Green and Scalable Fabrication of Sandwich-like NG/SiOx/NG Homogenous Hybrids for Superior Lithium-Ion Batteries
Abstract
:1. Introduction
2. Experimental Section
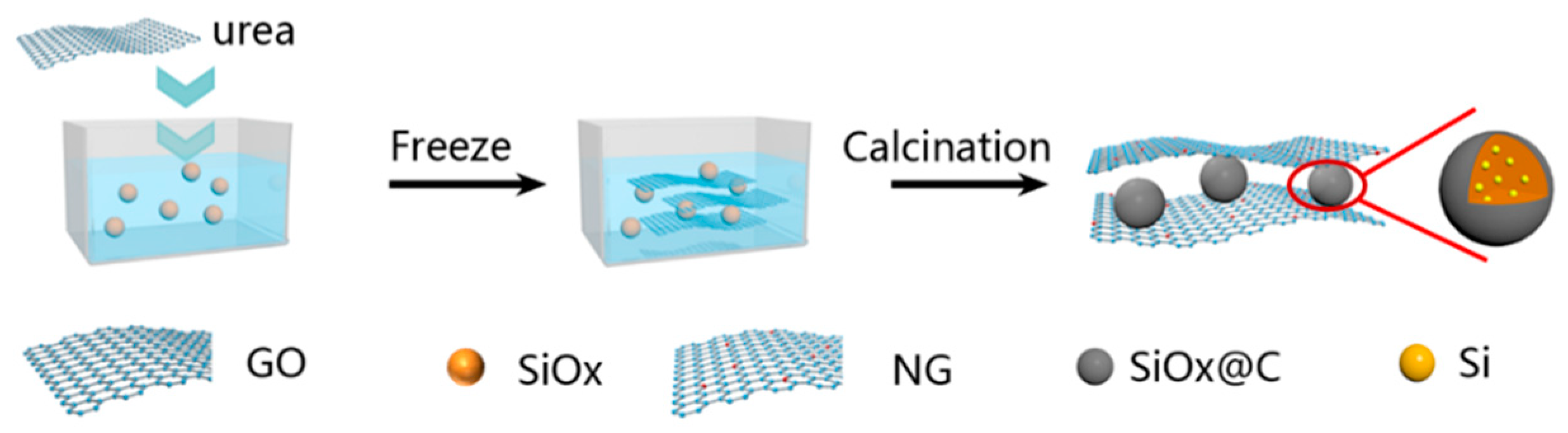
2.1. Material Preparation
2.2. Material Characterizations
2.3. Electrochemical Characterization
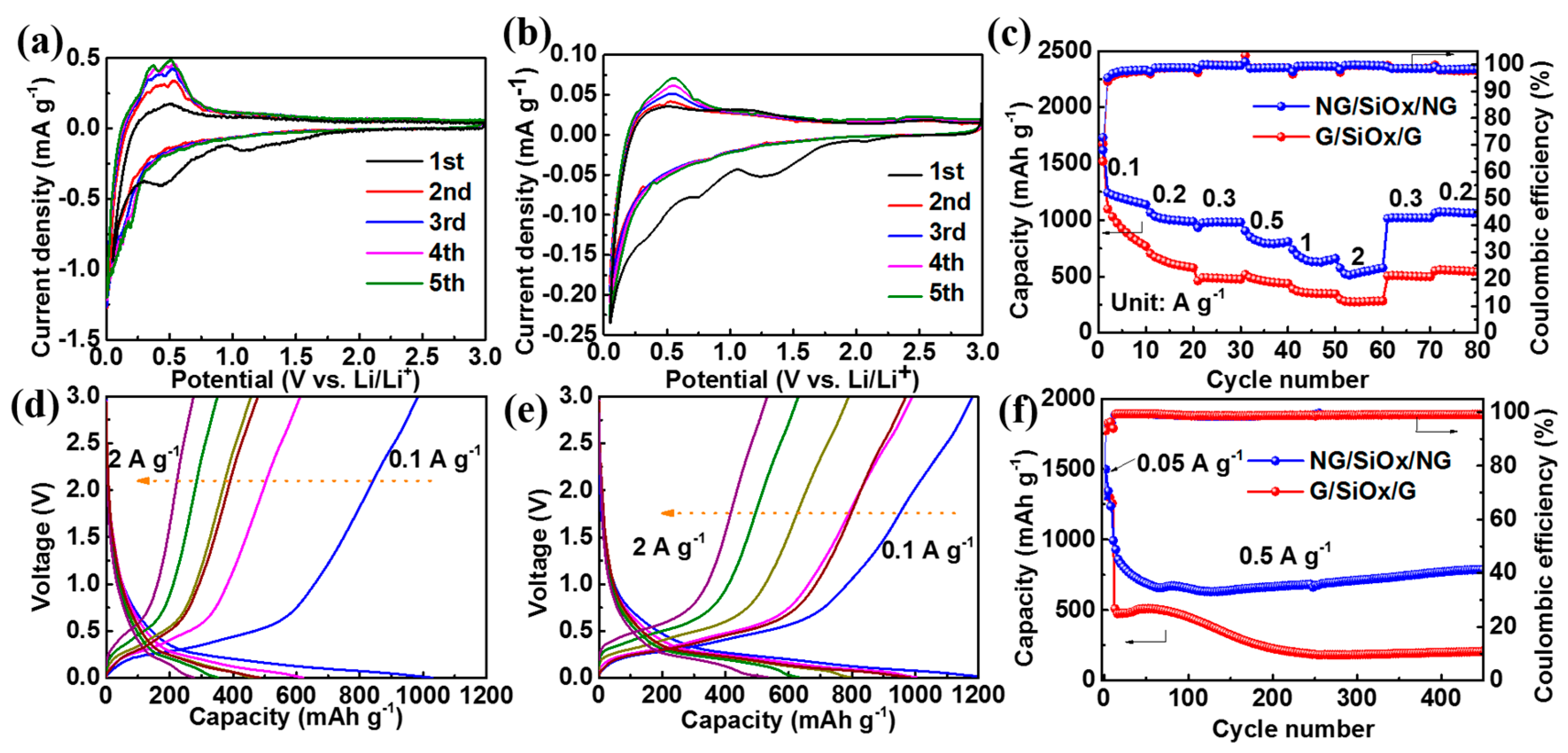
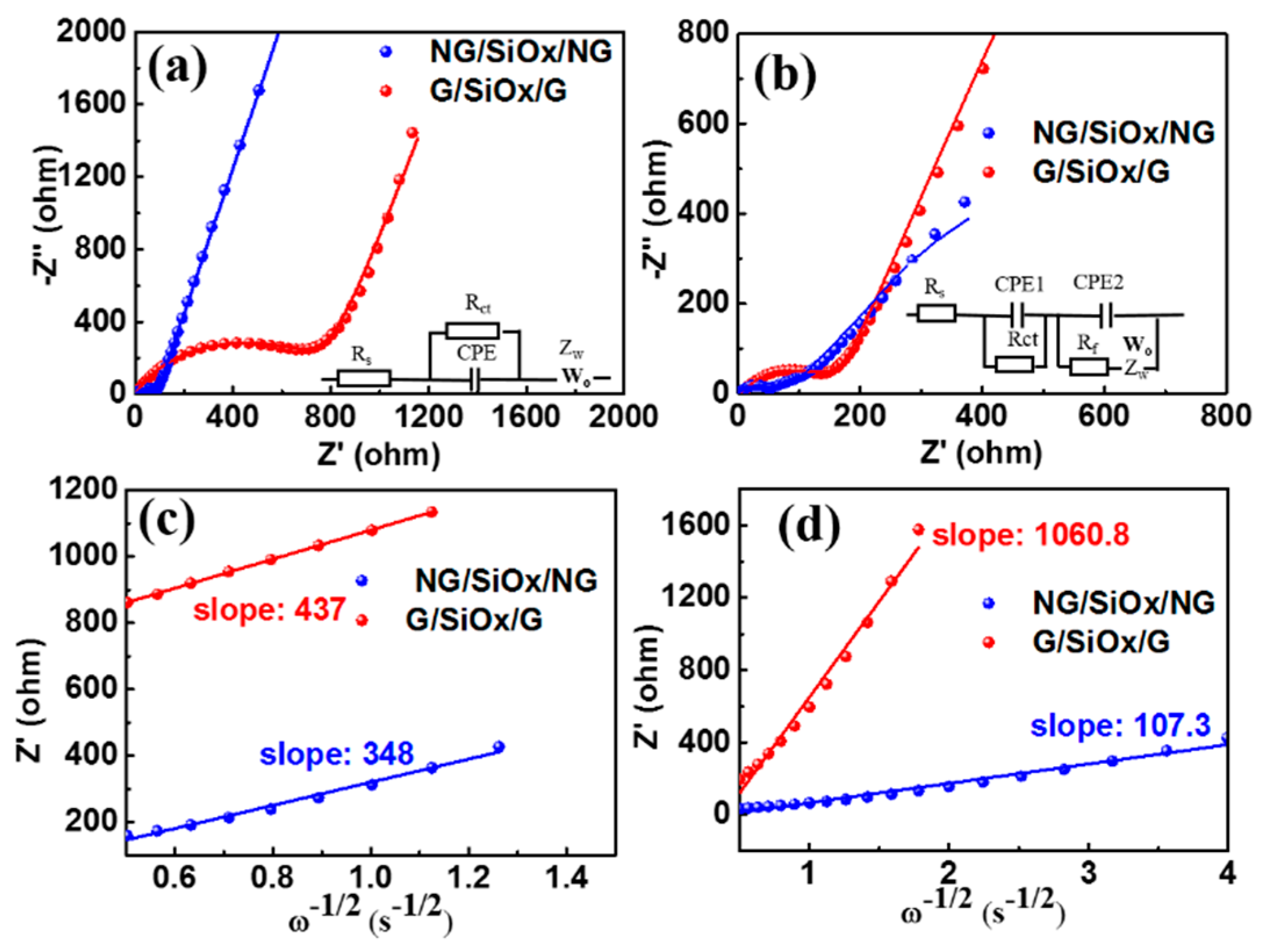
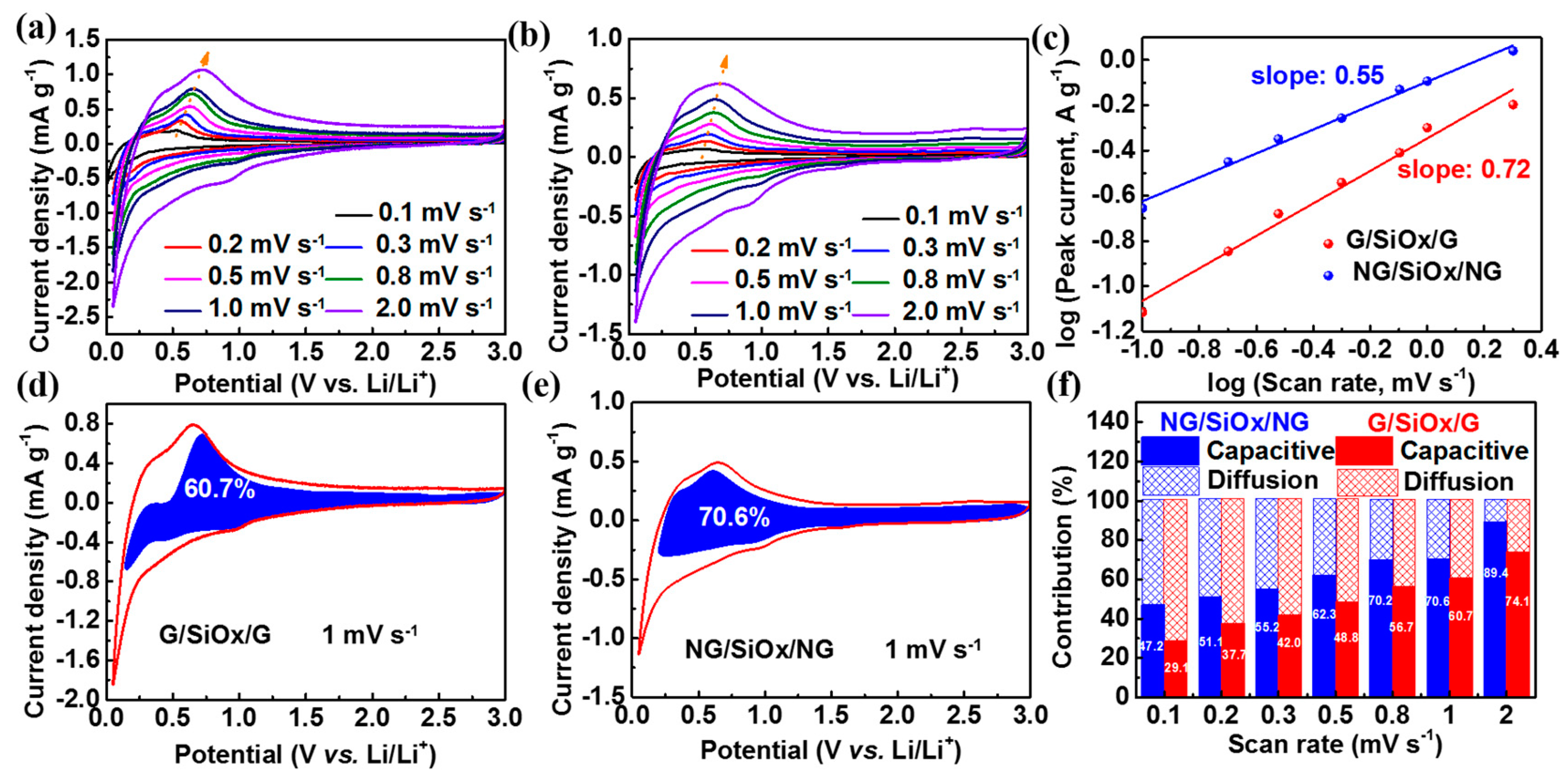
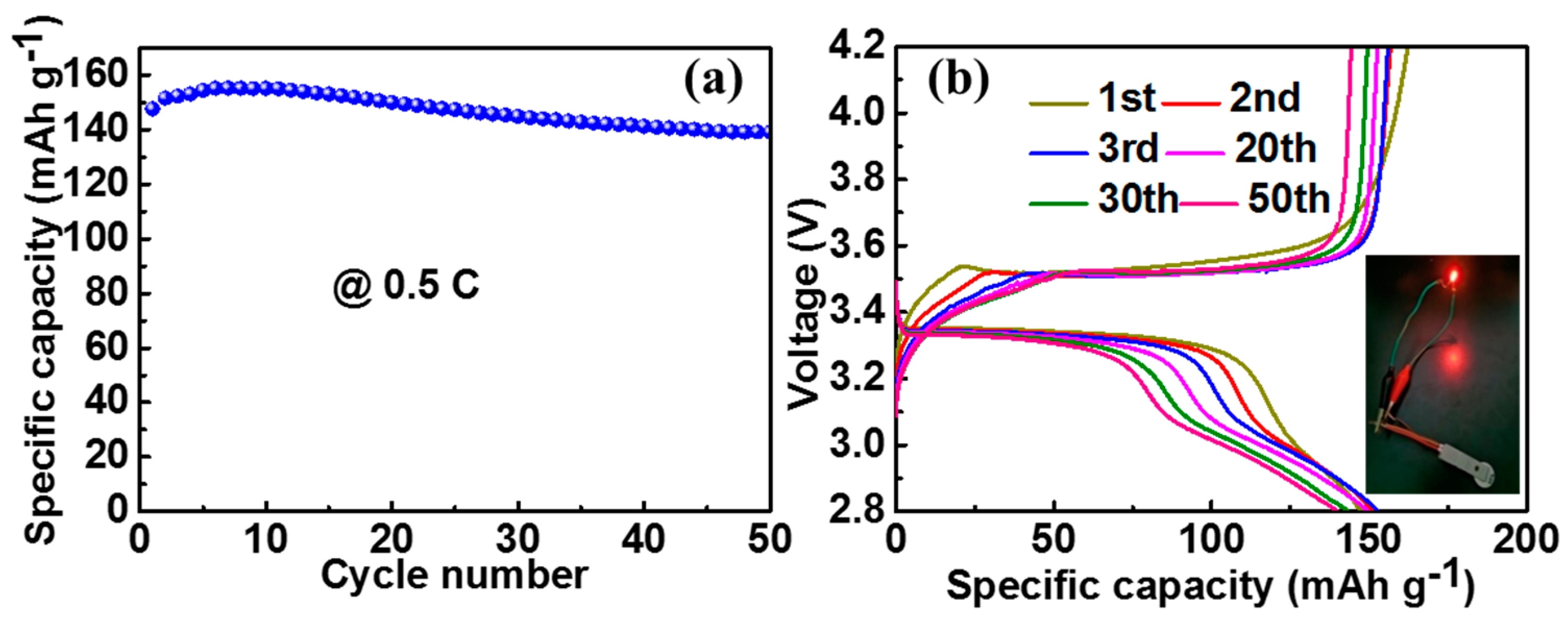
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xiao, Z.; Yu, C.; Lin, X.; Chen, X.; Zhang, C.; Jiang, H.; Zhang, R.; Wei, F. TiO2 as a multifunction coating layer to enhance the electrochemical performance of SiOx@TiO2@C composite as anode material. Nano Energy 2020, 77, 105082. [Google Scholar] [CrossRef]
- Park, E.; Yoo, H.; Lee, J.; Park, M.-S.; Kim, Y.-J.; Kim, H. Dual-Size Silicon Nanocrystal-Embedded SiOx Nanocomposite as a High-Capacity Lithium Storage Material. ACS Nano 2015, 9, 7690–7696. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, H.; Lv, P.; Zhang, Z.; Zhang, Y.; Du, Z.; Teng, Y.; Zhao, L.; Zhu, Z. Watermelon-Like Structured SiOx -TiO2 @C Nanocomposite as a High-Performance Lithium-Ion Battery Anode. Adv. Funct. Mater. 2018, 28, 1605711. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Y.; Wei, H.-J.; Li, T.-F.; Xiong, X.-H.; Wei, S.-Z.; Ren, F.-Z.; Volinsky, A.A. Recent advances and perspective in metal coordination materials-based electrode materials for potassium-ion batteries. Rare Met. 2021, 40, 448–470. [Google Scholar] [CrossRef]
- Wu, N.; Miao, D.; Zhou, X.; Zhang, L.; Liu, G.; Guo, D.; Liu, X. V3S4 Nanosheets Anchored on N, S Co-Doped Graphene with Pseudocapacitive Effect for Fast and Durable Lithium Storage. Nanomaterials 2019, 9, 1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.; Li, M.; Wu, N.; Cui, L.; Huang, X.; Liu, X.; Zhao, Y.; Chen, H.; Yuan, W.; Bai, Y. Single-Crystalline Particles: An Effective Way to Ameliorate the Intragranular Cracking, Thermal Stability, and Capacity Fading of the LiNi0.6Co0.2Mn0.2O2 Electrodes. J. Electrochem. Soc. 2018, 165, A3040–A3047. [Google Scholar] [CrossRef]
