Stability Studies of Magnetite Nanoparticles in Environmental Solutions
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Apparatus
2.2. Preparation of Nanoparticles
- (A)
- In this case (Fe3O4 NP’s), the so-called Massart synthesis was used. This method is based on the co-precipitation of Fe(III) and Fe(II) chlorides in ammonia aqueous solution at the temperature of 80 °C under an Ar atmosphere. In two flasks, a proper amount of FeCl3·6H2O and FeCl2·4H2O was dissolved in ammonia solution. In the next step, TBAOH was added to both flasks. The mixture with the Fe(II) chlorides was combined with the flask containing the Fe(III) chlorides, and the whole solution was heated to 80 °C.
- (B)
- To obtain magnetite with a silica shell (SiO2@Fe3O4 NP’s), in a similar manner to the previous nanoparticles, two round-bottom flasks were used. However, in this case, in the first flask, distilled water was used, and in the second flask, a solution of distilled water with 37% HCl was deoxygenated. Then, FeCl3·6H2O was added to the first flask, and FeCl2·4H2O was added to the second flask. Both solutions were heated to 50 °C, and at the same time, vigorous stirring in an argon flow was continued. The solution from the second flask was slowly combined with the solution from the first flask, and after the addition of 1 M NaOH, the mixture was heated to 95 °C. The resultant mixture was still blended and deoxygenated for the next 30 min [37].When the resultant precipitate was cooled down, the excess citric acid solution of 0.01 M was added to the mixture. Then, a TMAOH solution was poured until the pH reached value 7. With such a solution, a deoxygenated mixture of ethanol, distilled water, and 25% ammonia was combined and mixed for about 10 min. After that, a TEOS solution was slowly added and was left for 24 h at RT on the stirrer to start a reaction.
