Development of Patient Specific Conformal 3D-Printed Devices for Dose Verification in Radiotherapy
Abstract
1. Introduction
2. Materials and Methods
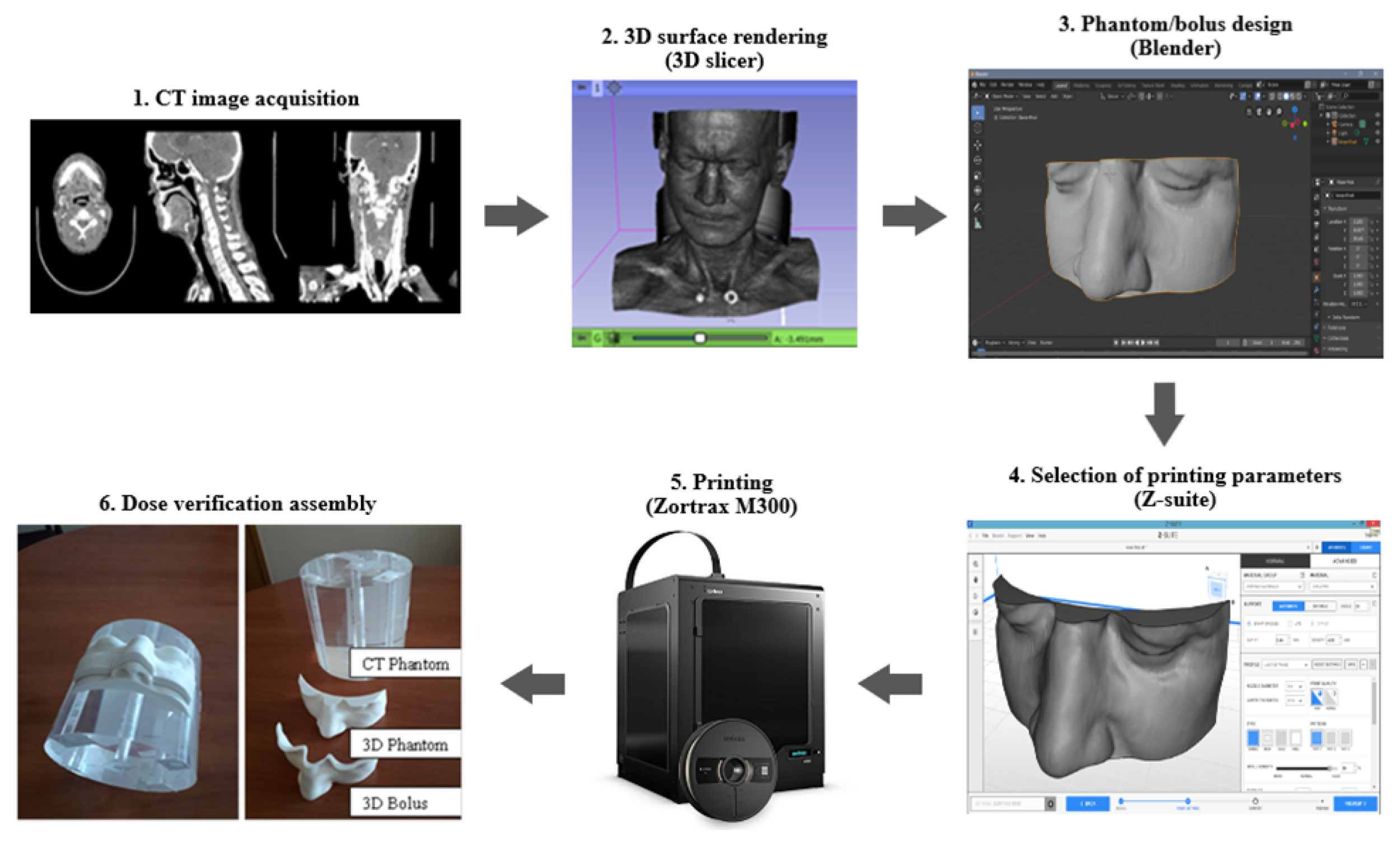
2.1. Development of Personalized 3D Devices
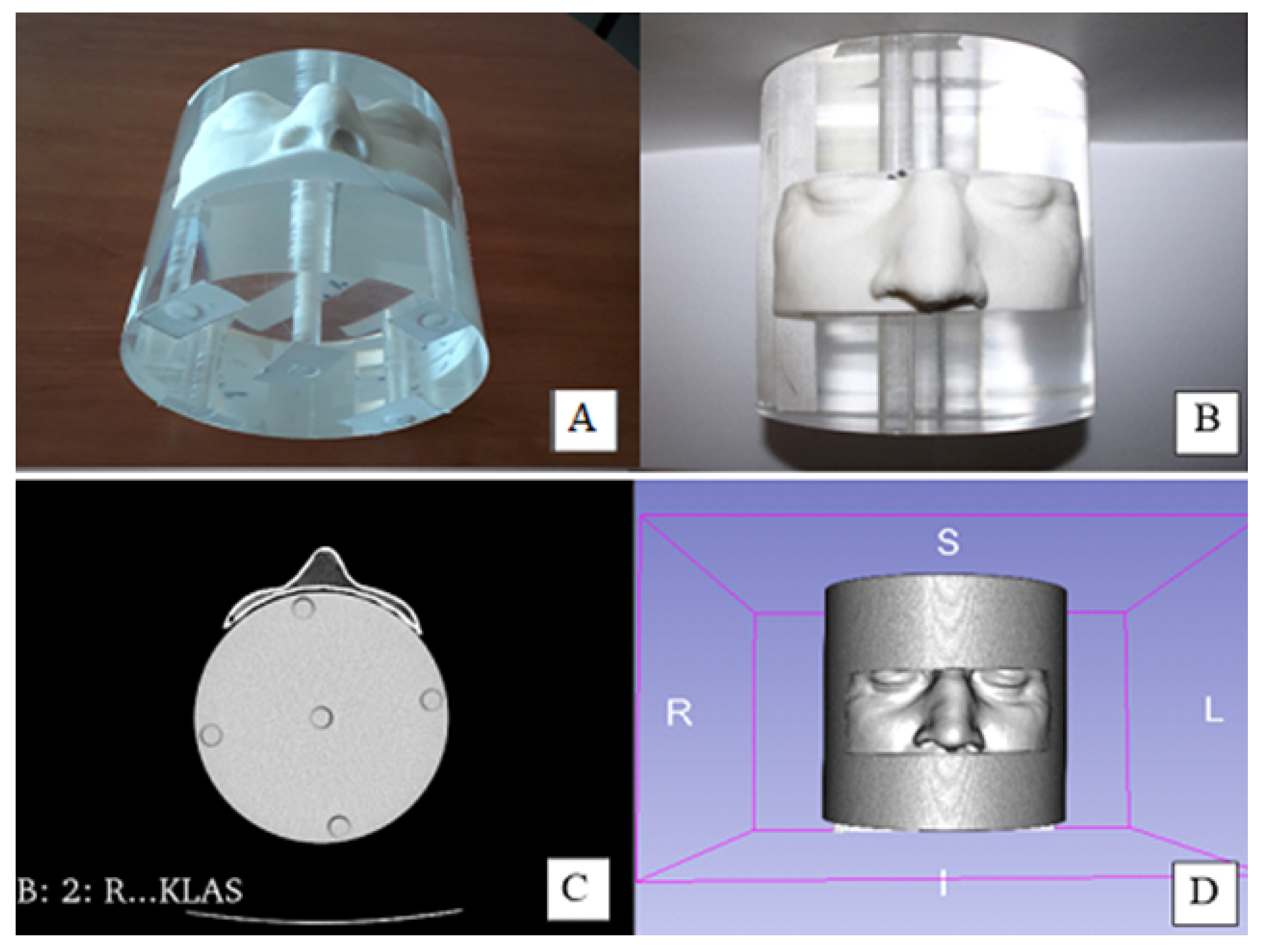
2.2. Physical Characterization of 3D-Printed Objects
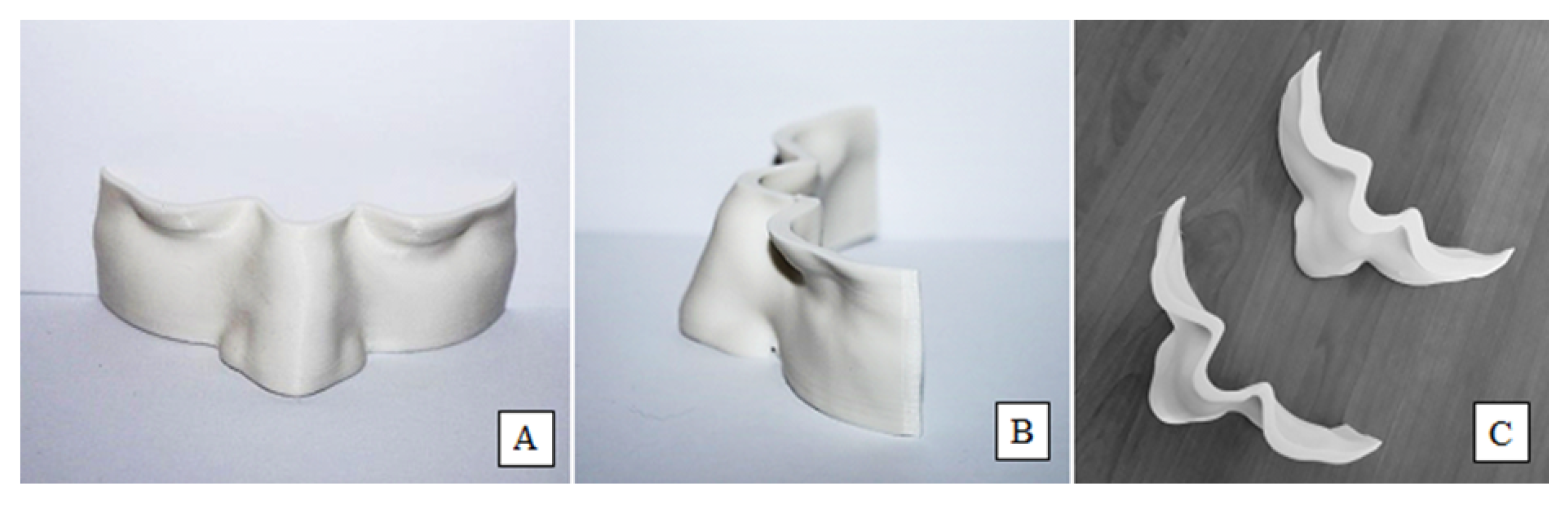
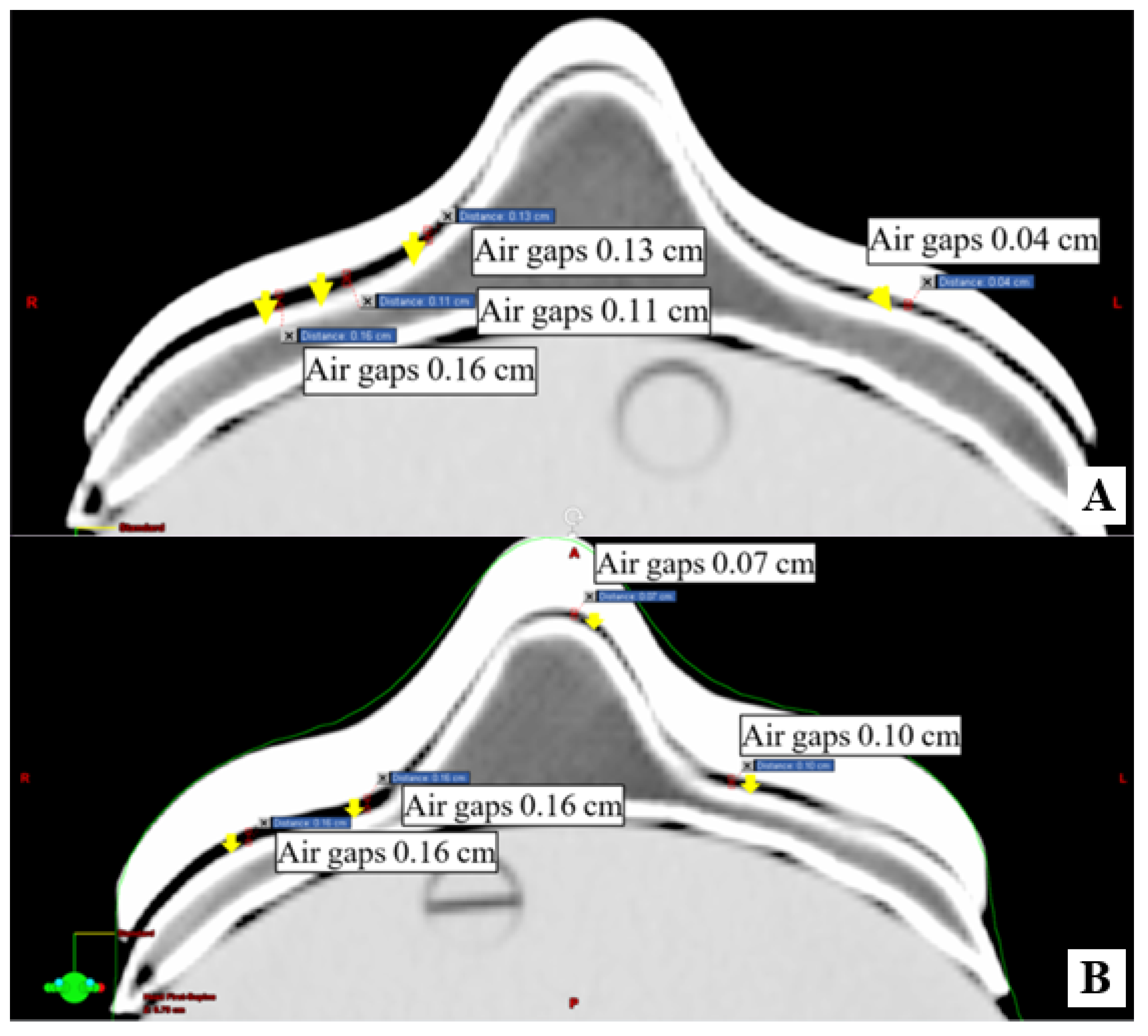
2.3. Conformity of 3D-Printed Boluses
2.4. Dosimetric Evaluation
3. Results and Discussion
3.1. Physical Characterization of 3D Printed Objects
3.2. Conformity of 3D-Printed Bolus
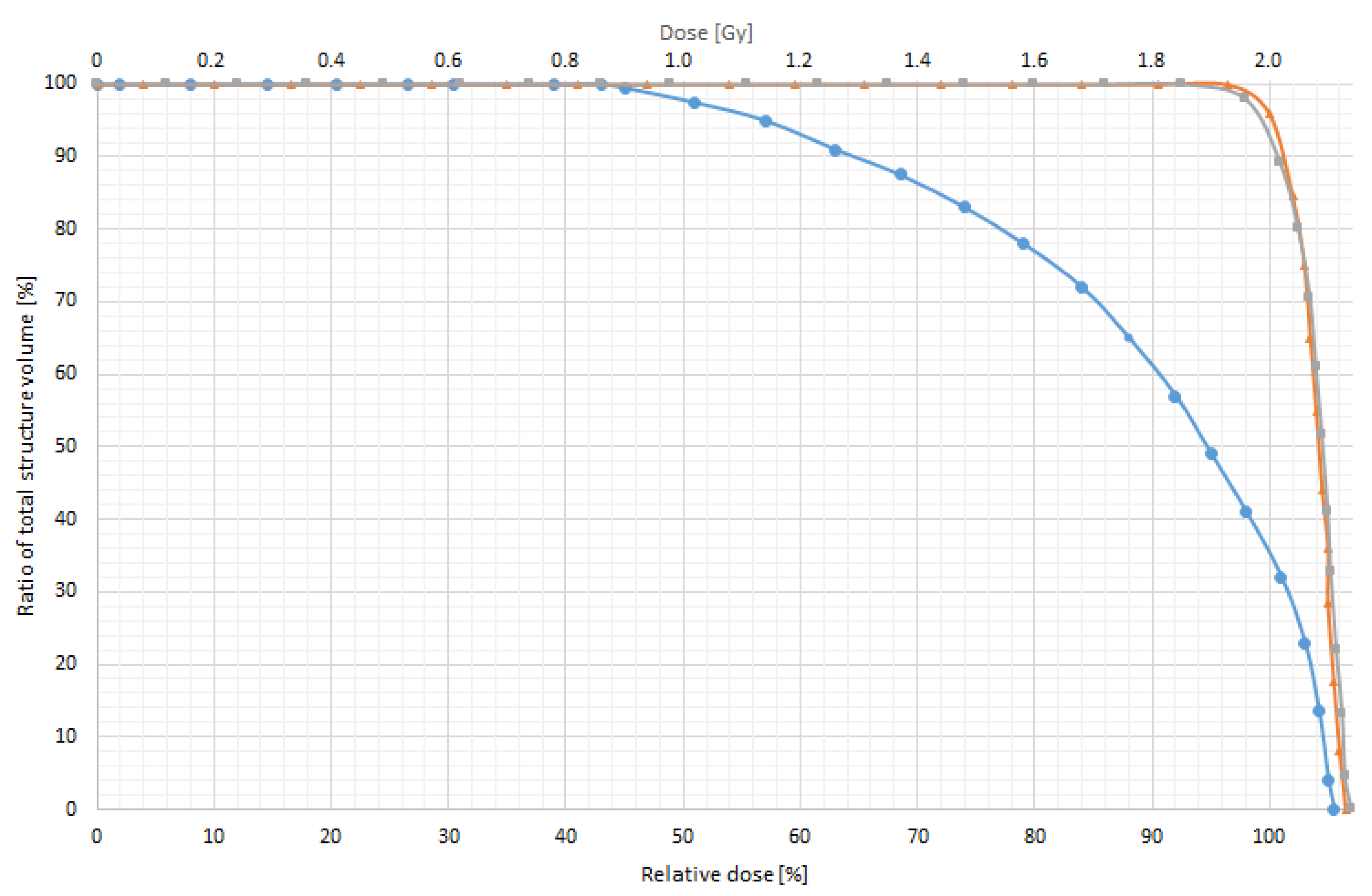
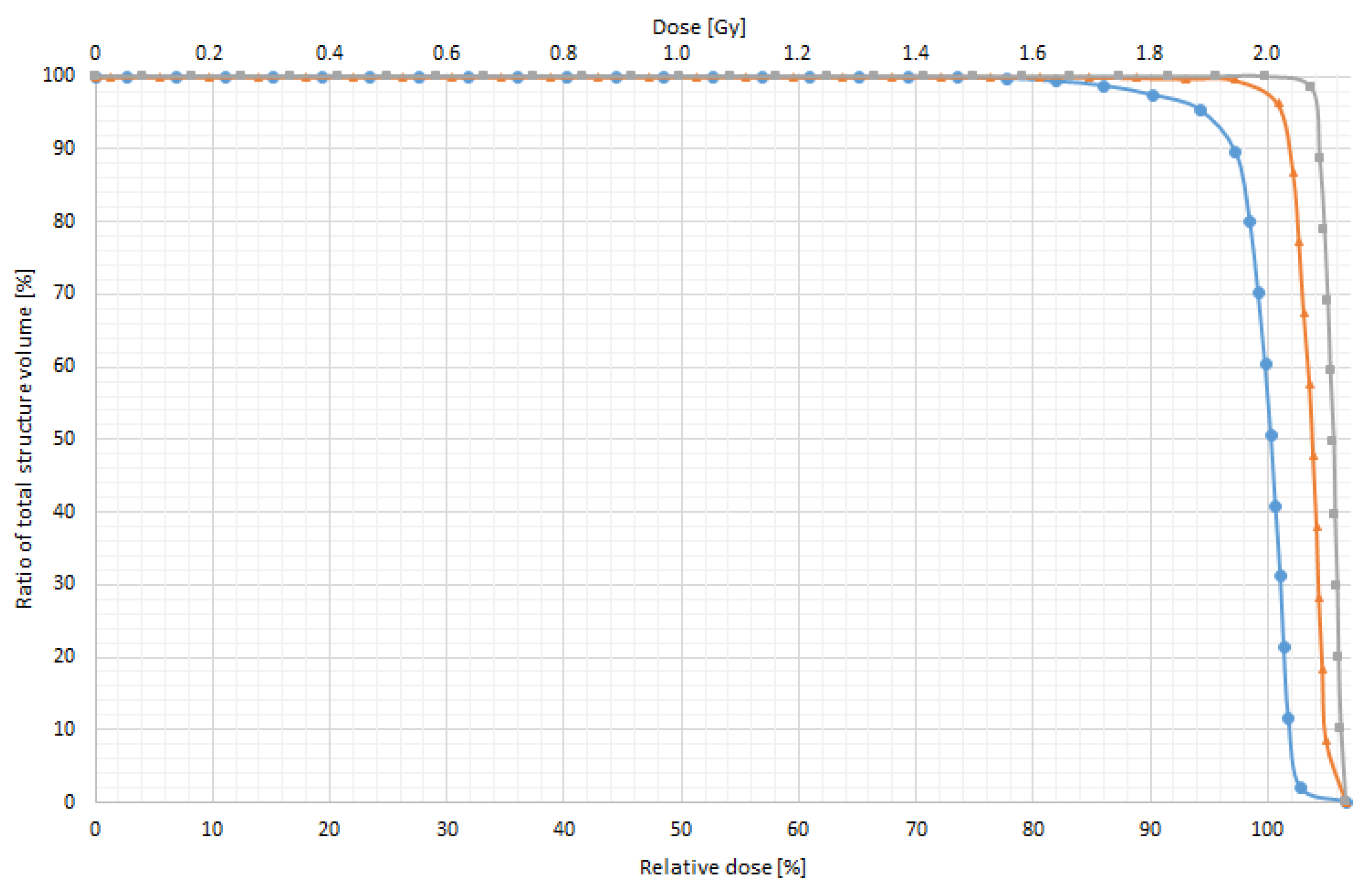
3.3. In Vitro Dose Verification Using 3D-Printed Devices
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Beadle, B.M.; Liao, K.P.; Elting, L.S.; Buchholz, T.A.; Ang, K.K.; Garden, A.S.; Guadagnolo, B.A. Improved survival using intensity-modulated radiation therapy in head and neck cancers: A SEER-Medicare analysis. Cancer 2014, 120, 702–710. [Google Scholar] [CrossRef]
- Mizowaki, T.; Norihisa, Y.; Takayama, K.; Ikeda, I.; Inokuchi, H.; Nakamura, K.; Kamba, T.; Inoue, T.; Kamoto, T.; Ogawa, O.; et al. Ten year outcomes of intensity-modulated radiation therapy combined with neoadjuvant hormonal therapy for intermediate- and high-risk patients with T1c–T2N0M0 prostate cancer. Int. J. Clin. Oncol. 2016, 21, 783–790. [Google Scholar] [CrossRef]
