Physiological and Psychological Responses to a Maximal Swimming Exercise Test in Adolescent Elite Athletes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Swimming Test
2.3. Hormonal Measurements
2.4. Lactate Measurements
2.5. Heart Rate Measurements
2.6. Psychological Tests
2.7. Statistical Analysis
- descriptive statistics (mean ± SD),
- Student t-test for independent samples to analyse gender differences
- Student t-test for dependent samples to analyse pre and post exercise differences
- Pearson’s linear correlation analysis to show associations of different variables
- Linear multiple regression analysis to predict hormonal changes as OT indicators
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- McArdle, W.D.; Katch, F.I.; Katch, V.L. Essentials of Exercise Physiology; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2006; pp. 407–531. [Google Scholar]
- Kremaer, W.J.; Rogol, A.D. Encyclopaedia of Sports Medicine XI: An IOC Medical Commission Publication. In The Endocrine System in Sports and Exercise; Wiley: Hoboken, NJ, USA, 2005; pp. 217–231. [Google Scholar]
- O’Connor, P.J.; Corrigan, D.L. Influence of short-term cycling on salivary cortisol level. Med. Sci. Sports Exerc. 1987, 19, 224–228. [Google Scholar]
- Stupnicki, R.; Obminski, Z. Glucocorticoid response to exercise as measured by serum and salivary cortisol. Eur. J. Appl. Physiol. Occup. Physiol. 1992, 65, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Azarbayjani, M.A.; Rahmani, M.; Rasaee, M.J.; Tojari, F.; Pournemati, P.; Ostojic, S.M.; Stannard, S.R. The effect of sport competition on salivarysteroids in amateur female karate athletes. J. Phys. Educ. Sport. 2010, 27, 44–48. [Google Scholar]