- (C)
- In the last synthesis (Fe3O4@Fe3O4 NP’s), Fe(acac)3 salt was used to obtain seed particles. The mixture of 8 mmol Fe(acac)3, 1,2-hexadecanediol, phenyl ether, oleic acid, and oleyl amine was stirred and heated to 260 °C for 30 min with continuous argon flow. When the solution had cooled to room temperature, Fe(acac)3, 1-octadecanol, oleic acid, and oleyl amine were added. After that, the whole mixture was heated once again, this time to 230 °C for 30 min [36,38].
3. Results and Discussion
3.1. TEM Studies
3.2. Gravimetric and FAAS Results
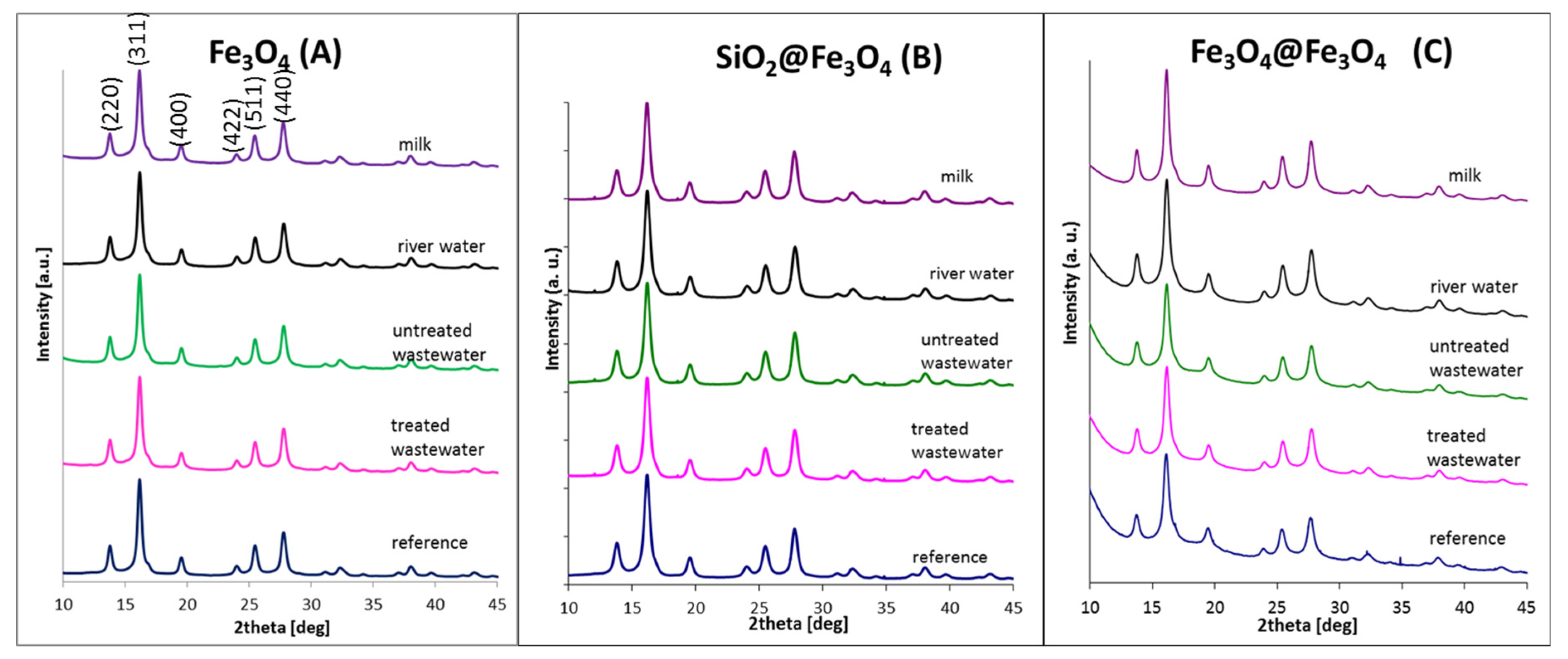
3.3. X-ray Studies
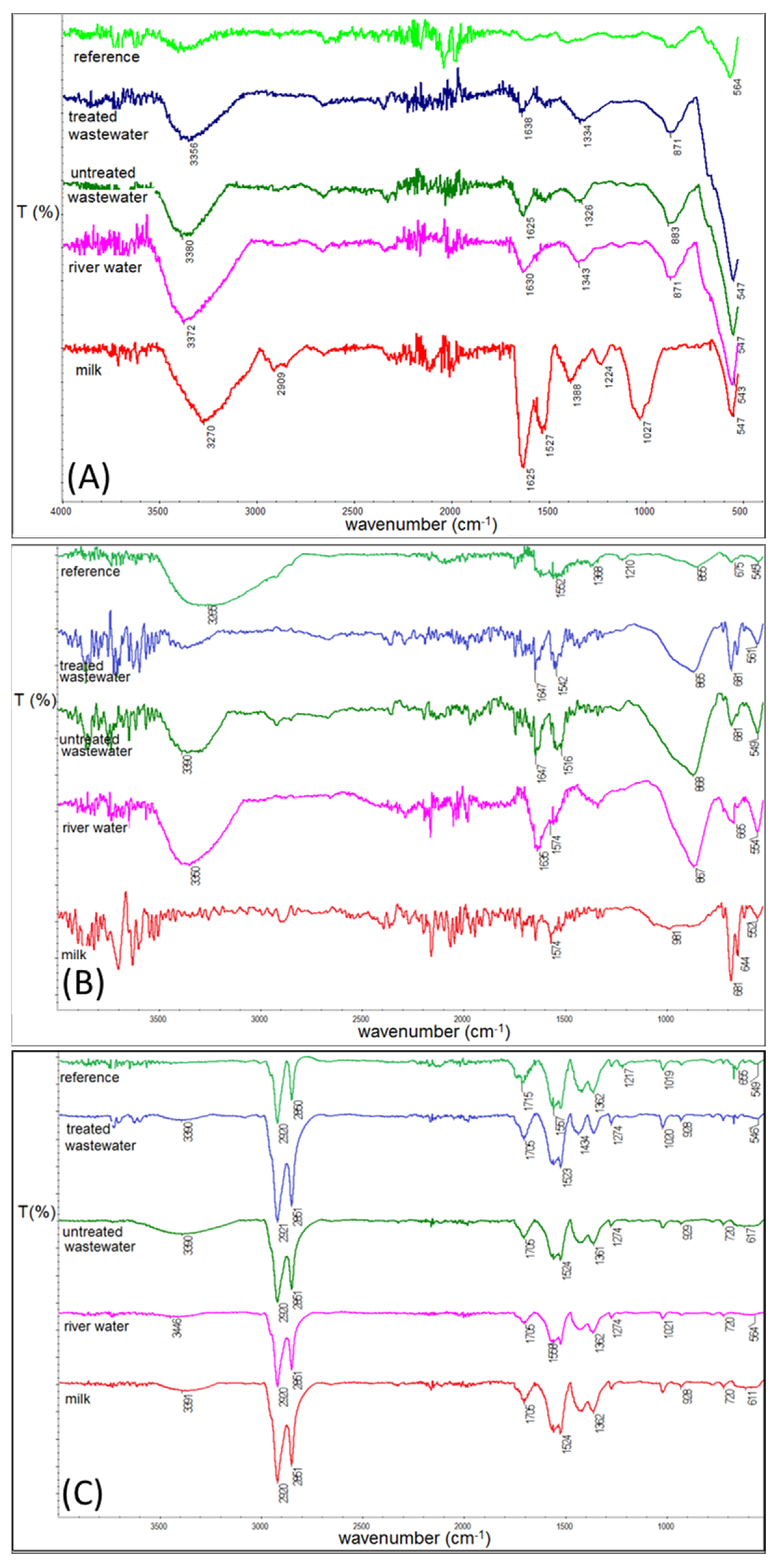
3.4. IR Studies
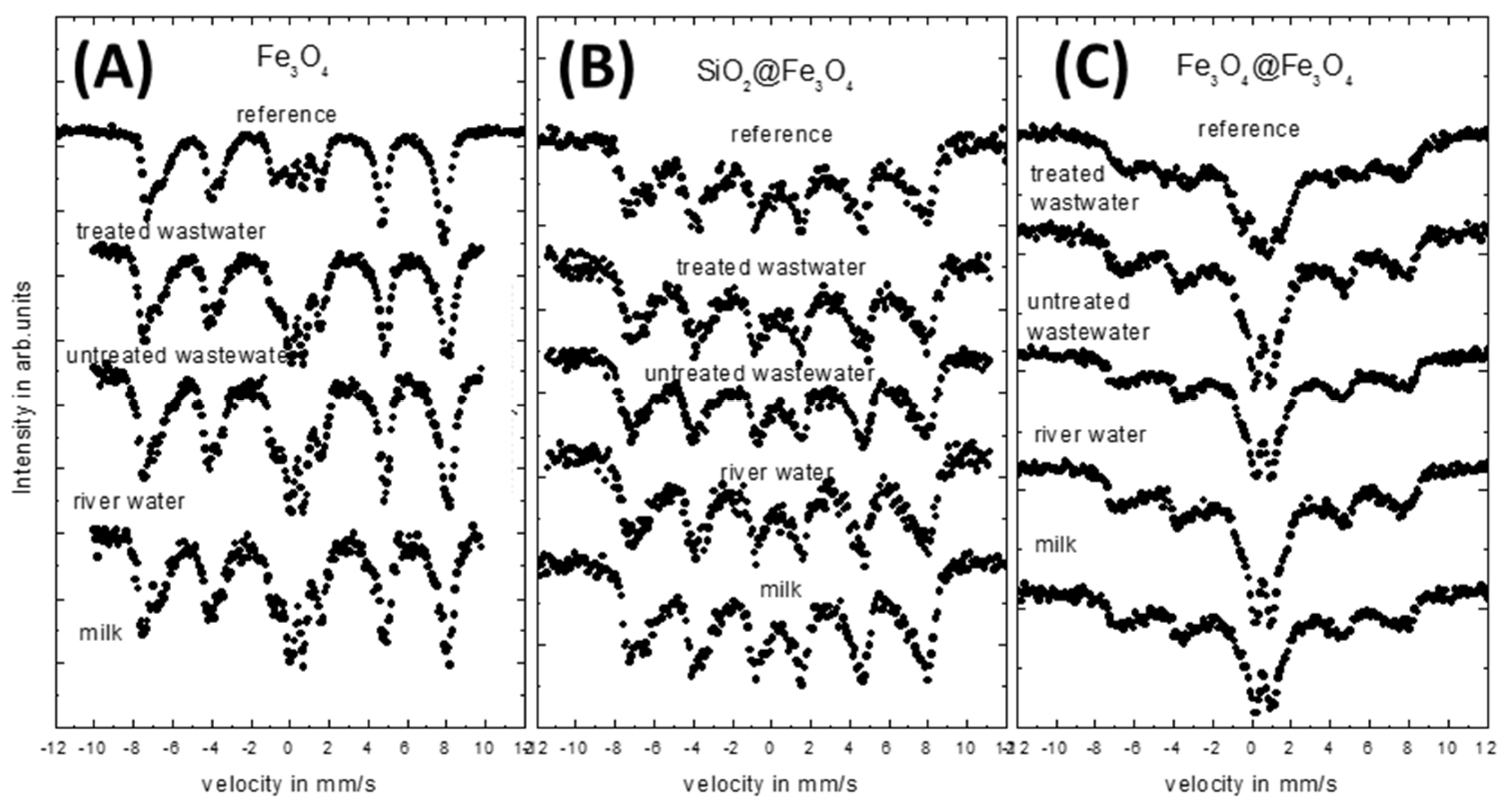
3.5. Mössbauer Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiao, D.; Lu, T.; Zeng, R.; Bi, Y. Preparation and highlighted applications of magnetic microparticles and nanoparticles: A review on recent advances. Microchim. Acta 2016, 183, 2655–2675. [Google Scholar] [CrossRef]
- Campos, E.A.; Stockler Pinto, D.V.B.; Sampaio de Oliviera, J.I.; da Costa Mattos, E.; de Cassia Lazzarini Dutra, R. Synthesis, Characterization and Applications of Iron Oxides Nanoparticles—A Short Reveiw. J. Aerosp. Technol. Maneg. 2015, 7, 267–276. [Google Scholar] [CrossRef] [Green Version]
- Machala, L.; Tuček, J.; Zbořil, R. Polymorphous transformations of nanometric iron(III) oxide: A review. Chem. Mater. 2011, 23, 3255–3272. [Google Scholar] [CrossRef]
- Amiri, M.; Pardakhti, A.; Ahmadi-Zeidabadi, M.; Akbari, A.; Salavati-Niasari, M. Magnetic nickel ferrite nanoparticles: Green synthesis by Urtica and therapeutic effect of frequency magnetic field on creating cytotoxic response in neural cell lines. Colloids Surfaces B Biointerfaces 2018, 172, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Blaney, L. Magnetite (Fe3O4): Properties, Synthesis, and Applications. Leigh Rev. 2007, 15, 32–81. [Google Scholar]
