed]
- Stuart, M.E.; Strite, S.A.; Gillard, K.K. A systematic evidence-based review of treatments for primary hyperhidrosis. J. Drug Assess. 2021, 10, 35–50. [Google Scholar] [CrossRef]
- Sim, W.S. Application of Botulinum Toxin in Pain Management. Korean J. Pain 2011, 24, 1–6. [Google Scholar] [CrossRef]
- Anandan, C.; Jankovic, J. Botulinum Toxin in Movement Disorders: An Update. Toxins 2021, 13, 42. [Google Scholar] [CrossRef]
- Small, R. Botulinum toxin injection for facial wrinkles. Am. Fam. Physician 2014, 90, 168–175. [Google Scholar]
- Kumar, R. Therapeutic use of botulinum toxin in pain treatment. Neuronal Signal. 2018, 2, NS20180058. [Google Scholar] [CrossRef] [Green Version]
- Bakheit, A.M.O. The possible adverse effects of intramuscular botulinum toxin injections and their management. Curr. Drug Saf. 2006, 1, 271–279. [Google Scholar] [CrossRef]
- Bellows, S.; Jankovic, J. Immunogenicity Associated with Botulinum Toxin Treatment. Toxins 2019, 11, 491. [Google Scholar] [CrossRef] [Green Version]
- Padda, I.S.; Tadi, P. Botulinum Toxin; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557387/ (accessed on 1 June 2021).
- Naldi, L. Botulinum toxin adverse effects. Focus Farmacovigilanza. 2012, 73, 2. [Google Scholar]
- Charitos, I.A.; Gagliano-Candela, R.; Santacroce, L.; Bottalico, L. Venoms and poisonings during the centuries. A narrative review. Endocrine, Metab. Immune Disord. Drug Targets 2020, 20, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Bottalico, L.; Haxhirexha, K.; Topi, S.; Charitos, I.A. Pre-Chemistry Concepts and Medical Therapy among Ancient Physicians through the Pre-Socratic Philosophers. Endocrine, Metab. Immune Disord. Drug Targets 2020, 20, 1470–1477. [Google Scholar] [CrossRef] [PubMed]
- UNODA–the United Nations. Biological Weapons Convention. Available online: https://www.un.org/disarmament/biological-weapons (accessed on 12 June 2021).
- Aken, J.V.; Hammond, E. Genetic engineering and biological weapons. New technologies, desires and threats from bio-logical research. EMBO Rep. 2003, 4 (Suppl. S1), S57–S60. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.; Armstrong, L.; Sizemore, D.C. Biologic, Chemical, and Radiation Terrorism Review; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK493217/ (accessed on 30 June 2021).
- Dhaked, R.K.; Singh, M.K.; Singh, P.; Gupta, P. Botulinum toxin: Bioweapon & magic drug. Indian J. Med. Res. 2010, 132, 489–503. [Google Scholar] [PubMed]
- CDC. Bioterrorism Agents/Diseases. Available online: https://emergency.cdc.gov/agent/agentlist-category.asp (accessed on 2 June 2021).
- Charitos, I.A.; Ballini, A.; Cantore, S.; Boccellino, M.; Domenico, M.D.; Borsani, E.; Nocini, R.; Cosola, M.D.; Santacroce, L.; Bottalico, L. Stem Cells: A Historical Review about Biological, Religious, and Ethical Issues. Stem Cells Int. 2021, 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).