- Tanner, A.; Day, S. The Effects of a 4-Week, Intensified Training, and Competition Period on Salivary Hormones, Immunoglobulin A, Illness Symptoms, and Mood State in Elite Synchronised Swimmers. Sports 2017, 5, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, R.A.; Beltran, O.A.B.; Zapata, D.M.; Silva, E.; Freitas, W.Z.; Junior, R.V.; Da Silva, F.F.; Higino, W.P. Heart rate variability, salivary cortisol and competitive state anxiety responses during pre-competition and pre-training moments. Biol. Sport 2019, 36, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Filaire, E.; Lac, G. Dehydroepiandrosterone (DHEA) rather than testosterone shows saliva androgen responses to exercise in elite female handball players. Int. J. Sports Med. 2000, 21, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, R.; Acevedo, E.; Synovitz, L.; Hebert, E.; Gimpel, T.; Castracane, V. Leptin and steroid hormone responses to exercise in adolescent female runners over a 7-week season. Graefe Arch. Clin. Exp. Ophthalmol. 2001, 86, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Navalta, J.W.; Sedlock, D.A.; Park, K.-S.; McFarlin, B.K. Neither gender nor menstrual cycle phase influences exercise-induced lymphocyte apoptosis in untrained subjects. Appl. Physiol. Nutr. Metab. 2007, 32, 481–486. [Google Scholar] [CrossRef]
- Loucks, A.B.; Mortola, J.F.; Girton, L.; Yen, S.S.C. Alterations in the Hypothalamic-Pituitary-Ovarian and the Hypothalamic-Pituitary-Adrenal Axes in Athletic Women. J. Clin. Endocrinol. Metabolism. 1989, 68, 402–411. [Google Scholar] [CrossRef]
- De Crée, C.; Ball, P.; Seidlitz, B.; Kranenburg, G.V.; Geurten, P.; Keizer, H.A. Responses of Catecholestrogen Metabolism to Acute Graded Exercise in Normal Menstruating Women before and after Training. J. Clin. Endocrinol. Metabolism. 1997, 82, 3342–3348. [Google Scholar]
- Skarda, S.T.; Burge, M.R. Prospective evaluation of risk factors for exercise-induced hypogonadism in male runners. West. J. Med. 1998, 169, 9–12. [Google Scholar]