- Lodhia, J.; Mandarano, G.; Ferris, N.; Eu, P.; Cowell, S. Development and use of iron oxide nanoparticles (Part 1): Synthesis of iron oxide nanoparticles for MRI. Biomed. Imaging Interv. J. 2010, 6, e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, H.L.; Nguyen, H.N.; Nguyen, H.H.; Luu, M.Q.; Nguyen, M.H. Nanoparticles: Synthesis and applications in life science and environmental technology. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015, 6, 015008. [Google Scholar] [CrossRef] [Green Version]
- Kong, J.; Coolahan, K.; Mugweru, A. Manganese based magnetic nanoparticles for heavy metal detection and environmental remediation. Anal. Methods 2013, 5, 5128. [Google Scholar] [CrossRef]
- Tang, S.C.N.; Lo, I.M.C. Magnetic nanoparticles: Essential factors for sustainable environmental applications. Water Res. 2013, 47, 2613–2632. [Google Scholar] [CrossRef]
- Toal, B.; McMillen, M.; Murphy, A.; Hendren, W.; Arredondo, M.; Pollard, R. Optical and magneto-optical properties of gold core cobalt shell magnetoplasmonic nanowire arrays. Nanoscale 2014, 6, 12905–12911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troitiño, N.F.; Rivas-Murias, B.; Rodríguez-González, B.; Salgueiriño, V. Exchange bias effect in CoO@Fe3O4 core—Shell octahedron-shaped nanoparticles. Chem. Mater. 2014, 26, 5566–5575. [Google Scholar] [CrossRef]
- Brollo, M.; Orozco-Henao, J.; López-Ruiz, R.; Muraca, D.; Dias, C.; Pirota, K.; Knobel, M. Magnetic hyperthermia in brick-like Ag@Fe3O4 core-shell nanoparticles. J. Magn. Magn. Mater. 2016, 397, 20–27. [Google Scholar] [CrossRef]
- Lee, B.; Koo, S. Preparation of silver nanoparticles on the surface of fine magnetite particles by a chemical reduction. J. Ind. Eng. Chem. 2011, 17, 762–766. [Google Scholar] [CrossRef]
