Influence of Selected Substrate Dosage on the Process of Biogas Installation Start-Up in Real Conditions
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
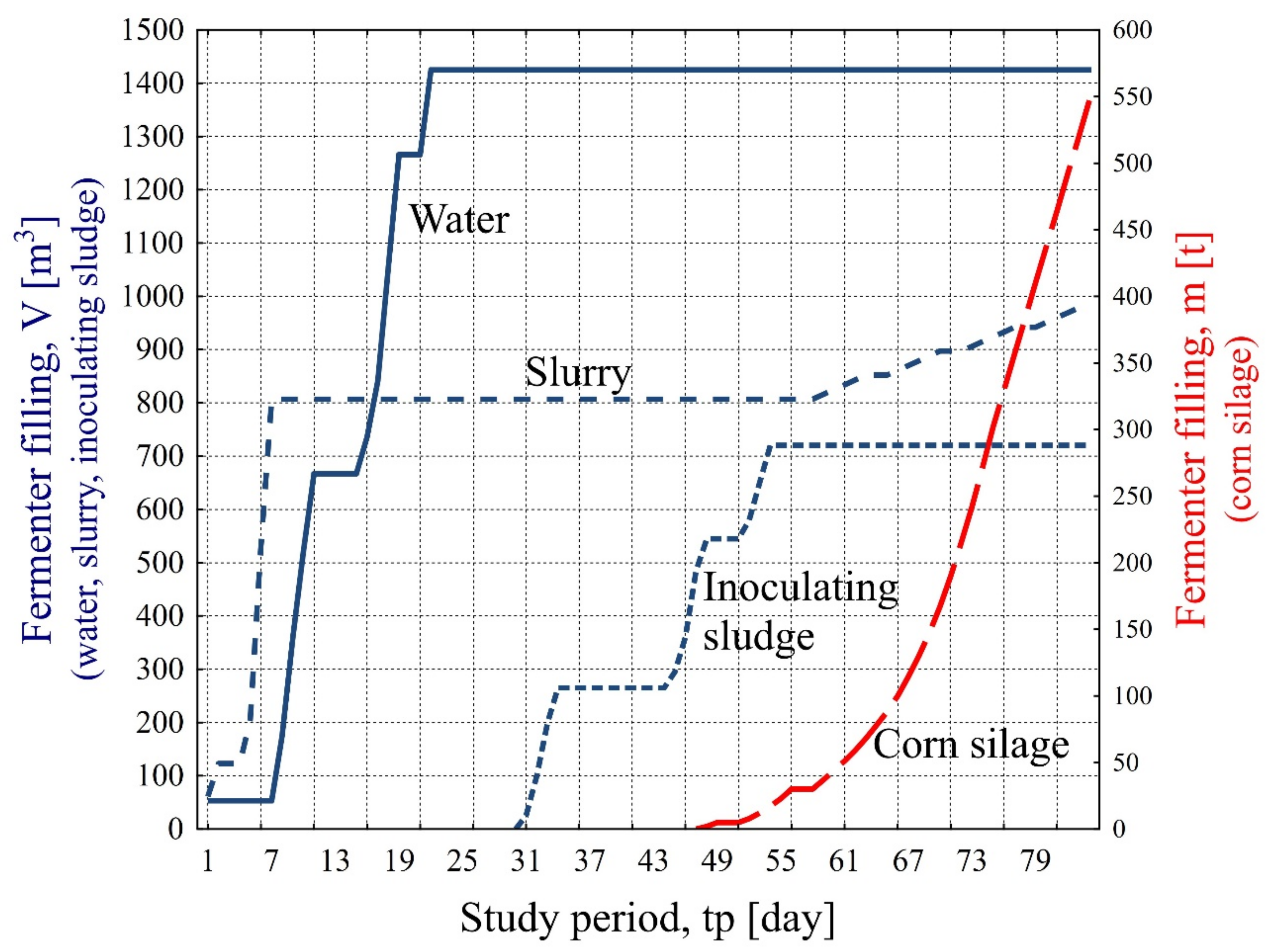
- The dosage of substrates in the form of corn silage as a source of protein and carbohydrates, providing a nutrient for methanogenic bacteria located in the inoculated sludge, results in the acceleration of the fermentation process and faster achievement of proper biogas parameters.