- Smilios, I.; Pilianidis, T.; Karamouzis, M.; Tokmakidis, S. Hormonal Responses after Various Resistance Exercise Protocols. Med. Sci. Sports Exerc. 2003, 35, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Rowland, T.W. Children’s Exercise Physiology; Human Kinetics Publishers: Champaign, IL, USA, 2005. [Google Scholar]
- Kilian, Y.; Engel, F.; Wahl, P.; Achtzehn, S.; Sperlich, B.; Mester, J. Markers of biological stress in response to a single session of high-intensity interval training and high-volume training in young athletes. Graefe Arch. Clin. Exp. Ophthalmol. 2016, 116, 2177–2186. [Google Scholar] [CrossRef] [PubMed]
- Olbrecht, J. The Science of Winning: Planning, Periodizing and Optimizing Swim Training; F&G Partners: Antwerp, Belgium, 2015. [Google Scholar]
- Beneke, R.; Hütler, M.; Jung, M.; Leithäuser, R. Modeling the blood lactate kinetics at maximal hort-term exercise conditions in children, adolescents, and adults. J. Appl. Physiol. Bethesda Md. 2005, 99, 499–504. [Google Scholar]
- Morgan, W.P.; Brown, D.R.; Raglin, J.S.; O’Connor, P.J.; Ellickson, A.K. Psychological monitoring of overtraining and staleness. Br. J. Sports Med. 1987, 21, 107–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenttä, G.; Hassmén, P.; Raglin, J.S. Training Practices and Overtraining Syndrome in Swedish Age-Group Athletes. Int. J. Sports Med. 2001, 22, 460–465. [Google Scholar] [CrossRef]
- Brandt, R.; Herrero, D.; Massetti, T.; Crocetta, T.; Guarnieri, R.; de Mello Monteiro, C.; da Silveira Viana, M.; Bevilacqua, G.; de Abreu, L.; Andrade, A. The Brunel Mood Scale Rating in Mental Health for Physically Active and Apparently Healthy Populations. Health 2016, 8, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Cadegiani, F.A.; Kater, C.E. Hormonal aspects of overtraining syndrome: A systematic review. BMC Sports Sci. Med. Rehabil. 