- Novakova, A.; Lanchinskaya, V.; Volkov, A.; Gendler, T.; Kiseleva, T.Y.; Moskvina, M.; Zezin, S. Magnetic properties of polymer nanocomposites containing iron oxide nanoparticles. J. Magn. Magn. Mater. 2003, 258, 354–357. [Google Scholar] [CrossRef]
- Harris, L.A.; Goff, J.D.; Carmichael, A.Y.; Riffle, J.S.; Harburn, J.J.; Pierre, T.S.; Saunders, M. Magnetite nanoparticle dispersions stabilized with triblock copolymers. Chem. Mater. 2003, 15, 1367–1377. [Google Scholar] [CrossRef]
- Nishad, K.K.; Tiwari, N.; Pandey, R.K. Synthesis and Characterization of Ferromagnetic Fe3O4-ZnO Hybrid Core-Shell Nanoparticles. J. Electron. Mater. 2018, 47, 3440–3450. [Google Scholar] [CrossRef]
- Dresco, P.A.; Zaitsev, V.S.; Gambino, R.J.; Chu, B. Preparation and properties of magnetite and polymer magnetite nanoparticles. Langmuir 1999, 15, 1945–1951. [Google Scholar] [CrossRef]
- Li, S.; Liang, Q.; Ahmed, S.A.H.; Zhang, J. Simultaneous Determination of Five Benzimidazoles in Agricultural Foods by Core-Shell Magnetic Covalent Organic Framework Nanoparticle–Based Solid-Phase Extraction Coupled with High-Performance Liquid Chromatography. Food Anal. Methods 2020, 13, 1111–1118. [Google Scholar] [CrossRef]
- Xu, Z.; Hou, Y.; Sun, S. Magnetic Core/Shell Fe3O4/Au and Fe3O4/Au/Ag Nanoparticles with Tunable Plasmonic Properties. J. Am. Chem. Soc. 2007, 129, 8698–8699. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Luo, J.; Fan, Q.; Suzuki, M.; Suzuki, I.S.; Engelhard, M.H.; Lin, Y.; Kim, N.; Wang, J.Q.; Zhong, C.J. Monodispersed Core−Shell Fe3O4@Au Nanoparticles. J. Phys. Chem. B 2005, 109, 21593–21601. [Google Scholar] [CrossRef] [PubMed]
- Salihov, S.V.; Ivanenkov, Y.A.; Krechetov, S.P.; Veselov, M.; Sviridenkova, N.V.; Savchenko, A.G.; Klyachko, N.L.; Golovin, Y.I.; Chufarova, N.V.; Beloglazkina, E.K.; et al. Recent advances in the synthesis of Fe3O4@AU core/shell nanoparticles. J. Magn. Magn. Mater. 2015, 394, 173–178. [Google Scholar] [CrossRef]
