Multistep Chemical Processing of Crickets Leading to the Extraction of Chitosan Used for Synthesis of Polymer Drug Carriers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
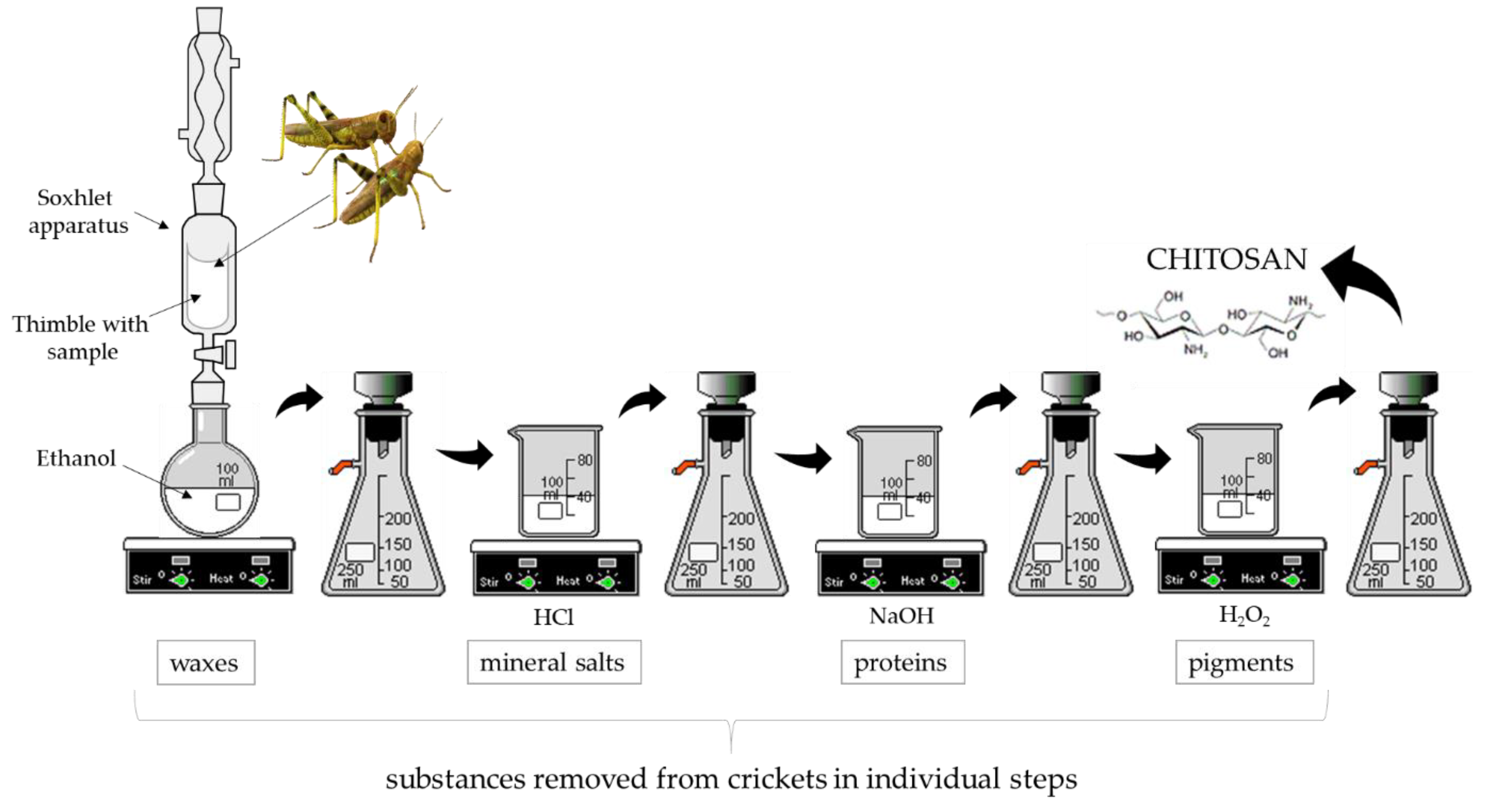
2.2. Preparation of Chitosan from Crickets
2.2.1. Removal of Waxes Using Soxhlet Extraction
2.2.2. Removal of Mineral Salts
2.2.3. Removal of Proteins (Deproteinization)
2.2.4. Removal of Natural Pigments
2.3. Determination of the Deacetylation Degree (DD) of Obtained Chitosan
- —concentration of NaOH solution used during the titration, mol/L;
- —mass of chitosan sample, g;
- —volume of NaOH solution resulting in the first equivalent point, mL;
- —volume of NaOH solution resulting in the second equivalent point, mL;
- 161—molar mass of monomeric unit of chitosan which is fully deacetylated, g/mol.
2.4. Synthesis of Polymer Capsules Based on Chitosan Obtained from Crickets
2.5. Studies on Polymer Capsules Based on Chitosan of Crickets’ Origin
2.5.1. Characterization of a Chemical Structure of Polymer Capsules via FT-IR Spectroscopy
2.5.2. Investigation on Swelling Properties of Polymer Capsules
2.5.3. Studies on the Release of Nisin from Capsules in Different Environments
3. Results and Discussion
3.1. Removal of Waxes from Crickets
3.2. Removal of Mineral Salts
3.3. Removal of Proteins
3.4. Removal of Pigments
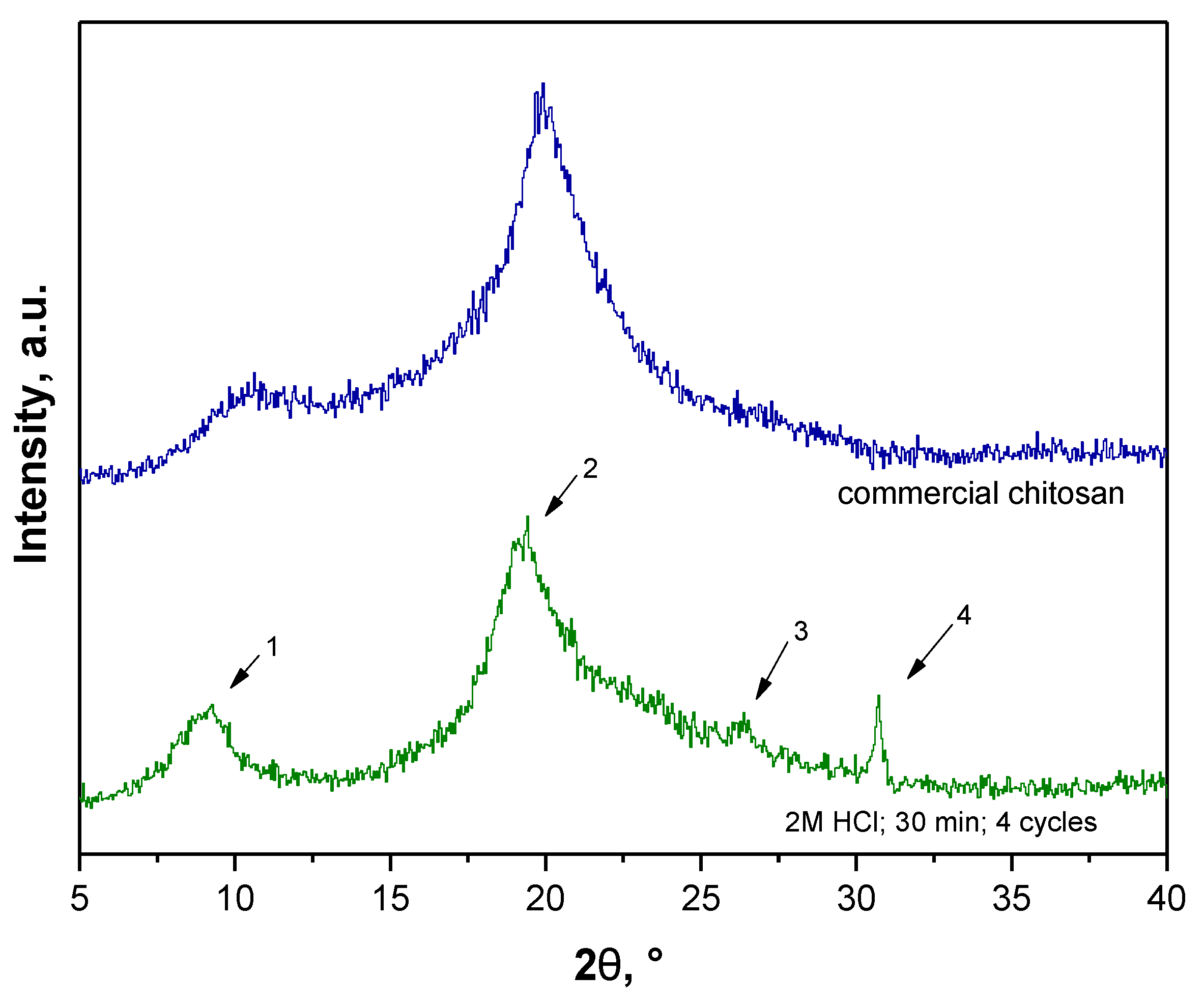
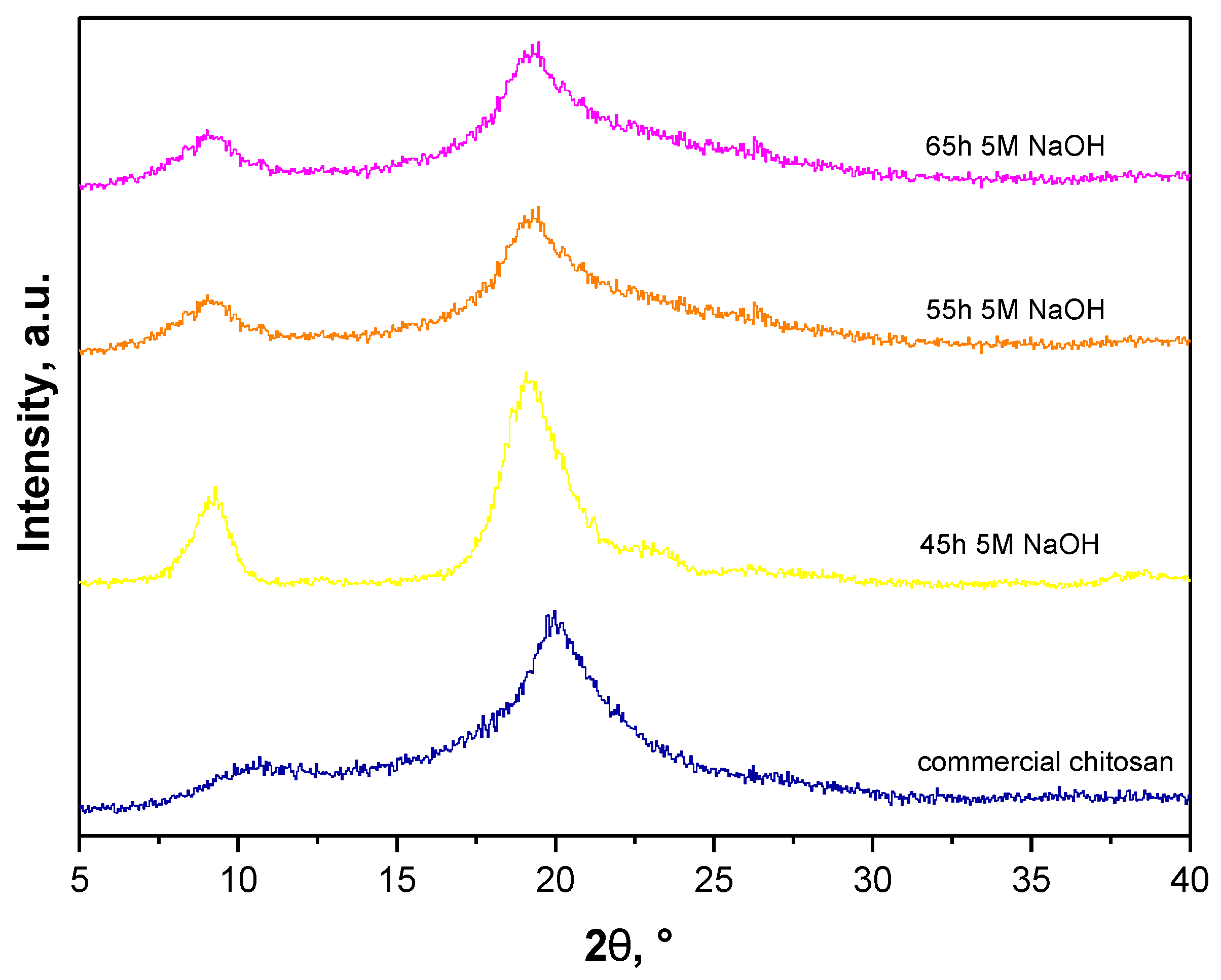
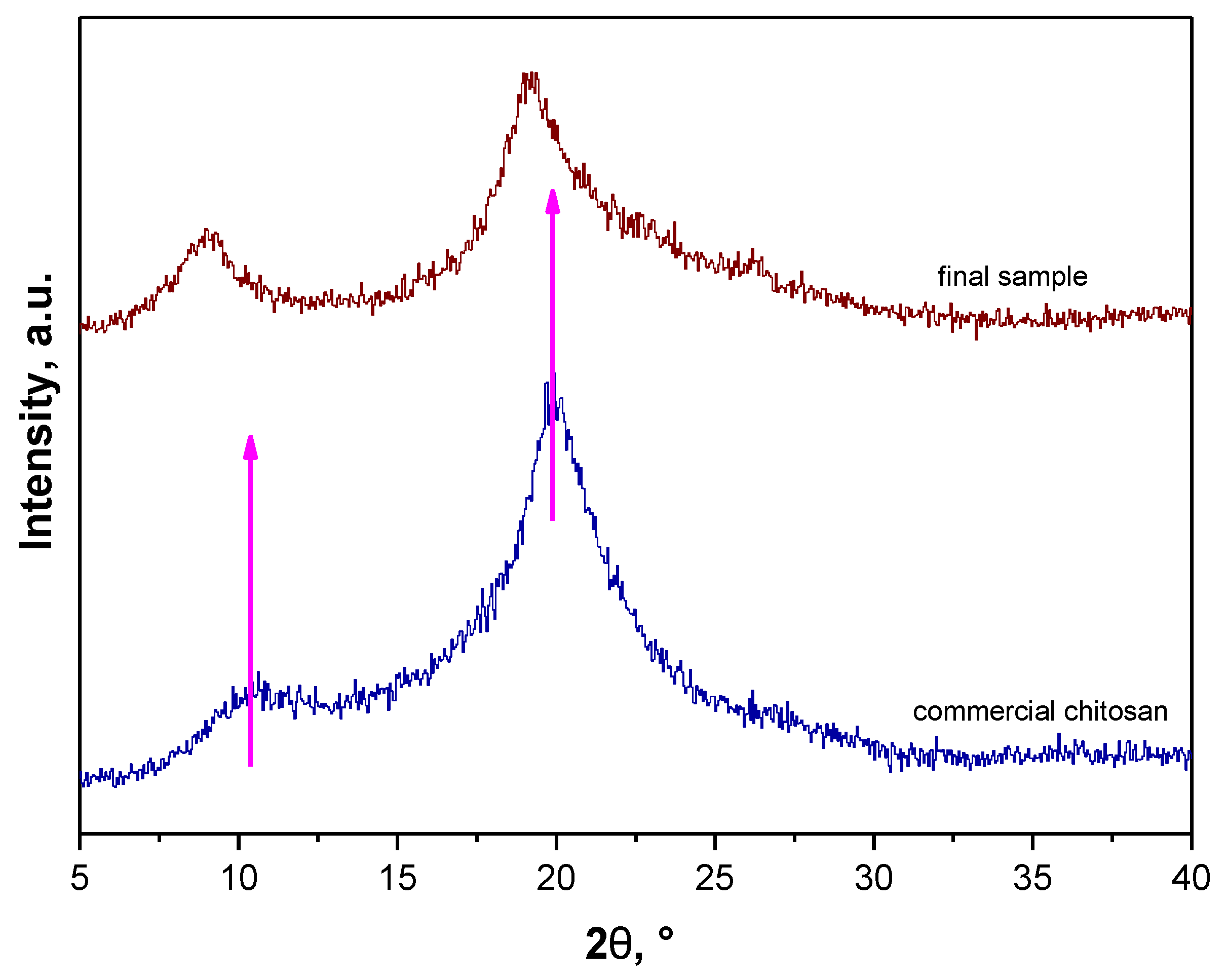
3.5. Determination of the Deacetylation Degree (DD)
3.6. Synthesis of Polymer Capsules
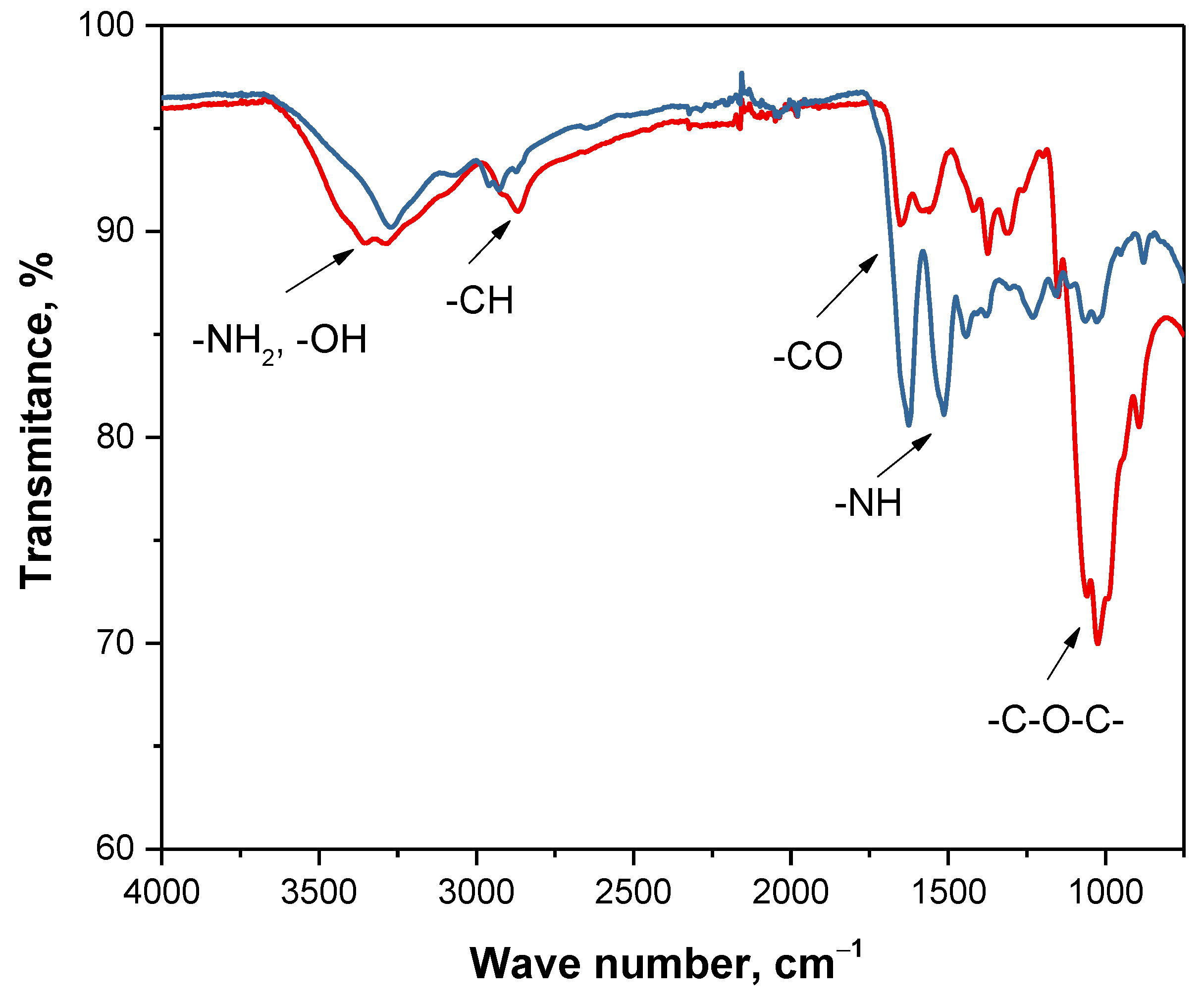
3.7. Analysis of Chemical Structure of Polymer Capsules via FT-IR Spectroscopy
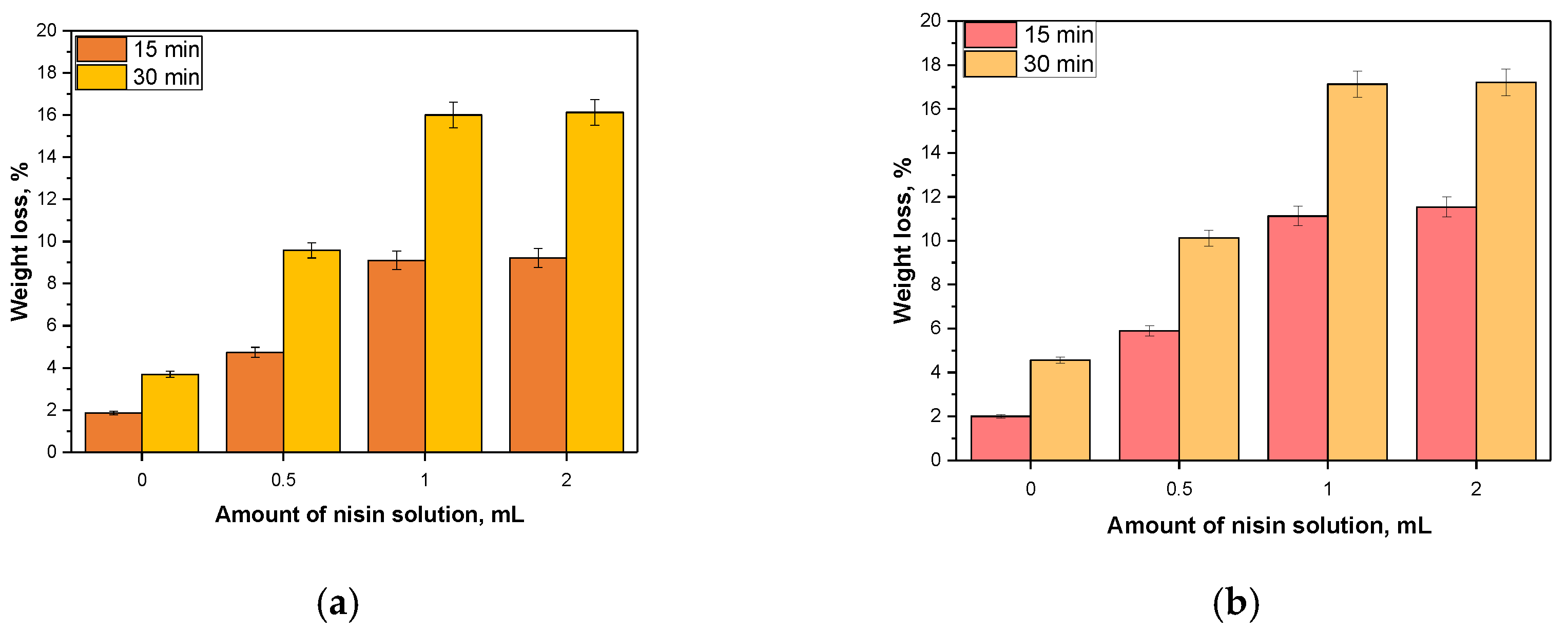
3.8. Sorption Capacity of Prepared Capsules
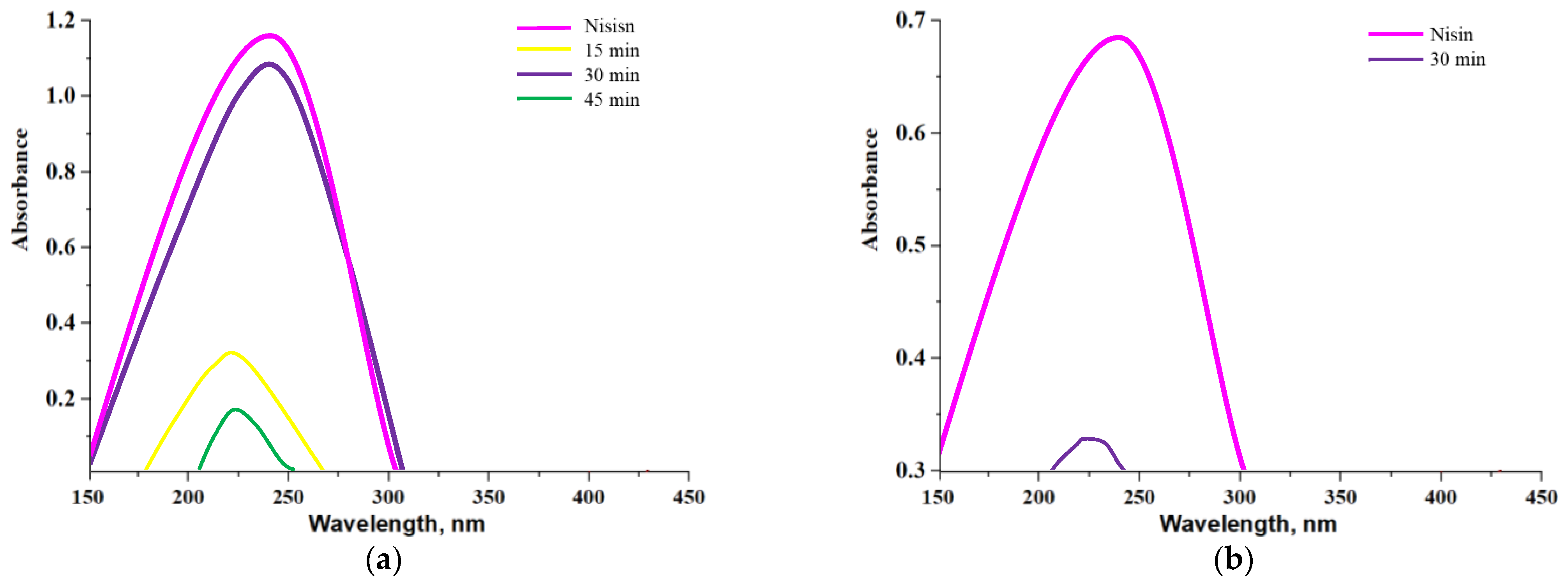
