Abstract
The chemical composition of rutile has been used as an indicator in magmatic and metamorphic-related diagenetic systems, but rarely in porphyry-style ore systems. The Tuwu deposit (557 Mt at 0.58% Cu) is a large porphyry-style Cu mineralization in Eastern Tianshan, Xinjiang, with typical disseminated, stockwork mineralized veins hosted in tonalite and diorite porphyry, and to a lesser extent in volcanic rocks of the Qi’eshan Group. We first present determination of rutile minerals coupled with chlorite identified in mineralized porphyries from Tuwu to reveal their geochemical features, thus providing new insights into the ore-forming processes and metal exploration. Petrographic and BSE observations show that the rutile generally occurs as large crystals (30 to 80 µm), in association with hydrothermal quartz, chlorite, pyrite, and chalcopyrite. The rutile grains display V, Fe, and Sn enrichment and flat LREE-MREE patterns, indicating a hydrothermal origin. Titanium in rutile (TiO2) is suggested to be sourced from the breakdown and re-equilibration of primary magmatic biotite and Ti-magnetite, and substituted by Sn4+, high field strength elements (HFSE; e.g., Zr4+ and Hf4+), and minor Mo4+ under hydrothermal conditions. The extremely low Mo values (average 30 ppm) in rutile may be due to rutile formation postdating that of Mo sulfides (MoS2) formation in hydrothermal fluids. Chlorite analyses imply that the ore-forming fluids of the main stage were weakly oxidized (logfO2 = −28.5 to −22.1) and of intermediate temperatures (308 to 372 °C), consistent with previous fluid inclusion studies. In addition, Zr-in-rutile geothermometer yields overestimated temperatures (>430 °C) as excess Zr is incorporated into rutile, which is likely caused by fast crystal growth or post crystallization modification by F-Cl-bearing fluid. Thus, application of this geothermometer to magmatic-hydrothermal ore systems is questionable. Based on the comparison of rutile characteristics of porphyry Cu with other types of ore deposits and barren rocks, we suggest that porphyry Cu-related rutile typically has larger grain size, is enriched in V (average 3408 ppm, compared to <1500 ppm of barren rocks) and to a lesser extent in W and Sn (average 121 and 196 ppm, respectively), and has elevated Cr + V/Nb + Ta ratios. These distinctive signatures can be used as critical indicators of porphyry-style Cu mineralization and may serve as a valuable tool in mineral exploration.
1. Introduction
Rutile (TiO2) as an accessory mineral is widely distributed in igneous, metamorphic, and sedimentary rocks [1,2,3,4]. It is also found in hydrothermal ore systems, such as porphyry Cu or Cu–Au, Carlin-type Au, and orogenic Au deposits [5,6,7]. Trace element compositions of different genetic rutile can provide insights into potential sources and evolution of rocks, as well as information for ore genesis [8,9]. Rutile is also evaluated as a powerful tool for detecting mineralization systems with its distinct grain structure (i.e., size and aspect ratio) and abundance of V, W, Sn, and Nb [10,11,12,13]. In addition, the Zr content in the rutile lattice can be used as a geothermometer [14,15], and rutile with sufficient uranium can be used for U-Pb isotope determination [3]. To date, rutile geochemistry has been determined and applied to magmatic and metamorphic-related diagenetic systems [2,4,16,17,18], but rarely to porphyry Cu systems [8,11,19,20].
The porphyry Cu deposit, as an economically important type of deposit supplies approximately 75 percent of global Cu [21,22]. The Early Carboniferous Tuwu deposit that formed in the Dananhu-Tousuquan Arc setting is one of the largest porphyry Cu deposits in the Eastern Tianshan Orogen, Xinjiang, northwestern China region [23,24,25]. Numerous studies have examined the ore genesis of Tuwu through mineralogy, geochemistry, tectonics, multiple isotopes, and fluid inclusions [23,26,27,28]. However, few studies have dealt with rutile minerals that are well developed and related to calc-alkalic porphyry alteration and mineralization in the Tuwu porphyry Cu deposit. In this research, we conducted a detailed textural and geochemical survey of rutile crystals from mineralizing porphyries of the Tuwu deposit, Xinjiang, using electron probe microanalyses (EPMA) and laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) method, and acquired back-scattered electron images and X-ray maps. The results provide new insights into the formation conditions and substitution mechanisms for rutile and related Cu mineralization. We also evaluated the applicability of a Zr-in-rutile geothermometer for hydrothermal deposits, together with a chlorite geothermometer and previous fluid inclusion studies. In addition, the compositional variations of rutile from Tuwu were compared with those from other deposits and barren rocks, which would be significant indicators for guiding ore exploration.
2. Geological Setting
2.1. Regional Geology
The Eastern Tianshan Orogen, located between the Junggar Basin in the north and the Tarim Basin in the south (Figure 1a), is primarily composed of Precambrian basement overlain by Paleozoic to Mesozoic volcanic-sedimentary sequences and intruded by mafic to felsic magmatic rocks [29,30]. It can be tectonically subdivided into the Bogeda-Haerlike and Dananhu-Tousuquan Arcs, Kanggur-Huangshan Shear Zone, and Aqishan-Yamansu Arc, separated by the approximately E-W-trending Kanggur and Yamansu faults, respectively (Figure 1b) [28]. The Eastern Tianshan Orogen experienced a complex tectonic evolution involving the subduction, collision-accretionary, strike-slip motion, post collisional and intracontinental extension during the Early Paleozoic to the Mesozoic [31,32,33,34,35]. In response to the complex geodynamic evolution, different types of mineral deposits developed in the region, including porphyry Cu or Mo, magmatic Cu-Ni sulfide (e.g., Huangshan and Xiangshan), orogenic Au, IOCG Fe (-Cu), VMS, and skarn-type deposits (Figure 1b) [25]. Among them, the porphyry-style Cu deposits (e.g., Tuwu, Yandong, Fuxing, Linglong, Sanchakou, and Yuhai) in the Eastern Tianshan Orogen, have been proposed to be associated with the subduction of the paleo-Tianshan ocean beneath the Dananhu-Tousuquan and Bogeda-Haerlike Arcs and accompanied regional magmatism [36,37,38]. The regional intrusions related to porphyry Cu mineralization are intermediate to felsic in composition, mainly consisting of tonalite porphyry, diorite porphyry, quartz diorite, and granodiorite [26,27,28]. They are widely distributed in the Dananhu-Tousuquan Arc (~42° N—43° N), dating from ca. 460 to 300 Ma [21,28].
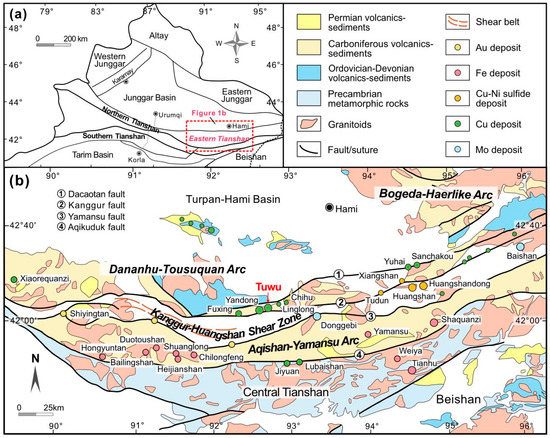
Figure 1.
(a) Geological map of NW China showing the main tectonic units, and (b) geological map of Eastern Tianshan Orogen showing major tectonic units, faults and ore deposits (modified from [28]).
To the south of the Eastern Tianshan Orogen, the Aqishan-Yamansu Arc predominantly contains Carboniferous basalt, andesite, dacite, and pyroclastic rocks, with the formation of important Fe (-Cu) deposits such as Hongyuntan, Bailingshan, Duotoushan, Chilongfeng, and Yamansu (Figure 1b). They are suggested to be generated by the southward subduction of north Tianshan oceanic crust beneath the Central Tianshan Block during the Carboniferous [39]. As an accretionary complex, the Kanggur-Huangshan Shear Zone was linked to long-term subduction and subsequent post collision tectonic processes [25,39]. The shear zone extends approximately 600 km and is dominated by Devonian to Carboniferous volcanic lavas, pyroclastic rocks, sandstones, and deep-sea turbidites with disrupted ophiolites [39,40,41,42]. A large number of Au (e.g., Kanggur and Shiyingtan) and Cu-Ni (e.g., Huangshan, Huangshandong, and Xiangshan) deposits, as well as two significant porphyry Mo deposits (i.e., Donggebi and Baishan), were formed in this zone (Figure 1b), related to syncollisional, post collisional, or intraplate extensional tectonics [40,41,42,43,44,45].
2.2. Geology of Tuwu Deposit
The Tuwu porphyry Cu deposit located in the southern part of Dananhu-Tousuquan Arc, Xinjiang (Figure 1) contains ore reserve of 557 Mt at average grade of 0.58 wt % Cu and 0.2 g/t Au [26,46]. The ore deposit is hosted in diorite porphyry and tonalite porphyry that were emplaced into the Carboniferous Qi’eshan Group, and mainly controlled by EW trending faults and several subordinate NW-trending faults (Figure 2). The Qi’eshan Group is primarily divided into three units from bottom to top, including the lower andesite and basalt lavas intercalated with tuff, the middle andesite and brecciated andesite lavas, and the upper pebbly lithic sandstone and minor tuffaceous siltstone intercalated with basalt, andesite and dacite lavas [26,47]. Previous U–Pb zircon dating for the diorite porphyry and tonalite porphyry indicates that they were emplaced at 338.6 ± 2.9 Ma and 332.3 ± 5.9 Ma, respectively [47,48]. Minor younger diorite and diabase dykes are present in the district, which intruded the earlier magmatic rocks.