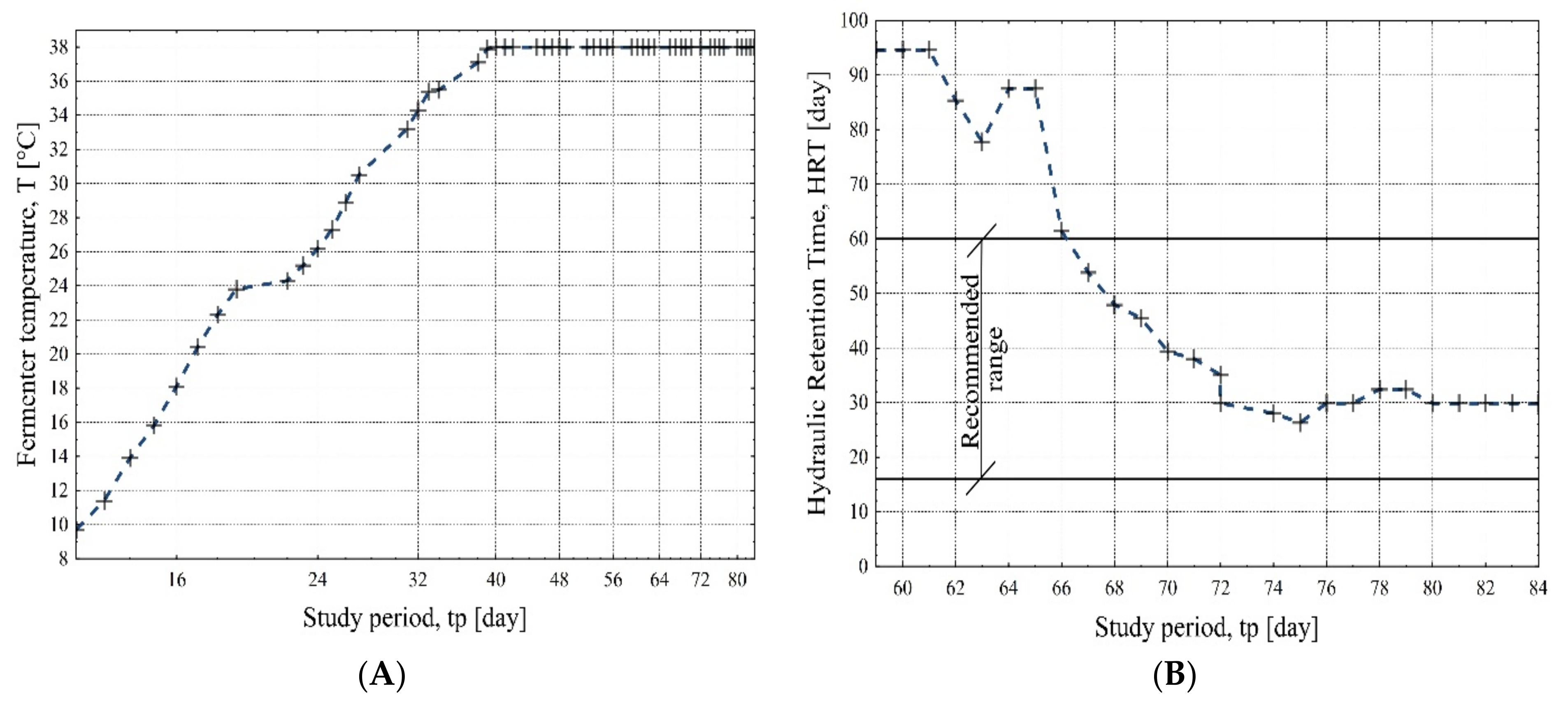
- The recommended reactor temperature of 38 °C for mesophilic conditions was safely reached on day 38 of the biogas plant start-up.
- On day 59 after the start of the biogas plant operation, the methane content increased to a maximum of about 62%. Finally, after about 80 days, the methane content stabilized at a constant level of about 55%.
- Carbon dioxide content increased from about 6% (day 32) to about 46% (day 84), with a clear linear correlation between carbon dioxide and methane content.