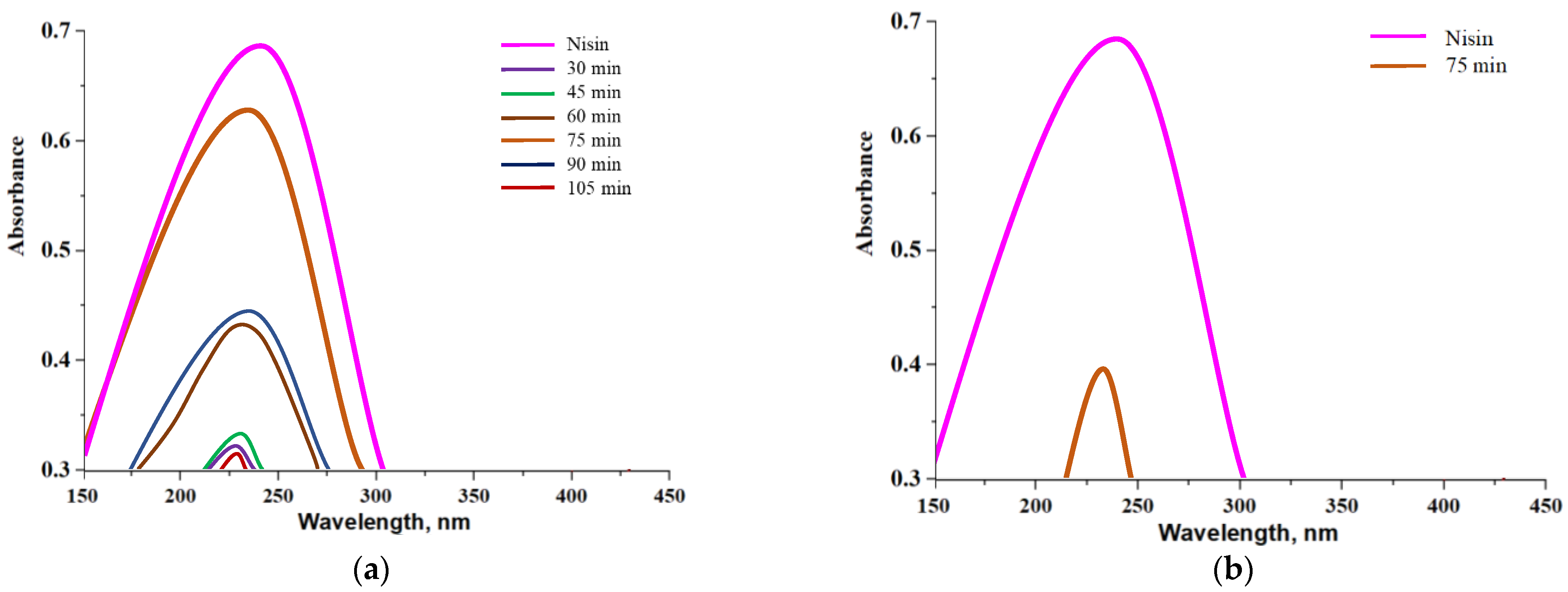
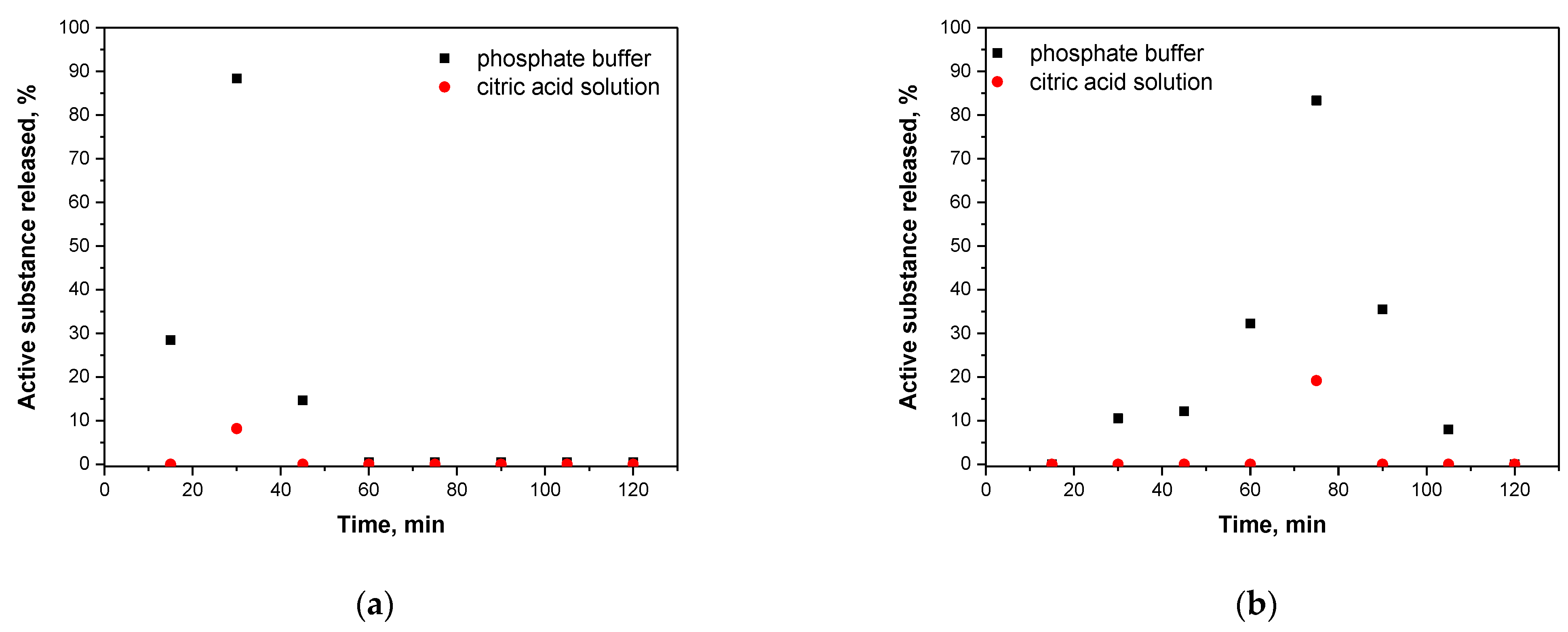
3.9. Studies on the Nisin Release from Capsules in Different Environments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Satitsri, S.; Muanprasat, C. Chitin and Chitosan Derivatives as Biomaterial Resources for Biological and Biomedical Applications. Molecules 2020, 25, 5961. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.G.; Kady, E.M.; Asker, M. Chitin, Chitosan and Glucan, Properties and Applications. WJASS 2019, 3, 1–19. [Google Scholar]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- Kim, C.H.; Park, S.J.; Yang, D.H.; Chun, H.J. Chitosan for Tissue Engineering. Nov. Biomater. Regen. Med. 2018, 475–485. [Google Scholar]
- Balagangadharan, K.; Dhivya, S.; Selvamurugan, N. Chitosan based nanofibers in bone tissue engineering. Int. J. Biol. Macromol. 2017, 104, 1372–1382. [Google Scholar] [CrossRef]
- Venkatesan, J.; Anil, S.; Kim, S.-K.; Shim, M.S. Chitosan as a vehicle for growth factor delivery: Various preparations and their applications in bone tissue regeneration. Int. J. Biol. Macromol. 2017, 104, 1383–1397. [Google Scholar] [CrossRef]
- Shih, P.Y.; Liao, Y.T.; Tseng, Y.K.; Deng, F.S.; Lin, C.H. A potential antifungal effect of chitosan against Candida albicans is mediated via the inhibition of SAGA complex component expression and the subsequent alteration of cell surface integrity. Front. Microbiol. 2019, 10, 602. [Google Scholar] [CrossRef]
- Lo, W.H.; Deng, F.S.; Chang, C.J.; Lin, C.H. Synergistic Antifungal Activity of Chitosan with Fluconazole against Candida albicans, Candida tropicalis, and Fluconazole-Resistant Strains. Molecules 2020, 25, 5114. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef]