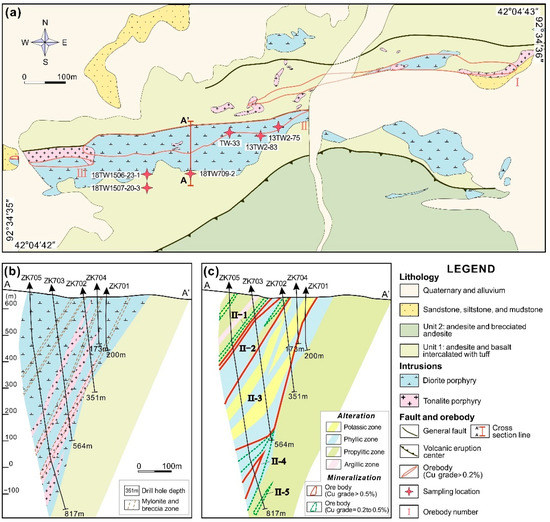
Figure 2.
(a) Geological map of the Tuwu porphyry Cu deposit (modified from [28]); (b) geologic cross section A-A’ looking north showing lithology, and (c) geological cross section A-A’ with alteration and mineralization.
The three recognized mineralized zones are referred to as I, II, and III ore zones (Figure 2a), where the ore zone II accounts for 90% of the total copper reserves. Hydrothermal alteration at Tuwu affecting porphyries, and wall-rocks can be divided into potassic, phyllic, propylitic, and limited argillic alteration zones, which are shown in Figure 2c [23,26]. Copper mineralization, mostly present as chalcopyrite and bornite, is closely related to the potassic and phyllic alteration zones. The mineralization process at Tuwu includes four stages based on mineral assemblages and crosscutting relationships [23,28,30]. Stage I is represented by two types of veins, including quartz-magnetite-biotite and quartz-magnetite ± K-feldspar ± albite ± pyrite veins associated with local potassic and late pervasive chlorite alterations. Stage II and III are composed of chalcopyrite, bornite, quartz, pyrite, sericite, chlorite, albite, with minor magnetite and enargite, some of which is locally intergrown with rutile minerals (Figure 3). Stage II and III veins are characterized by significant Cu and Mo mineralization and are considered to be the main hydrothermal stages at Tuwu. The copper enrichment is generally linked to phyllic and chlorite–sericite alterations. Three typical Cu-sulfide-bearing veins are recognized in the main stage, including quartz-chalcopyrite-magnetite ± pyrite, quartz-bornite ± chalcopyrite ± chlorite ± epidote ± pyrite, and quartz-chalcopyrite ± chlorite ± pyrite ± rutile veins [23]. Stage IV is defined by mineral associations of calcite, quartz, chlorite, anhydrite, epidote, and pyrite. The molybdenite Re–Os dating for Tuwu suggests that the main mineralization event occurred at 335.6 ± 4.1 Ma [28], in agreement with the emplacement age of the local porphyry intrusions [48,49,50].
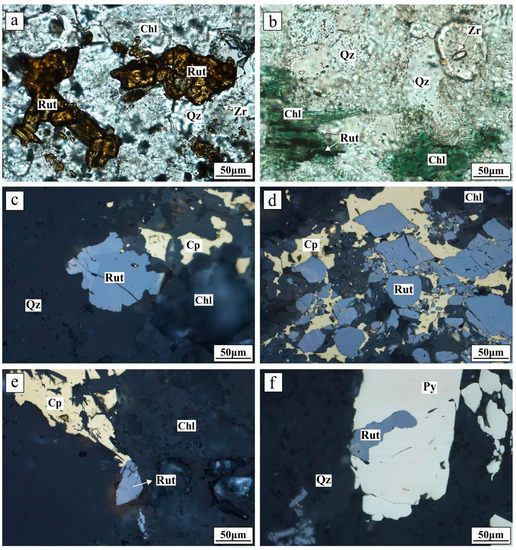
Figure 3.
Microphotographs of samples from the Tuwu porphyry Cu deposit. (a) Rutile enclosed within quartz (transmitted light); (b) rutile coexisting with quartz, zircon, and chlorite (transmitted light); (c) rutile crystal coexisting with chalcopyrite, quartz, and chlorite (reflected light); (d) fragmented rutile intergrown with chalcopyrite (reflected light); (e) subhedral rutile intergrown with chalcopyrite (reflected light); and (f) anhedral rutile included within pyrite (reflected light). Abbreviations: Chl = chlorite; Cp = chalcopyrite; Py = pyrite; Qz = quartz; Rut = rutile; Zr = zircon.
3. Sampling and Analytical Methods
Six representative mineralized samples analyzed in this paper were collected from ore zone II in the Tuwu porphyry Cu deposit (sample locations are shown in Figure 2a). The samples were polished and prepared for micro-observations and in situ chemical composition analyses, including EPMA and LA-ICP-MS. Detailed petrographic studies were carried out using an Olympus BX51 (Olympus Corporation, Tokyo, Japan) petrographic microscope at the China University of Geosciences, Beijing (CUGB), China.
Backscattered electron (BSE) images of rutile, chlorite and EPMA were taken on polished thin sections using a SIMADAZU EPMA-1720 instrument (Shimadzu Corporation, Kyoto, Japan) equipped with five wavelength dispersive spectrometers at the Resources Exploration Laboratory, CUGB. An accelerating voltage of 15 keV, a beam current of 10 nA, and a beam spot diameter of 5 μm were used for analyses. Different elements have different standard minerals, and the detection limits of each element are varied. The suite of analyzed elements mainly included Na, Cr, Si, Ti, Mg, Fe, Mn, Al, Ni, and K; the detection limits of these elements are shown in Table 1. Counting times were 20 s on the main peak and 10 s on the background. The standards used for calibration included albite for Na, chromite for Cr, diopside for Si and Mg, rutile for Ti, hematite for Fe, rhodonite for Mn, garnet for Al, synthetic NiO for Ni, and orthoclase for K. ZAF3 routine was used for data correction. The mineral formulas were recalculated using MINPET 2.0 software. Element mapping of one sample was performed using a spectrometer setup similar to the spot analyses, which consumed approximately 4 h of instrument time.

Table 1.
Summary of the major element compositions of rutile in Tuwu (wt %). dl = detection limits.
Trace elements in rutile samples (13TW2-75, 13TW2-83, and TW-33) were measured by LA-ICP-MS analyses, which were carried out on an ESI NWR 193 nm laser ablation system (Elemental Scientific, Montana, USA) coupled with an Agilent 7500a quadrupole ICP-MS (Agilent Technologies Inc., Tokyo, Japan) at the Beijing Kuangyan Geoanalysis Laboratory Co., Ltd., Beijing, China. Helium and argon were used as the carrier gas and make-up gas, respectively. Argon was mixed with helium via a Y-joint before entering the ICP. Each analysis included the acquisition of a background signal for approximately 15–20 s (gas blank) followed by 45 s of data acquisition from the samples. A spot size of 30 µm and a 10 Hz ablation frequency were used for single spot ablation. NIST SRM610 and NIST SRM612 standard silicate glass were used as the external standard minerals. Titanium content was used as the internal standard to correct the time-dependent drift of sensitivity and mass discrimination for the trace elements analyses [51]. The detection limits for each element are shown in Table 2. Quantification of element concentrations was obtained according to the GeoReM database [52]. Off-line selection and integration of background and analytic signals, time-drift correction, and quantitative calibration were performed using the ICPMSDataCal program [51,53].

Table 2.
LA-ICP-MS trace element analyses of different types of rutile in Tuwu (ppm). dl = detection limits.
4. Results
4.1. Mineralogical and Chemical Composition of Rutile
The samples analyzed in this study include mineralized porphyries containing disseminated chalcopyrite or quartz-chalcopyrite ± chlorite veins, with different alteration types (e.g., phyllic and propylitic). Rutile in mineralized porphyries is typically dark brownish-red or yellowish in color, varies from subhedral to anhedral in shape, and is approximately 30 to 60 μm in length (up to 80 μm; Figure 3). Most rutile occurs as aggregates, including 3 to 25 grains within a small area, and is commonly intergrown with quartz, chlorite, chalcopyrite, and/or pyrite, or formed prior to these minerals (Figure 4).
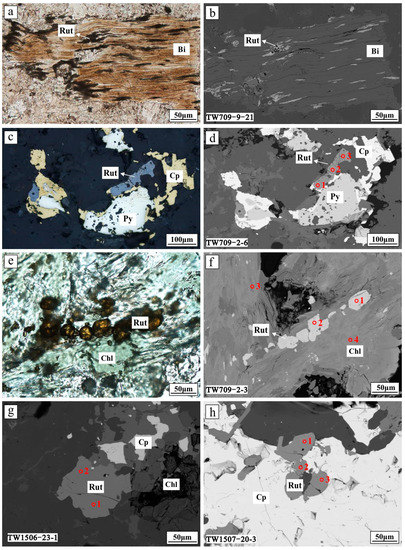
Figure 4.
