Phytosomes as an Emerging Nanotechnology Platform for the Topical Delivery of Bioactive Phytochemicals
Abstract
:1. Introduction
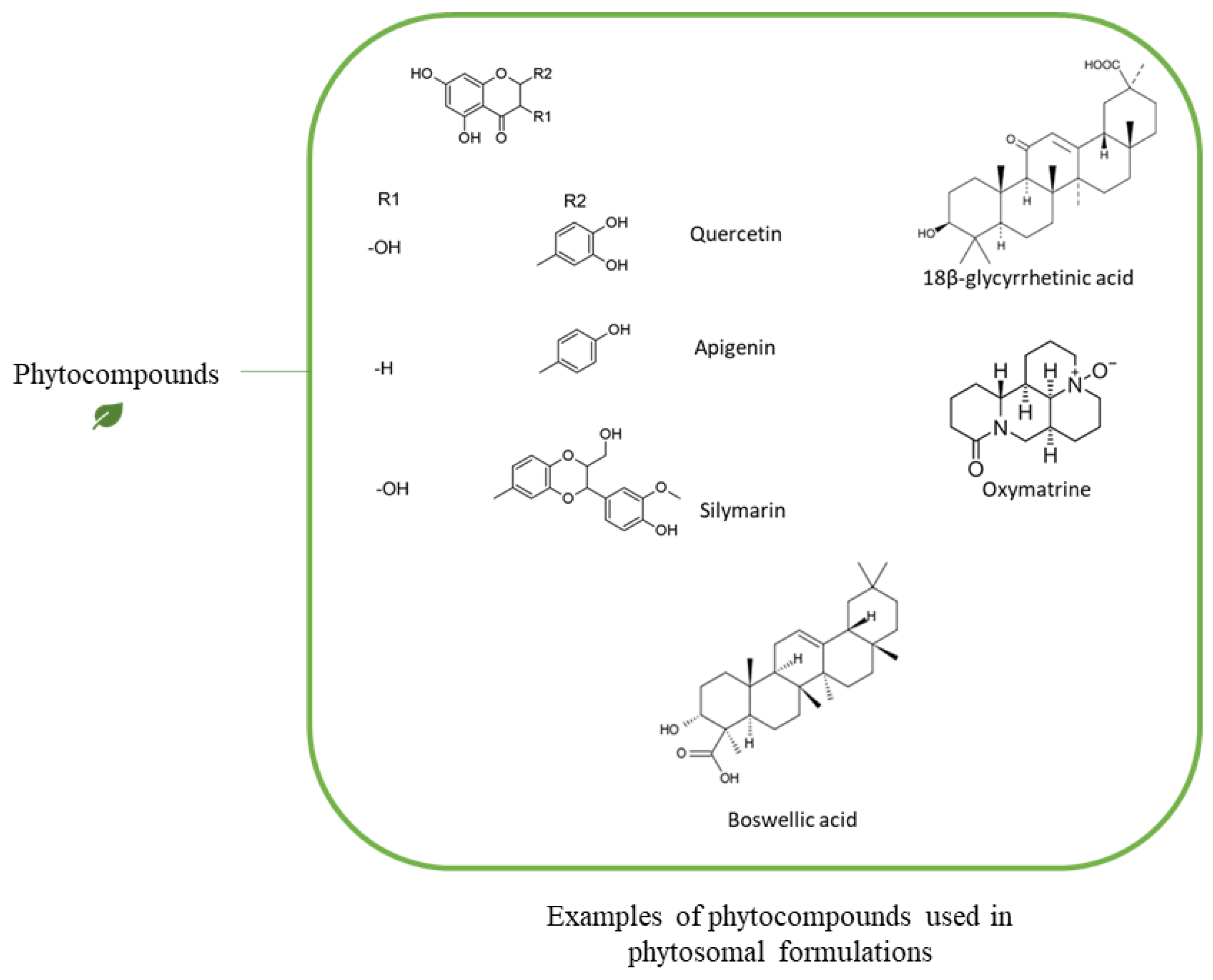
1.1. Phytochemicals
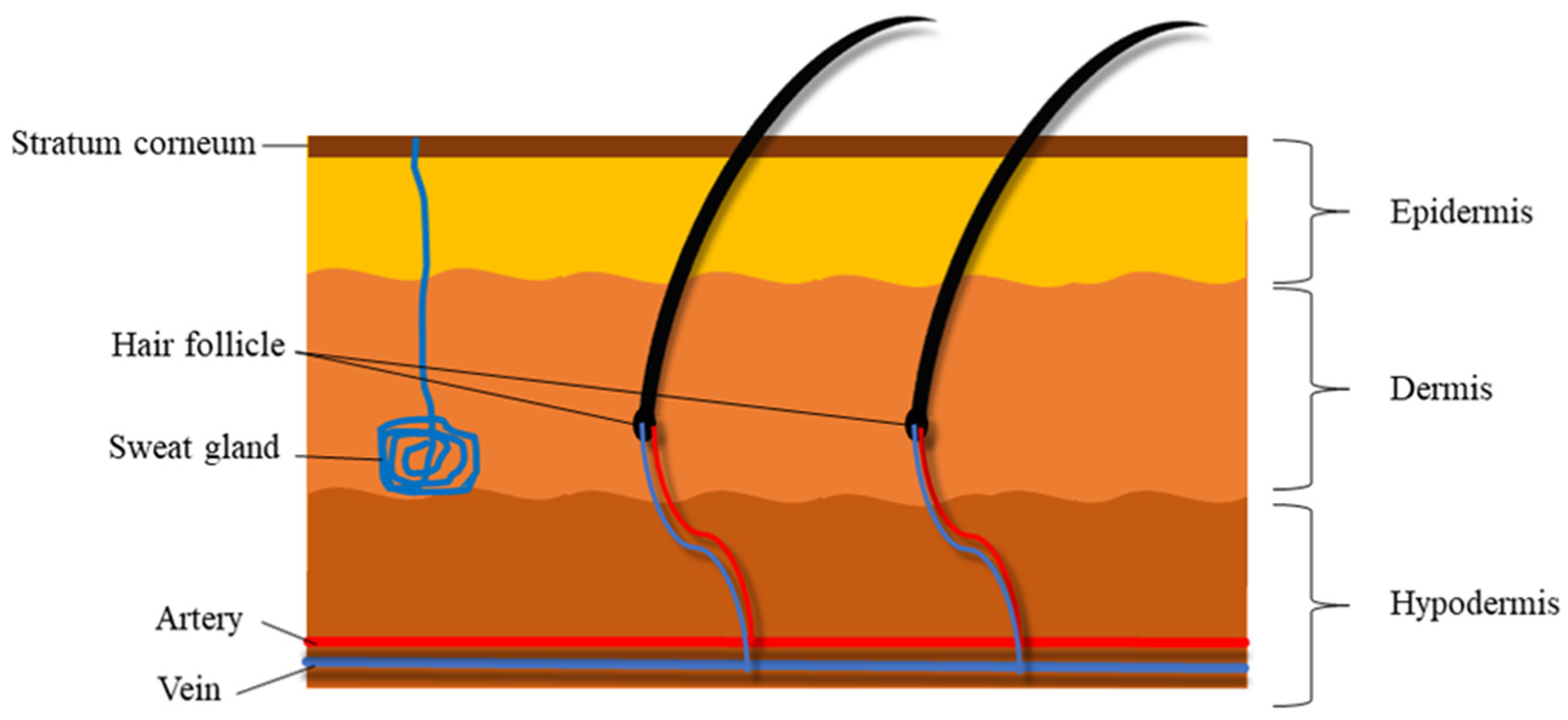
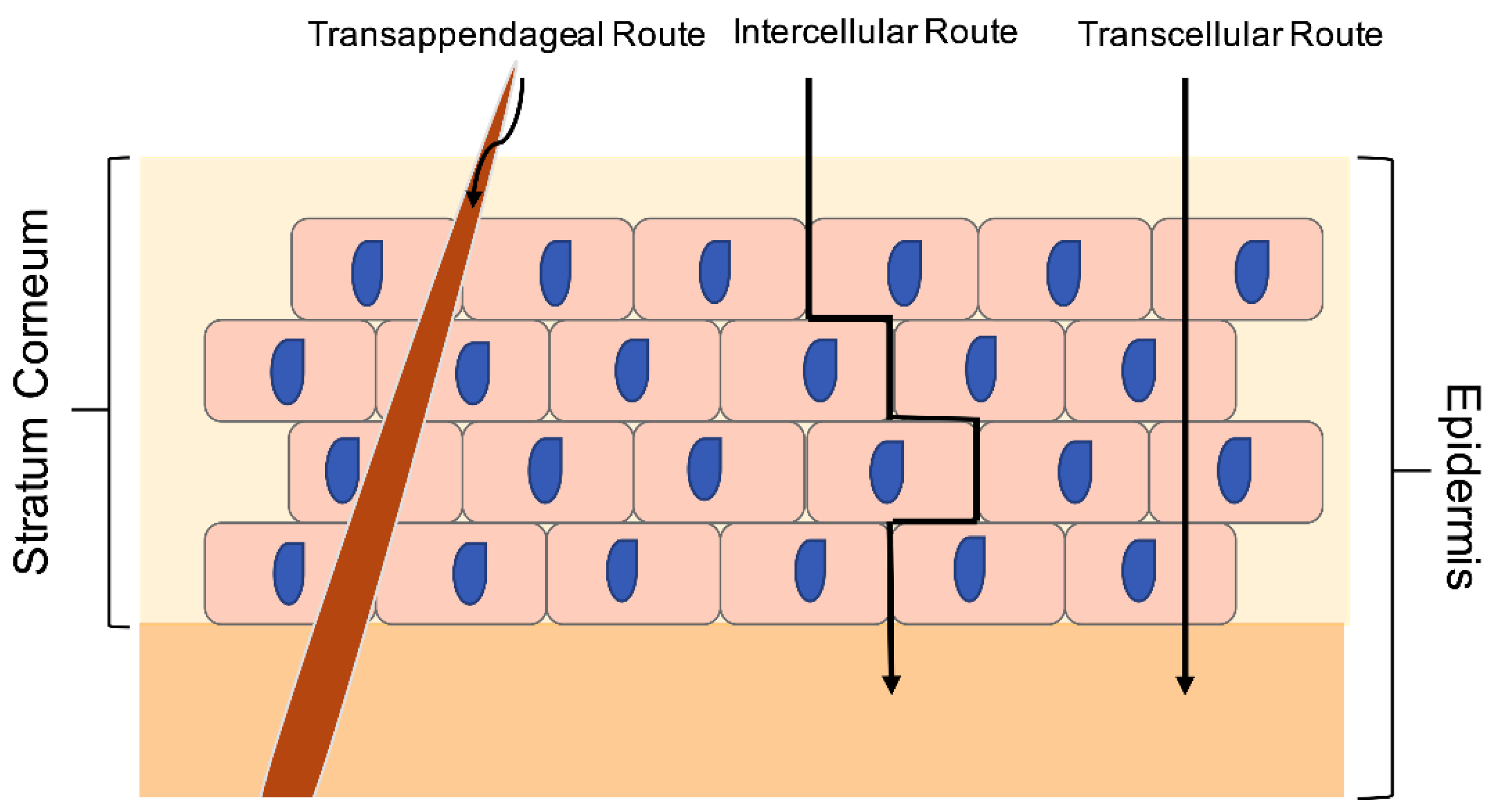
1.2. Structural Nature and Barrier Properties of the Skin
1.3. Nanotechnology Platform for the Delivery of Bioactive Phytochemicals
2. Phytosomes
2.1. Phytosome Significance in Drug Delivery
2.2. Phytosomes in Clinical Trials
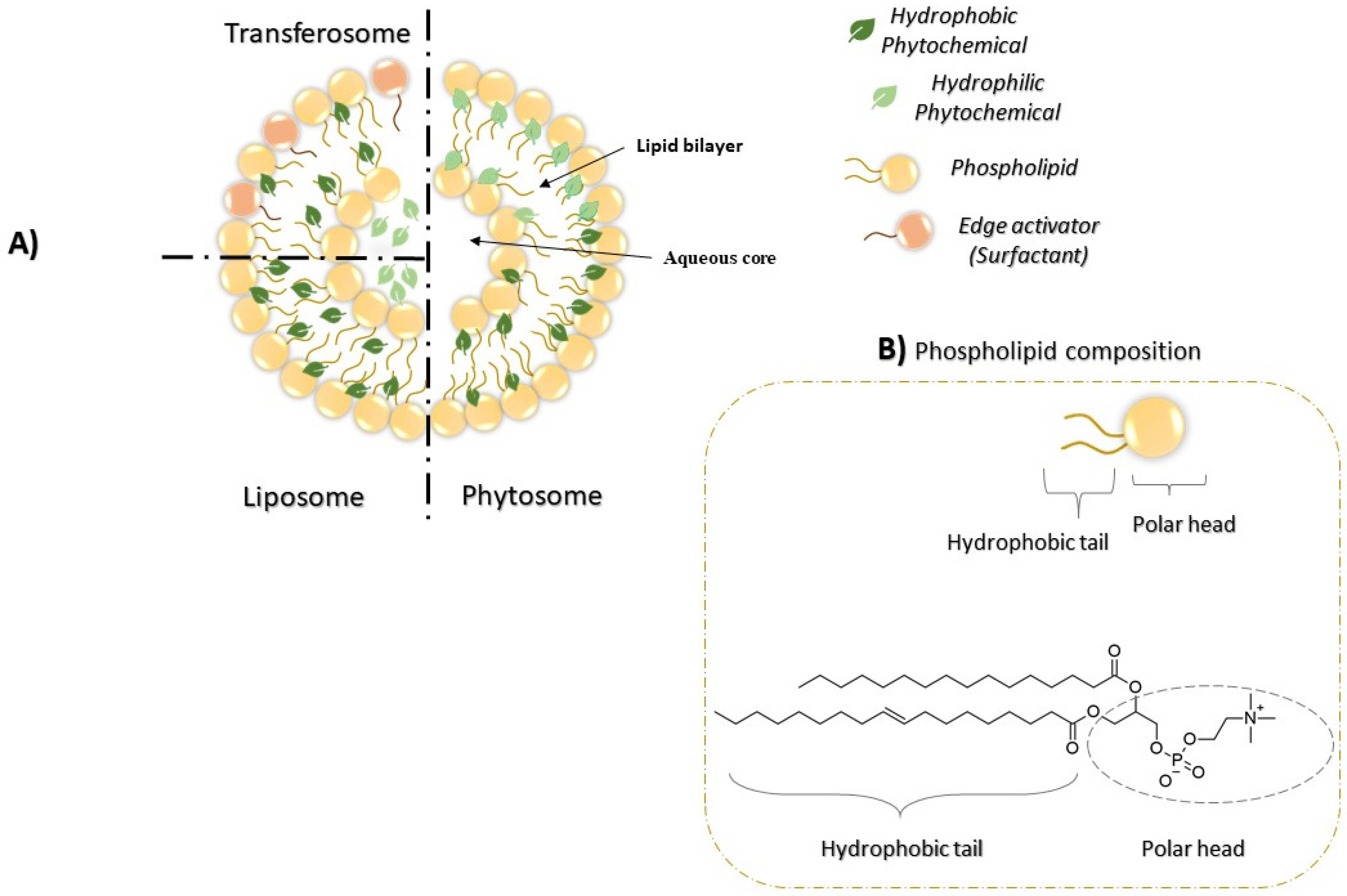
2.3. Phytosomes and Other Lipid-Based Nanocarriers: Similarities and Differences
3. Advantages of Phytosomes in Topical Applications
4. Conclusions and Future Prospects
Funding
Conflicts of Interest
References
- Jasemi, S.V.; Khazaei, H.; Aneva, I.Y.; Farzaei, M.H.; Echeverría, J. Medicinal Plants and Phytochemicals for the Treatment of Pulmonary Hypertension. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef]
- Oveissi, V.; Ram, M.; Bahramsoltani, R.; Ebrahimi, F.; Rahimi, R.; Naseri, R.; Belwal, T.; Devkota, H.P.; Abbasabadi, Z.; Farzaei, M.H. Medicinal plants and their isolated phytochemicals for the management of chemotherapy-induced neuropathy: Therapeutic targets and clinical perspective. DARU J. Pharm. Sci. 2019, 27, 389–406. [Google Scholar] [CrossRef]
- Kooti, W.; Servatyari, K.; Behzadifar, M.; Asadi-Samani, M.; Sadeghi, F.; Nouri, B.; Marzouni, H.Z. Effective Medicinal Plant in Cancer Treatment, Part 2: Review Study. J. Evid.-Based Integr. Med. 2017, 22, 982–995. [Google Scholar] [CrossRef]
- O’Hagan, D. Pyrrole, pyrrolidine, pyridine, piperidine and tropane alkaloids (1998 to 1999). Nat. Prod. Rep. 2000, 17, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Guler, G.O.; Aktumsek, A.; Ceylan, R.; Picot, C.M.N.; Mahomoodally, M.F. Enzyme Inhibitory Properties, Antioxidant Activities, and Phytochemical Profile of Three Medicinal Plants from Turkey. Adv. Pharmacol. Sci. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zarei, I.; Brown, D.G.; Nealon, N.J.; Ryan, E.P. Rice Bran Metabolome Contains Amino Acids, Vitamins & Cofactors, and Phytochemicals with Medicinal and Nutritional Properties. Rice 2017, 10, 24. [Google Scholar] [CrossRef] [Green Version]
- Dillard, C.J.; German, J.B. Phytochemicals: Nutraceuticals and human health. J. Sci. Food Agric. 2000, 80, 1744–1756. [Google Scholar] [CrossRef]
- Selby-Pham, S.N.B.; Miller, R.B.; Howell, K.; Dunshea, F.; Bennett, L.E. Physicochemical properties of dietary phytochemicals can predict their passive absorption in the human small intestine. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Gan, R.-Y.; Li, S.; Zhou, Y.; Li, A.-N.; Xu, D.-P.; Li, H.-B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef]
- Desborough, M.J.R.; Keeling, D.M. The aspirin story—From willow to wonder drug. Br. J. Haematol. 2017, 177, 674–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howes, M.R.; Perry, N.S.; Vásquez-Londoño, C.A.; Perry, E.K. Role of phytochemicals as nutraceuticals for cognitive functions affected in ageing. Br. J. Pharmacol. 2019, 177, 1294–1315. [Google Scholar] [CrossRef] [Green Version]
- Govindaraghavan, S.; Sucher, N.; Govindaraghavan, S.; Sucher, N. Quality assessment of medicinal herbs and their extracts: Criteria and prerequisites for consistent safety and efficacy of herbal medicines. Epilepsy Behav. 2015, 52, 363–371. [Google Scholar] [CrossRef]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-E. Molecular Targets of Phytochemicals for Skin Inflammation. Curr. Pharm. Des. 2018, 24, 1533–1550. [Google Scholar] [CrossRef] [PubMed]
- Bahramsoltani, R.; Farzaei, M.H.; Rahimi, R. Medicinal plants and their natural components as future drugs for the treatment of burn wounds: An integrative review. Arch. Dermatol. Res. 2014, 306, 601–617. [Google Scholar] [CrossRef]
- Dawid-Pac, R. Medicinal plants used in treatment of inflammatory skin diseases. Adv. Dermatol. Allergol. 2013, 3, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Laura, V.; Mattia, F.; Roberta, G.; Federico, I.; Emi, D.; Chiara, T.; Luca, B.; Elena, C. Potential of Curcumin in Skin Disorders. Nutrients 2019, 11, 2169. [Google Scholar] [CrossRef] [Green Version]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kidd, P.M. Bioavailability and activity of phytosome complexes from botanical polyphenols: The silymarin, curcumin, green tea, and grape seed extracts. Altern. Med. Rev. J. Clin. Ther. 2009, 14, 226–246. [Google Scholar]