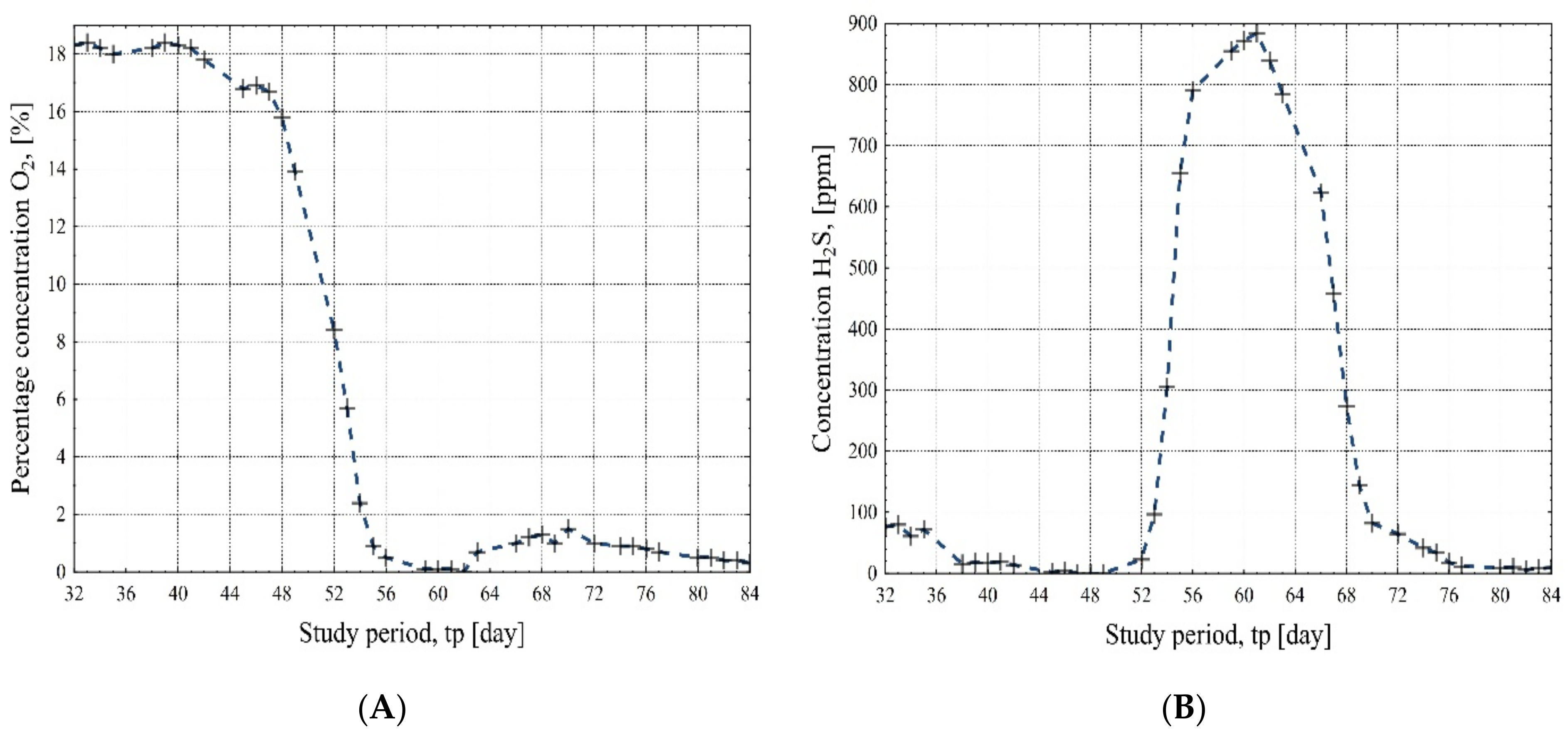
- The oxygen content decreased from about 18% (day 32) to about 0.3% (day 84) as the resulting gases displaced air from the reactor, and there was also a linear correlation between oxygen and methane content.
- The hydrogen sulfide content decreased from about 76 ppm (day 32) to about 0 ppm (day 47), after which, in a clear power correlation to the methane content, it maximally increased to 890 ppm (day 61). However, for the sake of safe engine operation, the desulfurization plant was started on day 63, which resulted in a hydrogen sulfide concentration below 50 ppm on day 74 of the experiment. The final hydrogen sulfide content was 9 ppm on day 84 of the biogas plant start-up.