Photomicrographs and backscattered electron images (BSE) of rutile. (a,b) Rutile replaced early formed biotite (reflected light and BSE, respectively); (c,d) rutile with chalcopyrite and pyrite (reflected light and BSE, respectively); (e,f) rutile enclosed in chlorite (transmitted light and BSE, respectively); (g) rutile crystal coexisted with chlorite and chalcopyrite (BSE); and (h) rutile enclosed within chalcopyrite (BSE). Abbreviations: Bi = biotite; Chl = chlorite; Cp = chalcopyrite; Py = pyrite; Qz = quartz; Rut = rutile.
Mineral chemical compositions of rutile determined by EPMA are listed in Table 1. Rutile analyses are high in TiO2 (96.66 to 98.92 wt %, average = 97.91 wt %), similar to those from the Zhunuo porphyry Cu-Mo deposit (TiO2 = 88.76 to 99.64 wt %) [20]. They contain minor FeO (0.23–2.34 wt %), CaO (0.01–0.95 wt %), Al2O3 (0.04–0.60 wt %), Cr2O3 (0.03–0.80 wt %) and CuO (0.01–0.58 wt %), and detectable SiO2 (0.01–0.11 wt %, corresponding to 51–519 ppm Si) contents. Other element concentrations were below the limit of detection (Table 1).
High-contrast BSE images reveal that rutile grains were not zoned or twinned (Figure 4), also verified in EPMA element mapping images (Figure 5). Single rutile crystals exhibited homogeneous distribution of Ti, V, Cr, and Sn, with slightly heterogeneous W distribution. Trace element contents of rutile crystals from samples of 13TW2-75, 13TW2-83, and TW-33 are present in Table 2. They displayed significant variation in trace elements, such as V (1476–5187 ppm), Cr (835–3734 ppm), Zr (50–519 ppm), W (12–627 ppm), Sn (73–346 ppm), Nb (18–270 ppm), Cu (5–45 ppm), and Mo (1–111 ppm). Two types of rare earth element (REE) patterns were recorded in the analyzed rutile minerals that are illustrated in Figure 6 and described below. Type 1 rutile had high total REE concentrations with most LREE and HREE at 1–10 times chondrite, showing a flat LREE–MREE pattern, decreasing MREE to HREE pattern, and negative Eu anomalies (Figure 6a). Types 2a and b rutile had lower REE concentrations relative to type 1 (Figure 6b,c), in which type 2a had the most REE values similar to chondrite and type 2b rutile has the most LREE and HREE at 0.05–0.1 times chondrite (Figure 6c). Some MREE in rutile were below the detection limits of the LA-ICP-MS method, such as Sm and Eu (Table 2).
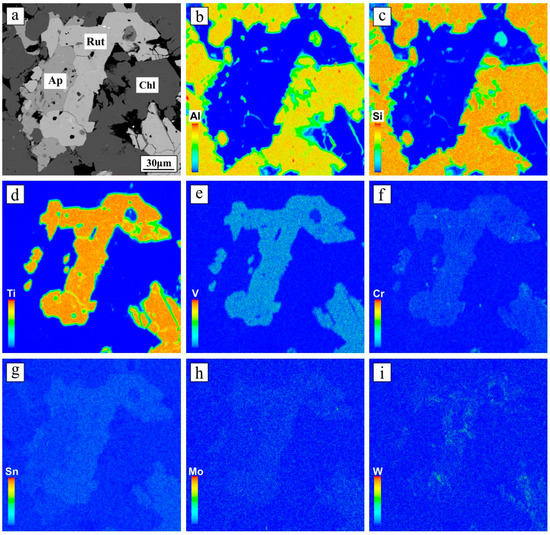
Figure 5.
BSE image (a) of Tuwu rutile and EPMA element mapping images (b–i) for Al, Si, Ti, V, Cr, Sn, Mo, and W. Warmer colors correspond to higher concentrations. Abbreviations: Ap = apatite; Chl = chlorite; Rut = rutile.
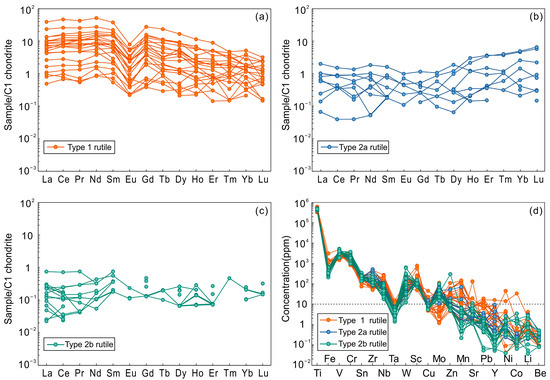
Figure 6.
REE patterns (a–c) and trace element contents (d) of three types of rutile from Tuwu.
4.2. Zr-in-Rutile Thermometry
The solubility of Zr in rutile coexisting with zircon and quartz turned out to be strongly temperature-dependent, and thus Zr content in rutile was confirmed as a geothermometer [14,15,54]. Rutile crystals from Tuwu generally occur in quartz-bearing veins of ore stage II with presence of minute zircon inside the quartz crystals (Figure 3a,b). The rutile samples had variable Zr concentrations ranging from 50 to 519 ppm (Table 2). Using the rutile Zr concentrations and equation of [14] without considering the pressure effect, the temperatures calculated for these samples were between 482 and 644 °C. Based on the estimation of fluid pressure (~90 bar) constrained by previous fluid inclusion analyses [23], the pressure-corrected temperatures were calculated to be 488–646 °C (Figure 7) using the experimentally calibrated method of Tomkins et al. [15], and 434–610 °C using the alternative method proposed by Kohn [54].
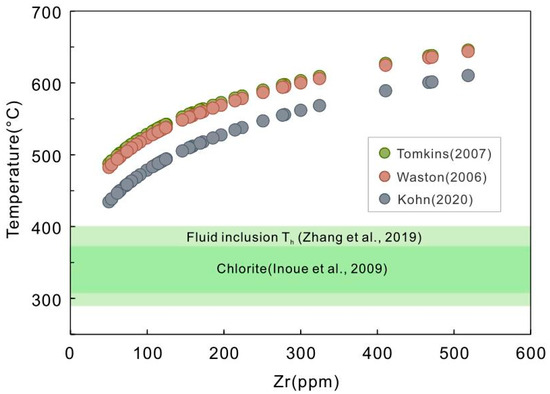
Figure 7.
Plot of Zr content variation and corresponding temperature (°C) based on Zr-in-rutile thermometers of [14,15,54]. For comparison, the homogenization temperatures estimated from quartz-hosted fluid inclusions [23] and temperatures yielded by chlorite thermometry are shown [55].
4.3. Chlorite Geothermometry
Chlorite group minerals from Tuwu were commonly intergrown with or occur later than the hydrothermal rutile of stage II (Figure 4). The major elements in chlorite coexisting with rutile were determined in this study, and the results are presented in Table 3. They yielded consistent SiO2 (25.40–26.51 wt %), TiO2 (0.09–0.24 wt %), Al2O3 (20.10–20.63 wt %), and FeO (14.92–22.79 wt %) contents. The (Na2O + K2O + CaO) contents were lower than 0.5 wt %, indicating negligible contamination [56,57,58]. The chemical formula of the analyzed chlorite was calculated based on 14 oxygen atoms per formula unit (a.p.f.u). The Si (a.p.f.u) and Al (a.p.f.u) values for chlorite range from 2.65 to 2.76 and 2.45 to 2.56, respectively (Table 3). The precipitation temperatures calculated for chlorite ranged from 308 to 330 °C (T87; Table 3), using semiempirical thermometry [59]. Alternatively, the temperatures were calculated to be in the same range of 338 to 372 °C (Figure 7), using the other two different empirical equations from [60] for T88 and [61] for T91.

Table 3.
Geochemical compositions of hydrothermal chlorite and calculated temperatures.
5. Discussion
5.1. Precipitation of Hydrothermal Rutile with Copper Mineralization
Rutile (TiO2) is a common accessory mineral in igneous, metamorphic, and sedimentary rocks, which also occurs as a secondary alteration mineral in magmatic-hydrothermal ore deposits [3,62]. In calc-alkaline porphyry-related systems, high pressure (1.3–1.5 GPa) is required for primary rutile crystallized from magma. Given the shallow formation depth of porphyry deposits, rutile generally formed during hydrothermal alteration instead of magmatic processes [8,63,64,65,66]. The origin of rutile can be distinguished through its paragenetic mineral assemblages, textures, and chemical compositions [67,68].
Primary rutile is commonly interstitial, intergrown with pyrite and/or phosphate minerals (e.g., monazite and xenotime), or rimmed by K-feldspar, quartz, ilmenite, and magnetite [2,4]. In contrast, rutile from the Tuwu porphyry Cu deposit mostly coexists with hydrothermal quartz, chlorite, pyrite, and chalcopyrite (Figure 3), and occurs as aggregates in mineralized porphyries (Figure 4). These features are similar to those of hydrothermal rutile associated with porphyry-type Cu-Mo and various types of gold mineralization, such as El Teniente porphyry Cu-Mo [8], Zhesang Carlin-type Au [6], orogenic Au from Precambrian terranes [7], and Wulong lode Au [68] deposits. Hydrothermal rutile can be distinguished from primary rutile hosted in igneous and metamorphic rocks with its high abundance of W, V, Fe, Cr, and Sn [6,11,16,69,70]. Enrichment of V (V2O3 = 0.22–0.76 wt %), Fe (FeO = 0.03–0.43 wt %), and Sn (SnO2 = 0.01–0.04 wt %) contents in rutile from Tuwu (Table 1) suggest that it is hydrothermal in origin. In addition, rutile displays U-shape REE patterns and has relatively high REE content for unaltered rutile (type 1; Figure 6a), which differs from igneous rutile [67,68]. As for type 2a and b rutile from Tuwu (Figure 6b,c), depletion of REE, especially for LREE, may result from post modification by later hydrothermal fluids due to higher mobilities of LREE than HREE. Taken together, the Tuwu rutile is interpreted to be hydrothermal and has spatial and genetic associations with Cu mineralization.