2017, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Angeli, A.; Minetto, M.; Dovio, A.; Paccotti, P. The overtraining syndrome in athletes: A stress-related disorder. J. Endocrinol. Investig. 2004, 27, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Meeusen, R.; Duclos, M.; Foster, C.; Fry, A.; Gleeson, M.; Nieman, D. European College of Sport Science, American College of Sports Medicine. Prevention, diagnosis, and treatment of the overtraining syndrome: Joint consensus statement of the European College of Sport Science and the American College of Sports Medicine. Med. Sci. Sports Exerc. 2013, 45, 186–205. [Google Scholar] [PubMed]
- Terry, P.C.; Lane, A.M.; Lane, H.J.; Keohane, L. Development and validation of a mood measure for adolescents: POMS-A. J. Sports Sci. 1999, 17, 861–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terry, P.C.; Lane, A.M.; Fogarty, G. Construct validity of the Profile of Mood States-A for use with adults. Psychol. Sport Exerc. 2003, 4, 125–139. [Google Scholar] [CrossRef] [Green Version]
- Lane, A.M.; Soós, I.; Leibinger, É.; Karsai, I.; Hamar, P. Validity of the Brunel Mood Scale for Use with UK, Italian and Hungarian Athletes; Lane, A.M., Ed.; Mood and Human Performance, Conceptual Measurement and Applied Issues; Nova Science Publishers Inc.: New York, NY, USA, 2007; pp. 119–130. [Google Scholar]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef]
- Stauder, A.; Konkolÿ, T.B. Az észlelt stressz kérdőív (PSS) magyar verziójának jellemzői [Characteristics of the Hungarian version of the Perceived Stress Scale (PSS)]. Mentálhigiéné és Pszichoszomatika 2006, 7, 203–216. [Google Scholar] [CrossRef] [Green Version]
- Currie, C. (Ed.) Social determinants of health and well-being among young people. In HBSC Study: International Report from the 2009/2010 Survey; WHO Regional Office for Europe: Copenhagen, Denmark, 2012. [Google Scholar]
