Lactoferrin, Quercetin, and Hydroxyapatite Act Synergistically against Pseudomonas fluorescens
Abstract
:1. Introduction
2. Results
2.1. Antimicrobial Activity of Que and Lacto Alone and Complexed with HA
2.2. Evaluation of HA Absorption Capacity
2.3. Synergistic Activity of the Complex
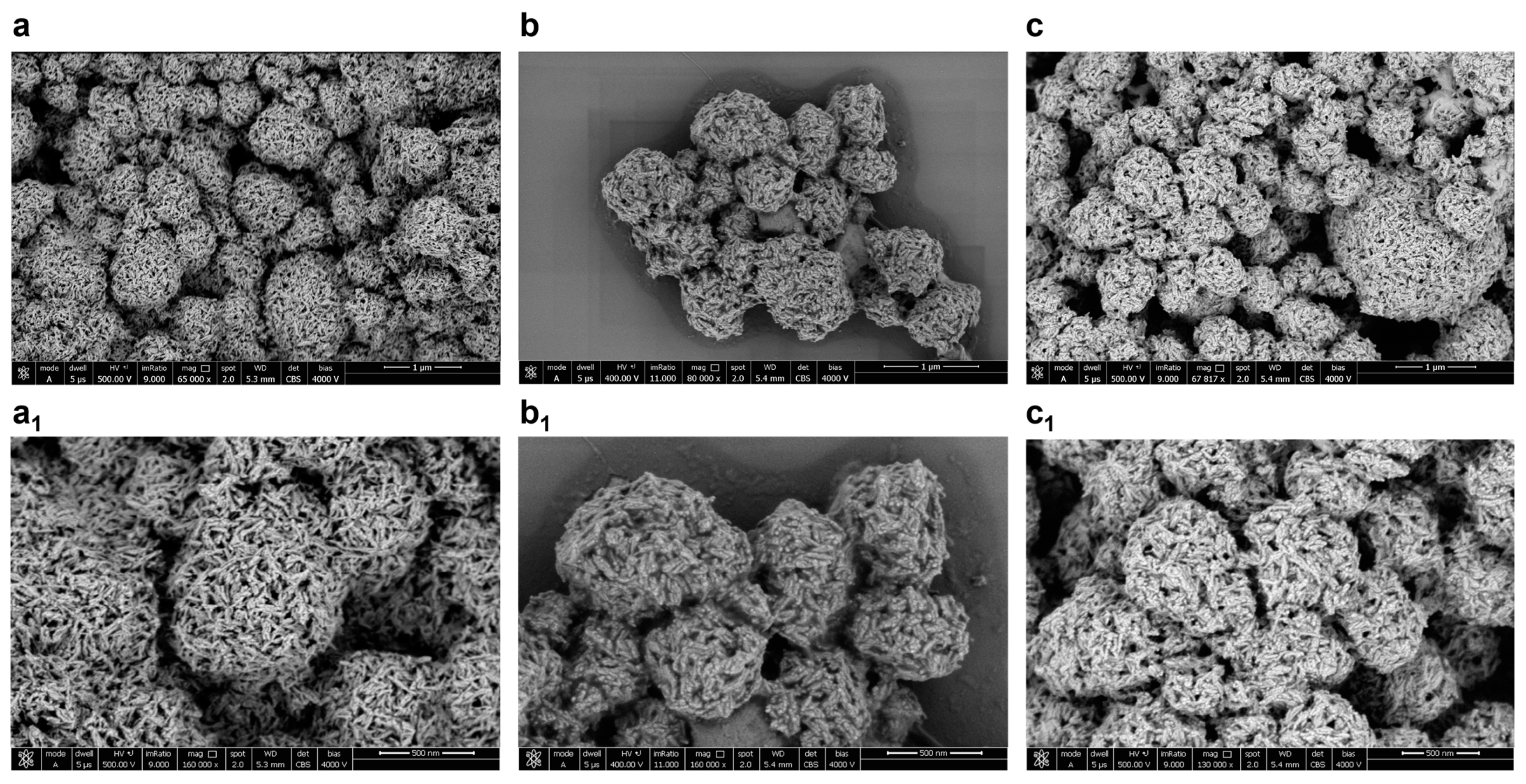