- Mandal, M.; Ghosh, S.K.; Kundu, S.; Esumi, K.; Pal, T. UV Photoactivation for Size and Shape Controlled Synthesis and Coalescence of Gold Nanoparticles in Micelles. Langmuir 2002, 18, 7792–7797. [Google Scholar] [CrossRef]
- Barrera, C.; Herrera, A.; Zayas, Y.; Rinaldi, C. Surface modification of magnetite nanoparticles for biomedical applications. J. Magn. Magn. Mater. 2009, 321, 1397–1399. [Google Scholar] [CrossRef]
- Neouze, M.-A.; Schubert, U. Surface Modification and Functionalization of Metal and Metal Oxide Nanoparticles by Organic Ligands. Mon. Chem.-Chem. Mon. 2008, 139, 183–195. [Google Scholar] [CrossRef]
- Bini, R.A.; Marques, R.F.C.; Santos, F.J.; Chaker, J.A.; Jafelicci, M., Jr. Synthesis and functionalization of magnetite nanoparticles with different amino-functional alkoxysilanes. J. Magn. Magn. Mater. 2011, 324, 534–539. [Google Scholar] [CrossRef] [Green Version]
- Pirouz, M.J.; Beyki, M.H.; Shemirani, F. Anhydride functionalised calcium ferrite nanoparticles: A new selective magnetic material for enrichment of lead ions from water and food samples. Food Chem. 2015, 170, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Shi, Q.; Fu, H.; Hu, O.; Fan, Y.; Xu, L.; Zhang, L.; Lan, W.; Sun, D.; Yang, T.; et al. Rapid detection of five pesticide residues using complexes of gold nanoparticle and porphyrin combined with ultraviolet visible spectrum. J. Sci. Food Agric. 2020, 100, 4464–4473. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, N.; Mehra, R.; Kumar, H.; Singh, V.P. Progress and challenges in the detection of residual pesticides using nanotechnology based colorimetric techniques. Trends Environ. Anal. Chem. 2020, 26, e00086. [Google Scholar] [CrossRef]
- Youssef, A.M.; Malhat, F.M. Selective Removal of Heavy Metals from Drinking Water Using Titanium Dioxide Nanowire. Macromol. Symp. 2014, 337, 96–101. [Google Scholar] [CrossRef]