- Verlee, A.; Mincke, S.; Stevens, V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Bano, I.; Arshad, M.; Yasin, T.; Ghauri, M.A.; Younus, M. Chitosan: A potential biopolymer for wound management. Int. J. Biol. Macomol. 2017, 102, 380–383. [Google Scholar] [CrossRef]
- Tyliszczak, B.; Drabczyk, A.; Kudłacik-Kramarczyk, S.; Bialik-Wąs, K.; Kijkowska, R.; Sobczak-Kupiec, A. Preparation and cytotoxicity of chitosan-based hydrogels modified with silver nanoparticles. Colloids Surf. B Biointerfaces 2017, 160, 325–330. [Google Scholar] [CrossRef]
- Li, J.; Cai, C.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-Based Nanomaterials for Drug Delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef] [Green Version]
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current Advances in Chitosan Nanoparticles Based Drug Delivery and Targeting. Adv. Pharm. Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Sundaramurthy, A.; Sundramoorthy, A.K. Polyelectrolyte capsules preloaded with interconnected alginate matrix: An effective capsule system for encapsulation and release of macromolecules. Int. J. Biol. Macromol. 2018, 107, 2251–2261. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Wang, R.; Ji, X.; Hu, M.; Wang, B.; Liu, J. Template-assisted synthesis of biodegradable and pH—responsive polymer capsules via RAFT polymerization for controlled drug release. Mater. Chem. Phys. 2014, 148, 87–95. [Google Scholar] [CrossRef]
- Amgoth, C.; Dharmapuri, G. Synthesis and Characterization of Polymeric Nanoparticles and Capsules as Payload for Anticancer Drugs and Nanomedicines. Mater. Today Proceed. 2016, 3, 3833–3837. [Google Scholar] [CrossRef]
- Belbekhouche, S.; Charaabi, S.; Picton, L.; Le, C.D.; Carbonnier, B. Glucose—Sensitive polyelectrolyte microcapsules based on (alginate/chitosan) pair. Carbohydr. Polym. 2018, 184, 144–153. [Google Scholar] [CrossRef]