- Wong, R.; Geyer, S.; Weninger, W.J.; Guimberteau, J.-C.; Wong, J.K. The dynamic anatomy and patterning of skin. Exp. Dermatol. 2015, 25, 92–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prausnitz, M.R.; Elias, P.M.; Franz, T.J.; Schmuth, M.; Tsai, J.-C.; Menon, G.K.; Holleran, W.M.; Feingold, K.R. Skin barrier and transdermal drug delivery. Dermatology 2012, 32, 760–769. [Google Scholar]
- Schnittger, S.; Sinha, M. The Materials Science of Cosmetics. MRS Bull. 2007, 32, 760–769. [Google Scholar] [CrossRef] [Green Version]
- Andrews, S.N.; Jeong, E.; Prausnitz, M.R. Transdermal Delivery of Molecules is Limited by Full Epidermis, Not Just Stratum Corneum. Pharm. Res. 2012, 30, 1099–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherratt, M.J. Tissue elasticity and the ageing elastic fibre. AGE 2009, 31, 305–325. [Google Scholar] [CrossRef] [Green Version]
- Hadgraft, J. Skin, the final frontier. Int. J. Pharm. 2001, 224, 1–18. [Google Scholar] [CrossRef]
- Kim, B.; Cho, H.-E.; Moon, S.H.; Ahn, H.-J.; Bae, S.; Cho, H.-D.; An, S. Transdermal delivery systems in cosmetics. Biomed. Dermatol. 2020, 4, 1–12. [Google Scholar] [CrossRef]
- Proksch, E.; Brandner, J.M.; Jensen, J.-M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef]
- Lu, M.; Qiu, Q.; Luo, X.; Liu, X.; Sun, J.; Wang, C.; Lin, X.; Deng, Y.; Song, Y. Phyto-phospholipid complexes (phytosomes): A novel strategy to improve the bioavailability of active constituents. Asian J. Pharm. Sci. 2018, 14, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Amin, T.; Bhat, S.V. A Review on Phytosome Technology as a Novel Approach to Improve the Bioavailability of Nutraceuticals. Int. J. Adv. Res. Technol. 2012, 1, 43. [Google Scholar]
- Gupta, A.; Ashawat, M.; Saraf, S.; Saraf, S. Phytosome: A novel approach towards functional cosmetics. J. Plant Sci. 2007, 2, 644–649. [Google Scholar]
- Magnusson, B.M.; Anissimov, Y.; Cross, S.E.; Roberts, M. Molecular Size as the Main Determinant of Solute Maximum Flux Across the Skin. J. Investig. Dermatol. 2004, 122, 993–999. [Google Scholar] [CrossRef] [Green Version]
- Schneider, M.; Stracke, F.; Hansen, S.; Schaefer, U.F. Nanoparticles and their interactions with the dermal barrier. Dermato-Endocrinol. 2009, 1, 197–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semalty, A.; Semalty, M.; Rawat, M.S.M.; Franceschi, F. Supramolecular phospholipids–polyphenolics interactions: The PHYTOSOME® strategy to improve the bioavailability of phytochemicals. Fitoterapia 2010, 81, 306–314. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [Green Version]
- Abubakar, A.R.; Haque, M. Preparation of medicinal plants: Basic extraction and fractionation procedures for experimental purposes. J. Pharm. Bioallied Sci. 2020, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.-P.; Pihlaja, K.; Kujala, A.T.S.; Heinonen, M. Antioxidant Activity of Plant Extracts Containing Phenolic Compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Upadhyay, S.; Dixit, M. Role of Polyphenols and Other Phytochemicals on Molecular Signaling. Oxid. Med. Cell. Longev. 2015, 2015, 1–15. [Google Scholar] [CrossRef]
- Boojar, M.M.A. An Overview of the Cellular Mechanisms of Flavonoids Radioprotective Effects. Adv. Pharm. Bull. 2019, 10, 13–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozkan, G.; Kamiloglu, S.; Ozdal, T.; Boyacioglu, D.; Capanoglu, E. Potential Use of Turkish Medicinal Plants in the Treatment of Various Diseases. Molecules 2016, 21, 257. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.; Katan, M. Absorption, metabolism and health effects of dietary flavonoids in man. Biomed. Pharmacother. 1997, 51, 305–310. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Ballesta, M.; Gil-Izquierdo, Á.; García-Viguera, C.; Domínguez-Perles, R. Nanoparticles and Controlled Delivery for Bioactive Compounds: Outlining Challenges for New “Smart-Food” for Health. Foods 2018, 7, 72. [Google Scholar] [CrossRef] [Green Version]
- Spanidi, E.; Karapetsas, A.; Voulgaridou, G.-P.; Letsiou, S.; Aligiannis, N.; Tsochantaridis, I.; Kynigopoulos, S.; Lambropoulou, M.; Mourtzinos, I.; Pappa, A.; et al. A New Controlled Release System for Propolis Polyphenols and Its Biochemical Activity for Skin Applications. Plants 2021, 10, 420. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, H.; Xu, C.; Gu, L. A review: Using nanoparticles to enhance absorption and bioavailability of phenolic phytochemicals. Food Hydrocoll. 2015, 43, 153–164. [Google Scholar] [CrossRef]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Vadhanam, M.V. Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Lett. 2013, 334, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Rizvi, S.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2017, 26, 64–70. [Google Scholar] [CrossRef]