- Kamomae, T.; Itoh, Y.; Okudaira, K.; Nakaya, T.; Tomida, M.; Miyake, Y.; Oguchi, H.; Shiinoki, T.; Kawamura, M.; Yamamoto, N.; et al. Dosimetric impact of dental metallic crown on intensity-modulated radiotherapy and volumetric- modulated arc therapy for head and neck cancer. J. Appl. Clin. Med. Phys. 2016, 17, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Kubo, K.; Monzen, H.; Ishii, K.; Tamura, M.; Kawamorita, R.; Sumida, I.; Mizuno, H.; Nishimura, Y. Dosimetric comparison of Rapid Plan and manually optimized plans in volumetric modulated arc therapy for prostate cancer. Phys. Med. 2017, 44, 199–204. [Google Scholar] [CrossRef]
- Didona, A.; Lancellotta, V.; Zucchetti, C.; Panizza, B.M.; Frattegiani, A.; Iacco, M.; Di Pilato, A.C.; Saldi, S.; Aristei, C. Is volumetric modulated arc therapy with constant dose rate a valid option in radiation therapy for head and neck cancer patients? Rep. Pract. Oncol. Radiother. 2018, 23, 175–182. [Google Scholar] [CrossRef]
- Puri, D.R.; Chou, W.; Lee, N. Intensity-modulated radiation therapy in head and neck cancers: Dosimetric advantages and update of clinical results. Am. J. Clin. Oncol. 2005, 28, 415–423. [Google Scholar] [CrossRef]