- Zakcharova, L.Y.; Vasilieva, E.A.; Gaynanova, G.A.; Mirgorodskaya, A.B.; Ibragimova, A.R.; Salnikov, V.V.; Uchegbu, I.F.; Konovalov, A.I.; Zuev, Y.F. The polyacrylic acid/modified chitosan capsules with tunable release of small hydrophobic probe and drug. Coll. Surf. A Physicochem. Eng. Asp. 2015, 471, 93–100. [Google Scholar] [CrossRef]
- Martín-López, H.; Pech-Cohuo, S.C.; Herrera-Pool, E.; Medina-Torres, N.; uevas-Bernardino, J.C.; Ayora-Talavera, T.; Espinosa-Andrews, H.; Ramos-Díaz, A.; Trombotto, S.; Pacheco, N. Structural and Physicochemical Characterization of Chitosan Obtained by UAE and Its Effect on the Growth Inhibition of Pythium ultimum. Agriculture 2020, 10, 464. [Google Scholar] [CrossRef]
- Agarwal, M.; Agarwal, M.K.; Shrivastav, N.; Pandey, S.; Gaur, P. A Simple and Effective Method for Preparation of Chitosan from Chitin. Int. J. Life. Sci. Sci. Res. 2018, 1721–1728. [Google Scholar] [CrossRef]
- Tyliszczak, B.; Drabczyk, A.; Kudłacik-Kramaaczyk, S.; Rudnicka, K.; Sobczak-Kupiec, A.; Jampilek, J. Beetosan/Chitosan from bees—Preparation and properties. Int. J. Adv. Sci. 2016, 4, 118–120. [Google Scholar]
- Tyliszczak, B.; Drabczyk, A.; Kudłacik-Kramarczyk, S.; Grabowska, B.; Kędzierska, M. Physicochemical properties and cytotoxicity of hydrogels based on Beetosan containing sage and bee pollen. Acta Biochim. Pol. 2017, 64, 709–712. [Google Scholar] [CrossRef]
- Juncioni de Arauz, L.; Jozala, A.F.; Mazzola, P.G.; Penna, T.C.V. Nisin biotechnological production and application: A review. Trends Food Sci. Technol. 2009, 20, 146–154. [Google Scholar] [CrossRef]
- Shin, J.M.; Gwak, J.W.; Kamarajan, P.; Fenno, J.C.; Rickard, A.H.; Kapila, Y.L. Biomedical applications of nisin. J. Appl. Microbiol. 2015, 120, 1449–1465. [Google Scholar] [CrossRef] [Green Version]
