Cyclodextrins as Anti-inflammatory Agents: Basis, Drugs and Perspectives
Abstract
:1. Introduction
2. Inflammation
2.1. Basic Principles
2.2. Inflammatory Diseases
2.3. Anti-Inflammatory Drugs
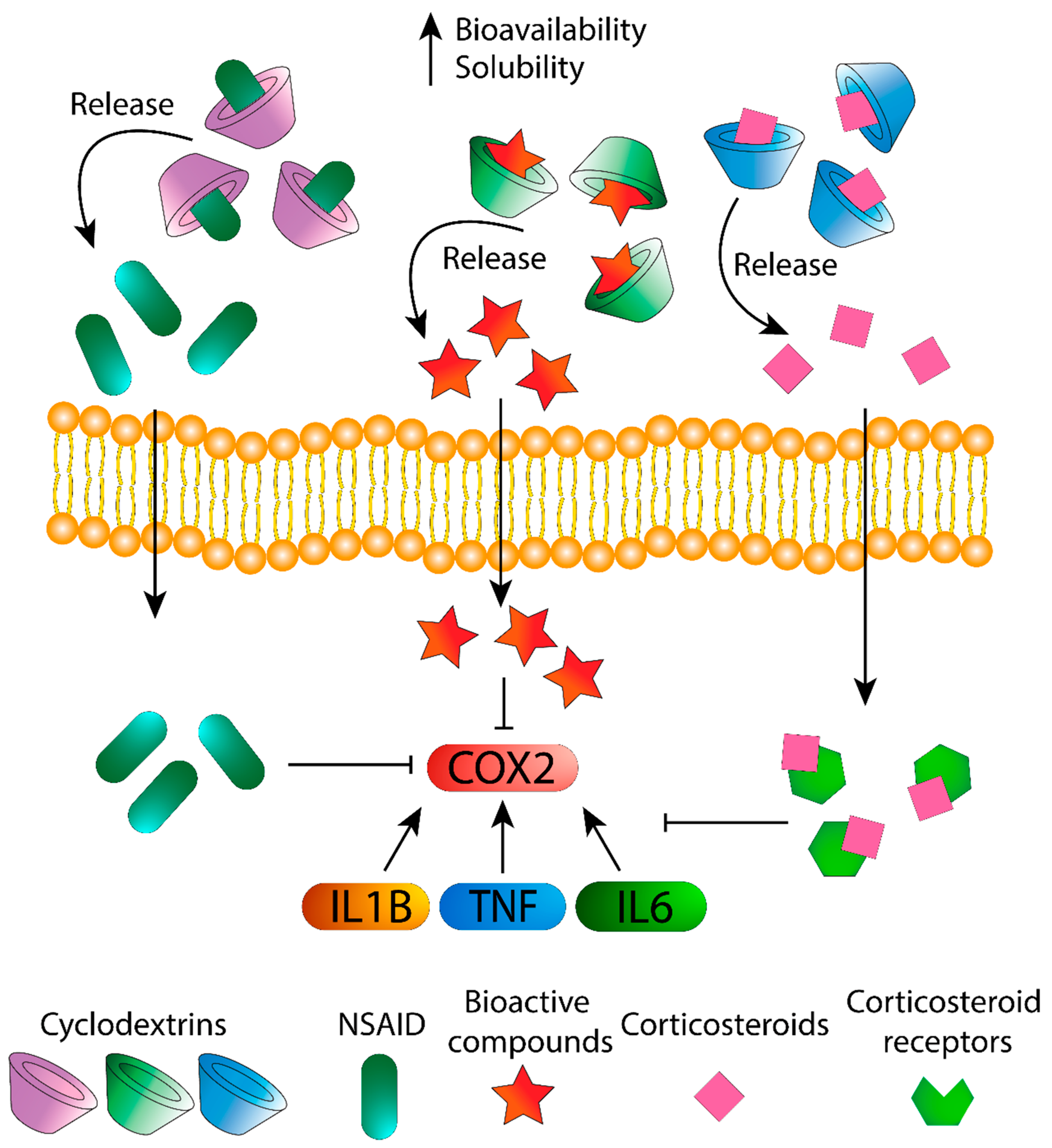
3. Cyclodextrins as Agents for Treatment
3.1. Drugs
3.2. Anti-Inflammatory Bioactive Compounds
| Type of CD | Bioactive Compound | Natural Source | Effect | Reference |
|---|---|---|---|---|
| HPβ-CD/PVP | Resveratrol | Wine, grapes and blackberries | Induced expression of inflammatory proteins, COX-2 and MMP-9 | [80] |
| HPβ-CD | Genistein | Soybeans | Down-regulate mRNA expression of anti-inflammatory cytokines | [82] |
| HPβ-, Mβ- and HPγ-CD | Curcumin | Tumeric | Treating inflammatory disorders | [84,85] |
| HPβ- and β-CD | Citral | Lemons and oranges | Reduced total leukocyte migration into the pleural cavity and TNF-α levels | [86] |
| HPβ-CD | Naringenin | Grapefruits and oranges | Reduced TNF- α levels | [87] |
| β-CD | Carvacrol | Oregano essential oil | Decrease level of IL-1β, IL-6, MIP-2 and TNF-α and higher of IL-10 | [88] |
| α-CD | Moringin | Moringa | Down-regulated pro-inflammatory cytokines TNF-α and IL-1β | [89] |
3.3. CD as Active Agents in Inflammatory Diseases
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CD | cyclodextrin |
| HPβ-CD | 2-hydroxypropyl β-cyclodextrin |
| HPγ-CD | 2-hydroxypropyl γ-cyclodextrin |
| Mβ-CD | methyl β-cyclodextrin |
| PVP | polyvinylpyrrolidone |
| PEG | polyethylene glycol |
| TNF-α | tumoral necrosis factor α |
| IL | interleukin |
| MPO | myeloperoxidase |
| MIP-2 | macrophage inflammatory protein |
| NF-κB | nuclear factor κB |
| LPS | Lipopolysaccharide |
| TLR | Toll-like receptor |
| PRRs | Pattern Recognition Receptor |
| CRP | C-reactive protein |
| NSAID | Nonsteroidal anti-inflammatory drug |
| LOX | Lipoxygenase |
| COX | Cyclooxygenase |
References
- Gregersen, P.K.; Behrens, T.W. Genetics of Autoimmune Diseases—Disorders of Immune Homeostasis. Nat. Rev. Genet. 2006, 7, 917–928. [Google Scholar] [CrossRef]
- Abdulkhaleq, L.A.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.H.; Hezmee, M.N.M. The Crucial Roles of Inflammatory Mediators in Inflammation: A Review. Vet. World 2018, 11, 627–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Hong, Y.; Huang, H. Triptolide Attenuates Inflammatory Response in Membranous Glomerulo-Nephritis Rat via Downregulation of NF-ΚB Signaling Pathway. Kidney Blood Press. Res. 2016, 41, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, Physicochemical Properties and Pharmaceutical Applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Brewster, M.E.; Loftsson, T. Cyclodextrins as Pharmaceutical Solubilizers. Adv. Drug Deliv. Rev. 2007, 59, 645–666. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.I.A.V.; Ribeiro, A.C.F.; Esteso, M.A. Drug Delivery Systems: Study of Inclusion Complex Formation between Methylxanthines and Cyclodextrins and Their Thermodynamic and Transport Properties. Biomolecules 2019, 9, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Matencio, A.; Navarro-Orcajada, S.; García-Carmona, F.; López-Nicolás, J.M. Applications of Cyclodextrins in Food Science. A Review. Trends Food Sci. Technol. 2020, 104, 132–143. [Google Scholar] [CrossRef]
- Matencio, A.; Bermejo-Gimeno, M.J.; García-Carmona, F.; López-Nicolás, J.M. Separating and Identifying the Four Stereoisomers of Methyl Jasmonate by RP-HPLC and Using Cyclodextrins in a Novel Way. Phytochem. Anal. 2017, 28, 151–158. [Google Scholar] [CrossRef]
- Matencio, A.; Caldera, F.; Pedrazzo, A.R.; Monfared, Y.K.; Dhakar, N.K.; Trotta, F. A Physicochemical, Thermodynamical, Structural and Computational Evaluation of Kynurenic Acid/Cyclodextrin Complexes. Food Chem. 2021, 356, 129639. [Google Scholar] [CrossRef]
- López-Nicolás, J.M.; García-Carmona, F. Effect of Hydroxypropyl-β-Cyclodextrin on the Aggregation of (E)-Resveratrol in Different Protonation States of the Guest Molecule. Food Chem. 2010, 118, 648–655. [Google Scholar] [CrossRef]