- Vincent, W.J.; Joseph, P.W. Statistics in Kinesiology, 4th ed.; Human Kinetics: Champaign, IL, USA, 2012. [Google Scholar]
- Forsyth, J.J.; Reilly, T. The Combined Effect of Time of Day and Menstrual Cycle on Lactate Threshold. Med. Sci. Sports Exerc. 2005, 37, 2046–2053. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Fry, A.C.; Warren, B.J.; Stone, M.H.; Fleck, S.J.; Kearney, J.T.; Conroy, B.P.; Maresh, C.M.; Weseman, C.A.; Triplett, N.T.; et al. Acute hormonal responses in elite junior weightlifters. Int. J. Sports Med. 1992, 13, 103–109. [Google Scholar] [CrossRef]
- Mahon, A.D.; Anderson, C.S.; Hipp, M.J.; Hunt, K.A. Heart Rate Recovery from Submaximal Exercise in Boys and Girls. Med. Sci. Sports Exerc. 2003, 35, 2093–2097. [Google Scholar] [CrossRef] [Green Version]
- Gottschall, J.S.; Davis, J.; Hastings, B.; Porter, H.J. Exercise Time and Intensity: How Much Is Too Much? Int. J. Sports Physiol. Perform. 2020, 15, 808–815. [Google Scholar] [CrossRef]
- Monfared, S.S.; Lebeau, J.C.; Mason, J.; Cho, S.K.; Basevitch, I.; Perry, I.; Baur, D.A.; Tenenbaum, G. A Bio-Physio-Psychological Investigation of Athletes’ Burnout. Res. Q. Exerc. Sport 2020, 28, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Prado-Gascó, V.; de la Barrera, U.; Sancho-Castillo, S.; de la Rubia-Ortí, J.E.; Montoya-Castilla, I. Perceived stress and reference ranges of hair cortisol in healthy adolescents. PLoS ONE 2019, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Aizawa, K.; Imai, T.; Kono, I.; Mesaki, N. Hormonal responses to resistance exercise during different menstrual cycle states. Med. Sci. Sports Exerc. 2011, 43, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Buchheit, M.; Duché, P.; Laursen, P.; Ratel, S. Postexercise heart rate recovery in children: Relationship with power output, blood pH, and lactate. Applied physiology, nutrition, and metabolism = Physiologie appliquée, nutrition et métabolisme. Appl. Physiol. Nutr. Metab. 