- Chan-Ick, C.; Yu-Ryang, P. Nisin biosynthesis and its properties. Biotechnol. Lett. 2005, 27, 1641–1648. [Google Scholar]
- De Queiroz Antonino, R.S.C.M.; Lia Fook, B.R.P.; De Oliveira Lima, V.A.; De Farias Rached, R.Í.; Lima, E.P.N.; Da Silva Lima, R.J.; Peniche Covas, C.A.; Lia Fook, M.V. Preparation and Characterization of Chitosan Obtained from Shells of Shrimp (Litopenaeus vannamei Bonne). Mar. Drugs 2017, 15, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalyoncuoglu, U.T.; Yilmaz, B.; Gungor, S.; Evis, Z.; Uyar, P.; Akca, G.; Kansu, G. Evaluation of the chitosan-coating effectiveness on a dental titanium alloy in terms of microbial and fibroblastic attachment and the effect of aging. Mater. Technol. 2015, 49, 925–931. [Google Scholar] [CrossRef]
- Kaya, M.; Baran, T.; Saman, I.; Ozusaglam, M.A.; Cakmak, Y.S.; Mentes, A. Physicochemical characterization of chitin and chitosan obtained from resting eggs of Ceriodaphnia Quadrangula (Branchiopoda: Cladocera: Daphnidae). J. Crust. Biol. 2014, 34, 283–288. [Google Scholar] [CrossRef] [Green Version]
- Honary, S.; Maleki, M.; Karami, M. The effect of chitosan molecular weight on the properties of alginate/chitosan microparticles containing prednisolone. Trop. J. Pharm. Res. 2009, 8, 53–61. [Google Scholar] [CrossRef]
- Thai, H.; Thuy Nguyen, C.; Thi Thach, L.; Thi Tran, M.; Duc Mai, H.; Thi Thu Nguyen, T.; Duc Le, G.; Van Can, M.; Tran, L.D.; Bach, G.L.; et al. Characterization of chitosan/ alginate/lovastatin nanoparticles and investigation of their toxic efects in vitro and in vivo. Sci. Rep. 2020, 10, 909. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, X.; Xiong, C. Effects of chitosan oligosaccharide-nisin conjugates formed by Maillard reaction on the intestinal microbiota of high-fat diet-induced obesity mice model. Food Qual. Saf. 2019, 3, 169–178. [Google Scholar] [CrossRef]