- Parkinson, G.S. Iron oxide surfaces. Surf. Sci. Rep. 2016, 71, 272–365. [Google Scholar] [CrossRef] [Green Version]
- Kalska-Szostko, B.; Wykowska, U.; Piekut, K.; Satuła, D. Stability of Fe3O4 nanoparticles in various model solutions. Colloids Surf. A Physicochem. Eng. Asp. 2014, 450, 15–24. [Google Scholar] [CrossRef]
- Kalska-Szostko, B.; Wykowska, U.; Satuła, D.; Zambrzycka, E. Stability of core-shell magnetite nanoparticles. Colloids Surf. B Biointerfaces 2014, 113, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Klekotka, U.; Zambrzycka-Szelewa, E.; Kalska-Szostko, B. Stability of nanowires in environmental aqueous solutions. J. Mol. Liq. 2019, 274, 477–483. [Google Scholar] [CrossRef]
- Li, J.; Zeng, H.; Sun, S.; Liu, J.P.; Wang, Z.L. Analyzing the structure of CoFe-Fe3O4 core-shell nanoparticles by electron imaging and diffraction. J. Phys. Chem. B 2004, 108, 14005–14008. [Google Scholar] [CrossRef]
- Wagner, J.; Autenrieth, T.; Hempelmann, R. Core shell particles consisting of cobalt ferrite and silica as model ferrofluids [CoFe2O4-SiO2 core shell particles]. J. Magn. Magn. Mater. 2002, 252, 4–6. [Google Scholar] [CrossRef]
- Sun, S.H.; Zeng, H. Size-controlled synthesis of magnetite nanoparticles. J. Am. Chem. Soc. 2002, 124, 8204–8205. [Google Scholar] [CrossRef] [PubMed]
- van Ewijk, G.; Vroege, G.; Philipse, A. Convenient preparation methods for magnetic colloids. J. Magn. Magn. Mater. 1999, 201, 31–33. [Google Scholar] [CrossRef]
- Kalska-Szostko, B.; Wykowska, U.; Satuła, D. Magnetic nanoparticles of core-shell structure. Colloids Surf. A Physicochem. Eng. Asp. 2015, 481, 527–536. [Google Scholar] [CrossRef]
- Thermo Elemental. AAS, GFAAS, ICP or ICP-MS? Which Technique Should I Use? Thermo Elemental (USA) S002B Rev 02/01. 2001.