- Wojcicka, J.B.; Lasher, D.E.; McAfee, S.S.; Fortier, G.A. Dosimetric comparison of three different treatment techniques in extensive scalp lesion irradiation. Radiother. Oncol. 2009, 91, 255–260. [Google Scholar] [CrossRef]
- Kudchadker, R.J.; Antolak, J.A.; Morrison, W.H.; Wong, P.F.; Hogstrom, K.R. Utilization of custom electron bolus in head and neck radiotherapy. J. Appl. Clin. Med. Phys. 2003, 4, 321–333. [Google Scholar] [CrossRef]
- Moyer, R.F.; King, G.A.; Hauser, J.F. Lead as surface bolus for high-energy photon and electron therapy. Med. Phys. 1986, 13, 263–266. [Google Scholar] [CrossRef]
- Vyas, V.; Palmer, L.; Mudge, R.; Jiang, R.; Fleck, A.; Schaly, B.; Osei, E.; Charl, P. On bolus for megavoltage photon and electron radiation therapy. Med. Dosim. 2013, 38, 268–273. [Google Scholar] [CrossRef]
- Butson, M.J.; Cheung, T.; Yu, P.; Metcalfe, P. Effects on skin dose from unwanted air gaps under bolus in photon beam radiotherapy. Radiat. Meas. 2000, 32, 201–204. [Google Scholar] [CrossRef]
- Khan, Y.; Villarreal-Barajas, J.E.; Udowicz, M.; Sinha, R.; Muhammad, W.; Abbasi, A.N.; Hussain, A. Clinical and dosimetric implications of air gaps between bolus and skin surface during radiation therapy. J. Cancer Ther. 2013, 4, 1251–1255. [Google Scholar] [CrossRef]
- Sharma, S.C.; Johnson, M.W. Surface dose perturbation due to air gap between patient and bolus for electron beams. Med. Phys. 1993, 20, 377–378. [Google Scholar] [CrossRef] [PubMed]