- Oliveira, T.V.d.; Freitas, P.A.V.d.; Pola, C.C.; Terra, L.R.; Silva, J.O.R.d.; Badaró, A.T.; Junior, N.S.; Oliveira, M.M.d.; Silva, R.R.A.; Soares, N.d.F.F. The Influence of Intermolecular Interactions between Maleic Anhydride, Cellulose Nanocrystal, and Nisin-Z on the Structural, Thermal, and Antimicrobial Properties of Starch-PVA Plasticized Matrix. Polysaccharides 2021, 2, 661–676. [Google Scholar] [CrossRef]
- Lawrie, G.; Keen, I.; Drew, B.; Chandler-Temple, A.; Rintoul, L.; Fredericks, P.; Grøndahl, L. Interactions between Alginate and Chitosan Biopolymers Characterized Using FTIR and XPS. Biomacromolecules 2007, 8, 2533–2541. [Google Scholar] [CrossRef]
- Kulig, D.; Zimoch-Korzycka, A.; Jarmoluk, A.; Marycz, K. Study on Alginate–Chitosan Complex Formed with Different Polymers Ratio. Polymers 2016, 8, 167. [Google Scholar] [CrossRef]
- Liu, X.; Xue, W.; Liu, Q.; Yu, W.; Fu, Y.; Xiong, X.; Ma, X.; Yuan, Q. Swelling behaviour of alginate–chitosan microcapsules prepared by external gelation or internal gelation technology. Carbohydr. Polym. 2004, 56, 459–464. [Google Scholar] [CrossRef]
- Shinde, U.A.; Nagarsenker, M.S. Characterization of Gelatin-Sodium Alginate Complex Coacervation System. Indian J. Pharm. Sci. 2009, 71, 313–317. [Google Scholar] [PubMed] [Green Version]
- Lopez-Garcia, M.; Martinez-Cabanas, M.; Vilarino, T.; Lodeiro, P.; Rodriguez-Barro, R.; Herrero, R.; Barriada, J.L. New polymeric/inorganic hybrid sorbents based on red mud and nanosized magnetite for large scale applications in As(V) removal. Chem. Eng. J. 2017, 311, 117–125. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahtat, D.; Mahlous, M.; Benamer, S.; Khodja, A.S.; Oussedik-Oumehdi, H.; Laraba-Djebari, F. Oral delivery of insulin from alginate/chitosan crosslinked by glutaraldehyde. Int. J. Biol. Macromol. 2013, 58, 160–168. [Google Scholar] [CrossRef] [PubMed]