- Salazar, S.; Guerra, D.; Yutronic, N.; Jara, P. Removal of Aromatic Chlorinated Pesticides from Aqueous Solution Using β-Cyclodextrin Polymers Decorated with Fe3O4 Nanoparticles. Polymers 2018, 10, 1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krabicová, I.; Appleton, S.L.; Tannous, M.; Hoti, G.; Caldera, F.; Pedrazzo, A.R.; Cecone, C.; Cavalli, R.; Trotta, F. History of Cyclodextrin Nanosponges. Polymers 2020, 12, 1122. [Google Scholar] [CrossRef] [PubMed]
- Matencio, A.; Dhakar, N.K.; Bessone, F.; Musso, G.; Cavalli, R.; Dianzani, C.; García-Carmona, F.; López-Nicolás, J.M.; Trotta, F. Study of Oxyresveratrol Complexes with Insoluble Cyclodextrin Based Nanosponges: Developing a Novel Way to Obtain Their Complexation Constants and Application in an Anticancer Study. Carbohydr. Polym. 2020, 231, 115763. [Google Scholar] [CrossRef]
- Chovatiya, R.; Medzhitov, R. Stress, Inflammation, and Defense of Homeostasis. Mol. Cell 2014, 54, 281–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medzhitov, R. Origin and Physiological Roles of Inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Schonbein, G.W. Analysis of Inflammation. Annu. Rev. Biomed. Eng. 2006, 8, 93–131. [Google Scholar] [CrossRef] [Green Version]
- Ortega-Gomez, A.; Perretti, M.; Soehnlein, O. Resolution of Inflammation: An Integrated View. EMBO Mol. Med. 2013, 5, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, P.W.; Vaidya, S.A.; Cheng, G. The Art of War: Innate and Adaptive Immune Responses. Cell. Mol. Life Sci. 2003, 60, 2604–2621. [Google Scholar] [CrossRef]
- Silva, M.T. When Two Is Better than One: Macrophages and Neutrophils Work in Concert in Innate Immunity as Complementary and Cooperative Partners of a Myeloid Phagocyte System. J. Leukoc. Biol. 2010, 87, 93–106. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, A. Neutrophils: Cinderella of Innate Immune System. Int. Immunopharmacol. 2010, 10, 1325–1334. [Google Scholar] [CrossRef]
- Beutler, B. Innate Immunity: An Overview. Mol. Immunol. 2004, 40, 845–859. [Google Scholar] [CrossRef] [PubMed]
- Mayadas, T.N.; Cullere, X.; Lowell, C.A. The Multifaceted Functions of Neutrophils. Annu. Rev. Pathol. 2014, 9, 181–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huether, S.E.; McCance, K.L. Understanding Pathophysiology, 6th ed.; Elsevier: St. Louis, MO, USA, 2017; ISBN 9780323354097. [Google Scholar]
- Serhan, C.N.; Levy, B.D. Resolvins in Inflammation: Emergence of the pro-Resolving Superfamily of Mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef]
- Serhan, C.N. Pro-Resolving Lipid Mediators Are Leads for Resolution Physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Abbas, A.K.; Aster, J.C.; Perkins, J.A. Robbins Basic Pathology, 10th ed.; Elsevier: Philadelphia, PA, USA, 2018; ISBN 9780323353175. [Google Scholar]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Bennett, J.M.; Reeves, G.; Billman, G.E.; Sturmberg, J.P. Inflammation—Nature’s Way to Efficiently Respond to All Types of Challenges: Implications for Understanding and Managing “the Epidemic” of Chronic Diseases. Front. Med. 2018, 5, 316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GBD 2017 Causes of Death Collaborators. Global, Regional, and National Age-Sex-Specific Mortality for 282 Causes of Death in 195 Countries and Territories, 1980–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef] [Green Version]
- Taniguchi, K.; Karin, M. NF-κB, Inflammation, Immunity and Cancer: Coming of Age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef]
- Kazankov, K.; Jorgensen, S.M.D.; Thomsen, K.L.; Moller, H.J.; Vilstrup, H.; George, J.; Schuppan, D.; Gronbaek, H. The Role of Macrophages in Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 145–159. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation, Metaflammation and Immunometabolic Disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef]
- Jin, C.; Henao-Mejia, J.; Flavell, R.A. Innate Immune Receptors: Key Regulators of Metabolic Disease Progression. Cell Metab. 2013, 17, 873–882. [Google Scholar] [CrossRef] [Green Version]
- Ridker, P.M. A Test in Context: High-Sensitivity C-Reactive Protein. J. Am. Coll. Cardiol. 2016, 67, 712–723. [Google Scholar] [CrossRef]