- Malik, H.H.; Darwood, A.R.; Shaunak, S.; Kulatilake, P.; Abdulrahman, A.; Mulki, O.; Baskaradas, A. Three-dimensional printing in surgery: A review of current surgical applications. J. Surg. Res. 2015, 199, 512–522. [Google Scholar] [CrossRef]
- Yan, Q.; Dong, H.; Su, J.; Han, J.; Song, B.; Wei, Q.; Shi, Y. A Review of 3D Printing Technology for Medical Applications. Engineering 2018, 4, 729–742. [Google Scholar] [CrossRef]
- Mavili, M.E.; Canter, H.I.; Saglam-Aydinatay, B.; Kamaci, S.; Kocadereli, I. Use of three-dimensional medical modeling methods for precise planning of orthognathic surgery. J. Craniofac. Surg. 2007, 18, 740–747. [Google Scholar] [CrossRef]
- Fisher, M.; Applegate, C.; Ryalat, M.; Laycock, S.; Hulse, M.; Emmens, D.; Bell, D. Evaluation of 3-D printed immobilisation shells for head and neck IMRT. Open J. Radiol. 2014, 4, 322–328. [Google Scholar] [CrossRef]
- Su, S.; Moran, K.; Robar, J.L. Design and production of 3D printed bolus for electron radiation therapy. J. Appl. Clin. Med. Phys. 2014, 15, 194–211. [Google Scholar] [CrossRef]
- Kim, S.W.; Shin, H.J.; Kay, C.S.; Son, S.H. A customized bolus produced using a 3-dimensional printer for radiotherapy. PLoS ONE 2014, 9, 1–8. [Google Scholar]