- Petcharoen, K.; Sirivat, A. Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater. Sci. Eng. B 2012, 177, 421–427. [Google Scholar] [CrossRef]
- Mahadevan, S.; Gnanaprakash, G.; Philip, J.; Rao, B.P.C.; Jayakumar, T. X-ray diffraction-based characterization of magnetite nanoparticles in presence of goethite and correlation with magnetic properties. Phys. E Low-Dimens. Syst. Nanostruct. 2007, 39, 20–25. [Google Scholar] [CrossRef]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R. Modified Scherrer equation to estimate more accurately nano-crystallite size using XRD. World J. Nano Sci. Eng. 2012, 2, 154. [Google Scholar] [CrossRef] [Green Version]
- Calero, V.L. Synthesis and Characterization of Cobalt-Substiuted Ferrite Nanoparticles Using Reverse Micelles. Ph.D. Thesis, University of Puerto Rico, San Juan, Puerto Rico, 2006. [Google Scholar]
- Namduri, H.; Nasrazadani, S. Quantitative analysis of iron oxides using Fourier transform infrared spectrophotometry. Corros. Sci. 2008, 50, 2493–2497. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2000. [Google Scholar] [CrossRef]
- Balasubramaniam, R.; Ramesh Kumar, A.V. Characterization of Delhi iron pillar rust by X-ray diffraction, Fourier transform infrared spectroscopy and Mossbauer spectroscopy. Corros. Sci. 2000, 42, 2085–2101. [Google Scholar] [CrossRef]
- Kalska, B. Magnetite particles studied by Mössbauer and magneto-optical Kerr effect. J. Appl. Phys. 2004, 95, 1343–1350. [Google Scholar] [CrossRef]
- Kalska-Szostko, B.; Cydzik, M.; Satuła, D.; Giersig, M. Mössbauer Studies of Core-Shell Nanoparticles. Acta Phys. Pol. A 2011, 119, 3–5. [Google Scholar] [CrossRef]
- Klekotka, U.; Winska, E.; Zambrzycka-Szelewa, E.; Satula, D.; Kalska-Szostko, B. Heavy-metal detectors based on modified ferrite nanoparticles. Beilstein J. Nanotechnol. 2018, 9, 762–770. [Google Scholar] [CrossRef] [PubMed]
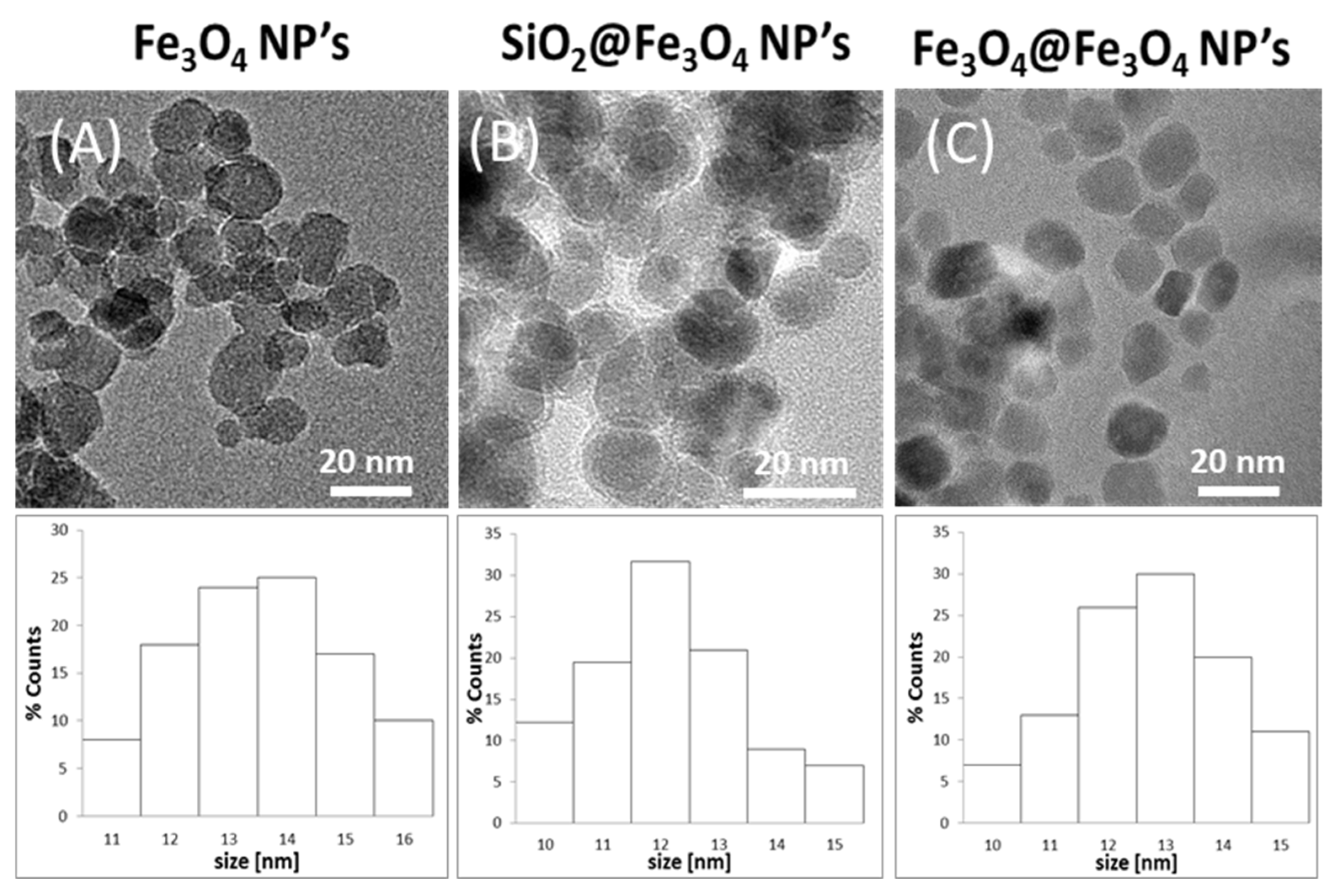
| Fe3O4 | SiO2@Fe3O4 | Fe3O4@Fe3O4 | ||||
|---|---|---|---|---|---|---|