- Li, P.; Kim, H.; Myung, S.-T.; Sun, Y.-K. Diverting Exploration of Silicon Anode into Practical Way: A Review Focused on Silicon-Graphite Composite for Lithium Ion Batteries. Energy Storage Mater. 2021, 35, 550–576. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, Q.; Zhao, Y.; He, R.; Xu, M.; Feng, S.; Li, S.; Zhou, L.; Mai, L. Silicon oxides: A promising family of anode materials for lithium-ion batteries. Chem. Soc. Rev. 2019, 48, 285–309. [Google Scholar] [CrossRef]
- Sui, D.; Chang, M.; Wang, H.; Qian, H.; Yang, Y.; Li, S.; Zhang, Y.; Song, Y. A Brief Review of Catalytic Cathode Materials for Na-CO2 Batteries. Catalysts 2021, 11, 603. [Google Scholar] [CrossRef]
- Zhang, Y.; Mu, Z.; Lai, J.; Chao, Y.; Yang, Y.; Zhou, P.; Li, Y.; Yang, W.; Xia, Z.; Guo, S. MXene/Si@SiOx@C Layer-by-Layer Superstructure with Autoadjustable Function for Superior Stable Lithium Storage. ACS Nano 2019, 13, 2167–2175. [Google Scholar] [CrossRef]
- Chang, W.-S.; Park, C.-M.; Kim, J.-H.; Kim, Y.-U.; Jeong, G.; Sohn, H.-J. Quartz (SiO2): A new energy storage anode material for Li-ion batteries. Energy Environ. Sci. 2012, 5, 6895–6899. [Google Scholar] [CrossRef]
- Liu, W.; Yao, T.; Xie, S.; She, Y.; Wang, H. Integrating TiO2/SiO2 into Electrospun Carbon Nanofibers towards Superior Lithium Storage Performance. Nanomaterials 2019, 9, 68. [Google Scholar] [CrossRef] [Green Version]
- Pang, H.; Zhang, W.; Yu, P.; Pan, N.; Hu, H.; Zheng, M.; Xiao, Y.; Liu, Y.; Liang, Y. Facile Synthesis of Core-Shell Structured SiO2@Carbon Composite Nanorods for High-Performance Lithium-Ion Batteries. Nanomaterials 2020, 10, 513. [Google Scholar] [CrossRef] [Green Version]
- Zeng, S.-Z.; Niu, Y.; Zou, J.; Zeng, X.; Zhu, H.; Huang, J.; Wang, L.; Kong, L.B.; Han, P. Green and scalable preparation of disproportionated SiO anode materials with cocoon-like buffer layer. J. Power Sources 2020, 466, 228234. [Google Scholar] [CrossRef]
- Meng, Q.; Li, G.; Yue, J.; Xu, Q.; Yin, Y.-X.; Guo, Y.-G. High-Performance Lithiated SiOx Anode Obtained by a Controllable and Efficient Prelithiation Strategy. ACS Appl. Mater. Interfaces 2019, 11, 32062–32068. [Google Scholar] [CrossRef] [PubMed]