- Unterhinninghofen, R.; Giesel, F.L.; Wade, M.; Kuypers, J.; Preuss, A.; Debus, J.; Sterzing, F. OC-0412: 3D printing of individual immobilization devices based on imaging ñ analysis of positioning accuracy. Radiother. Oncol. 2015, 115, S199–S200. [Google Scholar] [CrossRef]
- McCowan, P.; Sasaki, D.; Jensen, M.; Rickey, D.; Dubey, A.; Harris, C.; Aviles, J.A.; McCurdy, B. On The Physical and Dosimetric Properties of 3D Printed Electron Bolus Fabricated Using Polylactic Acid. Radiother. Ocol. 2016, 120, S47. [Google Scholar] [CrossRef]
- Mayer, R.; Liacouras, P.; Thomas, A.; Kang, M.; Lin, L.; Simone, C.B. 3D printer generated thorax phantom with mobile tumor for radiation dosimetry. Rev. Sci. Instrum. 2015, 86, 074301. [Google Scholar] [CrossRef]
- Lali, K.; Artūras, A.; Reda, Č.; Dimitrova, T.; Jurgita, L. 3D printed boluses usage in radiotherapy. In Proceedings of the 14th International Conference on Medical Physics, Kaunas, Lithuania, 7–9 November 2019; pp. 125–128. [Google Scholar]
- Sevcik, A.; Adliene, D.; Laurikaitiene, J.; Nedzinskiene, R.; Masiulyte, I. Low energy deposition patterns in irradiated phantom with metal artefacts inside: A comparison between FLUKA Monte Carlo simulation and GafChromic EBT2 film measurements. Nucl. Instrum. Methods Phys. Res. B 2020, 478, 142–149. [Google Scholar] [CrossRef]