| Mass Change ± 0.5 [mg] | FAAS <LOD | Mass Change ± 0.5 [mg] | FAAS <LOD | Mass Change ± 0.5 [mg] | FAAS <LOD | |
| treated wastewater | 14.9 | 0.00 | −18.0 | 0.00 | 14.3 | 0.03 |
| untreated wastewater | 20.3 | 0.00 | −18.9 | 0.00 | 3.2 | 0.00 |
| river water | 14.9 | 0.00 | −17.6 | 0.00 | −5.9 | 0.02 |
| milk | 51.9 | 0.05 | −14.7 | 0.05 | 13.2 | 0.00 |
| Type of Solution | Fe3O4 (A) | SiO2@Fe3O4 (B) | Fe3O4@Fe3O4 (C) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Grain Size ± 2 [nm] | Strain ± 0.5 [∙10−3] | Lattice Parameter ± 0.05 [Å] | Grain Size ± 2 [nm] | Strain ± 0.5 [∙10−3] | Lattice Parameter ± 0.05 [Å] | Grain Size ± 2 [nm] | Strain ± 0.5 [∙10−3] | Lattice Parameter ± 0.05 [Å] | |
| reference | 16 | 7.99 | 8.35 | 11 | 7.59 | 8.34 | 10 | 5.41 | 8.37 |
| treated wastewater | 17 | 7.90 | 8.36 | 10 | 6.11 | 8.33 | 10 | 6.38 | 8.36 |
| untreated wastewater | 17 | 7.76 | 8.36 | 10 | 5.69 | 8.33 | 10 | 6.56 | 8.36 |
| river water | 16 | 7.85 | 8.36 | 10 | 6.95 | 8.33 | 10 | 6.74 | 8.35 |
| milk | 16 | 7.87 | 8.37 | 10 | 6.35 | 8.34 | 11 | 6.82 | 8.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klekotka, U.; Zambrzycka-Szelewa, E.; Satuła, D.; Kalska-Szostko, B. Stability Studies of Magnetite Nanoparticles in Environmental Solutions. Materials 2021, 14, 5069. https://doi.org/10.3390/ma14175069
Klekotka U, Zambrzycka-Szelewa E, Satuła D, Kalska-Szostko B. Stability Studies of Magnetite Nanoparticles in Environmental Solutions. Materials. 2021; 14(17):5069. https://doi.org/10.3390/ma14175069
Chicago/Turabian StyleKlekotka, Urszula, Elżbieta Zambrzycka-Szelewa, Dariusz Satuła, and Beata Kalska-Szostko. 2021. "Stability Studies of Magnetite Nanoparticles in Environmental Solutions" Materials 14, no. 17: 5069. https://doi.org/10.3390/ma14175069
APA StyleKlekotka, U., Zambrzycka-Szelewa, E., Satuła, D., & Kalska-Szostko, B. (2021). Stability Studies of Magnetite Nanoparticles in Environmental Solutions. Materials, 14(17), 5069. https://doi.org/10.3390/ma14175069