- Khalid, K.; Tan, X.; Zaid, H.F.M.; Tao, Y.; Chew, C.L.; Chu, D.-T.; Lam, M.K.; Ho, Y.-C.; Lim, J.W.; Wei, L.C. Advanced in developmental organic and inorganic nanomaterial: A review. Bioengineered 2020, 11, 328–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.-L.; Liu, Y.-K.; Tsai, N.-M.; Hsieh, J.-H.; Chen, C.-H.; Lin, C.-M.; Liao, K.-W. A Lipo-PEG-PEI complex for encapsulating curcumin that enhances its antitumor effects on curcumin-sensitive and curcumin-resistance cells. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Das, R.K.; Kasoju, N.; Bora, U. Encapsulation of curcumin in alginate-chitosan-pluronic composite nanoparticles for delivery to cancer cells. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 153–160. [Google Scholar] [CrossRef]
- Singh, P.; Singh, H.; Ahn, S.; Castro-Aceituno, V.; Jiménez, Z.; Simu, S.Y.; Kim, Y.J.; Yang, D.C. Pharmacological importance, characterization and applications of gold and silver nanoparticles synthesized by Panax ginseng fresh leaves. Artif. Cells Nanomed. Biotechnol. 2016, 45, 1415–1424. [Google Scholar] [CrossRef] [Green Version]
- Anand, K.; Gengan, R.; Phulukdaree, A.; Chuturgoon, A. Agroforestry waste Moringa oleifera petals mediated green synthesis of gold nanoparticles and their anti-cancer and catalytic activity. J. Ind. Eng. Chem. 2015, 21, 1105–1111. [Google Scholar] [CrossRef]
- Sankar, R.; Maheswari, R.; Karthik, S.; Shivashangari, K.S.; Ravikumar, V. Anticancer activity of Ficus religiosa engineered copper oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, R.; Lalatsa, A.; Statts, L.; Bolás-Fernández, F.; Ballesteros, M.P.; Serrano, D.R. Transferosomes as nanocarriers for drugs across the skin: Quality by design from lab to industrial scale. Int. J. Pharm. 2019, 573, 118817. [Google Scholar] [CrossRef]
- Rajan, R.; Vasudevan, D.T.; Mukund, V.P.B.; Jose, S. Transferosomes—A vesicular transdermal delivery system for enhanced drug permeation. J. Adv. Pharm. Technol. Res. 2011, 2, 138–143. [Google Scholar] [CrossRef]
- Rai, S.; Pandey, V.; Rai, G. Transfersomes as versatile and flexible nano-vesicular carriers in skin cancer therapy: The state of the art. Nano Rev. Exp. 2017, 8, 1325708. [Google Scholar] [CrossRef] [PubMed]
- Al-Othman, M.; Al-Othman, A.M.; Al-Numair, K.S.; El-Desoky, G.E.; Yusuf, K.; Alothman, Z.; Aboul-Soud, M.A.M.; Giesy, J.P. Protection of -tocopherol and selenium against acute effects of malathion on liver and kidney of rats. Afr. J. Pharm. Pharmacol. 2011, 5, 1263–1271. [Google Scholar] [CrossRef] [Green Version]
- Sarwa, K.K.; Mazumder, B.; Rudrapal, M.; Verma, V.K. Potential of capsaicin-loaded transfersomes in arthritic rats. Drug Deliv. 2013, 22, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Hou, S.-X.; Zhang, L.-K.; Li, Y.; He, J.-Y.; Guo, D.-D. Transdermal and lymph targeting transfersomes of vincristine. Acta Pharm. Sin. 2007, 42, 1097–1101. [Google Scholar]
- El-Feky, G.S.; El-Naa, M.M.; Mahmoud, A.A. Flexible nano-sized lipid vesicles for the transdermal delivery of colchicine; in vitro/in vivo investigation. J. Drug Deliv. Sci. Technol. 2018, 49, 24–34. [Google Scholar] [CrossRef]
- Vargason, A.M.; Anselmo, A.C.; Mitragotri, S. The evolution of commercial drug delivery technologies. Nat. Biomed. Eng. 2021, 1–17. [Google Scholar] [CrossRef]
- Kumar, D.; Vats, N.; Saroha, K.; Rana, A.C. Phytosomes as Emerging Nanotechnology for Herbal Drug Delivery. In Sustainable Agriculture Reviews 43: Pharmaceutical Technology for Natural Products Delivery Vol. 1 Fundamentals and Applications; Saneja, A., Panda, A.K., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 217–237. [Google Scholar] [CrossRef]
- Rahman, H.; Othman, H.H.; Hammadi, N.I.; Yeap, S.K.; Amin, K.M.; Samad, N.A.; Alitheen, N.B. Novel Drug Delivery Systems for Loading of Natural Plant Extracts and Their Biomedical Applications. Int. J. Nanomed. 2020, 15, 2439–2483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandhi, A.; Dutta, A.; Pal, A.; Bakshi, P. Recent trends of phytosomes for delivering herbal extract with improved bioavailability. J. Pharmacogn. Phytochem. 2012, 1, 6–14. [Google Scholar]
- Shakeri, A.; Sahebkar, A. Opinion Paper: Phytosome: A Fatty Solution for Efficient Formulation of Phytopharmaceuticals. Recent Patents Drug Deliv. Formul. 2016, 10, 7–10. [Google Scholar] [CrossRef]
- Rathore, P.; Swami, G. Planterosomes: A potential phyto-phospholipid carriers for the bioavailability enhancement of herbal extracts. Int. J. Pharm. Sci. Res. 2012, 3, 737. [Google Scholar]
- Jain, N.; Gupta, B.P.; Thakur, N.; Jain, R.; Banweer, J.; Jain, D.K.; Jain, S. Phytosome: A novel drug delivery system for herbal medicine. Int. J. Pharm. Sci. Drug Res. 2010, 2, 224–228. [Google Scholar]