- As the mass of added corn silage increased, so did the fermentation mass index value, expressed as the VFA/TIC ratio. The adjustment of the corn silage dosage from 31 t/d to 27 t/d, applied on the 76th day after the start-up of the biogas plant, resulted in stabilization of the VFA/TIC value at a constant level of about 0.35.
- In order to guarantee the correct operation of the biogas plant, a comprehensive monitoring system is required, which will ensure increased efficiency and sustainable operation of the reactors through information about the parameter values of the resulting variables.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmad, S.; Razzaq, S.; Rehan, M.A.; Hashmi, M.A.; Ali, S.; Amjad, M.S.; Mehmood, U. Experimental Investigation of Novel Fixed Dome Type Biogas Plant using Gas Recovery Chamber in Rural Areas of Pakistan. Int. J. Renew. Energy Res. 2019, 9, 1537–1547. [Google Scholar]
- Csikos, N.; Schwanebeck, M.; Kuhwald, M.; Szilassi, P.; Duttmann, R. Density of Biogas Power Plants as An Indicator of Bioenergy Generat-ed Transformation of Agricultural Landscapes. Sustainability 2019, 9, 11. [Google Scholar]
- Kilic, M.K.; Abut, N.; Kilic, F.C.; Eyidoğan, M.; Kaya, D. An examination of measuring and control systems in an industrial biogas plant in turkey. Int. J. Ecosyst. Ecol. Sci. 2019, 9, 11–16. [Google Scholar] [CrossRef]
- Kogut, P.; Piekarski, J.; Dąbrowski, T.; Kaczmarek, F. Biogas Production Plants as a Method of Utilisation of Sewage Sludge in Relation to the Polish Legislation. Rocz. Ochr. Sr. 2012, 14, 534–545. [Google Scholar]
- Yu, Q.; Li, H.; Deng, Z.; Liao, X.; Liu, X.; Liu, J. Comparative assessment on two full-scale food waste treatment plants with different anaer-obic digestion processes. J. Clean. Prod. 2020, 263, 121625. [Google Scholar] [CrossRef]
- Kogut, P.; Piekarski, J.; Ignatowicz, K. Start-up of Biogas Plant with Inoculating Sludge Application. Rocz. Ochr. Sr. 2014, 16, 534–545. [Google Scholar]
- Refai, S. Development of Efficient Tools for Monitoring and Improvement of Biogas Production. Ph.D. Thesis, Universitäts-und Landesbibliothek Bonn, Bonn, Germany, 2016. [Google Scholar]
- Sarker, S.; Lamb, J.J.; Hjelme, D.R.; Lien, K.M. A Review of the Role of Critical Parameters in the Design and Operation of Biogas Production Plants. Appl. Sci. 2019, 9, 1915. [Google Scholar] [CrossRef]
- Stolze, Y.; Bremges, A.; Maus, I.; Pühler, A.; Sczyrba, A.; Schlüter, A. Targeted in situ metatranscriptomics for selected taxa from mesophilic and thermophilic biogas plants. Microb. Biotechnol. 2017, 11, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Heerenklage, J.; Rechtenbach, D.; Atamaniuk, I.; Alassali, A.; Raga, R.; Koch, K.; Kuchta, K. Development of a method to produce standardised and storable inocula for biomethane potential tests-Preliminary steps. Renew. Energy 2019, 143, 753–761. [Google Scholar] [CrossRef]
- Jarrar, L.; Ayadi, O.; Al, A.J. Techno-economic Aspects of Electricity Generation from a Farm Based Biogas Plant. J. Sustain. Dev. Energy Water Environ. Syst. 2020, 8, 476–492. [Google Scholar] [CrossRef]
- Ervasti, S.; Vainio, M.; Tampio, E. Use of local resources as co-substrates in a farm-scale biogas plant. Open Agric. 2019, 4, 650–660. [Google Scholar] [CrossRef]
- Uddin, M.E.; Larson, R.A.; Wattiaux, M.A.E. Efects of dairy cow breed and dietary forage on greenhouse gas emissions from manure during storage and after field application. J. Clean. Prod. 2020, 270, 122–461. [Google Scholar] [CrossRef]
- Wattiaux, M.A.; Uddin, M.E.; Letelier, P.; Jackson, R.D.; Larson, R.A. Invited Review: Emission and mitigation of greenhouse gases from dairy farms: The cow, the manure, and the field. Appl. Anim. Sci. 2019, 35, 238–254. [Google Scholar] [CrossRef]