5.2. Origin and Substitution Mechanisms of Ti in Rutile
Rutile is generally produced by breakdown and/or re-equilibration of Ti-rich and/or Ti-bearing minerals during water-rock interaction in porphyry Cu systems [8,11,19,20,71]. It occurs predominantly in potassic and phyllic alteration zones associated with chalcopyrite and molybdenite-bearing quartz veins. Given the low solubility of titanium in ore-forming fluids at temperatures below 700 °C [72], fluids cannot be a significant Ti source of the hydrothermal rutile at Tuwu. Four possible processes have been reported for rutile formation driven by the introduction of S and CO2 in fluids [11,73], including (1) biotite altered to phlogopite, (2) titanomagnetite altered to magnetite, (3) ilmenite altered to rutile in areas of intense alteration, and (4) titanite altered to calcite in propylitic zones. The relevant reactions are listed below:
K(Fe,Mg,Ti)3(Si3Al)O10(OH)2 (biotite) + S2 →
K(Mg,Fe)3(Si3Al)O10(OH)2 (phlogopitic biotite) + FeS2 (pyrite) + TiO2 (rutile)
K(Mg,Fe)3(Si3Al)O10(OH)2 (phlogopitic biotite) + FeS2 (pyrite) + TiO2 (rutile)
2(Fe,Ti)3O4 (Ti-magnetite) + S2 =
Fe3O4 (magnetite) + FeS2 (pyrite) + TiO2 (rutile)
Fe3O4 (magnetite) + FeS2 (pyrite) + TiO2 (rutile)
FeTiO3 (ilmenite) + S2 = FeS2 + TiO2 (rutile)
CaTiSiO5 (titanite) + CO2 = TiO2 (rutile) + CaCO3 + SiO2
No primary ilmenite and titanite were detected in porphyries at Tuwu. Hence, it is more likely that biotite and magnetite released Ti for rutile generation as a function of high temperature and influx of S-rich oxidized fluids [28,49]. Pan et al. [49] and Rui et al. [46] determined the compositions of primary biotite, and the results show that biotite has Mg/(Mg + Fe + Mn) values ranging from 0.35 to 0.60. Petrographic observations of the early-stage biotite replaced by later hydrothermal rutile (Figure 4a,b) and chlorite further suggest that biotite can be a source of Ti for rutile formation [49]. This interpretation is consistent with our unpublished EPMA results of hydrothermal biotite from the potassic alteration zone, in which the biotite was Fe-rich and had TiO2 ranging from 0.10–1.11 wt %. Moreover, the magmatic/early-stage magnetite at Tuwu is Ti-bearing (TiO2 = 0.04 to 1.09 wt %), as supported by EMPA results reported by Yuan et al. [24], implying that magnetite can be a candidate for releasing some Ti into hydrothermal fluids. During early potassic alteration, we propose that rutile was partially formed by breakdown and re-equilibration of primary biotite, which released residual Ti by exsolution under fluid-dominated conditions [74]. The Ti-magnetite breakdown also contributed some Ti, resulting in the formation of Ti-poor magnetite, pyrite, and rutile (reaction 2) [8,63]. In the phyllic and subsequent propylitic stages, owing to the instability of biotite [8], quartz–chlorite–rutile assemblage replaced the pre-existing biotite, during which abundant sulfides, mainly including chalcopyrite, pyrite, and molybdenite, precipitated from the hydrothermal system. This is consistent with the intimate relationships of rutile with copper mineralization and chlorite alteration in the study area (Figure 4).
The LA-CP-MS and EPMA analyses of rutile indicate that cations such as Fe, Ta, Nb, V, W, Zr, Sn, Ta, and Cr with different valences potentially substitute for the main Ti sites in rutile (Table 1 and Table 2; Figure 6d), which dominantly depends on their ionic size, charge neutrality, and cation mobility [75,76,77,78]. In the Tuwu porphyry Cu deposits, significant Sn4+, some high field strength elements (HFSE; e.g., Zr4+ and Hf4+), and minor Mo4+ may directly replace for Ti4+ (r = 0.67 Å) in rutile. Furthermore, two coupled-substitution mechanisms were proposed for trivalent, pentavalent, and hexavalent cations incorporating into the rutile structure [8,11] according to the following coupled substitutions (1) (Fe, V, Cr, Sc)3+ + (Nb, Ta)5+ = 2 Ti 4+, and (2) 2(Fe, V, Cr, Sc)3+ + W6+ = 3 Ti 4+. Considering the excess total content of trivalent ions compared to Nb5+, Ta5+, and W6+ ions, the incorporation of hydrogen in the rutile structure has been suggested for charge-balancing [79]. Low Cu and Mo concentrations in rutile indicate that these two elements are more incompatible with respect to hydrothermal rutile (Figure 8a,b), although previous experimental studies reported the DMorutile/fluid value could be up to 1.5 [74]. Importantly, it is easier for Mo than Cu to exchange Ti in Ti-rich mineral phases, as it can directly substitute Ti4+ as Mo4+ under low fO2 condition, or as Mo6+ via coupled substitutions such as W6+ under oxidized systems [74]. Rutile at Tuwu contains some Mo, but only up to 110 ppm (Figure 8a), which is controlled by Mo content in Ti-rich parental minerals and in hydrothermal fluids [8]. Previous studies have demonstrated that the ore-related porphyry melt at Tuwu was hydrous and highly oxidized without significant early sulfide saturation prior to fluid exsolution [28]. In this case, more Mo would have been removed from silicate melts into aqueous fluids, which would have limited the amount of Mo partitioned into Ti-bearing magmatic mineral phases. The oxidized, Mo-bearing hydrothermal fluids can be an alternative Mo source for hydrothermal rutile, which is supported by the low measured Mo content in rutile (Mo = 1–111 ppm) assuming a low DMorutile/fluid value of 1.5. The reason for the extremely low Mo values in rutile may be that its precipitations postdated that of molybdenite (MoS2).
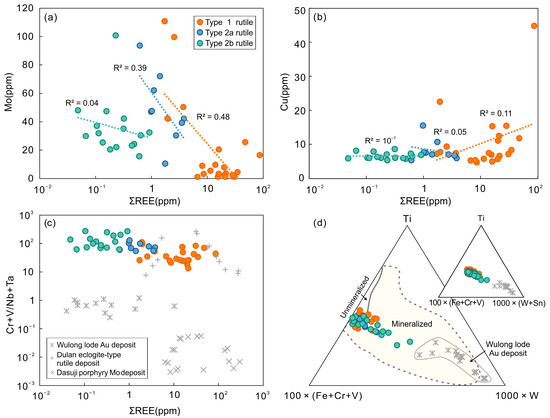
Figure 8.
(a) Correlation between REE and Mo in rutile. (b) Correlation between REE and Cu in rutile. (c) Cr + V/Nb + Ta versus sum REE in rutile. (d) Ti-100 × (Fe + Cr + V) − 1000 × W ternary diagram allowing to compare the composition of rutile in Tuwu with those from the literature [16].
5.3. Chlorite Classification and Ore Formation Conditions
Chlorite from Tuwu has consistent amounts of tetrahedral trivalent cation (R3+ = 1.24–1.35), octahedral divalent cation (R2+ = 4.77–4.98) and octahedral vacancy (□ = 0.01–0.04), with low Fe/(Fe + Mg) (0.26–0.42), AlIV (1.24–1.35), and AlVI (1.12–1.24) values (Table 3). They fall into the fields of ripidolite and pycnochlorite in the Si versus Fe (a.p.f.u) classification diagram (Figure 9a) [80]. In the (Al + □)–Mg–Fe ternary diagram (Figure 9b), they are defined as Mg-chlorite [81]. In the R2+ versus Si (a.p.f.u) diagram (Figure 9c) [82], they plot within the tritrioctahedral field, and fall between clinochlore/chamosite and sheridanite/ripidolite endmembers.
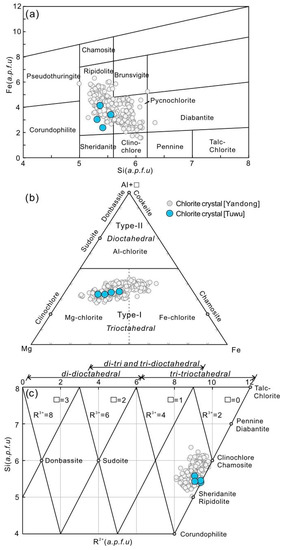
Figure 9.
(a) Si versus Fe diagram of chlorite (after [80]). (b) (Al + □)–Mg–Fe plot (after [81]). (c) R2+ versus Si (a.p.f.u) plot (after [82]).