- Emerging Risk Factors Collaboration. C-Reactive Protein Concentration and Risk of Coronary Heart Disease, Stroke, and Mortality: An Individual Participant Meta-Analysis. Lancet 2010, 375, 132–140. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Navarro, F.J.; Martinez-Menchon, T.; Mulero, V.; Galindo-Villegas, J. Models of Human Psoriasis: Zebrafish the Newly Appointed Player. Dev. Comp. Immunol. 2019, 97, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Navarro, F.J.; Martinez-Morcillo, F.J.; Lopez-Munoz, A.; Pardo-Sanchez, I.; Martinez-Menchon, T.; Corbalan-Velez, R.; Cayuela, M.L.; Perez-Oliva, A.B.; Garcia-Moreno, D.; Mulero, V. The Vitamin B6-Regulated Enzymes PYGL and G6PD Fuel NADPH Oxidases to Promote Skin Inflammation. Dev. Comp. Immunol. 2020, 108, 103666. [Google Scholar] [CrossRef] [PubMed]
- Patil, K.R.; Mahajan, U.B.; Unger, B.S.; Goyal, S.N.; Belemkar, S.; Surana, S.J.; Ojha, S.; Patil, C.R. Animal Models of Inflammation for Screening of Anti-Inflammatory Drugs: Implications for the Discovery and Development of Phytopharmaceuticals. Int. J. Mol. Sci. 2019, 20, 4367. [Google Scholar] [CrossRef] [Green Version]
- Kianmeher, M.; Ghorani, V.; Boskabady, M.H. Animal Model of Asthma, Various Methods and Measured Parameters: A Methodological Review. Iran. J. Allergy Asthma Immunol. 2016, 15, 445–465. [Google Scholar]
- Barnes, P.J. How Corticosteroids Control Inflammation: Quintiles Prize Lecture 2005. Br. J. Pharmacol. 2006, 148, 245–254. [Google Scholar] [CrossRef]
- Rice, J.B.; White, A.G.; Scarpati, L.M.; Wan, G.; Nelson, W.W. Long-Term Systemic Corticosteroid Exposure: A Systematic Literature Review. Clin. Ther. 2017, 39, 2216–2229. [Google Scholar] [CrossRef] [Green Version]
- Oyler, D.R.; Parli, S.E.; Bernard, A.C.; Chang, P.K.; Procter, L.D.; Harned, M.E. Nonopioid Management of Acute Pain Associated with Trauma: Focus on Pharmacologic Options. J. Trauma Acute Care Surg. 2015, 79, 475–483. [Google Scholar] [CrossRef]
- Shekelle, P.G.; Newberry, S.J.; FitzGerald, J.D.; Motala, A.; O’Hanlon, C.E.; Tariq, A.; Okunogbe, A.; Han, D.; Shanman, R. Management of Gout: A Systematic Review in Support of an American College of Physicians Clinical Practice Guideline. Ann. Intern. Med. 2017, 166, 37–51. [Google Scholar] [CrossRef] [Green Version]
- Dawood, M.Y. Primary Dysmenorrhea: Advances in Pathogenesis and Management. Obstet. Gynecol. 2006, 108, 428–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vane, J.R. Inhibition of Prostaglandin Synthesis as a Mechanism of Action for Aspirin-like Drugs. Nat. New Biol. 1971, 231, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Marcum, Z.A.; Hanlon, J.T. Recognizing the Risks of Chronic Nonsteroidal Anti-Inflammatory Drug Use in Older Adults. Ann. Longterm Care 2010, 18, 24–27. [Google Scholar]
- Campanati, A.; Giuliodori, K.; Ganzetti, G.; Liberati, G.; Offidani, A.M. A Patient with Psoriasis and Vitiligo Treated with Etanercept. Am. J. Clin. Dermatol. 2010, 11 (Suppl. 1), 46–48. [Google Scholar] [CrossRef]
- Ryan, C.; Korman, N.J.; Gelfand, J.M.; Lim, H.W.; Elmets, C.A.; Feldman, S.R.; Gottlieb, A.B.; Koo, J.Y.; Lebwohl, M.; Leonardi, C.L.; et al. Research Gaps in Psoriasis: Opportunities for Future Studies. J. Am. Acad. Dermatol. 2014, 70, 146–167. [Google Scholar] [CrossRef] [PubMed]
- Molinelli, E.; Campanati, A.; Ganzetti, G.; Offidani, A. Biologic Therapy in Immune Mediated Inflammatory Disease: Basic Science and Clinical Concepts. Curr. Drug Saf. 2016, 11, 35–43. [Google Scholar] [CrossRef]
- Otero-Espinar, F.J.; Torres-Labandeira, J.J.; Alvarez-Lorenzo, C.; Blanco-Méndez, J. Cyclodextrins in Drug Delivery Systems. J. Drug Deliv. Sci. Technol. 2010, 20, 289–301. [Google Scholar] [CrossRef]
- Crini, G. Review: A History of Cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef] [PubMed]
- Shende, P.K.; Gaud, R.S.; Bakal, R.; Patil, D. Effect of Inclusion Complexation of Meloxicam with β-Cyclodextrin- and β-Cyclodextrin-Based Nanosponges on Solubility, in Vitro Release and Stability Studies. Colloids Surf. B Biointerfaces 2015, 136, 105–110. [Google Scholar] [CrossRef]
- Santos, P.L.; Brito, R.G.; Quintans, J.S.S.; Araujo, A.A.S.; Menezes, I.R.A.; Brogden, N.K.; Quintans-Junior, L.J. Cyclodextrins as Complexation Agents to Improve the Anti-Inflammatory Drugs Profile: A Systematic Review and Meta-Analysis. Curr. Pharm. Des. 2017, 23, 2096–2107. [Google Scholar] [CrossRef] [PubMed]