- Mu, G.; Mu, D.; Wu, B.; Ma, C.; Bi, J.; Zhang, L.; Yang, H.; Wu, F. Microsphere-Like SiO2/MXene Hybrid Material Enabling High Performance Anode for Lithium Ion Batteries. Small 2019, 16, 1905430. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhang, H.; Li, X.; Du, Y.; Hou, G.; Xiang, M.; Lv, P.; Zhu, Q. In situ fabrication of dual coating structured SiO/1D-C/a-C composite as high-performance lithium ion battery anode by fluidized bed chemical vapor deposition. Carbon 2020, 168, 113–124. [Google Scholar] [CrossRef]
- Tang, C.; Liu, Y.; Xu, C.; Zhu, J.; Wei, X.; Zhou, L.; He, L.; Yang, W.; Mai, L. Ultrafine Nickel-Nanoparticle-Enabled SiO2 Hierarchical Hollow Spheres for High-Performance Lithium Storage. Adv. Funct. Mater. 2018, 28, 1704561. [Google Scholar] [CrossRef]
- Xu, Q.; Sun, J.; Yu, Z.; Yin, Y.; Xin, S.; Yu, S.; Guo, Y. SiOx Encapsulated in Graphene Bubble Film: An Ultrastable Li-Ion Battery Anode. Adv. Mater. 2018, 30, e1707430. [Google Scholar] [CrossRef]
- Lee, J.; Moon, J.; Han, S.A.; Kim, J.; Malgras, V.; Heo, Y.-U.; Kim, H.; Lee, S.-M.; Liu, H.K.; Dou, S.X.; et al. Everlasting Living and Breathing Gyroid 3D Network in Si@SiOx/C Nanoarchitecture for Lithium Ion Battery. ACS Nano 2019, 13, 9607–9619. [Google Scholar] [CrossRef]
- Xu, T.; Wang, Q.; Zhang, J.; Xie, X.; Xia, B. Green Synthesis of Dual Carbon Conductive Network-Encapsulated Hollow SiOx Spheres for Superior Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 19959–19967. [Google Scholar] [CrossRef]
- Liu, Q.; Cui, Z.; Zou, R.; Zhang, J.; Xu, K.; Hu, J. Surface Coating Constraint Induced Anisotropic Swelling of Silicon in Si-Void@SiOxNanowire Anode for Lithium-Ion Batteries. Small 2017, 13, 1603754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Weng, Y.; Shen, W.; Lv, R.; Kang, F.; Huang, Z.-H. Scalable synthesis of lotus-seed-pod-like Si/SiOx@CNF: Applications in freestanding electrode and flexible full lithium-ion batteries. Carbon 2020, 158, 163–171. [Google Scholar] [CrossRef]