2010, 35, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Guilkey, J.P.; Overstreet, M.; Mahon, A.D. Heart rate recovery and parasympathetic modulation in boys and girls following maximal and submaximal exercise. Eur. J. Appl. Physiol. 2015, 115, 2125–2133. [Google Scholar] [CrossRef]
- Inchley, J.; Currie, D.; Budisavljevic, S.; Torsheim, T.; Jåstad, A.; Cosma, A.; Samdal, O. Spotlight on adolescent health and well-being. Findings from the 2017/2018 Health Behaviour in School-aged Children (HBSC) survey in Europe and Canada. Int. Rep. 2020, 1, 67. [Google Scholar]
- Meiser, S.; Esser, G. Interpersonal Stress Generation--A Girl Problem? The Role of Depressive Symptoms, Dysfunctional Attitudes, and Gender in Early Adolescent Stress Generation. J. Early Adolesc. 2019, 39, 41–66. [Google Scholar] [CrossRef]
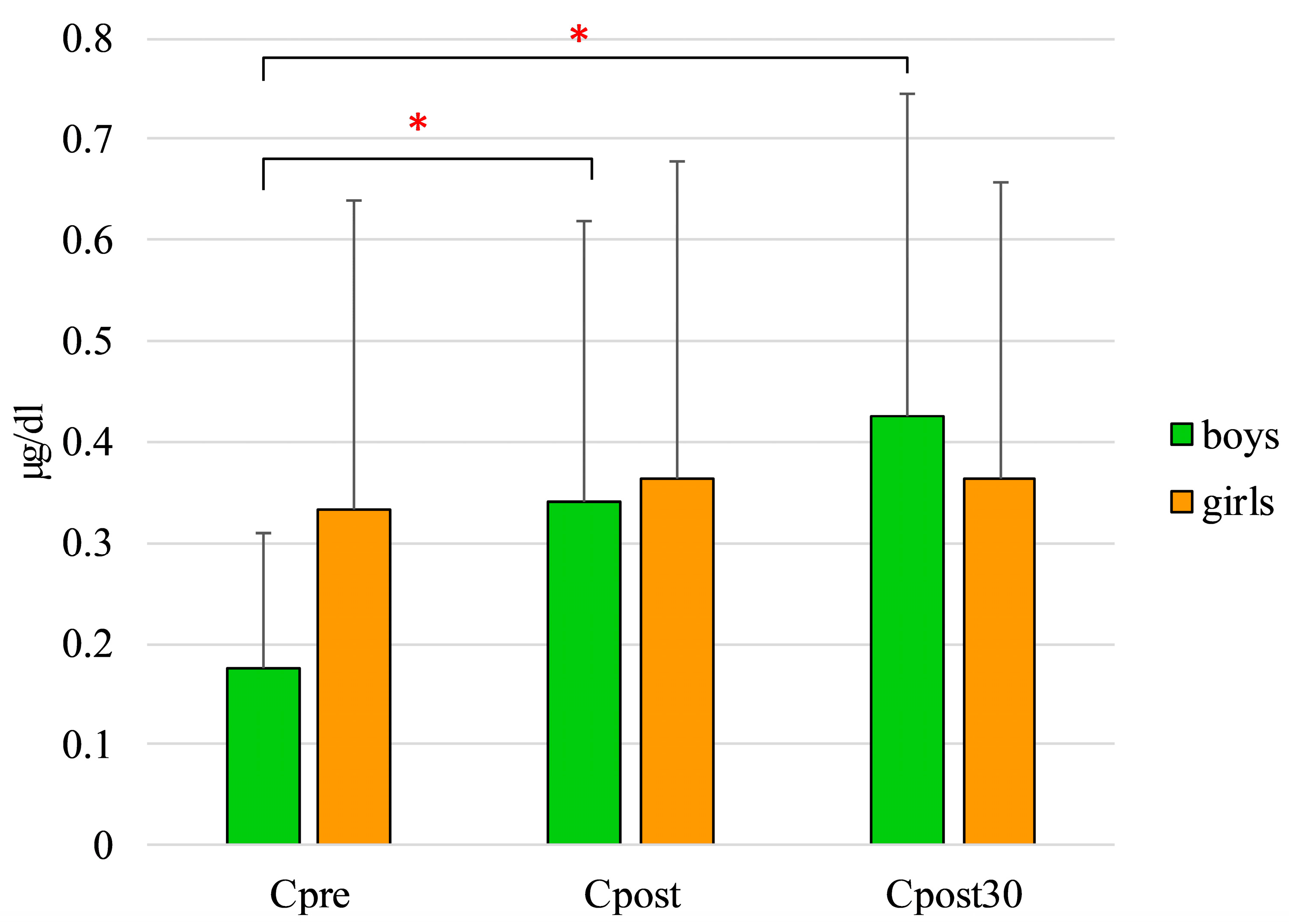


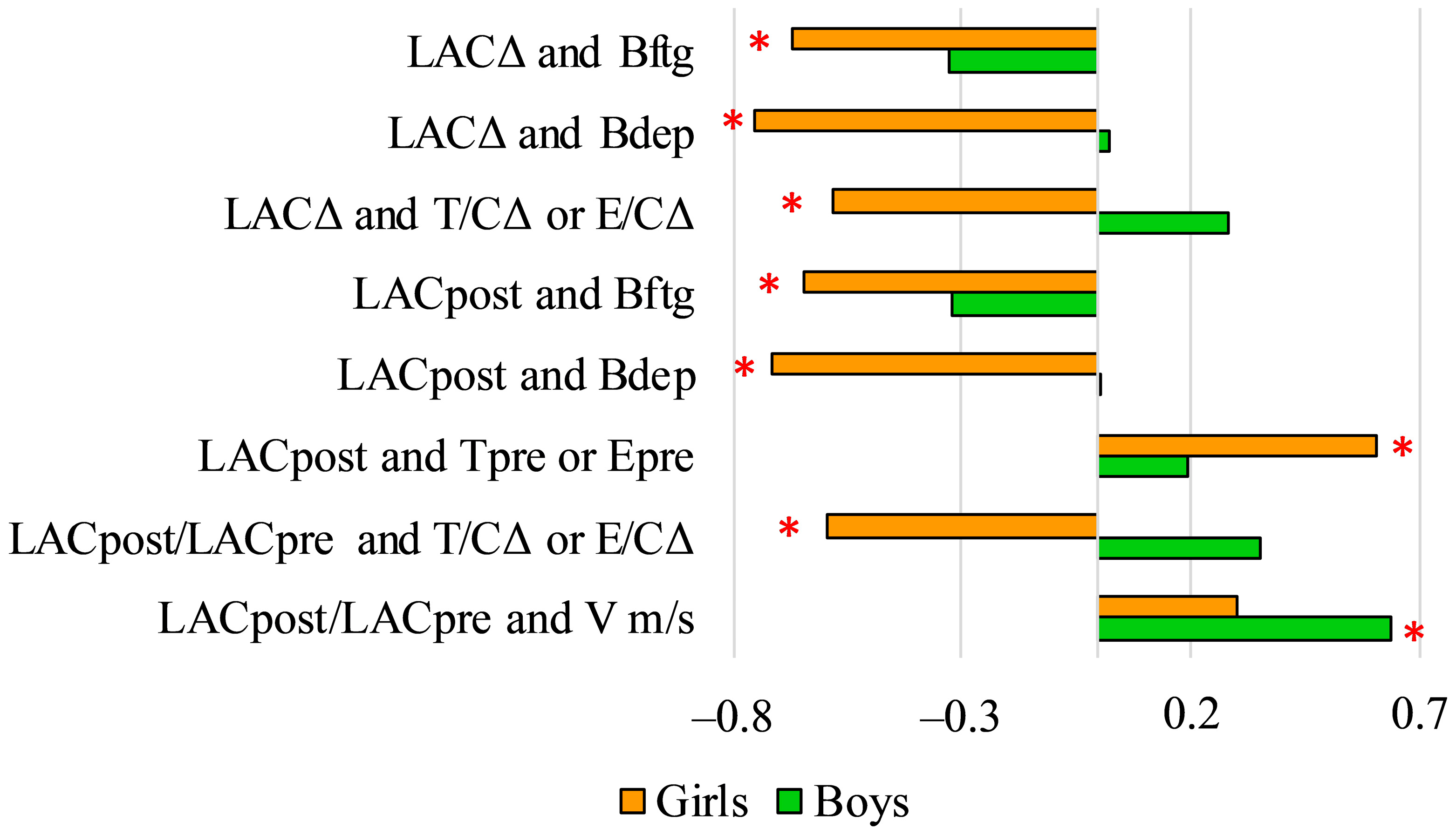
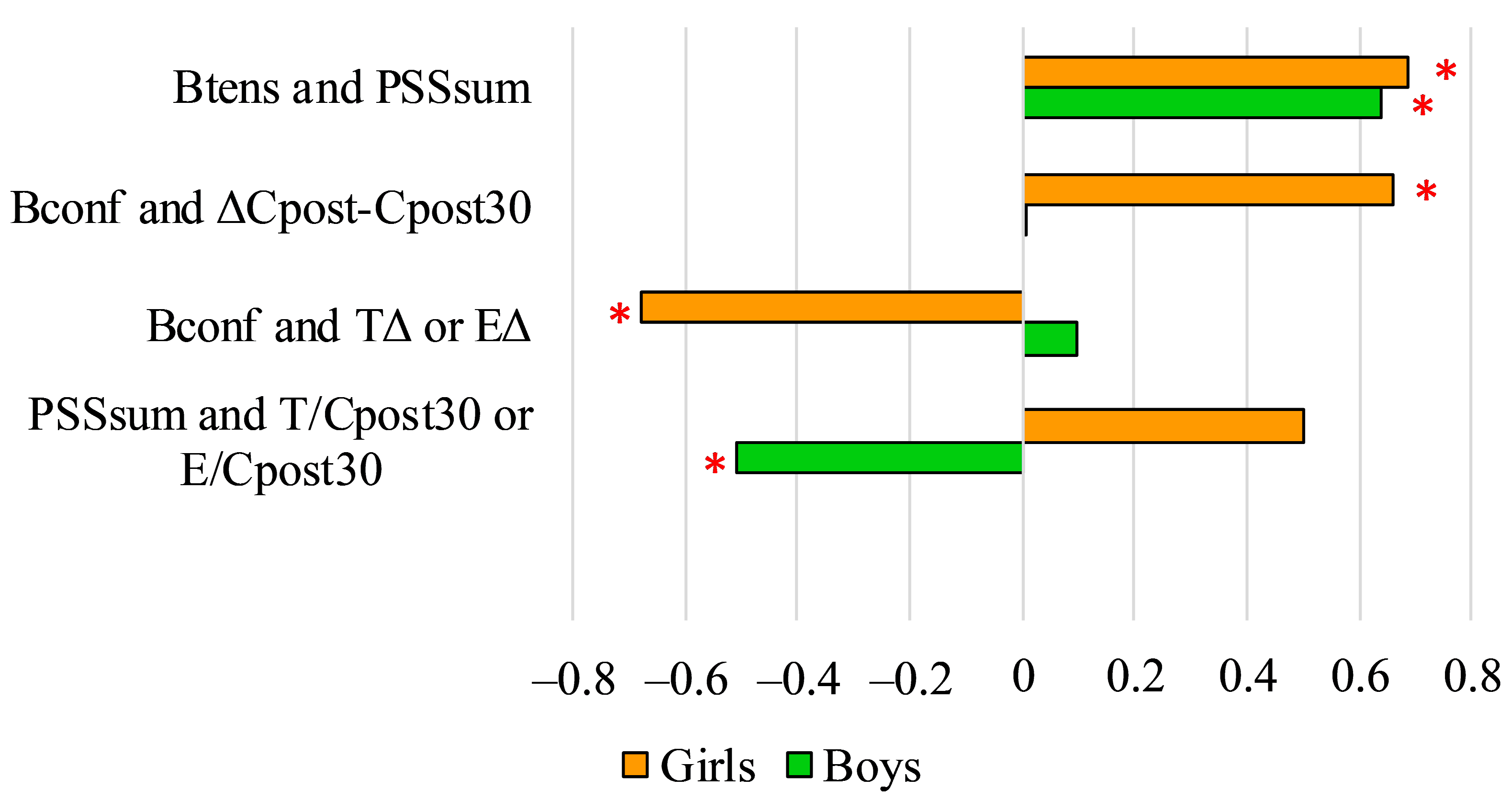
| Boys | |||||
|---|---|---|---|---|---|
| Pre-FT | SD | Post-FT | SD | p | |
| T * (µg/dL) | 0.006 | 0.003 | 0.005 | 0.002 | 0.0164 |
| T/C *** | 0.047 | 0.032 | 0.020 | 0.015 | 0.0004 |
| LAC *** (mmol/L) | 1.011 | 0.259 | 8.189 | 3.237 | >0.0001 |
| Girls | |||||
| Pre-FT | SD | Post-FT | SD | p | |
| E (µg/dL) | 0.0003 | 0.0001 | 0.0004 | 0.0001 | 0.1062 |
| E/C | 0.0016 | 0.0010 | 0.0021 | 0.0025 | 0.5135 |
| LAC *** (mmol/L) | 0.742 | 0.228 | 5.833 | 2.476 | >0.0001 |
| Girls | SD | Boys | SD | p | |
| LACpre * | 0.742 | 0.228 | 1.021 | 0.255 | 0.0043 |
| LACpost * | 5.833 | 2.476 | 8.189 | 3.237 | 0.0417 |
| Girls E/Cpost30 | |||||||
|---|---|---|---|---|---|---|---|
| M 1 | LACΔ | HRrest. | R2 | ||||
| B * | −0.51 * | 0.61 * | 0.661 * | ||||