- Miranda, G.M.; Bessa, J.R.; Teles, Y.C.F.; Cocou, S.; Alexandre, M.; Goncalves, M.S.; Ribeiro-filho, J. Inclusion Complexes of Non-Steroidal Anti-Inflammatory Drugs with Cyclodextrins: A Systematic Review. Biomolecules 2021, 11, 361. [Google Scholar] [CrossRef]
- Rein, S.M.T.; Lwin, W.W.; Tuntarawongsa, S.; Phaechamud, T. Meloxicam-Loaded Solvent Exchange-Induced in Situ Forming Beta-Cyclodextrin Gel and Microparticle for Periodontal Pocket Delivery. Mater. Sci. Eng. C 2020, 117, 111275. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, D.A.; Ernst, C.C.; Kramer, W.G.; Madden, D.; Lang, E.; Liao, E.; Lacouture, P.G.; Ramaiya, A.; Carr, D.B. Pharmacokinetics of Diclofenac and Hydroxypropyl-β-Cyclodextrin (HPβCD) Following Administration of Injectable HPβCD-Diclofenac in Subjects With Mild to Moderate Renal Insufficiency or Mild Hepatic Impairment. Clin. Pharmacol. Drug Dev. 2018, 7, 110–122. [Google Scholar] [CrossRef] [Green Version]
- Shinde, U.A.; Joshi, P.N.; Jain, D.D.; Singh, K. Preparation and Evaluation of N-Trimethyl Chitosan Nanoparticles of Flurbiprofen for Ocular Delivery. Curr. Eye Res. 2019, 44, 575–582. [Google Scholar] [CrossRef]
- Volkova, T.; Surov, A.; Terekhova, I. Metal–Organic Frameworks Based on β-Cyclodextrin: Design and Selective Entrapment of Non-Steroidal Anti-Inflammatory Drugs. J. Mater. Sci. 2020, 55, 13193–13205. [Google Scholar] [CrossRef]
- Mishra, M.; Chawla, V.; Chawla, P. Pectin, Beta-Cyclodextrin, Chitosan and Albumin Based Gastroprotective Systems for Piroxicam Maleate: Synthesis, Characterization and Biological Evaluation. Int. J. Biol. Macromol. 2019, 122, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Kaur, M.; Raza, K.; Thakur, K.; Katare, O.P. Aceclofenac-β-Cyclodextrin-Vesicles: A Dual Carrier Approach for Skin with Enhanced Stability, Efficacy and Dermatokinetic Profile. RSC Adv. 2016, 6, 20713–20727. [Google Scholar] [CrossRef]
- Maestrelli, F.; Mura, P.; Cirri, M.; Mennini, N.; Ghelardini, C.; Mannelli, L.D.C. Development and Characterization of Fast Dissolving Tablets of Oxaprozin Based on Hybrid Systems of the Drug with Cyclodextrins and Nanoclays. Int. J. Pharm. 2017, 531, 640–649. [Google Scholar] [CrossRef]
- Beech, E.; Rodwell, A.; Squires, M. Pharmaceutical Formulation Comprising NSAID and Cyclodextrin. U.S. Patent 9,138,482, 22 September 2015. [Google Scholar]
- Schwarz, D.H.; Engelke, A.; Wenz, G. Solubilizing Steroidal Drugs by β-Cyclodextrin Derivatives. Int. J. Pharm. 2017, 531, 559–567. [Google Scholar] [CrossRef]
- Tan, L.; Li, J.; Liu, Y.; Zhou, H.; Zhang, Z.; Deng, L. Synthesis and Characterization of β-Cyclodextrin-Conjugated Alginate Hydrogel for Controlled Release of Hydrocortisone Acetate in Response to Mechanical Stimulation. J. Bioact. Compat. Polym. 2015, 30, 584–599. [Google Scholar] [CrossRef]
- Kesavan, K.; Kant, S.; Singh, P.; Pandit, J.K. Effect of Hydroxypropyl-β-Cyclodextrin on the Ocular Bioavailability of Dexamethasone from a PH-Induced Mucoadhesive Hydrogel. Curr. Eye Res. 2011, 36, 918–929. [Google Scholar] [CrossRef]
- Hwang, J.; Rodgers, K.; Oliver, J.; Schluep, T. Alphamethylprednisolone Conjugated Cyclodextrin Polymer-Based Nanoparticles for Rheumatoid Arthritis Therapy. Int. J. Nanomed. 2008, 3, 359–371. [Google Scholar]
- Yano, H.; Hirayama, F.; Arima, H.; Uekama, K. Prednisolone-Appended Alpha-Cyclodextrin: Alleviation of Systemic Adverse Effect of Prednisolone after Intracolonic Administration in 2,4,6-Trinitrobenzenesulfonic Acid-Induced Colitis Rats. J. Pharm. Sci. 2001, 90, 2103–2112. [Google Scholar] [CrossRef]
- Yano, H.; Hirayama, F.; Kamada, M.; Arima, H.; Uekama, K. Colonspecific Delivery of Prednisolone-Appended Alpha-Cyclodextrin Conjugate: Alleviation of Systemic Side Effect after Oral Administration. J. Control. Release 2002, 79, 103–112. [Google Scholar] [CrossRef]
- Tanito, M.; Hara, K.; Takai, Y.; Matsuoka, Y.; Nishimura, N.; Jansook, P. Topical Dexamethasone-Cyclodextrin Microparticle Eye Drops for Diabetic Macular Edema. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7944–7948. [Google Scholar] [CrossRef]