- Huang, X.; Tang, J.; Luo, B.; Knibbe, R.; Lin, T.; Hu, H.; Rana, M.; Hu, Y.; Zhu, X.; Gu, Q.; et al. Sandwich-Like Ultrathin TiS2 Nanosheets Confined within N, S Codoped Porous Carbon as an Effective Polysulfide Promoter in Lithium-Sulfur Batteries. Adv. Energy Mater. 2019, 9. [Google Scholar] [CrossRef]
- Jiang, Y.; Song, D.; Wu, J.; Wang, Z.; Huang, S.; Xu, Y.; Chen, Z.; Zhao, B.; Zhang, J. Sandwich-like SnS2/Graphene/SnS2 with Expanded Interlayer Distance as High-Rate Lithium/Sodium-Ion Battery Anode Materials. ACS Nano 2019, 13, 9100–9111. [Google Scholar] [CrossRef]
- Huang, J.; Meng, R.; Zu, L.; Wang, Z.; Feng, N.; Yang, Z.; Yu, Y.; Yang, J. Sandwich-like Na0.23TiO2 nanobelt/Ti3C2 MXene composites from a scalable in situ transformation reaction for long-life high-rate lithium/sodium-ion batteries. Nano Energy 2018, 46, 20–28. [Google Scholar] [CrossRef]
- Liu, R.; Shen, C.; Dong, Y.; Qin, J.; Wang, Q.; Iocozzia, J.; Zhao, S.; Yuan, K.; Han, C.; Li, B.; et al. Sandwich-like CNTs/Si/C nanotubes as high performance anode materials for lithium-ion batteries. J. Mater. Chem. A 2018, 6, 14797–14804. [Google Scholar] [CrossRef]
- Guo, X.; Wang, S.; Yang, B.; Xu, Y.; Liu, Y.; Pang, H. Porous pyrrhotite Fe7S8 nanowire/SiO/nitrogen-doped carbon matrix for high-performance Li-ion-battery anodes. J. Colloid Interface Sci. 2020, 561, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Im, J.; Jang, E.K.; Kim, S.; Yoon, S.; Kim, D.-H.; Cho, K.Y. Two-dimensional, P-doped Si/SiOx alternating veneer-like microparticles for high-capacity lithium-ion battery composite. Chem. Eng. J. 2020, 402, 126292. [Google Scholar] [CrossRef]
- Sui, D.; Xu, L.; Zhang, H.; Sun, Z.; Kan, B.; Ma, Y.; Chen, Y. A 3D cross-linked graphene-based honeycomb carbon composite with excellent confinement effect of organic cathode material for lithium-ion batteries. Carbon 2020, 157, 656–662. [Google Scholar] [CrossRef]
- Guo, C.; Wang, D.; Liu, T.; Zhu, J.; Lang, X. A three dimensional SiOx/C@RGO nanocomposite as a high energy anode material for lithium-ion batteries. J. Mater. Chem. A 2014, 2, 3521–3527. [Google Scholar] [CrossRef]