| Process | Amount of Cycles | Weight Loss [%] |
|---|---|---|
| 1 | 4 | 33.2 |
| 2 | 35.5 | |
| 3 | 34.8 | |
| 4 | 34.3 | |
| 5 | 33.6 | |
| 6 | 5 | 34.2 |
| 7 | 34.6 |
| Sample | Time [min] | Cycles | HCl Concentration [mol/L] | Weight Loss [%] |
|---|---|---|---|---|
| 1 | 60 | 4 | 1 | 18.5 |
| 2 | 30 | 2 | 13.4 | |
| 3 | 60 | 2 | 17.9 | |
| 4 | 30 | 5 | 1 | 17.7 |
| 5 | 60 | 1 | 20.7 | |
| 6 | 30 | 2 | 11.7 | |
| 7 | 60 | 2 | 20.1 |
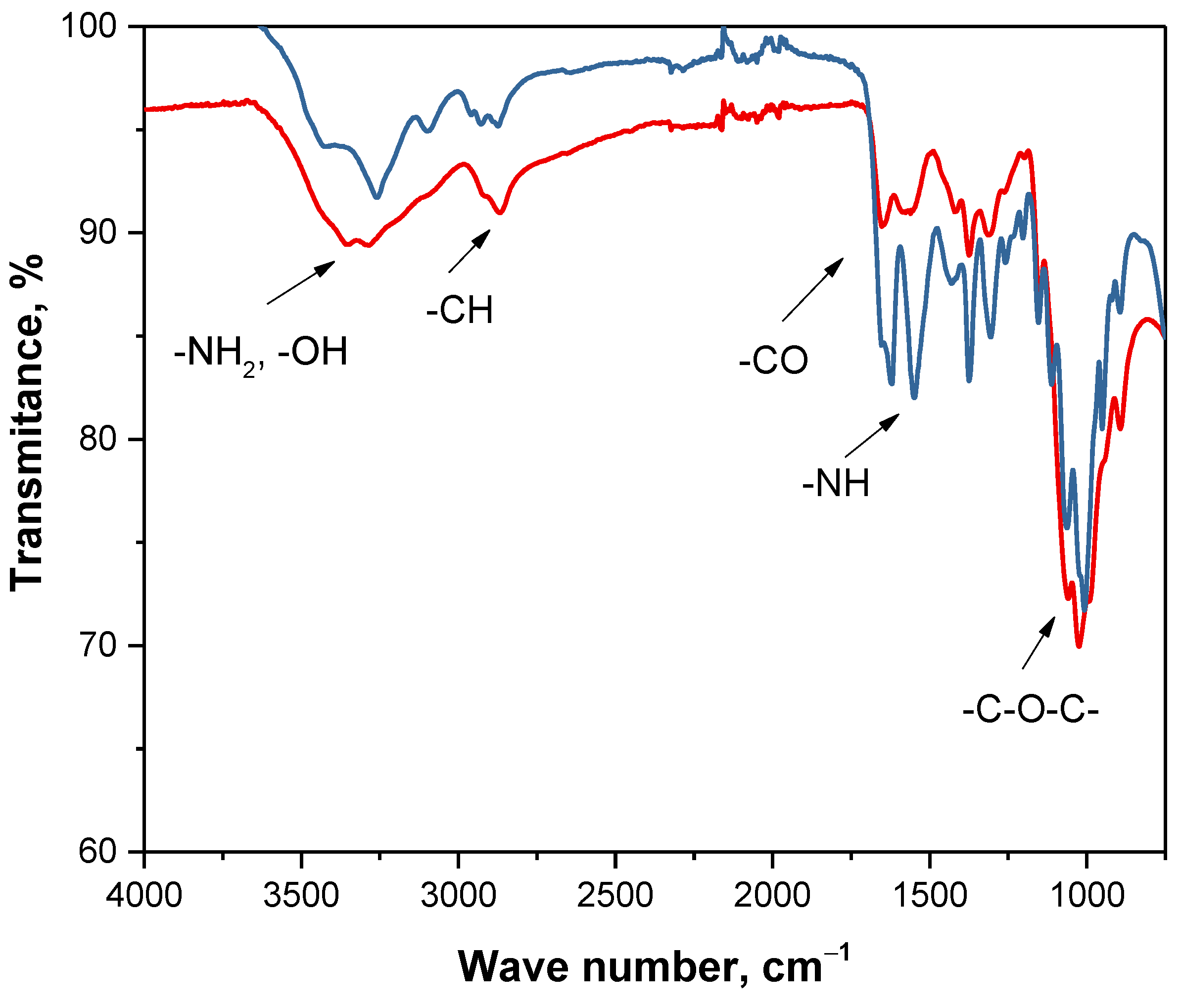
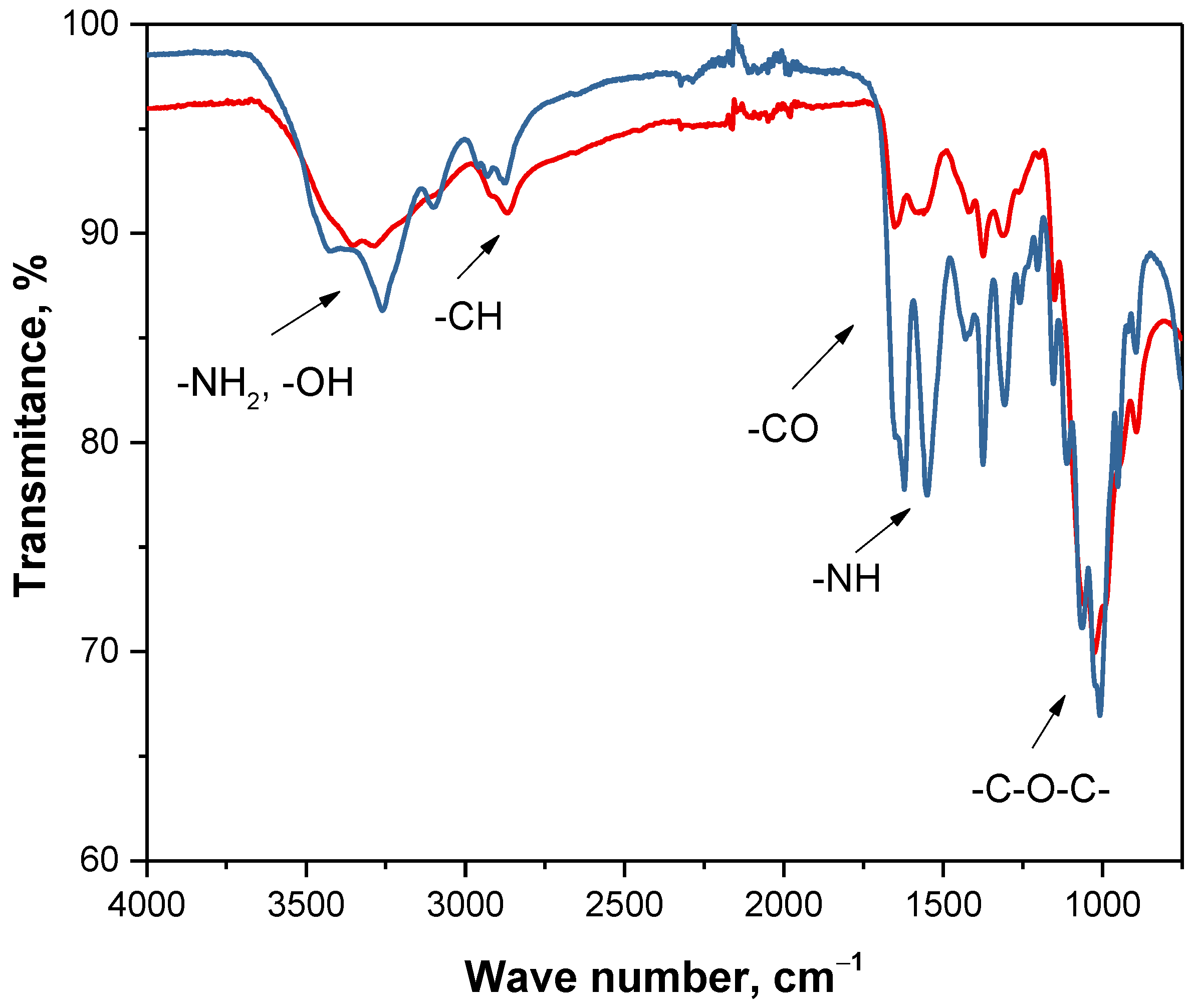
| Region of Absorption [cm−1] | Functional Group | Type of Vibration |
|---|---|---|
| 3250–3500 | -NH2, -OH | stretching |
| 2900–3000 | -CH | stretching |
| 1600–1650 | -CO | stretching |
| 1500–1600 | -NH | deformation |
| 1370–1390 | -CH | deformation |
| 1300–1350 | -CN | stretching |
| 1000–1250 | -C-O-C- | stretching |
| 800–870 | -CH | deformation |
| Sample | Yield [%] | Time [h] | Sample | Yield [%] | Time [h] | Sample | Yield [%] | Time [h] | Sample | Yield [%] | Time [h] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 17.18 | 45 | 6 | 18.11 | 20 | ||||||
| 2 | 16.21 | 7 | 20.64 | 11 | 16.42 | 14 | 16.32 | ||||
| 3 | 16.14 | 8 | 16.91 | 12 | 16.67 | 55 | 15 | 16.47 | 65 | ||
| 4 | 17.66 | 9 | 21.32 | 13 | 16.85 | 16 | 16.17 | ||||
| 5 | 16.56 | 10 | 21.88 |
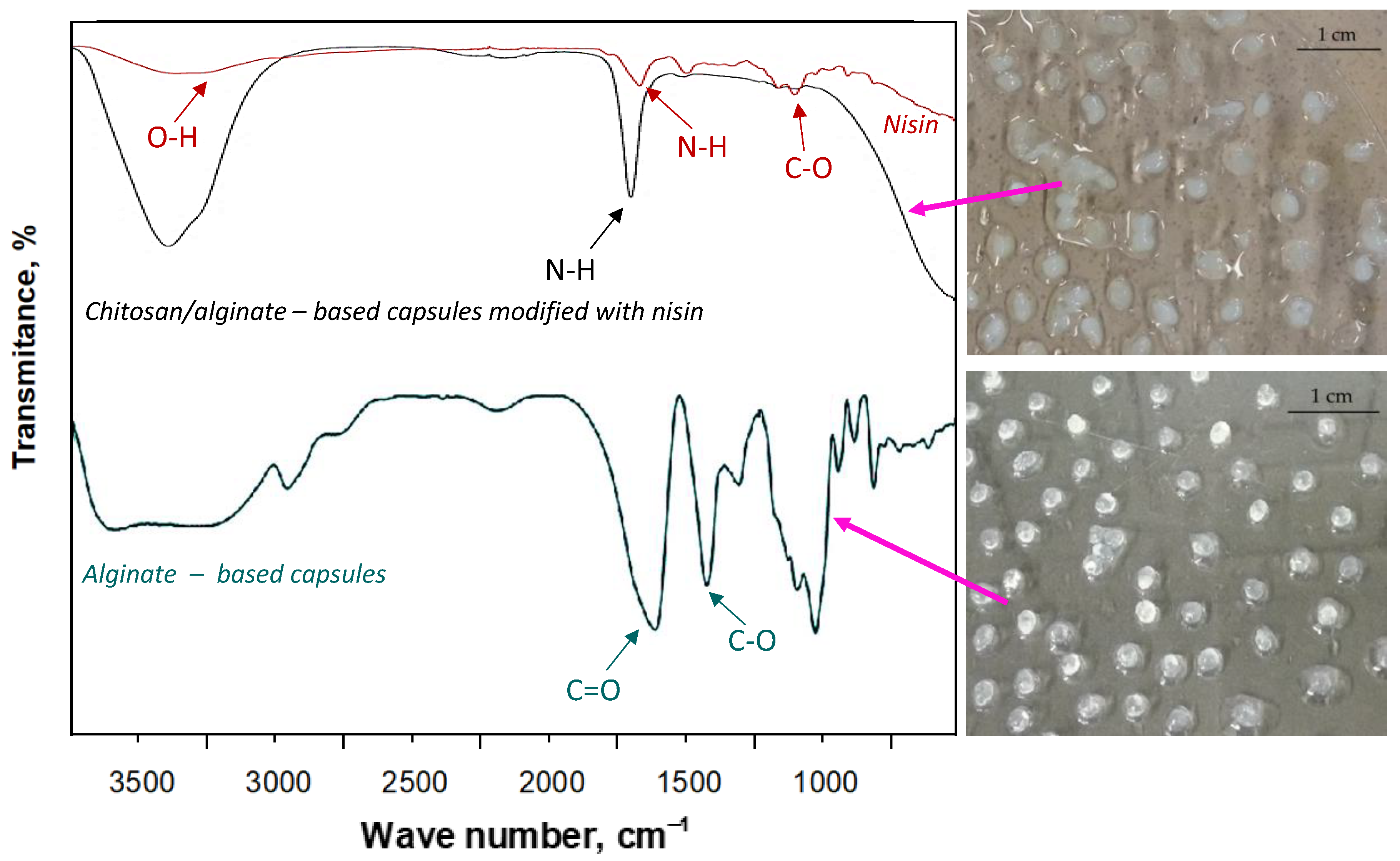
| Wavenumber [cm−1] | Functional Group | Type of Vibration |
|---|---|---|
| 3350 | -OH; -NH2 | stretching |
| 2870 | -CH3; -CH2- | stretching |
| 1650 | C=O; N-H | stretching |