- Yang, B.; Dong, Y.; Wang, F.; Zhang, Y. Nanoformulations to Enhance the Bioavailability and Physiological Functions of Polyphenols. Molecules 2020, 25, 4613. [Google Scholar] [CrossRef] [PubMed]
- Kidd, P.; Head, K. A review of the bioavailability and clinical efficacy of milk thistle phytosome: A silybin-phosphatidylcholine complex (Siliphos). Altern. Med. Rev. J. Clin. Ther. 2005, 10, 193–203. [Google Scholar]
- Gaikwad, A.R.; Ahire, K.D.; Gosavi, A.A.; Salunkhe, K.S.; Khalkar, A.; Gaikwad Abhijeet, R. Phytosome as a Novel Drug Delivery System for Bioavailability Enhancement of Phytoconstituents and its Applications: A Review. J. Drug Deliv. Ther. 2021, 11, 138–152. [Google Scholar] [CrossRef]
- Arora, S.; Sharma, A.; Kaur, P. Preparation and Characterization of Phytosomal-Phospholipid Complex of P. Amarus and its Tablet Formulation. J. Pharm. Technol. Res. Manag. 2013, 1, 1–18. [Google Scholar] [CrossRef]
- Bijak, M. Silybin, a Major Bioactive Component of Milk Thistle (Silybum marianum L. Gaernt.)—Chemistry, Bioavailability, and Metabolism. Molecules 2017, 22, 1942. [Google Scholar] [CrossRef] [Green Version]
- Loguercio, C. Silybin and the liver: From basic research to clinical practice. World J. Gastroenterol. 2011, 17, 2288–2301. [Google Scholar] [CrossRef]
- Yanyu, X.; Yunmei, S.; Zhipeng, C.; Qineng, P. The preparation of silybin–phospholipid complex and the study on its pharmacokinetics in rats. Int. J. Pharm. 2006, 307, 77–82. [Google Scholar] [CrossRef]
- Naik, S.R.; Pilgaonkar, V.W.; Panda, V. Evaluation of antioxidant activity of Ginkgo biloba phytosomes in rat brain. Phytother. Res. 2006, 20, 1013–1016. [Google Scholar] [CrossRef]
- Shivanand, P.; Kinjal, P. Phytosomes: Technical revolution in phytomedicine. Int. J. PharmTech Res. 2010, 2, 627–631. [Google Scholar]
- Sbrini, G.; Brivio, P.; Fumagalli, M.; Giavarini, F.; Caruso, D.; Racagni, G.; Dell’Agli, M.; SanGiovanni, E.; Calabrese, F. Centella asiatica L. Phytosome Improves Cognitive Performance by Promoting Bdnf Expression in Rat Prefrontal Cortex. Nutrients 2020, 12, 355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belcaro, G.; Ledda, A.; Hu, S.; Cesarone, M.R.; Feragalli, B.; Dugall, M. Greenselect Phytosome for Borderline Metabolic Syndrome. Evid.-Based Complement. Altern. Med. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Das, M.K.; Kalita, B. Design and Evaluation of Phyto-Phospholipid Complexes (Phytosomes) of Rutin for Transdermal Application. J. Appl. Pharm. Sci. 2014, 4, 51–57. [Google Scholar] [CrossRef]
- Tung, B.T.; Hai, N.T.; Son, P.K. Hepatoprotective effect of Phytosome Curcumin against paracetamol-induced liver toxicity in mice. Braz. J. Pharm. Sci. 2017, 53. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Shi, M.; Li, N.; Xu, R. Application of Functional Biocompatible Nanomaterials to Improve Curcumin Bioavailability. Front. Chem. 2020, 8, 589957. [Google Scholar] [CrossRef]
- Burger, A.M.; Mengs, U.; Kelter, G.; Schüler, J.B.; Fiebig, H.H. No evidence of stimulation of human tumor cell proliferation by a standardized aqueous mistletoe extract in vitro. Anticancer Res. 2003, 23, 3801–3806. [Google Scholar]
- Kumar, A.; Kumar, B.; Singh, S.K.; Kaur, B.; Singh, S. A review on phytosomes: Novel approach for herbal phytochemicals. Asian J. Pharm. Clin. Res. 2017, 10, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Saharan, V.A.; Singh, M.; Bhandari, A. Phytosome: Drug Delivery System for Polyphenolic Phytoconstituents. Iran. J. Pharm. Sci. 2011, 7, 209–219. [Google Scholar]
- Agarwal, R.; Agarwal, C.; Ichikawa, H.; Singh, R.P.; Aggarwal, B.B. Anticancer potential of silymarin: From bench to bed side. Anticancer Res. 2007, 26, 4457–4498. [Google Scholar]
- Flaig, T.W.; Glodé, M.; Gustafson, D.; van Bokhoven, A.; Tao, Y.; Wilson, S.; Su, L.-J.; Li, Y.; Harrison, G.; Agarwal, R.; et al. A study of high-dose oral silybin-phytosome followed by prostatectomy in patients with localized prostate cancer. Prostate 2010, 70, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Flaig, T.W.; Gustafson, D.L.; Su, L.-J.; Zirrolli, J.A.; Crighton, F.; Harrison, G.S.; Pierson, A.S.; Agarwal, R.; Glodé, L.M. A phase I and pharmacokinetic study of silybin-phytosome in prostate cancer patients. Investig. New Drugs 2007, 25, 139–146. [Google Scholar] [CrossRef]