Inoue et al. [83] proposed that redox conditions (logfO2) can be estimated following the method of Walshe [84], based on a semiempirical thermometer [55]. The logfO2 for the main stage calculated from chlorite compositions varied from −28.5 to −22.1, which is consistent with a weakly oxidized environment. In addition, the precipitation temperature was calculated to be 308 °C to 372 °C (average 345 °C) using the chlorite geothermometer [59,60,61], which overlapped with the temperature (290–400 °C) determined by fluid inclusion measurements (Figure 7) [23]. Therefore, we suggest the main ore stage at Tuwu with the formation of Cu-bearing sulfides and associated chlorite occurred in intermediate temperature and weakly oxidized conditions.
5.4. Application of Zr-in-Rutile Thermometer to Magmatic-Hydrothermal Ore System
The zircon-in-rutile temperatures calculated in this study were based on the measurements of Zr contents in rutile and empirical equations from [14,15,54]. They yielded a variable formation temperature of 434–646 °C with an average of 531 °C (Table 1), largely higher than those estimated by chlorite geothermometer (Figure 7) [55], as well as fluid inclusion analyses for Tuwu [23]. Several factors are potentially responsible for the overestimation of temperatures from rutile, including (i) absence of quartz in veins, (ii) incorporation of Zr-rich mineral inclusions (e.g., zircon and zirconolite), and (iii) excess Zr incorporation into rutile caused by a high crystal growth rate [7,68,85]. In the case of Tuwu, petrographic observations support that the rutile is related to quartz-bearing veins, and thus the first factor can be excluded. No zircon inclusions (ZrSiO4) were identified in the analyzed rutile samples, excluding the influence of mineral inclusions on temperature estimations. The Zr and Si concentrations in rutile are up to 519 ppm and 31,072 ppm, respectively (Table 2), but are negatively correlated, further indicating the absence of zircon inclusions. In addition, the correlations between the Ca and Zr are weak (Table 2), differing from the case for Zr-rich rutile in the study of [7], which suggests absence of zirconolite (CaZrTi2O7).
The best reason for overestimated temperatures at Tuwu is excess Zr4+ substituting for Ti4+ in rutile. Agangi et al. [7] proposed that the equilibrium between rutile, zircon, and quartz is a prerequisite to use the rutile thermometer, a condition that can be met in slowly heating and cooling geological processes, especially at medium to high temperatures, under which the element diffusion is weak. Existing fluid inclusion studies have revealed the occurrence of a boiling event during the main ore stage [23], evidenced by the coexisting low-salinity vapor-rich and moderate- to low-salinity liquid-rich inclusions within individual fluid inclusion assemblage hosted in quartz. The rutile from the Tuwu porphyry Cu deposit is suggested to be generated in an intensively boiled magmatic-hydrothermal system that is characterized by pulsating fluid flow at rapidly changing temperatures [23], which would have promoted excess Zr incorporating into rutile crystal. Besides, the occurrence of fluorapatite suggests the ore-forming fluids at Tuwu are F-bearing [27,47,86], which can dissolve, transport, and precipitate remarkable quantities of Zr and Ti [87,88,89,90]. Similar halogen-rich (e.g., F and Cl) aqueous fluids have been reported in other deposits that significantly affect redistribution of Zr in rutile [68]. Large variations in temperatures estimated from the Zr-in-rutile geothermometry have been addressed in other studies [7,18,68,91]. Therefore, the application of a Zr-in-rutile thermometer to magmatic-hydrothermal ore systems should be approached with caution. Detailed microinvestigations coupled with multiple mineral thermobarometers would be helpful for evaluating formation temperatures and other conditions.
5.5. Implications for Mineralization Potential and Exploration
In recent years, rutile geochemistry has attracted more and more attention as an indicator or fertility tool for mineral deposits, especially when sulfides from deposits have been leached out, as they can retain chemical signatures through post-weathering and most hydrothermal events [3,11,16,62,63,92]. For example, previous work on the E26N porphyry Cu-Au deposit in Australia indicates that rutile grains from proximal orebody (less than 100 m) are larger in size (length × width > 4000 µm2) and enriched in V (commonly > 2000 ppm), compared to those from distal locations (length × width < 1500 µm2; V concentration < 1500 ppm) [11,12,13,16]. Such rutile is the product of relatively early hydrothermal fluid activity close to the potassic zone, corresponding to some Cu mineralization in porphyry-style systems [11,19].
In this study, petrographic and BSE observations on rutile from the Tuwu porphyry Cu deposit show that the rutile collected from proximal orebody (Figure 2) had large sizes of 30 to 80 µm (Figure 4). Furthermore, we compiled available chemical compositions of rutile from 12 mineral deposits in different genetic types and metal associations, and sediments and metamorphic rocks (Figure 10), including Duobuza porphyry Cu, Duolong porphyry Cu, Zhunuo porphyry Cu-Mo, Dasuji porphyry Mo, Northparkes porphyry Cu-Au, Panasqueira wolframite, Zhesang Carlin-type Au, Precambrian orogenic Au, Wulong lode Au, barren granite-related hydrothermal rutile in implacer from Menderes massif, Dulan eclogite-type rutile deposits, and siliciclastic rocks from Capricorn orogen. The results suggest that rutile from porphyry Cu and hydrothermal W deposits generally contain higher V concentrations (up to approximately 8000 ppm for porphyry deposit; Figure 10). Elemental mapping of rutile from Tuwu indicates homogenous and enriched V distribution (Figure 5). In contrast, rutile from Au-dominant deposits (e.g., lode, Carlin, and orogenic-type) have variable V concentrations, and rutile formed in sedimentary rocks and metamorphic deposits unrelated to metal mineralization record very low V content (Figure 10). Plotting samples of rutile from the Tuwu porphyry Cu and Wulong lode Au deposits in the Ti − 100 × (Fe + Cr + V) − 1000 × W ternary diagram (Figure 8d) indicate that the porphyry-related rutile has intermediate W and Sn content (Sn = 73–346 ppm). In addition, the Cr + V/Nb + Ta ratios of rutile from porphyry Cu deposit, such as Tuwu, are higher than those of rutile formed in porphyry Mo or other Au types and rutile deposits (Figure 8c), which can be used as an indicting proxy to discriminate porphyry Cu systems. In summary, we suggest that hydrothermal rutile from porphyry Cu deposits is V-rich, has intermediate W + Sn contents, and is high in Cr + V/Nb + Ta, criteria that can be used as critical indicators for copper exploration.
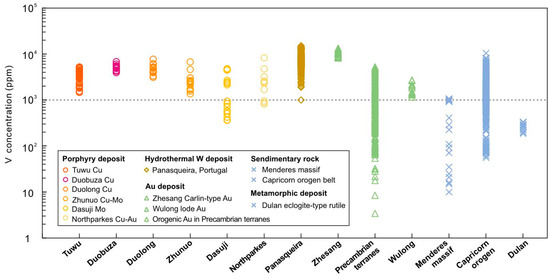
Figure 10.
Vanadium contents of rutile of the Tuwu porphyry copper deposit compared with several other deposits. The compiled porphyry deposits include Duobuza Cu [12], Duolong Cu [93], Zhunuo Cu-Mo [20], Dasuji Mo [94], and Northparkes Cu-Au [11]. The hydrothermal W deposit includes the Panasqueira deposit in Portugal [17]. The Au deposits include the Zhesang Carlin-type Au [6], the orogenic Au deposit in Precambrian terranes [7], and the Wulong lode Au [68]. Data from sedimentary rocks are from Menderes massif [70] and Capricorn orogen belt [95]. The metamorphic deposit includes Dulan eclogite-type rutile [9].
6. Conclusions
(1) Rutile in the Tuwu porphyry Cu deposit generally occurs as individual crystals and aggregate grains coexisting with hydrothermal quartz, chlorite, pyrite, and chalcopyrite. It contains an abundance of high V, Fe, and Sn, and displays flat LREE–MREE patterns, suggesting a hydrothermal origin and association with Cu mineralization.
(2) Chlorite at Tuwu is Fe-poor and formed at intermediate temperatures (308 to 372 °C) and weakly oxidized conditions (logfO2 = −28.5 to −22.1) during the main ore stage.
(3) Titanium for rutile formation was made available by the breakdown and re-equilibration of primary biotite and Ti-magnetite in phyllic and subsequent propylitic stages. Sn4+, some high field strength elements, and minor Mo4+ may directly replace for Ti4+ in rutile.
(4) Rutile of larger grain size and high V (up to 8000 ppm), intermediate W + Sn, and high Cr + V/Nb + Ta, is typical of porphyry Cu mineralization. The combined parameters can be considered as useful proxies for ore exploration in Eastern Tianshan and other regions.
Author Contributions
Conceptualization, F.Z.; sample collection, Y.L., H.Z. and Y.C.; methodology and data curation, X.W., M.S. and W.Z.; writing and organizing the paper, X.W.; writing, review, and editing, F.Z. and Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (42072102, 41702079, and 41772073), the 111 Project of the Ministry of Science and Technology of China (BP0719021), the MOST Special Fund from the State Key Laboratory of Geological Processes and Mineral Resources, China University of Geosciences of China (MSFGPMR201804), and the Fundamental Research Funds for the Central Universities (2-9-2019-061).
Data Availability Statement
Not applicable.