- Adlienė, D.; Jaselskė, E.; Urbonavičius, B.G.; Laurikaitienė, J.; Rudžianskas, V.; Didvalis, T. Development of 3D printed phantom for dose verification in radiotherapy for the patient with metal artefacts inside. In Proceedings of the World Congress on Medical Physics and Biomedical Engineering, Prague, Czech Republic, 3–8 June 2018; Springer: Berlin/Heidelberg, Germany, 2019; Volume 68, pp. 643–647. [Google Scholar]
- Taha, A.A.; Hanbury, A. Metrics for evaluating 3D medical image segmentation: Analysis, selection, and tool. BMC Med. Imaging 2015, 15, 29. [Google Scholar] [CrossRef]
- Zou, K.H.; Warfield, S.K.; Bharatha, A.; Tempany, C.M.; Kaus, M.R.; Haker, S.J.; Wells, I.I.I.W.M.; Jolesz, F.A.; Kikinis, R. Statistical validation of image segmentation quality based on a spatial overlap index. Acad. Radiol. 2004, 11, 178–189. [Google Scholar] [CrossRef]
- Lawton, T. DSC ImageCalc—Software for Determining Similarity Coefficients for the Analysis of Image Segmentations. J. Open Res. Softw. 2017, 5, 28. [Google Scholar] [CrossRef]
- Tino, R.; Yeo, A.; Leary, M.; Brandt, M.; Kron, T. A Systematic Review on 3D-Printed Imaging and Dosimetry Phantoms in Radiation Therapy. Technol. Cancer Res. Treat. 2019, 18, 1533033819870208. [Google Scholar] [CrossRef]