| M 2 | HRmax | LACpost/LACpre | HRrest | Bvig | PSsympt | R2 | |
| B * | −0.44 * | −0.32 | 0.64 * | 0.28 | 0.21 | 0.898 * | |
| Boys T/Cpost30 | |||||||
| M 1 | HRR60 | LACpre/LACpost | R2 | ||||
| B * | 0.73 * | 0.43 | 0.375 * | ||||
| M 2 | HRR60 | LACpre/LACpost | HRrest | PSSsum | Btens | Bftg | R2 |
| B * | 0.96 * | 0.51 | −0.45 | −0.49 | 0.62 | −0.41 | 0.661 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almási, G.; Bosnyák, E.; Móra, Á.; Zsákai, A.; Fehér, P.V.; Annár, D.; Nagy, N.; Sziráki, Z.; Kemper, H.C.G.; Szmodis, M. Physiological and Psychological Responses to a Maximal Swimming Exercise Test in Adolescent Elite Athletes. Int. J. Environ. Res. Public Health 2021, 18, 9270. https://doi.org/10.3390/ijerph18179270
Almási G, Bosnyák E, Móra Á, Zsákai A, Fehér PV, Annár D, Nagy N, Sziráki Z, Kemper HCG, Szmodis M. Physiological and Psychological Responses to a Maximal Swimming Exercise Test in Adolescent Elite Athletes. International Journal of Environmental Research and Public Health. 2021; 18(17):9270. https://doi.org/10.3390/ijerph18179270
Chicago/Turabian StyleAlmási, Gábor, Edit Bosnyák, Ákos Móra, Annamária Zsákai, Piroska V. Fehér, Dorina Annár, Nikoletta Nagy, Zsófia Sziráki, Han C. G. Kemper, and Márta Szmodis. 2021. "Physiological and Psychological Responses to a Maximal Swimming Exercise Test in Adolescent Elite Athletes" International Journal of Environmental Research and Public Health 18, no. 17: 9270. https://doi.org/10.3390/ijerph18179270
APA StyleAlmási, G., Bosnyák, E., Móra, Á., Zsákai, A., Fehér, P. V., Annár, D., Nagy, N., Sziráki, Z., Kemper, H. C. G., & Szmodis, M. (2021). Physiological and Psychological Responses to a Maximal Swimming Exercise Test in Adolescent Elite Athletes. International Journal of Environmental Research and Public Health, 18(17), 9270. https://doi.org/10.3390/ijerph18179270