Acknowledgments
We thank Lian-Hui Dong and Jing Feng from the Xinjiang Bureau of Geology and Mineral Exploration. We also appreciate the kind help of Li Liu from the Beijing Zhongke Mining Research Testing Technology Co.; Ltd. and Jinhua Hao from the China University of Geosciences (Beijing) during LA-ICP-MS element, electron microprobe, and element mapping analyses. Thorough and constructive reviews by two anonymous reviewers were very helpful in our revision and are gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, D.Q.; Chen, Y.C.; Wang, D.H.; Tang, Y.L.; Zhou, R.H.; Wang, J.L.; Li, H.Q.; Chen, F.W. A discussion on problems related to mineralization of Tuwu-Yandong Cu-Mo ore field in Hami, Xinjiang. Miner. Depos. 2003, 22, 334–344. (In Chinese) [Google Scholar]
- Carruzzo, S.; Clarke, D.B.; Pelrine, K.M.; MacDonald, M.A. Texture, composition, and origin of rutile in the South Mountain batholith, Nova Scotia. Can. Mineral. 2006, 44, 715–729. [Google Scholar] [CrossRef]
- Meinhold, G. Rutile and its applications in earth sciences. Earth-Sci. Rev. 2010, 102, 1–28. [Google Scholar] [CrossRef]
- Plavsa, D.; Reddy, S.M.; Agangi, A.; Clark, C.; Kylander-Clark, A.; Tiddy, C.J. Microstructural, trace element and geochronological characterisation of TiO2 polymorphs and implications for mineral exploration. Chem. Geol. 2018, 476, 130–149. [Google Scholar] [CrossRef]
- Doyle, M.G.; Rasmussen, B.; Fletcher, I.R.; Muhling, J.R.; Foster, J.; Large, R.R.; Meffre, S.; Mathur, R.; McNaughton, N.J.; Phillips, D. Geochronological constraints on the tropicana gold deposit and albany-fraser Orogen, Western Australia. Econ. Geol. 2015, 110, 355–386. [Google Scholar] [CrossRef]
- Pi, Q.H.; Hu, R.Z.; Xiong, B.; Li, Q.L.; Zhong, R.C. In situ SIMS U-Pb dating of hydrothermal rutile: Reliable age for the Zhesang Carlin-type gold deposit in the golden triangle region, SW China. Miner. Depos. 2017, 52, 1179–1190. [Google Scholar] [CrossRef]
- Agangi, A.; Reddy, S.M.; Plavsa, D.; Fougerouse, D.; Clark, C.; Roberts, M.; Johnson, T.E. Antimony in rutile as a pathfinder for orogenic gold deposits. Ore Geol. Rev. 2019, 106, 1–11. [Google Scholar] [CrossRef]
- Rabbia, O.M.; Hernández, L.B.; French, D.H.; King, R.W.; Ayers, J.C. The El Teniente porphyry Cu-Mo deposit from a hydrothermal rutile perspective. Miner. Depos. 2009, 44, 849–866. [Google Scholar] [CrossRef]
- Chen, X.; Xu, R.K.; Zheng, Y.Y.; Gao, S.B.; Cai, P.J.; Yu, J.Z.; Wang, Q.M. The geodynamic setting of Dulan eclogite-type rutile deposits in the North Qaidam orogen, western China. Ore Geol. Rev. 2019, 110, 1–21. [Google Scholar] [CrossRef]
- Tiepolo, M.; Vannucci, R.; Oberti, R.; Foley, S.; Bottazzi, P.; Zanetti, A. Nb and Ta incorporation and fractionation in titanian pargasite and kaersutite: Crystal–chemical constraints and implications for natural systems. Earth Planet. Sci. Lett. 2000, 176, 185–201. [Google Scholar] [CrossRef]
- Scott, K.M. Rutile geochemistry as a guide to porphyry Cu-Au mineralization, Northparkes, New South Wales, Australia. Geochem. Explor. Environ. Anal. 2005, 5, 247–253. [Google Scholar] [CrossRef]
- Li, J.X.; Qin, K.Z.; Li, G.M.; Xiao, B.; Zhang, T.P.; Lei, X.G. Characteristics of rutiles from Duobuza gold-rich porphyry copper deposit in Bangong Lake Belt of northern Tibet and their significance. Miner. Depos. 2008, 27, 209–219. (In Chinese) [Google Scholar]
- Xie, F.W.; Tang, J.X.; Lang, X.H. Ore potential of the porphyry in No.I deposit of the Xiongcun ore district, Tibet: Evidence from hydrothermal and accessory minerals. Acta Petrol. Mineral. 2015, 34, 51–64. (In Chinese) [Google Scholar]
- Watson, E.B.; Wark, D.A.; Thomas, J.B. Crystallization thermometers for zircon and rutile. Contrib. Mineral. Petrol. 2006, 151, 413. [Google Scholar] [CrossRef]
- Tomkins, H.S.; Powell, R.; Ellis, D.J. The pressure dependence of the zirconium-in-rutile thermometer. J. Metamorph. Geol. 2007, 25, 703–713. [Google Scholar] [CrossRef]
- Clark, J.R.; Williams, J. Rutile as a potential indicator mineral for metamorphosed metallic ore deposits. Québec Explor. 2004, 1, 1–17. [Google Scholar]
- Carocci, E.; Marignac, C.; Cathelineau, M.; Truche, L.; Poujol, M.; Boiron, M.C.; Pinto, F. Incipient Wolframite Deposition at Panasqueira (Portugal): W Rutile and Tourmaline Compositions as Proxies for the Early Fluid Composition. Econ. Geol. 2021, 116, 123–146. [Google Scholar] [CrossRef]
- Moore, J.; Beinlich, A.; Porter, J.K.; Talavera, C.; Berndt, J.; Piazolo, S.; Austrheim, H.; Putnis, A. Microstructurally controlled trace element (Zr, U-Pb) concentrations in metamorphic rutile: An example from the amphibolites of the Bergen Arcs. J. Metamorph. Geol. 2021, 38, 103–127. [Google Scholar] [CrossRef]
- Czamanski, G.K.; Force, E.R.; Moore, W.J. Some geologic and potential resource aspects of rutile in porphyry copper deposits. Econ. Geol. 1981, 76, 2240–2256. [Google Scholar] [CrossRef]
- Dai, J.; Ni, S.J.; Huang, Y.; Ding, J.; Chou, I.M. Genesisi of Rutile from Metallogenic Porphyry in the Zhunuo Porphyry-type Cu-Mo Deposit, Tibet, China, and Its Significance for Prospecting. Acta Geol. Sin. 2018, 92, 1228–1239. (In Chinese) [Google Scholar]
- Cooke, D.R.; Hollings, P.; Walsh, J.L. Giant porphyry deposits: Characteristics, distribution, and tectonic controls. Econ. Geol. 2005, 100, 801–818. [Google Scholar] [CrossRef]
- Chiaradia, M.; Schaltegger, U.; Spikings, R.; Wotzlaw, J.F.; Ovtcharova, M. How accurately can we date the duration of magmatic-hydrothermal events in porphyry systems?—An invited paper. Econ. Geol. 2013, 108, 565–584. [Google Scholar] [CrossRef]
- Zhang, F.F.; Wang, Y.H.; Xue, C.J.; Liu, J.J.; Zhang, W. Fluid inclusion and isotope evidence for magmatic-hydrothermal fluid evolution in the Tuwu porphyry copper deposit, Xinjiang, NW China. Ore Geol. Rev. 2019, 113, 1–23. [Google Scholar] [CrossRef]
- Yuan, H.Q.; Shen, P.; Pan, H.D.; An, Z.H.; Ma, G.; Li, W.G. Geochemistry and mineral chemical behavior of hydrothermal alteration of the Tuwu porphyry copper deposit, Eastern Tianshan, Northwest China. Geol. J. 2020, 55, 786–805. [Google Scholar] [CrossRef]
- Chen, H.Y.; Wan, B.; Pirajno, F.; Chen, Y.J.; Xiao, B. Metallogenesis of the Xinjiang orogens, NW China—New discoveries and ore genesis. Ore Geol. Rev. 2018, 100, 1–11. [Google Scholar] [CrossRef]
- Shen, P.; Pan, H.D.; Zhou, T.F.; Wang, J.B. Petrography, geochemistry and geo-chronology of the host porphyries and associated alteration at the Tuwu Cu deposit, NW China: A case for increased depositional efficiency by reaction with mafic hostrock? Miner. Depos. 2014, 49, 709–731. [Google Scholar] [CrossRef]
- Xiao, B.; Chen, H.Y.; Hollings, P.; Han, J.S.; Wang, Y.F.; Yang, J.T.; Cai, K. Magmatic evolution of the Tuwu-Yandong porphyry Cu belt, NW China: Constraints from geochronology, geochemistry and Sr-Nd-Hf isotopes. Gondwana Res. 2017, 43, 74–91. (In Chinese) [Google Scholar] [CrossRef]
- Wang, Y.H.; Zhang, F.F.; Xue, C.J.; Liu, J.J.; Zhang, Z.Z.; Sun, M. Geology and Genesis of the Tuwu Porphyry Cu Deposit, Xinjiang, Northwest China. Econ. Geol. 2021, 116, 471–500. [Google Scholar] [CrossRef]
- Xiao, W.J.; Windley, B.F.; Allen, M.B.; Han, C.M. Paleozoic multiple accretionary and collisional tectonics of the Chinese Tianshan orogenic collage. Gondwana Res. 2013, 23, 1316–1341. [Google Scholar] [CrossRef]
- Xiao, W.J.; Sun, M.; Santosh, M. Continental reconstruction and metallogeny of the Circum-Junggar areas and termination of the southern Central Asian Orogenic Belt. Geosci. Front. 2015, 6, 137–140. [Google Scholar] [CrossRef] [Green Version]
- Xiao, W.J.; Zhang, L.C.; Qin, K.Z.; Sun, S.; Li, J.L. Paleozoic accretionary and collisional tectonics of the Eastern Tianshan (China): Implications for the continental growth of Central Asia. Am. J. Sci. 2004, 304, 370–395. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.J.; Shu, L.S.; Santosh, M. Late Paleozoic post-collisional magmatism in the Eastern Tianshan Belt, northwest China: New insights from geochemistry, geochronology and petrology of bimodal volcanic rocks. Lithos 2011, 127, 581–598. [Google Scholar] [CrossRef]