- Ehler, E.D.; Barney, B.M.; Higgins, P.D.; Dusenbery, K.E. Patient specific 3D printed phantom for IMRT quality assurance. Phys. Med. Biol. 2014, 59, 5763–5773. [Google Scholar] [CrossRef] [PubMed]
- Burleson, S.; Baker, J.; Hsia, A.T.; Xu, Z. Use of 3D printers to create a patient-specific 3D bolus for external beam therapy. J. Appl. Clin. Med. Phys. 2015, 16, 166–178. [Google Scholar] [CrossRef]




| Settings | Values |
|---|---|
| Bed temperature | 30 °C |
| Extruder temperature | 207 °C |
| Layer height | 0.14 mm |
| Fill density (bolus) | 100% |
| Fill density (phantom) | 90% |
| Fill pattern | Rectilinear |
| Nozzle diameter | 0.4 mm |
| PLA Filament diameter | 1.75 mm |
| Criteria | Value |
|---|---|
| Sorensen–Dice Coefficient (DICE) | 0.957 |
| Jaccard Coefficient (JAC) | 0.917 |
| Proportional Agreement (PA) | 0.998 |
| Cohen‘s Kappa (KAP) | 0.956 |
| Goodman & Kruskal‘s Gamma (GKG) | 0.913 |
| Rogot–Goldberg Agreement (RGA) | 0.978 |
| A Single-Field Plan | Difference from Prescribed Dose 2 Gy | |||||
|---|---|---|---|---|---|---|
| D95% | D97% | D99% | ||||
| Gy | % | Gy | % | Gy | % | |
| Without bolus | 0.85 | 40.0 | 0.98 | 50.0 | 1.04 | 52.0 |
| With 0.5 cm bolus | 0.01 | 0.5 | 0.04 | 2.0 | 0.06 | 3.0 |
| With 1.0 cm bolus | 0.00 | 0.0 | 0.01 | 0.5 | 0.03 | 1.5 |
| IMRT Plan | Difference from Prescribed Dose 2 Gy | |||||
|---|---|---|---|---|---|---|
| D95% | D97% | D99% | ||||