- Gilardini, L.; Pasqualinotto, L.; Di Pierro, F.; Risso, P.; Invitti, C. Effects of Greenselect Phytosome® on weight maintenance after weight loss in obese women: A randomized placebo-controlled study. BMC Complement. Altern. Med. 2016, 16, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tampucci, S.; Burgalassi, S.; Chetoni, P.; Monti, D. Cutaneous Permeation and Penetration of Sunscreens: Formulation Strategies and In Vitro Methods. Cosmetics 2018, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Karimi, N.; Ghanbarzadeh, B.; Hamishehkar, H.; Keivani, F.; Pezeshki, A.; Gholian, M.M. Phytosome and Liposome: The Beneficial Encapsulation Systems in Drug Delivery and Food Application. Appl. Food Biotechnol. 2015, 2, 17–27. [Google Scholar] [CrossRef]
- Pierre, M.B.R.; Costa, I.M. Liposomal systems as drug delivery vehicles for dermal and transdermal applications. Arch. Dermatol. Res. 2011, 303, 607–621. [Google Scholar] [CrossRef]
- Kaul, S.; Gulati, N.; Verma, D.; Mukherjee, S.; Nagaich, U. Role of Nanotechnology in Cosmeceuticals: A Review of Recent Advances. J. Pharm. 2018, 2018, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Rafiee, Z.; Jafari, S.M. Application of Lipid Nanocarriers for the Food Industry. In Bioactive Molecules in Food; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 623–665. [Google Scholar] [CrossRef]
- Ahmad, M.U.; Ali, S.M.; Ahmad, I. Applications of nanotechnology in pharmaceutical development. In Lipids in Nanotechnology; Ahmad, M.U., Ed.; AOCS Press: Urbana, IL, USA, 2012; pp. 171–190. [Google Scholar] [CrossRef]
- Morrow, D.I.J.; Garland, M.J.; McCarron, P.; Woolfson, A.D.; Donnelly, R. Innovative Drug Delivery Strategies for Topical Photodynamic Therapy using Porphyrin Precursors. J. Environ. Pathol. Toxicol. Oncol. 2007, 26, 105–116. [Google Scholar] [CrossRef]
- Hoque, M.; Agarwal, S.; Gupta, S.; Garg, S.; Syed, I.; Rupesh, A.; Mohapatra, N.; Bose, S.; Sarkar, P. 3.34—Lipid Nanostructures in Food Applications. In Innovative Food Processing Technologies; Knoerzer, K., Muthukumarappan, K., Eds.; Elsevier: Oxford, UK, 2021; pp. 565–579. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadidi, N.; Saffari, M.; Faizi, M. Optimized Transferosomal Bovine Lactoferrin (BLF) as a Promising Novel Non-Invasive Topical Treatment for Genital Warts Caused by Human Papiluma Virus (HPV). Iran. J. Pharm. Res. 2018, 17, 12–23. [Google Scholar] [PubMed]
- Fathi-Azarbayjani, A.; Ng, K.X.; Chan, Y.W.; Chan, S.Y. Lipid Vesicles for the Skin Delivery of Diclofenac: Cerosomes vs. Other Lipid Suspensions. Adv. Pharm. Bull. 2015, 5, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Asghar, A.; Randhawa, M.A.; Masood, M.M.; Abdullah, M.; Irshad, M.A. Chapter 10-nutraceutical formulation strategies to enhance the bioavailability and efficiency: An overview. In Role of Materials Science in Food Bioengineering; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Waltham, MA, USA, 2018; pp. 329–352. [Google Scholar] [CrossRef]
- Naik, U.S. The Synthesis and Characterisation of Novel Ultra-Flexible Lipidic Vesicles Using Propanol; University of Central Lancashire: Preston, UK, 2013. [Google Scholar]
- Bhushan Rajendra, R.; Nayan Ashok, G. Transfersomes and Protransfersome: Ultradeformable Vesicular System. In Novel Approaches for Drug Delivery; Raj, K.K., Anil, K.S., Rajesh Kumar, K., Eds.; IGI Global: Hershey, PA, USA, 2017; pp. 149–169. [Google Scholar] [CrossRef]
- Kumar, S.; Baldi, A.; Sharma, D. Phytosomes: A Modernistic Approach for Novel Herbal Drug Delivery—Enhancing Bioavailability and Revealing Endless Frontier ofPhytopharmaceuticals. J. Dev. Drugs 2019, 9, 1–8. [Google Scholar]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mouhid, L.; Corzo-Martínez, M.; Torres, C.; Vázquez, L.; Reglero, G.; Fornari, T.; de Molina, A.R. ImprovingIn VivoEfficacy of Bioactive Molecules: An Overview of Potentially Antitumor Phytochemicals and Currently Available Lipid-Based Delivery Systems. J. Oncol. 2017, 2017, 1–34. [Google Scholar] [CrossRef] [Green Version]
- Fernández-García, R.; Statts, L.; De Jesus, J.A.; Ayuela, M.A.D.; Bautista, L.; Simão, R.; Bolás-Fernández, F.; Ballesteros, M.P.; Laurenti, M.D.; Passero, L.F.D.; et al. Ultradeformable Lipid Vesicles Localize Amphotericin B in the Dermis for the Treatment of Infectious Skin Diseases. ACS Infect. Dis. 2020, 6, 2647–2660. [Google Scholar] [CrossRef]
- Zhang, H. Thin-Film Hydration Followed by Extrusion Method for Liposome Preparation. In Liposomes: Methods and Protocols; D’Souza, G.G.M., Ed.; Springer: New York, NY, USA, 2017; pp. 17–22. [Google Scholar] [CrossRef]
- Olga Popovska, J.; Kavrakovski, Z.; Rafajlovska, V. An Overview: Methods for Preparation and Characterization of Liposomes as Drug Delivery Systems. Int. J. Pharm. Phytopharmacol. 2013, 3, 13–20. [Google Scholar]
- Karole, S.; Gupta, G. Preparation and evaluation of phytosomes containing ethanolic extract of leaves of Bombax ceiba for hepatoprotective activity. Evaluation 2019, 6, 1–5. [Google Scholar]