- Wu, N.; Tian, W.; Shen, J.; Qiao, X.; Sun, T.; Wu, H.; Zhao, J.; Liu, X.; Zhang, Y. Facile fabrication of a jarosite ultrathin KFe3(SO4)2(OH)6@rGO nanosheet hybrid composite with pseudocapacitive contribution as a robust anode for lithium-ion batteries. Inorg. Chem. Front. 2018, 6, 192–198. [Google Scholar] [CrossRef]
- Bai, X.; Yu, Y.; Kung, H.H.; Wang, B.; Jiang, J. Si@SiOx/graphene hydrogel composite anode for lithium-ion battery. J. Power Sources 2016, 306, 42–48. [Google Scholar] [CrossRef]
- Alam, S.N.; Sharma, N.; Kumar, L. Synthesis of Graphene Oxide (GO) by Modified Hummers Method and Its Thermal Reduction to Obtain Reduced Graphene Oxide (rGO). Graphene 2017, 06, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Sharma, N.; Sharma, V.; Jain, Y.; Kumari, M.; Gupta, R.; Sharma, S.K.; Sachdev, K. Synthesis and Characterization of Graphene Oxide (GO) and Reduced Graphene Oxide (rGO) for Gas Sensing Application. Macromol. Symp. 2017, 376, 1700006. [Google Scholar] [CrossRef]
- Majeed, M.K.; Ma, G.; Cao, Y.; Mao, H.; Ma, X.; Ma, W. Metal–Organic Frameworks-Derived Mesoporous Si/SiOx@NC Nanospheres as a Long-Lifespan Anode Material for Lithium-Ion Batteries. Chem. A Eur. J. 2019, 25, 11991–11997. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Li, Y.; Wu, Y.; Shen, C.; Fan, C.; Liu, Z. Controlling siloxene oxidization to tailor SiOx anodes for high performance lithium ion batteries. J. Power Sources 2019, 432, 65–72. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, M.; Tan, G.; Chen, S.; Wu, F.; Lu, J.; Amine, K. Encapsulating micro-nano Si/SiOx into conjugated nitrogen-doped carbon as binder-free monolithic anodes for advanced lithium ion batteries. Nanoscale 2015, 7, 8023–8034. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Zhang, S.; Niu, Y.; Song, H.; Chen, X.; Zhou, J.; Cao, B. Direct amination of Si nanoparticles for the preparation of Si@ultrathin SiOx@graphene nanosheets as high performance lithium-ion battery anodes. J. Mater. Chem. A 2015, 3, 19892–19900. [Google Scholar] [CrossRef]