- Xue, B.; Wang, L.; Yan, T. Methane emission inventories for enteric fermentation and manure management of yak, buffalo and dairy and beef cattle in China from 1988 to 2009. Agric. Ecosyst. Environ. 2014, 195, 202–210. [Google Scholar] [CrossRef]
- Ignatowicz, K. Occurrence Study of Agro-Chemical Pollutants in Waters of Supraśl Catchment. Arch. Environ. Prot. 2009, 35, 69–77. [Google Scholar]
- Piñas, J.A.V.; Venturini, O.J.; Silva Lora, E.E.; del Olmo, O.A.; Calle Roalcaba, O.D. An economic holistic feasibility assessment of centralized and decentralized biogas plants with mono-digestion and co-digestion systems. Renew. Energy 2019, 139, 40–51. [Google Scholar] [CrossRef]
- Lenort, R.; Stas, D.; Wicher, P.; Holman, D.; Ignatowicz, K. Comparative Study of Sustainable Key Performance Indicators in Metallurgical Industry. Rocz. Ochr. Sr. 2017, 19, 36–51. [Google Scholar]
- Wang, Y.; Zhang, W.; Dong, H.; Zhu, Z.; Li, B. Performance Evaluation of a Large-Scale Swine Manure Mesophilic Biogas Plant in China. Trans. ASABE 2017, 60, 1713–1720. [Google Scholar] [CrossRef]
- Puchlik, M.; Ignatowicz, K.; Dąbrowski, W. Influence of bio-preparation on wastewater purification process in constructed wetlands. J. Ecol. Eng. 2015, 16, 159–163. [Google Scholar] [CrossRef]
- Appels, L.; Lauwers, J.; Degrève, J.; Helsen, L.; Lievens, B.; Willems, K.A.; Van Impe, J.; Dewil, R. Anaerobic digestion in global bio-energy production: Potential and research challenges. Renew. Sustain. Energy Rev. 2011, 15, 4295–4301. [Google Scholar] [CrossRef]
- Ibrahim, U.M.; Ahmed, S.I.; Gutti, B.; Muhammad, I.M.; Hamza, U.D.; Ibrahim, M.D. Biogas Production from Combined Irish Potato and Poultry Wastes: Optimization and Kinetic Studies. Int. J. Eng. Technol. 2018, 7, 170–176. [Google Scholar] [CrossRef][Green Version]
- Han, Y.; Green, H.; Tao, W. Reversibility of propionic acid inhibition to anaerobic digestion: Inhibition kinetics and micro-bial mechanism. Chemosphere 2020, 255, 126840. [Google Scholar] [CrossRef]
- Nsair, A.; Onen Cinar, S.; Alassali, A.; Abu Qdais, H.; Kuchta, K. Operational Parameters of Biogas Plants: A Review and Evaluation Study. Energies 2020, 13, 3761. [Google Scholar] [CrossRef]
- Gattermann, H.; Kaltschmitt, M.; Niebaum, A.; Schattauer, A.; Scholwin, F.; Weiland, P. Biogaserzeugung und-Nutzung; Institut fur Energetik und Umwelt gGMBH: Leipzig, Germany, 2006. [Google Scholar]
- Jin, Y.; Wen, J.; Nie, Y.; Chen, H.; Wang, G. Biomass-biogas recycling technique studies of municipal food waste disposal. Rocz. Ochr. Środowiska 2012, 14, 21–55. [Google Scholar]
- Piekutin, J.; Puchlik, M.; Haczykowski, M.; Dyczewska, K. The Efficiency of the Biogas Plant Operation Depending on the Substrate Used. Energies 2021, 14, 3157. [Google Scholar] [CrossRef]
- Lemmer, A.; Naegele, H.-J.; Sondermann, J. How Efficient are Agitators in Biogas Digesters? Determination of the Efficiency of Submersible Motor Mixers and Incline Agitators by Measuring Nutrient Distribution in Full-Scale Agricultural Biogas Digesters. Energies 2013, 6, 6255–6273. [Google Scholar] [CrossRef]
- Weiland, P. Biogas production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2009, 85, 849–860. [Google Scholar] [CrossRef]
- Wiedemann, L.; Conti, F.; Janus, T.; Sonnleitner, M.; Zörner, W.; Goldbrunner, M. Mixing in Biogas Digesters and Development of an Artificial Substrate for Laboratory-Scale Mixing Optimization. Chem. Eng. Technol. 2016, 40, 238–247. [Google Scholar] [CrossRef]
- Last, S. The Anaerobic Digestion Biofuels Blog. Available online: https://blog.anaerobic-digestion.com/digester-cleaning-services/ (accessed on 28 January 2019).