- Pirajno, F.; Seltmann, R.; Yang, Y.Q. A review of mineral systems and associated tectonic settings of northern Xinjiang, NW China. Geosci. Front. 2011, 2, 157–185. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.H.; Xue, C.J.; Liu, J.J.; Zhang, F.F. Geological, geochronological, geochemical, and Sr–Nd–O–Hf isotopic constraints on origins of intrusions associated with the Baishan porphyry Mo deposit in Eastern Tianshan, NW China. Miner. Depos. 2016, 51, 953–969. [Google Scholar] [CrossRef]
- Wang, Y.H.; Xue, C.J.; Gao, J.B.; Zhang, F.F.; Liu, J.J.; Wang, J.P.; Wang, J.C. The genesis of the ores and granitic rocks at the Hongshi Au deposit in Eastern Tianshan, China: Constraints from zircon U-Pb geochronology, geochemistry and isotope systematics. Ore Geol. Rev. 2016, 74, 122–138. [Google Scholar] [CrossRef]
- Mao, J.W.; Goldfarb, R.T.; Wang, Y.T.; Hart, C.J.; Wang, Z.L.; Yang, J.M. Late Paleozoic base and precious metal deposits, East Tianshan, Xinjiang, China, characteristics and geodynamic setting. Episodes 2005, 28, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.C.; Qin, K.Z.; Xiao, W.J. Multiple mineralization events in the Eastern Tianshan district, NW China: Isotopic geochronology and geological significance. J. Asian Earth Sci. 2008, 32, 236–246. [Google Scholar] [CrossRef]
- Wang, Y.H.; Zhang, F.F.; Liu, J.J.; Que, C.Y. Genesis of the Fuxing porphyry Cu deposit in Eastern Tianshan, China: Evidence from fluid inclusions and C–H–O–S–Pb isotope systematics. Ore Geol. Rev. 2016, 79, 46–61. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Sun, M.; Yuan, C.; Long, X.P.; Jiang, Y.D.; Li, P.F.; Huang, Z.Y.; Du, L. Alternating trench advance and retreat: Insights from Paleozoic magmatism in the Eastern Tianshan, Central Asian Orogenic Belt. Tectonics 2018, 37, 2142–2164. [Google Scholar] [CrossRef]
- Wang, Y.H.; Zhang, F.F.; Li, B.C. Genesis of the Yandong porphyry Cu deposit in Eastern Tianshan, NW China: Evidence from geology, fluid inclusions and isotope systematics. Ore Geol. Rev. 2017, 86, 280–296. [Google Scholar] [CrossRef]
- Qin, K.Z.; Fang, T.H.; Wang, S.L.; Zhu, B.Q.; Feng, Y.M.; Yu, H.F.; Xiu, Q.Y. Plate tectonic division, evolution and metallogenic settings in Eastern Tianshan Mountains, NW-China. Xinjiang Geol. 2002, 20, 302–308. (In Chinese) [Google Scholar]
- Su, B.X.; Qin, K.Z.; Sun, H.; Tang, D.M.; Xiao, Q.H.; Liu, P.P. Olivine compositional mapping of mafic-ultramafic complexes in Eastern Xinjiang (NW China): Implications for Cu–Ni mineralization and tectonic dynamics. J. Earth Sci. 2012, 23, 41–53. [Google Scholar] [CrossRef]
- Huang, X.W.; Qi, L.; Gao, J.F.; Zhou, M.F. First reliable Re–Os Ages of pyrite and stable isotope compositions of Fe(-Cu) deposits in the Hami Region, Eastern Tianshan Orogenic Belt, NW China. Resour. Geol. 2013, 63, 166–187. [Google Scholar] [CrossRef]
- Han, C.M.; Xiao, W.J.; Zhao, G.C.; Mao, J.W.; Li, S.Z.; Yan, Z.; Mao, Q.G. Majortypes, characteristics and geodynamic mechanism of upper Paleozoic copper deposits in northern Xinjiang, north western China. Ore Geol. Rev. 2006, 28, 308–328. [Google Scholar] [CrossRef]
- Zhang, F.F.; Wang, Y.H.; Liu, J.J.; Wang, J.P. Zircon U-Pb and molybdenite Re–Os geochronology, Hf isotope analyses, and whole-rock geochemistry of the Donggebi Mo deposit, Eastern Tianshan, Northwest China, and their geological significance. Int. Geol. Rev. 2015, 57, 446–462. [Google Scholar] [CrossRef]
- Rui, Z.Y.; Wang, L.S.; Wang, Y.T.; Liu, Y.L. Discussion on metallogenic epoch of Tuwu and Yandong porphyry copper deposits in East Tianshan Mountains, Xinjiang. Miner. Depos. 2002, 21, 16–22. (In Chinese) [Google Scholar]
- Wang, Y.H.; Xue, C.J.; Liu, J.J.; Wang, J.P.; Yang, J.T.; Zhang, F.F.; Zhao, Z.N.; Zhao, Y.J.; Liu, B. Early Carboniferous adakitic rocks in the area of the Tuwu deposit, Eastern Tianshan, NW China: Slab melting and implications for porphyry copper mineralization. J. Asian Earth Sci. 2015, 103, 332–349. [Google Scholar] [CrossRef]
- Wang, Y.H.; Xue, C.J.; Liu, J.J.; Wang, J.P.; Yang, J.T.; Zhang, F.F.; Zhao, Z.N.; Zhao, Y.J. Geochemistry, geochronology, Hf isotope, and geological significance of the Tuwu porphyry copper deposit in Eastern Tianshan, Xinjiang. Acta Petrol. Sin. 2014, 30, 3383–3399. (In Chinese) [Google Scholar]
- Pan, H.D.; Shen, P.; Chen, G.; Yang, J.T.; Zhao, Y.J.; Dai, H.W. Volcanic-plutonic complex, ore-forming rocks and their alterations in Tuwu porphyry Cu deposit of Xinjiang. Deposit 2013, 32, 794–808. (In Chinese) [Google Scholar]
- Zhang, D.Y.; Zhou, T.F.; Yuan, F.; Fan, Y.; Liu, S.; Peng, M.X. Geochemical characters, metallogenic chronology and geological significance of the Yanxi copper deposit in Eastern Tianshan, Xinjiang. Acta Pet. Sin 2010, 26, 3327–3338. (In Chinese) [Google Scholar]
- Liu, Y.S.; Gao, S.; Hu, Z.C.; Gao, C.G.; Zong, K.Q.; Wang, D.B. Continental and oceanic crust recycling-induced melt-peridotite interactions in the Trans-North China Orogen: U-Pb dating, Hf isotopes and trace elements in zircons from mantle xenoliths. J. Petrol. 2010, 51, 537–571. [Google Scholar] [CrossRef]
- GeoReM Database. Available online: http://georem.mpch-mainz.gwdg.de/ (accessed on 5 September 2021).
- Hou, Z.H.; Xiao, Y.L.; Shen, J.; Yu, C.L. In situ rutile U-Pb dating based on zircon calibration using LA-ICP-MS, geological applications in the Dabie orogen, China. J. Asian Earth Sci. 2020, 192, 104261. [Google Scholar] [CrossRef]
- Kohn, M.J. A refined zirconium-in-rutile thermometer. Am. Mineral. 2020, 105, 963–971. [Google Scholar] [CrossRef]
- Inoue, A.; Meunier, A.; Patrier-Mas, P.; Rigault, C.; Beaufort, D.; Vieillard, P. Application of chemical geothermometry to low-temperature trioctahedral chlorites. Clays Clay Miner. 2009, 57, 371–382. [Google Scholar] [CrossRef]
- Hiller, S.; Velde, B. Octahedral occupancy and the chemical composition of diagenetic (low-temperature) chlorite. Clay Miner. 1991, 26, 149–168. [Google Scholar] [CrossRef]
- Inoue, A.; Kurokawa, K.; Hatta, T. Application of chlorite geothermometry to hydrothermal alteration in Toyoha geothermal system, Southwestern Hokkaido, Japan. Resour. Geol. 2010, 60, 52–70. [Google Scholar] [CrossRef]
- Zu, B.; Xue, C.; Seltmann, R. Geology, geochronology, and S-Pb-Os geochemistry of the Alastuo gold deposit, West Tianshan, NW China. Miner. Depos. 2020, 55, 1407–1424. [Google Scholar] [CrossRef]
- Kranidiotis, P.; MacLean, W.H. Systematics of chlorite alteration at the Phelps Dodge massive sulfide deposit, Matagami, Quebec. Econ. Geol. 1987, 82, 1898–1911. [Google Scholar] [CrossRef]
- Cathelineau, M. Cation site occupancy in chlorites and illites as a function of temperature. Clay Miner. 1988, 23, 471–485. [Google Scholar] [CrossRef]
- Jowett, E.C. Fitting iron and magnesium into the hydrothermal chlorite geothermometer. In Proceedings of the GAC-MAC-SEG Joint Annual Meeting, Toronto, ON, Canada, 27–29 May 1991; Volume 16, p. A62, Unpublished paper. [Google Scholar]
- Porter, J.K.; McNaughton, N.J.; Evans, N.J.; McDonald, B.J. Rutile as a pathfinder for metals exploration. Ore Geol. Rev. 2020, 120, 1–22. [Google Scholar] [CrossRef]
- Williams, S.A.; Cesbron, F.P. Rutile and apatite: Useful prospecting guides for porphyry copper deposits. Mineral. Mag. 1977, 41, 288–292. [Google Scholar] [CrossRef]
- Emst, W.G.; Liu, J. Experimental phase equilibrium study of Al and Ti contents of calcic amphibole in MORB: A semiquantitative thermobarometer. Am. Mineral. 1998, 83, 952–969. [Google Scholar]
- Ai, H.; Zhao, X.M.; Tang, C.; Guo, W.B.; Huang, C. Review of the application of accessory minerals in the study of porphyry copper deposit. Mordern Mining. 2017, 573, 32–50. (In Chinese) [Google Scholar]
- Force, E.R.; Djaswadi, S.; Leeuwen, T.V. Exploration for Porphyry Metal Deposits Based on Rutile Distribution—A Test in Sumatera; United States Government Publishing Office: Washington, DC, USA, 1984; pp. A1–A13.