- Dini, I.; Laneri, S. The New Challenge of Green Cosmetics: Natural Food Ingredients for Cosmetic Formulations. Molecules 2021, 26, 3921. [Google Scholar] [CrossRef] [PubMed]
- Chanchal, D.; Swarnlata, S. Novel approaches in herbal cosmetics. J. Cosmet. Dermatol. 2008, 7, 89–95. [Google Scholar] [CrossRef]
- Tessema, E.N.; Gebre-Mariam, T.; Neubert, R.H.; Wohlrab, J. Potential Applications of Phyto-Derived Ceramides in Improving Epidermal Barrier Function. Skin Pharmacol. Physiol. 2017, 30, 115–138. [Google Scholar] [CrossRef] [Green Version]
- Haque, T.; Talukder, M.U. Chemical Enhancer: A Simplistic Way to Modulate Barrier Function of the Stratum Corneum. Adv. Pharm. Bull. 2018, 8, 169–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patzelt, A.; Antoniou, C.; Sterry, W.; Lademann, J. Skin penetration from the inside to the outside: A review. Drug Discov. Today Dis. Mech. 2008, 5, e229–e235. [Google Scholar] [CrossRef]
- Bombardelli, E.; Spelta, M. Phospholipid-polyphenol complexes: A new concept in skin care ingredients. Cosmet. Toilet. 1991, 106, 69–76. [Google Scholar]
- Tripathy, S.; Patel, D.K.; Barob, L.; Naira, S.K. A review on phytosomes, their characterization, advancement & potential for transdermal application. J. Drug Deliv. Ther. 2013, 3, 147–152. [Google Scholar] [CrossRef]
- Droy-Lefaix, M.T. Effect of the antioxidant action of Ginkgo biloba extract (EGb 761) on aging and oxidative stress. Age 1997, 20, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Loggia, R.d.; Sosa, S.; Tubaro, A.; Morazzoni, P.; Bombardelli, E.; Griffini, A. Anti-inflammatory activity of some Ginkgo biloba constituents and of their phospholipid-complexes. Fitoterapia 1996, 67, 257–264. [Google Scholar]
- Chen, Z.-P.; Sun, J.; Chen, H.-X.; Xiao, Y.-Y.; Liu, D.; Chen, J.; Cai, H.; Cai, B.-C. Comparative pharmacokinetics and bioavailability studies of quercetin, kaempferol and isorhamnetin after oral administration of Ginkgo biloba extracts, Ginkgo biloba extract phospholipid complexes and Ginkgo biloba extract solid dispersions in rats. Fitoterapia 2010, 81, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.; Haskell, C.F.; Mauri, P.; Scholey, A.B. Acute cognitive effects of standardised Ginkgo biloba extract complexed with phosphatidylserine. Hum. Psychopharmacol. Clin. Exp. 2007, 22, 199–210. [Google Scholar] [CrossRef]
- Maramaldi, G.; Togni, S.; Pagin, I.; Giacomelli, L.; Cattaneo, R.; Eggenhöffner, R.; Burastero, S.E. Clin. CosmetSoothing and anti-itch effect of quercetin phytosome in human subjects: A single-blind study. Clin. Cosmet. Investig. Dermatol. 2016, 9, 55–62. [Google Scholar] [CrossRef] [Green Version]
- El-Fattah, A.I.A.; Fathy, M.M.; Ali, Z.Y.; El-Garawany, A.E.-R.A.; Mohamed, E.K. Enhanced therapeutic benefit of quercetin-loaded phytosome nanoparticles in ovariectomized rats. Chem. Interact. 2017, 271, 30–38. [Google Scholar] [CrossRef]
- Okonogi, S.; Riangjanapatee, P. Physicochemical characterization of lycopene-loaded nanostructured lipid carrier formulations for topical administration. Int. J. Pharm. 2015, 478, 726–735. [Google Scholar] [CrossRef]
- Sharma, P.K.S.P.; Saxena, P.; Jaswanth, A.; Chalamaiah, M.; Tekade, K.R.; Balasubramaniam, A. Novel encapsulation of lycopene in niosomes and assessment of its anticancer activity. J. Bioequivalence Bioavailab. 2016, 8, 224–232. [Google Scholar]
- Ghazi, A.M.; Al-Bayati, M.A. Anti-proliferative of the phytosome propolis, phytosome lycopene and synergistic effect on the benign prostatic hyperplasia cells in-vitro. Plant Arch. 2020, 20, 6579–6589. [Google Scholar]
- De Granada-Flor, A.; Sousa, C.; Filipe, H.A.L.; Santos, M.S.C.S.; De Almeida, R.F.M. Quercetin dual interaction at the membrane level. Chem. Commun. 2019, 55, 1750–1753. [Google Scholar] [CrossRef] [PubMed]
- Pawlikowska-Pawlęga, B.; Gruszecki, W.I.; Misiak, L.; Paduch, R.; Piersiak, T.; Zarzyka, B.; Pawelec, J.; Gawron, A. Modification of membranes by quercetin, a naturally occurring flavonoid, via its incorporation in the polar head group. Biochim. Biophys. Acta Biomembr. 2007, 1768, 2195–2204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emiliano, A.; Veronica, B.; Walter, V.; Elena Del, B.; Marzia, C. Oral Curcumin (Meriva) Is Effective as an Adjuvant Treatment and Is Able to Reduce IL-22 Serum Levels in Patients with Psoriasis Vulgaris. BioMed Res. Int. 2015, 3, 1–7. [Google Scholar] [CrossRef]
- Djekic, L.; Krajišnik, D.; Mićic, Z.; Čalija, B. Formulation and physicochemical characterization of hydrogels with 18β-glycyrrhetinic acid/phospholipid complex phytosomes. J. Drug Deliv. Sci. Technol. 2016, 35, 81–90. [Google Scholar] [CrossRef]