- Nsair, A.; Cinar, S.Ö.; Abu-Qdais, H.; Kuchta, K. Optimizing the performance of a large-scale biogas plant by controlling stirring process: A case study. Energy Convers. Manag. 2019, 198, 111931. [Google Scholar] [CrossRef]
- Frey, J.; Grüssing, F.; Nägele, H.-J.; Oechsner, H. Cutting the electric power consumption of biogas plants: The impact of new technologies. Landtechnik. Agric. Eng. 2013, 68, 58–63. [Google Scholar]
- Aboudi, K.; Álvarez-Gallego, C.J.; García, L.I.R. Semi-continuous anaerobic co-digestion of sugar beet byproduct and pig manure: Effect of the organic loading rate (OLR) on process performance. Bioresour. Technol. 2015, 194, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Dhar, H.; Kumar, P.; Kumar, S.; Mukherjee, S.; Vaidya, A.N. Effect of organic loading rate during anaerobic digestion of municipal solid waste. Bioresour. Technol. 2016, 217, 56–61. [Google Scholar] [CrossRef]
- Żyłka, R.; Dąbrowski, W.; Malinowski, P.; Karolinczak, B. Modeling of Electric Energy Consumption during Dairy Wastewater Treatment Plant Operation. Energies 2020, 13, 3769. [Google Scholar] [CrossRef]
- Bindergroup Measurement & Control. Available online: http://www.bindergroup.info/binder/downloads/EN/BIDE-M-D-COMBIMASS-GA-m-EN-R08-Datenblatt-COMBIMASS-GA-m.pdf (accessed on 16 September 2021).
- Alternative Energies Solution. Available online: http://www.alternative-energies.fr/site/wp-content/uploads/2012/07/FOS-TAC-2000-EN.pdf (accessed on 16 September 2021).
- Jabłoński, S.; Rodowicz, P.; Łukaszewicz, M.; Ski, S.J.J.O.; Lukaszewicz, M. Methanogenic archaea database containing physiological and biochemical characteristics. Int. J. Syst. Evol. Microbiol. 2015, 65, 1360–1368. [Google Scholar] [CrossRef] [PubMed]
- Mondal, C.; Biswas, G.K. Effect of Temperature on Kinetic Constants in Anaerobic Bio-digestion. Chitkara Chem. Rev. 2013, 1, 19–28. [Google Scholar] [CrossRef]
- Adekunle, K.F.; Okolie, J.A. A Review of Biochemical Process of Anaerobic Digestion. Adv. Biosci. Biotechnol. 2015, 6, 205–212. [Google Scholar] [CrossRef]
- Hans, B. Enzyme Kinetics Principles and Methods; Wiley Vch Valag: Weinheim, Germany, 2008. [Google Scholar]
- Caballero-Arzápalo, N. Untersuchungen zum Anaeroben Abbauprozess Ausgewählter Abfallsubstrate mit Hilfe Spezieller Mikroorganismen und Enzyme; Technische Universität München: Munich, Germany, 2015. [Google Scholar]
- Li, C.; Champagne, P.; Anderson, B.C. Biogas production performance of mesophilic and thermophilic anaerobic co-digestion with fat, oil, and grease in semi-continuous flow digesters: Effects of temperature, hydraulic retention time, and organic loading rate. Environ. Technol. 2013, 34, 2125–2133. [Google Scholar] [CrossRef]
- Kaosol, T.; Sohgrathok, N. Influence of Hydraulic Retention Time on Biogas Production from Frozen Seafood Wastewater Using Decanter Cake as Anaerobic Co-digestion Material. Int. J. Environ. Ecol. Eng. 2012, 6, 303–307. [Google Scholar]
- Dareioti, M.A.; Kornaros, M. Anaerobic mesophilic co-digestion of ensiled sorghum, cheese whey and liquid cow manure in a two-stage CSTR system: Effect of hydraulic retention time. Bioresour. Technol. 2015, 175, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Dareioti, M.A.; Kornaros, M. Effect of hydraulic retention time (HRT) on the anaerobic co-digestion of agro-industrial wastes in a two-stage CSTR system. Bioresour. Technol. 2014, 167, 407–415. [Google Scholar] [CrossRef]