- Saari, K.; Nelimarkka, L.; Ahola, V.; Loftsson, T.; Stefánsson, E. Comparison of Topical 0.7% Dexamethasone-Cyclodextrin with 0.1% Dexamethasone Sodium Phosphate for Postcataract Inflammation. Graefe’s Arch. Clin. Exp. Ophthalmol. 2006, 244, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Mura, P. Advantages of the Combined Use of Cyclodextrins and Nanocarriers in Drug Delivery: A Review. Int. J. Pharm. 2020, 579, 119181. [Google Scholar] [CrossRef] [PubMed]
- Appleton, S.L.; Tannous, M.; Rosa, A.C.; Argenziano, M.; Muntoni, E.; Rossi, D.; Caldera, F.; Scomparin, A.; Trotta, F.; Cavalli, R. Nanosponges as Protein Delivery Systems: Insulin, a Case Study. Int. J. Pharm. 2020, 590, 119888. [Google Scholar] [CrossRef] [PubMed]
- Trotta, F.; Zanetti, M.; Cavalli, R. Cyclodextrin-Based Nanosponges as Drug Carriers. Beilstein J. Org. Chem. 2012, 8, 2091–2099. [Google Scholar] [CrossRef]
- Cavalli, R.; Trotta, F.; Tumiatti, W. Cyclodextrin-Based Nanosponges for Drug Delivery. J. Incl. Phenom. Macrocycl. Chem. 2006, 56, 209–213. [Google Scholar] [CrossRef]
- Deshmukh, K.; Shende, P. Toluene Diisocyanate Cross-Linked β-Cyclodextrin Nanosponges as a PH-Sensitive Carrier for Naproxen. Mater. Res. Express 2018, 5, 075008. [Google Scholar] [CrossRef]
- Ferro, M.; Castiglione, F.; Pastori, N.; Punta, C.; Melone, L.; Panzeri, W.; Rossi, B.; Trotta, F.; Mele, A. Dynamics and Interactions of Ibuprofen in Cyclodextrin Nanosponges by Solid-State NMR Spectroscopy. Beilstein J. Org. Chem. 2017, 13, 182–194. [Google Scholar] [CrossRef]
- Ferro, M.; Castiglione, F.; Punta, C.; Melone, L.; Panzeri, W.; Rossi, B.; Trotta, F.; Mele, A. Anomalous Diffusion of Ibuprofen in Cyclodextrin Nanosponge Hydrogels: An HRMAS NMR Study. Beilstein J. Org. Chem. 2014, 10, 2715–2723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suvarna, V.; Gujar, P.; Murahari, M. Complexation of Phytochemicals with Cyclodextrin Derivatives—An Insight. Biomed. Pharmacother. 2017, 88, 1122–1144. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Hu, S.C.-S.; Huang, P.-H.; Lin, T.-C.; Yen, F.-L. Electrospun Resveratrol-Loaded Polyvinylpyrrolidone/Cyclodextrin Nanofibers and Their Biomedical Applications. Pharmaceutics 2020, 12, 552. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.R.I.; Preshaw, P.M.; Lim, L.P.; Ong, M.M.A.; Lin, H.-S.; Tan, K.S. Pterostilbene Complexed with Cyclodextrin Exerts Antimicrobial and Anti-Inflammatory Effects. Sci. Rep. 2020, 10, 9072. [Google Scholar] [CrossRef]
- Choi, D.J.; Cho, U.M.; Choi, D.H.; Hwang, H.S. Effects of Anti-inflammation and Skin Barrier by Genistein Cyclodextrin Complex. J. Soc. Cosmet. Sci. Korea 2018, 44, 171–181. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhong, L.; Wei, X.; Dou, W.; Chou, G.; Wang, Z. Baicalein and Hydroxypropyl-γ-Cyclodextrin Complex in Poloxamer Thermal Sensitive Hydrogel for Vaginal Administration. Int. J. Pharm. 2013, 454, 125–134. [Google Scholar] [CrossRef]
- Yadav, V.R.; Suresh, S.; Devi, K.; Yadav, S. Effect of Cyclodextrin Complexation of Curcumin on Its Solubility and Antiangiogenic and Anti-Inflammatory Activity in Rat Colitis Model. AAPS PharmSciTech 2009, 10, 752–762. [Google Scholar] [CrossRef] [Green Version]
- Neven, P.; Serteyn, D.; Delarge, J.; Scheer, E.; Kiss, R.; Mathieu, V.; Cataldo, D.; Rocks, N. Water Soluble Curcumin Compositions for Use in Anti-Cancer and Anti-Inflammatory Therapy. U.S. Patent No. 8,772,265, 8 July 2014. [Google Scholar]
- Campos, C.A.; Lima, B.S.; Trindade, G.G.G.; Souza, E.P.B.S.S.; Mota, D.S.A.; Heimfarth, L.; Quintans, J.S.S.; Quintans-Júnior, L.J.; Sussuchi, E.M.; Sarmento, V.H.V.; et al. Anti-Hyperalgesic and Anti-Inflammatory Effects of Citral with β-Cyclodextrin and Hydroxypropyl-β-Cyclodextrin Inclusion Complexes in Animal Models. Life Sci. 2019, 229, 139–148. [Google Scholar] [CrossRef]
- Gratieri, T.; Pinho, L.A.G.; Oliveira, M.A.; Sa-Barreto, L.L.; Marreto, R.N.; Silva, I.C.; Gelfuso, G.M.; Quintans, J.D.S.S.; Quintans-Junior, L.J.; Cunha-Filho, M. Hydroxypropyl-β-Cyclodextrin-Complexed Naringenin by Solvent Change Precipitation for Improving Anti-Inflammatory Effect in Vivo. Carbohydr. Polym. 2020, 231, 115769. [Google Scholar] [CrossRef]