- Kim, N.; Park, H.; Yoon, N.; Lee, J.K. Zeolite-Templated Mesoporous Silicon Particles for Advanced Lithium-Ion Battery Anodes. ACS Nano 2018, 12, 3853–3864. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Pang, C.; Chen, S.; Wang, M.; Wang, K.; Tan, Z.; Gao, P.; Ren, J.; Huang, Y.; Peng, H.; et al. Vertical Graphene Growth on SiO Microparticles for Stable Lithium Ion Battery Anodes. Nano Lett. 2017, 17, 3681–3687. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Cao, Y.; Suo, Y.; Liu, Y.; Zhang, J.; Zheng, X. Facile Fabrication of 3D SiO2@Graphene Aerogel Composites as Anode Material for Lithium Ion Batteries. Electrochim. Acta 2015, 176, 1001–1009. [Google Scholar] [CrossRef]
- Liu, G.; Wu, H.-H.; Meng, Q.; Zhang, T.; Sun, D.; Jin, X.; Guo, D.; Wu, N.; Liu, X.; Kim, J.-K. Role of the anatase/TiO2 (B) heterointerface for ultrastable high-rate lithium and sodium energy storage performance. Nanoscale Horiz. 2019, 5, 150–162. [Google Scholar] [CrossRef]
- Guo, C.; Xie, Y.; Pan, K.; Li, L. MOF-derived hollow SiOx nanoparticles wrapped in 3D porous nitrogen-doped graphene aerogel and their superior performance as the anode for lithium-ion batteries. Nanoscale 2020, 12, 13017–13027. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Qiao, X.; Shen, J.; Liu, G.; Sun, T.; Wu, H.; Hou, H.; Liu, X.; Zhang, Y.; Ji, X. Anatase inverse opal TiO2-x@N-doped C induced the dominant pseudocapacitive effect for durable and fast lithium/sodium storage. Electrochim. Acta 2019, 299, 540–548. [Google Scholar] [CrossRef]
- Zhao, L.; Wu, H.-H.; Yang, C.; Zhang, Q.; Zhong, G.; Zheng, Z.; Chen, H.; Wang, J.; He, K.; Wang, B.; et al. Mechanistic Origin of the High Performance of Yolk@Shell Bi2S3@N-Doped Carbon Nanowire Electrodes. ACS Nano 2018, 12, 12597–12611. [Google Scholar] [CrossRef]
- Liu, G.; Cui, J.; Luo, R.; Liu, Y.; Huang, X.; Wu, N.; Jin, X.; Chen, H.; Tang, S.; Kim, J.-K.; et al. 2D MoS2 grown on biomass-based hollow carbon fibers for energy storage. Appl. Surf. Sci. 2019, 469, 854–863. [Google Scholar] [CrossRef]