| Gy | % | Gy | % | Gy | % | |
| Without bolus | 0.1 | 5.14 | 0.17 | 8.64 | 0.52 | 26.04 |
| With 0.5 cm bolus | 0.007 | 0.35 | 0.008 | 0.4 | 0.02 | 0.98 |
| With 1.0 cm bolus | 0 | 0 | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jreije, A.; Keshelava, L.; Ilickas, M.; Laurikaitiene, J.; Urbonavicius, B.G.; Adliene, D. Development of Patient Specific Conformal 3D-Printed Devices for Dose Verification in Radiotherapy. Appl. Sci. 2021, 11, 8657. https://doi.org/10.3390/app11188657
Jreije A, Keshelava L, Ilickas M, Laurikaitiene J, Urbonavicius BG, Adliene D. Development of Patient Specific Conformal 3D-Printed Devices for Dose Verification in Radiotherapy. Applied Sciences. 2021; 11(18):8657. https://doi.org/10.3390/app11188657
Chicago/Turabian StyleJreije, Antonio, Lalu Keshelava, Mindaugas Ilickas, Jurgita Laurikaitiene, Benas Gabrielis Urbonavicius, and Diana Adliene. 2021. "Development of Patient Specific Conformal 3D-Printed Devices for Dose Verification in Radiotherapy" Applied Sciences 11, no. 18: 8657. https://doi.org/10.3390/app11188657
APA StyleJreije, A., Keshelava, L., Ilickas, M., Laurikaitiene, J., Urbonavicius, B. G., & Adliene, D. (2021). Development of Patient Specific Conformal 3D-Printed Devices for Dose Verification in Radiotherapy. Applied Sciences, 11(18), 8657. https://doi.org/10.3390/app11188657