- Souza, A.C.A.; Abreu, F.F.; Diniz, L.R.L.; Grespan, R.; DeSantana, J.M.; Quintans-Júnior, L.J.; Menezes, P.P.; Araújo, A.A.S.; Correa, C.B.; Teixeira, S.A.; et al. The Inclusion Complex of Carvacrol and β-Cyclodextrin Reduces Acute Skeletal Muscle Inflammation and Nociception in Rats. Pharmacol. Rep. 2018, 70, 1139–1145. [Google Scholar] [CrossRef]
- Giacoppo, S.; Rajan, T.S.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. The α-Cyclodextrin Complex of the Moringa Isothiocyanate Suppresses Lipopolysaccharide-Induced Inflammation in RAW 264.7 Macrophage Cells through Akt and P38 Inhibition. Inflamm. Res. 2017, 66, 487–503. [Google Scholar] [CrossRef] [PubMed]
- Bulani, V.D.; Kothavade, P.S.; Kundaikar, H.S.; Gawali, N.B.; Chowdhury, A.A.; Degani, M.S.; Juvekar, A.R. Inclusion Complex of Ellagic Acid with β-Cyclodextrin: Characterization and In Vitro Anti-Inflammatory Evaluation. J. Mol. Struct. 2016, 1105, 308–315. [Google Scholar] [CrossRef]
- Matencio, A.; Navarro-Orcajada, S.; Garcia-Carmona, F.; López-Nicolás, J.M. Ellagic Acid-Borax Fluorescence Interaction. Application to a Novel Cyclodextrin-Borax Nanosensor for Analyzing Ellagic Acid in Food Samples. Food Funct. 2018, 9, 3683–3687. [Google Scholar] [CrossRef] [PubMed]
- Vidal, P.J.; López-Nicolás, J.M.; Gandía-Herrero, F.; García-Carmona, F. Inactivation of Lipoxygenase and Cyclooxygenase by Natural Betalains and Semi-Synthetic Analogues. Food Chem. 2014, 154, 246–254. [Google Scholar] [CrossRef]
- Matencio, A.; Guerrero-Rubio, M.A.; Gandía-Herrero, F.; García-Carmona, F.; López-Nicolás, J.M. Nanoparticles of Betalamic Acid Derivatives with Cyclodextrins. Physicochemistry, Production Characterization and Stability. Food Hydrocoll. 2020, 106176. [Google Scholar] [CrossRef]
- da Silva, S.A.V.; Clemente, A.; Rocha, J.; Direito, R.; Marques, H.C.; Sepodes, B.; Figueira, M.-E.; Ribeiro, M.H. Anti-Inflammatory Effect of Limonin from Cyclodextrin (Un)Processed Orange Juices in in Vivo Acute Inflammation and Chronic Rheumatoid Arthritis Models. J. Funct. Foods 2018, 49, 146–153. [Google Scholar] [CrossRef]
- Kim, M.; Choi, S.-Y.; Lee, P.; Hur, J. Neochlorogenic Acid Inhibits Lipopolysaccharide-Induced Activation and Pro-Inflammatory Responses in BV2 Microglial Cells. Neurochem. Res. 2015, 40, 1792–1798. [Google Scholar] [CrossRef]
- Navarro-Orcajada, S.; Matencio, A.; Vicente-Herrero, C.; García-Carmona, F.; López-Nicolás, J.M. Study of the Fluorescence and Interaction between Cyclodextrins and Neochlorogenic Acid, in Comparison with Chlorogenic Acid. Sci. Rep. 2021, 11, 3275. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.G.; Oliveira, M.A.; Alves, R.D.S.; Menezes, P.D.P.; Serafini, M.R.; de Souza Araújo, A.A.; Bezerra, D.P.; Júnior, L.J.Q. Encapsulation of Carvacrol, a Monoterpene Present in the Essential Oil of Oregano, with β-Cyclodextrin, Improves the Pharmacological Response on Cancer Pain Experimental Protocols. Chem. Biol. Interact. 2015, 227, 69–76. [Google Scholar] [CrossRef]
- Quintans, J.D.S.S.; Menezes, P.P.; Santos, M.R.V.; Bonjardim, L.R.; Almeida, J.R.G.S.; Gelain, D.P.; Araújo, A.A.D.S.; Quintans-Júnior, L.J. Improvement of P-Cymene Antinociceptive and Anti-Inflammatory Effects by Inclusion in β-Cyclodextrin. Phytomedicine 2013, 20, 436–440. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Zhang, W.; Yang, H.; Sun, W.; Gong, X.; Zhao, J.; Sun, Y.; Diao, G. A Water-Soluble Inclusion Complex of Pedunculoside with the Polymer β-Cyclodextrin: A Novel Anti-Inflammation Agent with Low Toxicity. PLoS ONE 2014, 9, e101761. [Google Scholar] [CrossRef] [PubMed]
- Quintans-Júnior, L.J.; Barreto, R.S.S.; Menezes, P.P.; Almeida, J.R.G.S.; Viana, A.F.S.C.; Oliveira, R.C.M.; Oliveira, A.P.; Gelain, D.P.; Júnior, W.D.L.; Araújo, A.A.S. β-Cyclodextrin-Complexed (−)-Linalool Produces Antinociceptive Effect Superior to That of (−)-Linalool in Experimental Pain Protocols. Basic Clin. Pharmacol. Toxicol. 2013, 113, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, S.S.; Camargo, E.A.; DeSantana, J.M.; Araújo, A.A.S.; Menezes, P.P.; Lucca-Júnior, W.; Albuquerque-Júnior, R.L.C.; Bonjardim, L.R.; Quintans-Júnior, L.J. Linalool and Linalool Complexed in β-Cyclodextrin Produce Anti-Hyperalgesic Activity and Increase Fos Protein Expression in Animal Model for Fibromyalgia. Naunyn-Schmiedebergs Arch. Pharmacol. 2014, 387, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Hădărugăa, D.I.; Hădărugăa, N.G.; Miclea, L.M.; Vlaia, L.; Mircioiu, C. Bioactive Compounds (Hepatoprotective or Anti-Inflammatory Xenobiotics)/Cyclodextrin Nanoparticles: A Comparative Study. J. Agroaliment. Process. Technol. 2009, 15, 478–483. [Google Scholar]