- Pe-Piper, G.; Nagle, J.; Piper, D.J.W.; McFarlane, C.R.M. Geochronology and trace element mobility in rutile from a Carboniferous syenite pegmatite and the role of halogens. Am. Mineral. 2019, 104, 501–513. [Google Scholar] [CrossRef]
- Feng, H.X.; Shen, P.; Zhu, R.X.; Ma, G.; Li, C.H.; Li, J.P. SIMS U-Pb dating of vein-hosted hydrothermal rutile and carbon isotope of fluids in the Wulong lode gold deposit, NE China: Linking gold mineralization with craton destruction. Ore Geol. Rev. 2020, 127, 1–20. [Google Scholar] [CrossRef]
- Scott, K.M.; Radford, N.W. Rutile compositions at the Big Bell Au deposit as a guide for exploration. Geochem. Explor. Environ. Anal. 2007, 7, 353–361. [Google Scholar] [CrossRef]
- Kuscu, M.; Cengiz, O.; Isik, K.; Gul, E.K. The origin and geochemical characteristics of rutile in eluvial and fluvial-alluvial placers and quartz veins of the Menderes Massif from the Neoproterozoic Pan-African Belt, Western Turkey. J. Afr. Earth. Sci. 2018, 143, 10–27. [Google Scholar] [CrossRef]
- Beane, R.E.; Titley, S.R. Porphyry copper deposits. Part II. Econ. Geol. 1981, 75, 214–269. [Google Scholar]
- Ayers, J.C.; Waston, E.B. Rutile solubility and mobility in supercritical aqueous fluids. Contrib. Mineral. Petrol. 1993, 114, 321–330. [Google Scholar] [CrossRef]
- Force, E.R. Is the Untied States geologically dependent on imported rutile? In Proceedings of the 4th Industrial Minerals International Congress, Atlanta, GA, USA, 28–30 May 1981; pp. 43–48. [Google Scholar]
- Rabbia, O.M. Crystal Chemistry of Rutile and Anatase in Anddam Porphyry Deposits, Evaluation of Its Use as Monitor of Metal Activity in Crustal Hydrothermal Fluids. Ph.D. Thesis, Universidad de Chile, Santiago, Chile, 2002; pp. 1–147. [Google Scholar]
- Graham, J.; Morris, R.C. Tungsten- and antimony-substituted rutile. Mineral. Mag. 1973, 39, 470–473. [Google Scholar] [CrossRef] [Green Version]
- Urban, A.J.; Hoskins, B.F.; Grey, I.E. Characterization of V-Sb-W-bearing rutile from the Hemlo gold deposit, Ontario. Can. Mineral. 1992, 30, 319–326. [Google Scholar]
- Brenan, J.M.; Shaw, H.F.; Phinney, D.L.; Ryerson, F.J. Rutile-aqueous fluid partitioning of Nb, Ta, Hf, Zr, U and Th: Implications for the high field strength element depletions in island-arc basalts. Earth Planet. Sci. Lett. 1994, 128, 327–339. [Google Scholar] [CrossRef]
- Mueller, A.G.; Lawrence, L.M.; Muhling, J.; Pooley, G.D. Mineralogy and PTX relationships of the Archean South Au-Cu (Co-Bi) deposit, Kalgoorlie, Western Australia: Thermodynamic constraints on the formation of a zoned intrusion-related Skarn. Econ. Geol. 2012, 107, 1–24. [Google Scholar] [CrossRef]
- Rabbia, O.M.; Hernandez, L.B. Mineral Chemistry and Potential Applications of Natural Multi-Doped Hydrothermal Rutile from Porphyry Copper Deposits. In Rutile: Properties, Synthesis and Applications; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2012; pp. 209–228. [Google Scholar]
- Hey, M.H. A new review of the chlorites. Mineral. Mag. 1954, 30, 277–292. [Google Scholar] [CrossRef]
- Zane, A.; Weiss, Z. A procedure for classifying rock-forming chlorites based on microprobe data. Rend. Lincei 1998, 9, 51–56. [Google Scholar] [CrossRef]
- Wiewióra, A.; Weiss, Z. Crystallochemical classifications of phyllosilicates based on the unified system of projection of chemical composition: II. The chlorite group. Clay Miner. 1990, 25, 83–92. [Google Scholar] [CrossRef]
- Inoue, A.; Inoué, S.; Utada, M. Application of chlorite thermometry to estimation of formation temperature and redox conditions. Clay Miner. 2018, 53, 143–158. [Google Scholar] [CrossRef]
- Walshe, J.L. A six-component chlorite solid solution model and the conditions of chlorite formation in hydrothermal and geothermal system. Econ. Geol. 1986, 81, 681–703. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Gao, X.Y.; Chen, R.X.; Gao, T.S. Zr-inrutile thermometry of eclogite in the Dabie orogen: Constraints on rutile growth during continental subduction-zone metamorphism. J. Asian Earth Sci. 2011, 40, 427–451. [Google Scholar] [CrossRef]
- Yuan, H.Q.; Shen, P.; Pan, H.D.; Liu, Y. Mineralogy and mineral geochemistry of the Tuwu porphyry Cu deposit, Eastern Tianshan, NW China: Implication for the ore-forming condition and Cu mineralization. Arabian J. Geosci. 2020, 13, 1–19. [Google Scholar] [CrossRef]
- John, T.; Klemd, R.; Gao, J.; Garbe-Schönberg, C.D. Trace-element mobilization in slabs due to non steady-state fluid–rock interaction: Constraints from an eclogite-facies transport vein in blueschist (Tianshan, China). Lithos 2008, 103, 1–24. [Google Scholar] [CrossRef]
- Rapp, J.F.; Klemme, S.; Butler, I.B.; Harley, S.L. Extremely high solubility of rutile in chloride and fluoride-bearing metamorphic fluids: An experimental investigation. Geology 2010, 38, 323–326. [Google Scholar] [CrossRef]
- Tanis, E.A.; Simon, A.; Zhang, Y.X.; Chow, P.; Xiao, Y.M.; Hanchar, J.M.; Tschauner, O.; Shen, G.Y. Rutile solubility in NaF-NaCl-KCl-bearing aqueous fluids at 0.5–2.79 GPa and 250–650 °C. Geochim. Cosmochim. Acta 2016, 177, 170–181. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, R.J.; Harley, S.L. Zr-in-rutile resetting in aluminosilicate bearing ultra-high temperature granulites: Refining the record of cooling and hydration in the Napier Complex, Antarctica. Lithos 2017, 272, 128–146. [Google Scholar] [CrossRef] [Green Version]
- Cabral, A.R.; Rios, F.J.; de Oliveira, L.A.R.; de Abreu, F.R.; Lehmann, B.; Zack, T.; Laufek, F. Fluid-inclusion microthermometry and the Zr-in-rutile thermometer for hydrothermal rutile. Int. J. Earth Sci. 2015, 104, 513–519. [Google Scholar] [CrossRef]
- Zack, T.; Moraes, R.; Kronz, A. Temperature dependence of Zr in rutile: Empirical calibration of a rutile thermometer. Contrib. Mineral. Petrol. 2004, 148, 471–488. [Google Scholar] [CrossRef]
- Li, J.X.; Li, G.M.; Qin, K.Z.; Xiao, B.; Chen, L.; Zhao, J.X. Mineralogy and Mineral Chemistry of the Cretaceous Duolong Gold-Rich Porphyry Copper Deposit in the Bangongco Arc, Northern Tibet. Resour. Geol. 2011, 62, 19–41. [Google Scholar] [CrossRef]
- Chen, P.W.; Zeng, Q.D.; Guo, W.K.; Chen, J.Q. Insights into the formation of the Dasuji porphyry Mo deposit (North China Craton) gained from mineral chemistry data. Ore Geol. Rev. 2019, 112, 1–15. [Google Scholar] [CrossRef]
- Agangi, A.; Plavsa, D.; Reddy, S.M.; Olierook, H.; Kylander-Clark, A. Compositional modification and trace element decoupling in rutile: Insight from the Capricorn Orogen, Western Australia. Precambrian Res. 2020, 345, 1–17. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).