- Schmidt, T.; Ziganshin, A.M.; Nikolausz, M.; Scholwin, F.; Nelles, M.; Kleinsteuber, S.; Pröter, J. Effects of the reduction of the hydraulic retention time to 1.5 days at constant organic loading in CSTR, ASBR, and fixed-bed reactors—Performance and methanogenic community composition. Biomass Bioenergy 2014, 69, 241–248. [Google Scholar] [CrossRef]
- Shi, X.-S.; Dong, J.-J.; Yu, J.-H.; Yin, H.; Hu, S.-M.; Huang, S.-X.; Yuan, X.-Z. Effect of Hydraulic Retention Time on Anaerobic Digestion of Wheat Straw in the Semicontinuous Continuous Stirred-Tank Reactors. BioMed Res. Int. 2017, 2017, 2457805. [Google Scholar] [CrossRef] [PubMed]
- Botheju, D. Oxygen Effects in Anaerobic Digestion—A Review. Open Waste Manag. J. 2011, 4, 1–19. [Google Scholar] [CrossRef]
- Rohstoffe, F.N. Guide to Biogas from Production to Use; Federal Ministry of Food, Agriculture and Consumer Protection: Gülzow, Germany; Fachagentur Nachwachsende Rohstoffe e. V. (FNR): Gülzow, Germany, 2012.
- Ioelovich, M. Recent findings and the energetic potential of plant biomass as a renewable source of biofuels—A review. Bio. Resour. 2015, 10, 1879–1914. [Google Scholar]
- Ahring, B.K.; Sandberg, M.; Angelidaki, I. Volatile fatty acids as indicators of process imbalance in anaerobic digestors. Appl. Microbiol. Biotechnol. 1995, 43, 559–565. [Google Scholar] [CrossRef]
- Agrahari, R.P.; Tiwari, G.N. The Production of Biogas Using Kitchen Waste. Int. J. Energy Sci. 2013, 3, 408. [Google Scholar] [CrossRef]
- Achinas, S.; Achinas, V.; Euverink, G.J.W. A Technological Overview of Biogas Production from Biowaste. BioRixv 2017, 3, 299–307. [Google Scholar] [CrossRef]
- Zhang, C.; Su, H.; Baeyens, J.; Tan, T. Reviewing the anaerobic digestion of food waste for biogas production. Renew. Sustain. Energy Rev. 2014, 38, 383–392. [Google Scholar] [CrossRef]
- Voß, E. Prozessanalyse und Optimierung von Landwirtschaftlichen Biogasanlagen. Ph.D. Thesis, Institut für Siedlungswasserwirtschaft und Abfalltechnik, Hanover, Germany, 2015. [Google Scholar]
- Hamzah, M.A.F.; Jahim, J.M.; Abdul, P.M.; Asis, A.J. Investigation of Temperature Effect on Start-Up Operation from Anaerobic Digestion of Acidified Palm Oil Mill Effluent. Energies 2019, 12, 2473. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ignatowicz, K.; Piekarski, J.; Kogut, P. Influence of Selected Substrate Dosage on the Process of Biogas Installation Start-Up in Real Conditions. Energies 2021, 14, 5948. https://doi.org/10.3390/en14185948
Ignatowicz K, Piekarski J, Kogut P. Influence of Selected Substrate Dosage on the Process of Biogas Installation Start-Up in Real Conditions. Energies. 2021; 14(18):5948. https://doi.org/10.3390/en14185948
Chicago/Turabian StyleIgnatowicz, Katarzyna, Jacek Piekarski, and Paweł Kogut. 2021. "Influence of Selected Substrate Dosage on the Process of Biogas Installation Start-Up in Real Conditions" Energies 14, no. 18: 5948. https://doi.org/10.3390/en14185948
APA StyleIgnatowicz, K., Piekarski, J., & Kogut, P. (2021). Influence of Selected Substrate Dosage on the Process of Biogas Installation Start-Up in Real Conditions. Energies, 14(18), 5948. https://doi.org/10.3390/en14185948