- McNulty, H.P.; Byun, J.; Lockwood, S.F.; Jacob, R.F.; Mason, R.P. Differential effects of carotenoids on lipid peroxidation due to membrane interactions: X-ray diffraction analysis. Biochim. Biophys. Acta Biomembr. 2007, 1768, 167–174. [Google Scholar] [CrossRef] [Green Version]
- Khan, J.; Alexander, A.; Uddin, A.; Saraf, S.; Saraf, S. Recent advances and future prospects of phyto-phospholipid complexation technique for improving pharmacokinetic profile of plant actives. J. Control. Release 2013, 168, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, P.; Strzalka, K.; Kostecka-Gugala, A. Comparative X-Ray Studies on the Interaction of Carotenoids with a Model Phosphatidylcholine Membrane. Z. Nat. C 2002, 57, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Djekic, L.; Krajisnik, D.; Micic, Z. Polyphenolics-Phospholipid Complexes as Natural Cosmetic Ingredients: Properties and Application. Tenside Surfactants Deterg. 2015, 52, 186–192. [Google Scholar] [CrossRef]
- Cao, F.-H.; Ouyang, W.-Q.; Wang, Y.-P.; Yue, P.-F.; Li, S.-P. A combination of a microemulsion and a phospholipid complex for topical delivery of oxymatrine. Arch. Pharmacal Res. 2011, 34, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.; Yang, Y.; Wang, Q.; Wang, Y.; Wen, J.; Zhang, Y. Anti-inflammatory effects of oxymatrine on rheumatoid arthritis in rats via regulating the imbalance between Treg and Th17 cells. Mol. Med. Rep. 2017, 15, 3615–3622. [Google Scholar] [CrossRef] [Green Version]
- Iram, F.; Khan, S.; Husain, A. Phytochemistry and potential therapeutic actions of Boswellic acids: A mini-review. Asian Pac. J. Trop. Biomed. 2017, 7, 513–523. [Google Scholar] [CrossRef]
- Hüsch, J.; Gerbeth, K.; Fricker, G.; Setzer, C.; Zirkel, J.; Rebmann, H.; Schubert-Zsilavecz, M.; Abdel-Tawab, M. Effect of Phospholipid-Based Formulations of Boswellia serrataExtract on the Solubility, Permeability, and Absorption of the Individual Boswellic Acid Constituents Present. J. Nat. Prod. 2012, 75, 1675–1682. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, N.K.; Dixit, V.K. Complexation with phosphatidyl choline as a strategy for absorption enhancement of boswellic acid. Drug Deliv. 2010, 17, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.J.; Sung, J.J.; Cheon, K.K.; Tae, H.J. Anti-inflammatory effect of Centella asiatica phytosome in a mouse model of phthalic anhydride-induced atopic dermatitis. Phytomedicine 2018, 43, 110–119. [Google Scholar] [CrossRef]
- Gray, N.E.; Magana, A.A.; Lak, P.; Wright, K.M.; Quinn, J.; Stevens, J.F.; Maier, C.S.; Soumyanath, A. Centella asiatica: Phytochemistry and mechanisms of neuroprotection and cognitive enhancement. Phytochem. Rev. 2017, 17, 161–194. [Google Scholar] [CrossRef] [PubMed]
- Bombardelli, E.; Cristoni, A.; Morazzoni, P. Phytosome® s in functional cosmetics. Fitoterapia 1994, 65, 387–401. [Google Scholar]
- Darvishi, B.; Manoochehri, S.; Kamalinia, G.; Samadi, N.; Amini, M.; Mostafavi, S.H.; Maghazei, S.; Atyabi, F.; Dinarvand, R. Preparation and Antibacterial Activity Evaluation of 18-β-glycyrrhetinic Acid Loaded PLGA Nanoparticles. Iran. J. Pharm. Res. 2015, 14, 373–383. [Google Scholar]
- Anitha, V.; Reddy, P.D.; Ramkanth, S. Phytosomes: A promising technology in novel herbal drug delivery system. PharmaTutor 2019, 7, 18–25. [Google Scholar]
- Agarwal, A.; Chakraborty, P.; Chakraborty, D.D.; Saharan, V.A.S. Phytosomes: Complexation, Utilisation and Commerical Status. J. Biol. Act. Prod. Nat. 2012, 2, 65–77. [Google Scholar] [CrossRef]
- Babazadeh, A.; Zeinali, M.; Hamishehkar, H. Nano-Phytosome: A Developing Platform for Herbal Anti-Cancer Agents in Cancer Therapy. Curr. Drug Targets 2018, 19, 170–180. [Google Scholar] [CrossRef]

| Phytosome Formulation | Source Plant | Use | Reference |
|---|---|---|---|
| Silybin | Silymarin marium | Hepatoprotective and antioxidant activities | [73,74,75] |
| Ginkgo | Ginkgo biloba | Brain and vascular protection | [29,76] |
| Olive oil | Europaea oil | Anti-inflammatory, antioxidant, anti-hyperlipidemic activities and cardiovascular protection | [77] |
| Centella | Centella asiatica | Vein and skin disorders | [78] |
| Greenselect | Camellia sinensis | Antioxidant activity | [79] |
| Rutin | Ruta graveolens Sophora japonica | Rheumatoid arthritis | [80] |
| Curcumin | Curcuma longa | Hepatoprotective activity | [81,82] |
| Leucoselect | Vitis vinifera | Antioxidant activity | [28] |
| Ecdhinacea | Echinacea augustifolia | Immunomodulator | [83] |
| Cartaegus | Cartaegus mexicana | Antioxidant activity | [84] |