- Yu, Q.; Ge, P.; Liu, Z.; Xu, M.; Yang, W.; Zhou, L.; Zhao, D.; Mai, L. Ultrafine SiOx/C nanospheres and their pomegranate-like assemblies for high-performance lithium storage. J. Mater. Chem. A 2018, 6, 14903–14909. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, J.; Xue, L.; Huang, T.; Yu, A. Carbon-coated SiO2 nanoparticles as anode material for lithium ion batteries. J. Power Sources 2011, 196, 10240–10243. [Google Scholar] [CrossRef]
- Zhu, Y.; Hu, W.; Zhou, J.; Cai, W.; Lu, Y.; Liang, J.; Li, X.; Zhu, S.; Fu, Q.-Q.; Qian, Y. Prelithiated Surface Oxide Layer Enabled High-Performance Si Anode for Lithium Storage. ACS Appl. Mater. Interfaces 2019, 11, 18305–18312. [Google Scholar] [CrossRef]
- Hu, J.; Fu, L.; Rajagopalan, R.; Zhang, Q.; Luan, J.; Zhang, H.; Tang, Y.; Peng, Z.; Wang, H. Nitrogen Plasma-Treated Core–Bishell Si@SiOx@TiO2−δ: Nanoparticles with Significantly Improved Lithium Storage Performance. ACS Appl. Mater. Interfaces 2019, 11, 27658–27666. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, S.; Ding, Y.; Jiang, J.; Li, W.; Huang, S.; Chen, Z.; Zhao, B.; Zhang, J. Modification based on primary particle level to improve the electrochemical performance of SiO-based anode materials. J. Power Sources 2020, 467, 228301. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, X.; Tu, K.; Liu, D.; Tang, H.; Li, J.; Li, X.; Xie, Z.-Z.; Qu, D. Synthesis of carbon-SiO2 hybrid layer @ SiO2 @ CNT coaxial nanotube and its application in lithium storage. Electrochim. Acta 2020, 354, 136726. [Google Scholar] [CrossRef]
- Guo, D.; Yang, M.; Zhang, L.; Li, Y.; Wang, J.; Liu, G.; Wu, N.; Kim, J.-K.; Liu, X. Cr2O3 nanosheet/carbon cloth anode with strong interaction and fast charge transfer for pseudocapacitive energy storage in lithium-ion batteries. RSC Adv. 2019, 9, 33446–33453. [Google Scholar] [CrossRef] [Green Version]
- Xiao, T.; Zhang, W.; Xu, T.; Wu, J.; Wei, M. Hollow SiO2 microspheres coated with nitrogen doped carbon layer as an anode for high performance lithium-ion batteries. Electrochim. Acta 2019, 306, 106–112. [Google Scholar] [CrossRef]