- Bianchi, S.E.; Machado, B.E.K.; da Silva, M.G.C.; da Silva, M.M.A.; Bosco, L.D.; Marques, M.S.; Horn, A.P.; Persich, L.; Geller, F.C.; Argenta, D.; et al. Coumestrol/Hydroxypropyl-β-Cyclodextrin Association Incorporated in Hydroxypropyl Methylcellulose Hydrogel Exhibits Wound Healing Effect: In Vitro and in Vivo Study. Eur. J. Pharm. Sci. 2018, 119, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Sawatdee, S.; Choochuay, K.; Chanthorn, W.; Srichana, T. Evaluation of the Topical Spray Containing Centella asiatica Extract and Its Efficacy on Excision Wounds in Rats. Acta Pharm. 2016, 66, 233–244. [Google Scholar] [CrossRef] [Green Version]
- Rode, T.; Frauen, M.; Müller, B.W.; Düsing, H.J.; Schönrock, U.; Mundt, C.; Wenck, H. Complex Formation of Sericoside with Hydrophilic Cyclodextrins: Improvement of Solubility and Skin Penetration in Topical Emulsion Based Formulations. Eur. J. Pharm. Biopharm. 2003, 55, 191–198. [Google Scholar] [CrossRef]
- Rimbach, G.; Fischer, A.; Schloesser, A.; Jerz, G.; Ikuta, N.; Ishida, Y.; Matsuzawa, R.; Matsugo, S.; Huebbe, P.; Terao, K. Anti-Inflammatory Properties of Brazilian Green Propolis Encapsulated in a γ-Cyclodextrin Complex in Mice Fed a Western-Type Diet. Int. J. Mol. Sci. 2017, 18, 1141. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, L.B.; Martins, A.O.B.P.B.; Ribeiro-Filho, J.; Cesário, F.R.A.S.; e Castro, F.F.; de Albuquerque, T.R.; Fernandes, M.N.M.; da Silva, B.A.F.; Júnior, L.J.Q.; Araújo, A.A.D.S.; et al. Anti-Inflammatory Activity of the Essential Oil Obtained from Ocimum Basilicum Complexed with β-Cyclodextrin (β-CD) in Mice. Food Chem. Toxicol. 2017, 109, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, J.G.D.O.; Tavares, E.D.A.; da Silva, S.S.; Silva, J.F.; de Carvalho, Y.M.B.G.; Ferreira, M.R.A.; Araújo, A.A.D.S.; Barbosa, E.G.; Pedrosa, M.D.F.F.; Soares, L.A.L.; et al. Inclusion Complexes of Copaiba (Copaifera Multijuga Hayne) Oleoresin and Cyclodextrins: Physicochemical Characterization and Anti-Inflammatory Activity. Int. J. Mol. Sci. 2017, 18, 2388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonglairoum, P.; Chuchote, T.; Ngawhirunpat, T.; Rojanarata, T.; Opanasopit, P. Encapsulation of Plai Oil/2-Hydroxypropyl-β-Cyclodextrin Inclusion Complexes in Polyvinylpyrrolidone (PVP) Electrospun Nanofibers for Topical Application. Pharm. Dev. Technol. 2014, 19, 430–437. [Google Scholar] [CrossRef]
- Matencio, A.; Navarro-Orcajada, S.; González-Ramón, A.; García-Carmona, F.; López-Nicolás, J.M. Recent Advances in the Treatment of Niemann Pick Disease Type C: A Mini-Review. Int. J. Pharm. 2020, 584, 119440. [Google Scholar] [CrossRef]
- Matencio, A.; Caldera, F.; Cecone, C.; López-Nicolás, J.M.; Trotta, F. Cyclic Oligosaccharides as Active Drugs, an Updated Review. Pharmaceuticals 2020, 13, 281. [Google Scholar] [CrossRef]
- Barros, M.C.F.; Ribeiro, A.C.F.; Esteso, M.A. Cyclodextrins in Parkinson’s Disease. Biomolecules 2019, 9, 3. [Google Scholar] [CrossRef] [Green Version]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and Atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef]
- Kritharides, L.; Kus, M.; Brown, A.J.; Jessup, W.; Dean, R.T. Hydroxypropyl-β-Cyclodextrin-Mediated Efflux of 7-Ketocholesterol from Macrophage Foam Cells. J. Biol. Chem. 1996, 271, 27450–27455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmer, S.; Grebe, A.; Bakke, S.S.; Bode, N.; Halvorsen, B.; Ulas, T.; Skjelland, M.; De Nardo, D.; Labzin, L.I.; Kerksiek, A.; et al. Cyclodextrin Promotes Atherosclerosis Regression via Macrophage Reprogramming. Sci. Transl. Med. 2016, 8, 333ra50. [Google Scholar] [CrossRef] [Green Version]