| Haw thorn | Carteagus species | Antihypertensive activity | [65] |
| Roscugenin | Ruscus aculeatus | Anti-inflammatory activity | [85] |
| Phytosome Formulation | Condition | Clinical Trial Phase and No. | Sudy Outcome | Reference |
|---|---|---|---|---|
| Silybin | Prostate cancer | Phase II (NCT00487721) | High blood concentration of silybin | [87,88] |
| Silybin | EGFR mutant lung adenocarcinoma | Phase II (NCT02146118) | Under investigation | - |
| Green tea extract | Obesity | Phase IV (NCT02542449) | Maintaing weight following weight loss | [89] |
| Grape seeds extract | Early stages lung cancer | Phase II (NCT04515004) | Delay planned surgery of >14 days | - |
| Bergamot | Hypercholesterolemia | Not applicable (NCT04697121) | Anti-hypercholesterolemic activity | - |
| Quercetin | COVID-19 | Phase III (NCT04578158) | Under investigation | - |
| Property | Phytosomes | Liposomes | Transferosomes | Reference |
|---|---|---|---|---|
| Structure | Lipid bilayer vesicles composed of different type of phospholipids that can chemically bound to phytochemicals | Lipid bilayer visecles composed of wider range of lipids including cationic, anionic, and neutral lipids | Lipid bilayer viscles composed of phospholipids, an edge activator (i.e., surfactant or bile salt ranging from 10–25%), low percentage of ethanol, and water as a vehicle | [55,91] |
| Encapsulation | The bioactive molecules are fixed by H-bond to the polar tip of the phospholipids | The active materials are incorporated in the aqueous core of the vesicles or in the lipid bilayer membrane | The active materials are incorporated in the aqueous core of the vesicles or in the lipid bilayer membrane | [55,97,98] |
| Preparation | The phosphatidylcholine and the phytochemicals actually form a 1:1 or 2:1 molecular complex that contains chemical bonds | The lipid compositions mixed alone, then the loaded materials added to the lipid thin film to form the complex with no chemical bonds formed | The lipid compositions mixed alone, then the loaded materials added to the lipid thin film to form the complex with no chemical bonds formed | [55,109,110] |
| Hydration buffer | Act with aprotic solvents such as acetone,1,4-dioxane, hexane, metyhlenechlorideand ethylacetate | Formed in the presence of a water or buffer solution | Formed in the presence of a water or buffer solution | [55,110] |
| Skin absorption | High skin absorption | Lower skin absorption | High skin absorption | [55,101] |
| Stability | High stability | Lower stability | High stability | [55,106] |
| Phytochemical | Type of Phytochemical | Type of Phospholipid | Chemical Interaction | Analysis Method | Reference |
|---|---|---|---|---|---|
| Quercetin | Polyphenols | PC | H-bonds with the polar group of the phosphplipid | 1H-NMR, 31P-NMR, 13C-NMR | [127] |
| Polyphenols | DPPC 1 (PC) | (1) Electrostatic interactions, (2) H-bonds with the polar group of the phosphplipid, (3) Hydrophobic interaction with fatty acyl chains | 1H-NMR, 31P-NMR, 13C-NMR | [128] | |
| Lycopene | Carotenoids (Terpenoid) | DPPC (PC) | Hydrophobic interaction with the acyl fatty acid chain | X-Ray | [133] |
| β-carotene, Lycopene | Carotenoids (Terpenoid) | POPC 2 (PC) | Hydrophobic interaction with the acyl fatty acid chain | X-Ray | [131] |
| Tyrosol, Verbascoside, Hydroxytyrosol | Polyphenols | PC | H-bonds with the polar group of the phosphplipid | 1H-NMR, 31P-NMR, 13C-NMR | [33] |
| Saponin | Triterpene glycosides | PC | H-bonds with the polar group of the phosphplipid | 1H-NMR, 31P-NMR, 13C-NMR | [15] |
| 18-β-glycyrrhetinic Acid | Triterpenoids | Soy lecithin (PC) | H-bonds with the polar group of the phosphplipid | DSC | [132] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharbi, W.S.; Almughem, F.A.; Almehmady, A.M.; Jarallah, S.J.; Alsharif, W.K.; Alzahrani, N.M.; Alshehri, A.A. Phytosomes as an Emerging Nanotechnology Platform for the Topical Delivery of Bioactive Phytochemicals. Pharmaceutics 2021, 13, 1475. https://doi.org/10.3390/pharmaceutics13091475
Alharbi WS, Almughem FA, Almehmady AM, Jarallah SJ, Alsharif WK, Alzahrani NM, Alshehri AA. Phytosomes as an Emerging Nanotechnology Platform for the Topical Delivery of Bioactive Phytochemicals. Pharmaceutics. 2021; 13(9):1475. https://doi.org/10.3390/pharmaceutics13091475
Chicago/Turabian StyleAlharbi, Waleed S., Fahad A. Almughem, Alshaimaa M. Almehmady, Somayah J. Jarallah, Wijdan K. Alsharif, Nouf M. Alzahrani, and Abdullah A. Alshehri. 2021. "Phytosomes as an Emerging Nanotechnology Platform for the Topical Delivery of Bioactive Phytochemicals" Pharmaceutics 13, no. 9: 1475. https://doi.org/10.3390/pharmaceutics13091475