- Guo, D.; Yang, M.; Li, Y.; Xue, Y.; Liu, G.; Wu, N.; Kim, J.-K.; Liu, X. Hydrogel-derived VPO4/porous carbon framework for enhanced lithium and sodium storage. Nanoscale 2020, 12, 3812–3819. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Hu, X.; Liu, Y.; Wen, Z. One-Step Low-Temperature Molten Salt Synthesis of Two-Dimensional Si@SiOx@C Hybrids for High-Performance Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 55844–55855. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, D.; Xu, Z.; Xu, J.; Lu, J.; Cao, J.; Ni, S. A scalable synthesis of 2D laminate Li3VO4/C for robust pseudocapacitive Li-ion storage. J. Mater. Chem. A 2020, 8, 21122–21130. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, Z.; Zhou, Z.; Xi, S.; Xia, Y.; Zhang, Q.; Huang, L.; Mei, L.; Jiang, Y.; Gao, J.; et al. A Safe Flexible Self-Powered Wristband System by Integrating Defective MnO2−x Nanosheet-Based Zinc-Ion Batteries with Perovskite Solar Cells. ACS Nano 2021, 15, 10597–10608. [Google Scholar] [CrossRef] [PubMed]
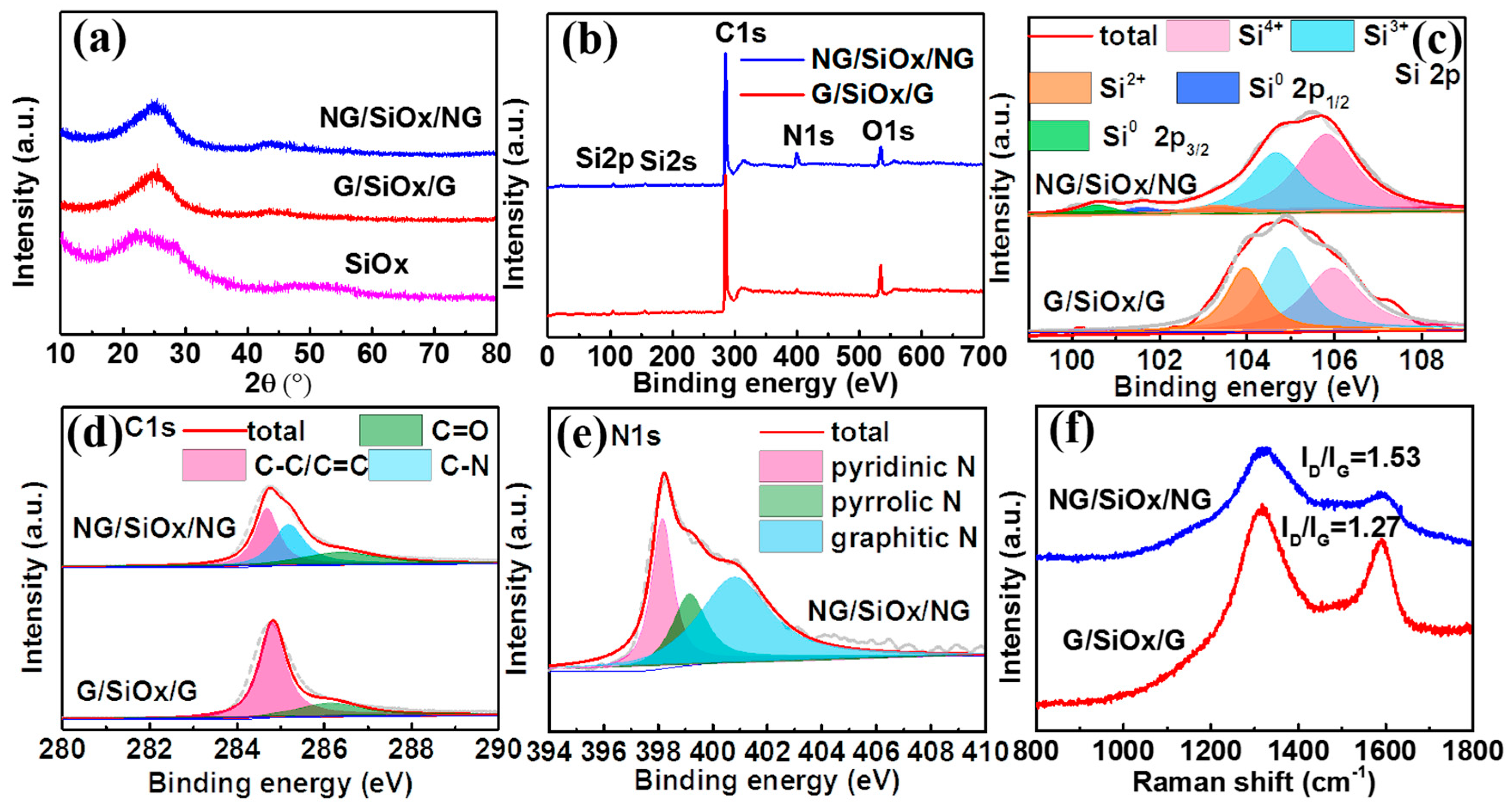

| Sample | Si4+ | Si3+ | Si2+ | Si0 | O/Si Ratio |
|---|---|---|---|---|---|
| G/SiOx/G | 36.4% | 37.9% | 25.7% | 0% | 1.55 |
| NG/SiOx/NG | 53.2% | 38.1% | 4.6% | 4.1% | 1.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Wei, Y.; Li, T.; Gu, Y.; Guo, D.; Wu, N.; Qin, A.; Liu, X. Green and Scalable Fabrication of Sandwich-like NG/SiOx/NG Homogenous Hybrids for Superior Lithium-Ion Batteries. Nanomaterials 2021, 11, 2366. https://doi.org/10.3390/nano11092366
Liu G, Wei Y, Li T, Gu Y, Guo D, Wu N, Qin A, Liu X. Green and Scalable Fabrication of Sandwich-like NG/SiOx/NG Homogenous Hybrids for Superior Lithium-Ion Batteries. Nanomaterials. 2021; 11(9):2366. https://doi.org/10.3390/nano11092366
Chicago/Turabian StyleLiu, Guilong, Yilin Wei, Tiantian Li, Yingying Gu, Donglei Guo, Naiteng Wu, Aimiao Qin, and Xianming Liu. 2021. "Green and Scalable Fabrication of Sandwich-like NG/SiOx/NG Homogenous Hybrids for Superior Lithium-Ion Batteries" Nanomaterials 11, no. 9: 2366. https://doi.org/10.3390/nano11092366
APA StyleLiu, G., Wei, Y., Li, T., Gu, Y., Guo, D., Wu, N., Qin, A., & Liu, X. (2021). Green and Scalable Fabrication of Sandwich-like NG/SiOx/NG Homogenous Hybrids for Superior Lithium-Ion Batteries. Nanomaterials, 11(9), 2366. https://doi.org/10.3390/nano11092366