| 1580 | N-H | bending |
| 1375 | C-H | deformation |
| 1125–1025 | -C-O-C- | Stretching |
| 880 | C-N | Stretching |
| Sample | Alginate Solution [g] | Chitosan Solution [g] | Nisin [g] |
|---|---|---|---|
| 1 | 10.578 | 2.975 | 0 |
| 2 | 0.485 | ||
| 3 | 0.970 | ||
| 4 | 1.940 | ||
| 5 | 10.578 | - | 0 |
| 6 | 0.485 | ||
| 7 | 0.970 | ||
| 8 | 1.940 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Głąb, M.; Kudłacik-Kramarczyk, S.; Drabczyk, A.; Guigou, M.D.; Sobczak-Kupiec, A.; Mierzwiński, D.; Gajda, P.; Walter, J.; Tyliszczak, B. Multistep Chemical Processing of Crickets Leading to the Extraction of Chitosan Used for Synthesis of Polymer Drug Carriers. Materials 2021, 14, 5070. https://doi.org/10.3390/ma14175070
Głąb M, Kudłacik-Kramarczyk S, Drabczyk A, Guigou MD, Sobczak-Kupiec A, Mierzwiński D, Gajda P, Walter J, Tyliszczak B. Multistep Chemical Processing of Crickets Leading to the Extraction of Chitosan Used for Synthesis of Polymer Drug Carriers. Materials. 2021; 14(17):5070. https://doi.org/10.3390/ma14175070
Chicago/Turabian StyleGłąb, Magdalena, Sonia Kudłacik-Kramarczyk, Anna Drabczyk, Martin Duarte Guigou, Agnieszka Sobczak-Kupiec, Dariusz Mierzwiński, Paweł Gajda, Janusz Walter, and Bożena Tyliszczak. 2021. "Multistep Chemical Processing of Crickets Leading to the Extraction of Chitosan Used for Synthesis of Polymer Drug Carriers" Materials 14, no. 17: 5070. https://doi.org/10.3390/ma14175070
APA StyleGłąb, M., Kudłacik-Kramarczyk, S., Drabczyk, A., Guigou, M. D., Sobczak-Kupiec, A., Mierzwiński, D., Gajda, P., Walter, J., & Tyliszczak, B. (2021). Multistep Chemical Processing of Crickets Leading to the Extraction of Chitosan Used for Synthesis of Polymer Drug Carriers. Materials, 14(17), 5070. https://doi.org/10.3390/ma14175070