- Bakke, S.S.; Aune, M.H.; Niyonzima, N.; Pilely, K.; Ryan, L.; Skjelland, M.; Garred, P.; Aukrust, P.; Halvorsen, B.; Latz, E.; et al. Cyclodextrin Reduces Cholesterol Crystal–Induced Inflammation by Modulating Complement Activation. J. Immunol. 2017, 199, 2910–2920. [Google Scholar] [CrossRef] [Green Version]
- Pilely, K.; Bakke, S.S.; Palarasah, Y.; Skjoedt, M.-O.; Bartels, E.D.; Espevik, T.; Garred, P. Alpha-Cyclodextrin Inhibits Cholesterol Crystal-Induced Complement-Mediated Inflammation: A Potential New Compound for Treatment of Atherosclerosis. Atherosclerosis 2019, 283, 35–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carneiro, S.B.; Costa Duarte, F.Í.; Heimfarth, L.; Siqueira Quintans, J.D.S.; Quintans-Júnior, L.J.; Veiga Júnior, V.F.D.; Neves de Lima, Á.A. Cyclodextrin–Drug Inclusion Complexes: In Vivo and In Vitro Approaches. Int. J. Mol. Sci. 2019, 20, 642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, P.S.S.; Lucchese, A.M.; Araújo-Filho, H.G.; Menezes, P.P.; Araújo, A.A.S.; Quintans-Júnior, L.J.; Quintans, J.S.S. Inclusion of Terpenes in Cyclodextrins: Preparation, Characterization and Pharmacological Approaches. Carbohydr. Polym. 2016, 151, 965–987. [Google Scholar] [CrossRef]
- Matencio, A.; Hoti, G.; Monfared, Y.K.; Rezayat, A.; Pedrazzo, A.R.; Caldera, F.; Trotta, F. Cyclodextrin Monomers and Polymers for Drug Activity Enhancement. Polymers 2021, 13, 1684. [Google Scholar] [CrossRef] [PubMed]
| Type of CD | NSAID | Objective of the study | Reference |
|---|---|---|---|
| β-CD | Meloxicam | In vitro evaluation for periodontitis treatment | [56] |
| HP-β-CD | Diclofenac | Clinical evaluation of the pharmacokinetics of diclofenac in patients with mild or moderate renal insufficiency or mild hepatic impairment | [57] |
| HP-β-CD | Flurbiprofen | In vitro drug release, mucoadhesion, and irritation potential study for ocular delivery | [58] |
| β-CD | Ibuprofen | In vitro evaluation of ibuprofen properties in metal organic frameworks | [59] |
| β-CD | Piroxicam | In vivo evaluation of the analgesic activity and anti-ulcerogenic potential of piroxicam in rats | [60] |
| β-CD | Aceclofenac | Ex vivo evaluation of stability and transdermal delivery to the inflammatory sites in osteoarthritis | [61] |
| β-CD | Oxaprozin | In vivo evaluation of the anti-inflammatory activity on adjuvant-induced arthritis in rats | [62] |
| Type of CD | Steroid | Objective of the Study | Reference |
|---|---|---|---|
| β-CD | Hydrocortisone acetate | Determination of the anti-inflammatory effect on LPS-stimulated RAW267 | [65] |
| HPβ-CD | Dexamethasone | Determination of the anti-inflammatory effect on rabbits affected by uveitis | [66] |
| γ-CD | Dexamethasone | Clinical assessment of the anti-inflammatory effect on diabetic macular edema and cataract | [70,71] |
| HPβ-CD | Dexamethasone | Clinical assessment of the anti-inflammatory effect on diabetic macular edema and cataract | [70,71] |
| α-CD | Prednisolone 21-hemisuccinate | Determination of the anti-inflammatory effect on rats with inflammatory bowel disease | [68,69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucia Appleton, S.; Navarro-Orcajada, S.; Martínez-Navarro, F.J.; Caldera, F.; López-Nicolás, J.M.; Trotta, F.; Matencio, A. Cyclodextrins as Anti-inflammatory Agents: Basis, Drugs and Perspectives. Biomolecules 2021, 11, 1384. https://doi.org/10.3390/biom11091384
Lucia Appleton S, Navarro-Orcajada S, Martínez-Navarro FJ, Caldera F, López-Nicolás JM, Trotta F, Matencio A. Cyclodextrins as Anti-inflammatory Agents: Basis, Drugs and Perspectives. Biomolecules. 2021; 11(9):1384. https://doi.org/10.3390/biom11091384
Chicago/Turabian StyleLucia Appleton, Silvia, Silvia Navarro-Orcajada, Francisco Juan Martínez-Navarro, Fabrizio Caldera, José Manuel López-Nicolás, Francesco Trotta, and Adrián Matencio. 2021. "Cyclodextrins as Anti-inflammatory Agents: Basis, Drugs and Perspectives" Biomolecules 11, no. 9: 1384. https://doi.org/10.3390/biom11091384
APA StyleLucia Appleton, S., Navarro-Orcajada, S., Martínez-Navarro, F. J., Caldera, F., López-Nicolás, J. M., Trotta, F., & Matencio, A. (2021). Cyclodextrins as Anti-inflammatory Agents: Basis, Drugs and Perspectives. Biomolecules, 11(9), 1384. https://doi.org/10.3390/biom11091384