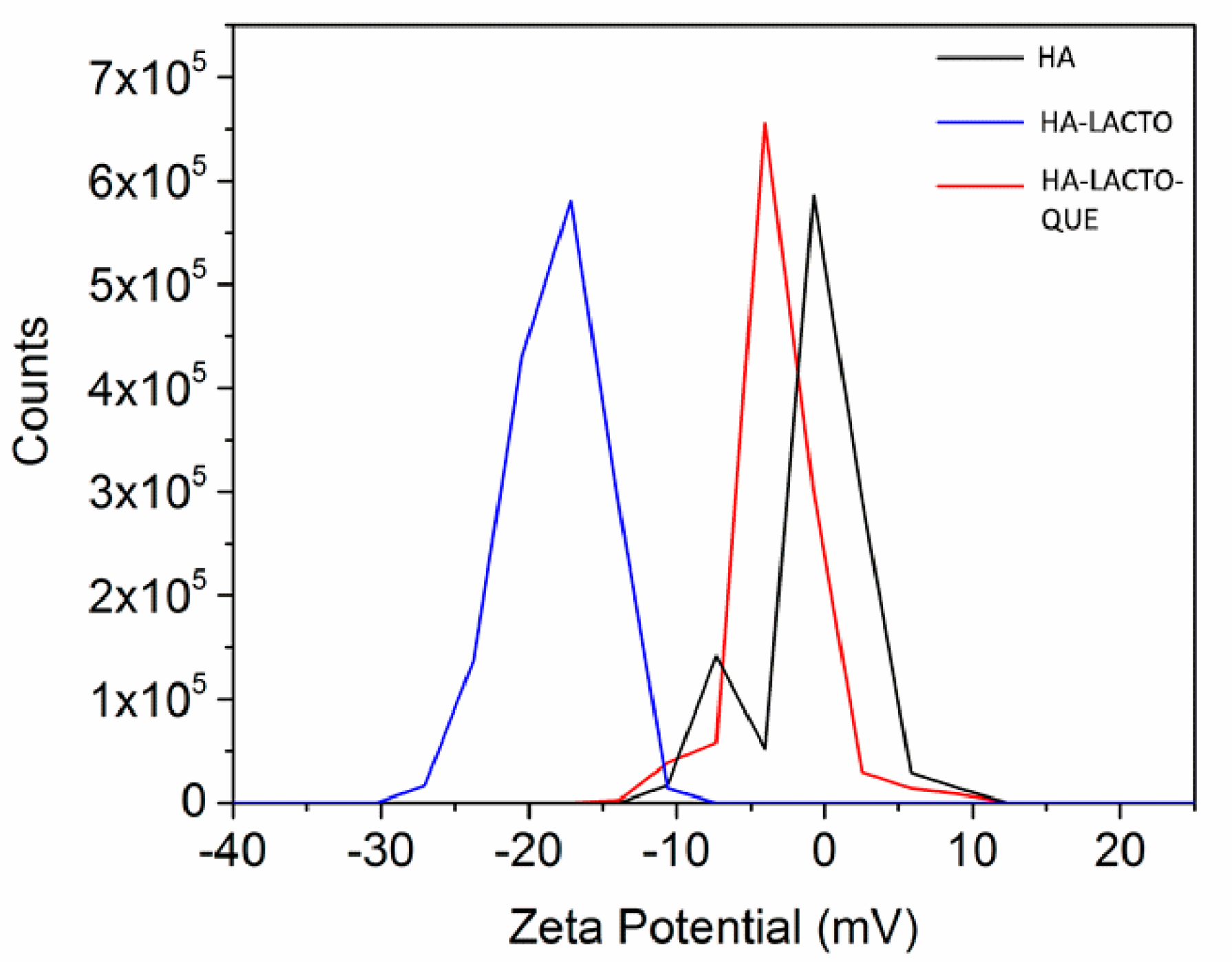
2.4. Characterization of the HA–Lacto Complex in Presence of Que
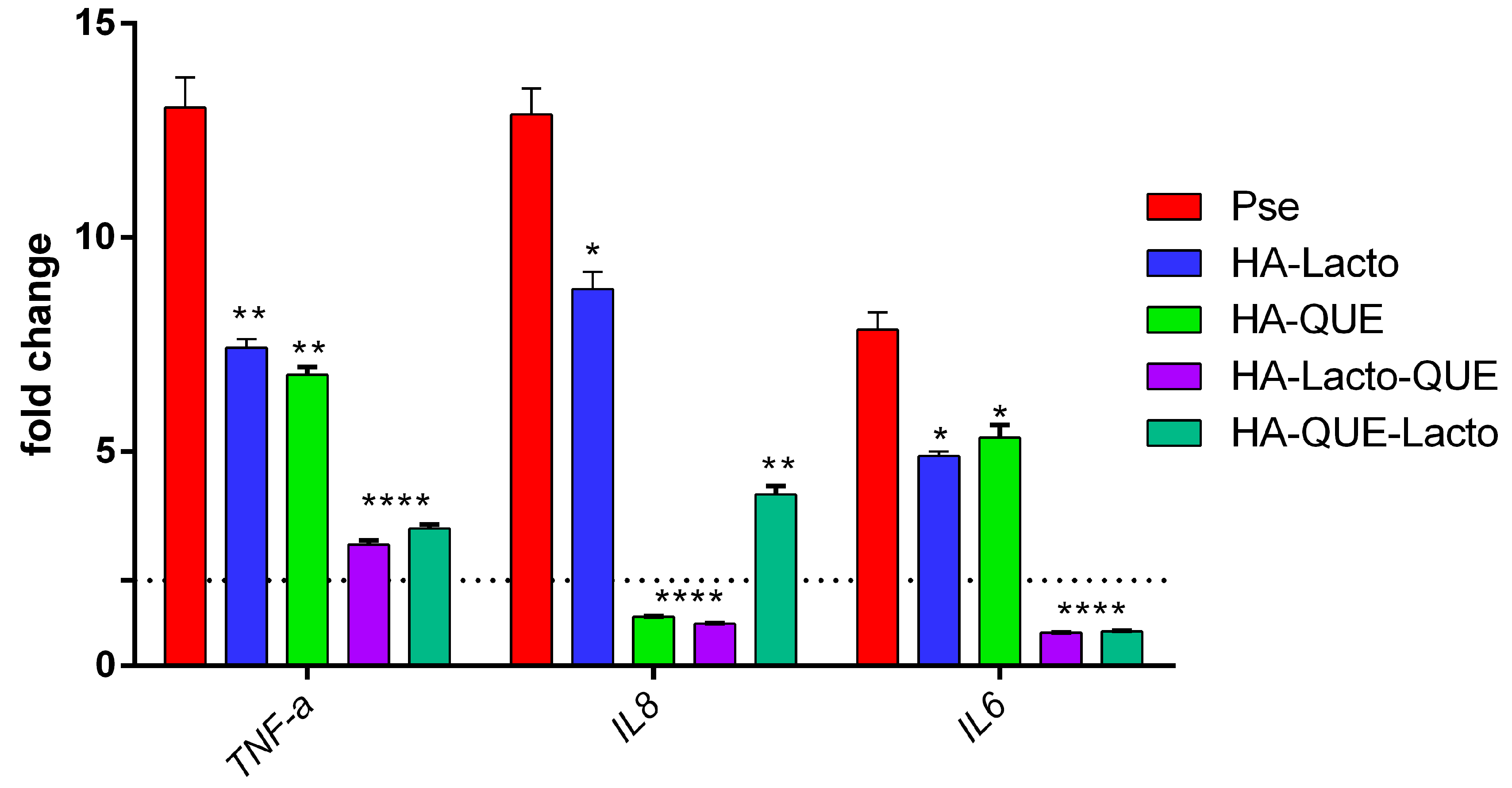
2.5. Anti-Inflammatory Activity of the Complex
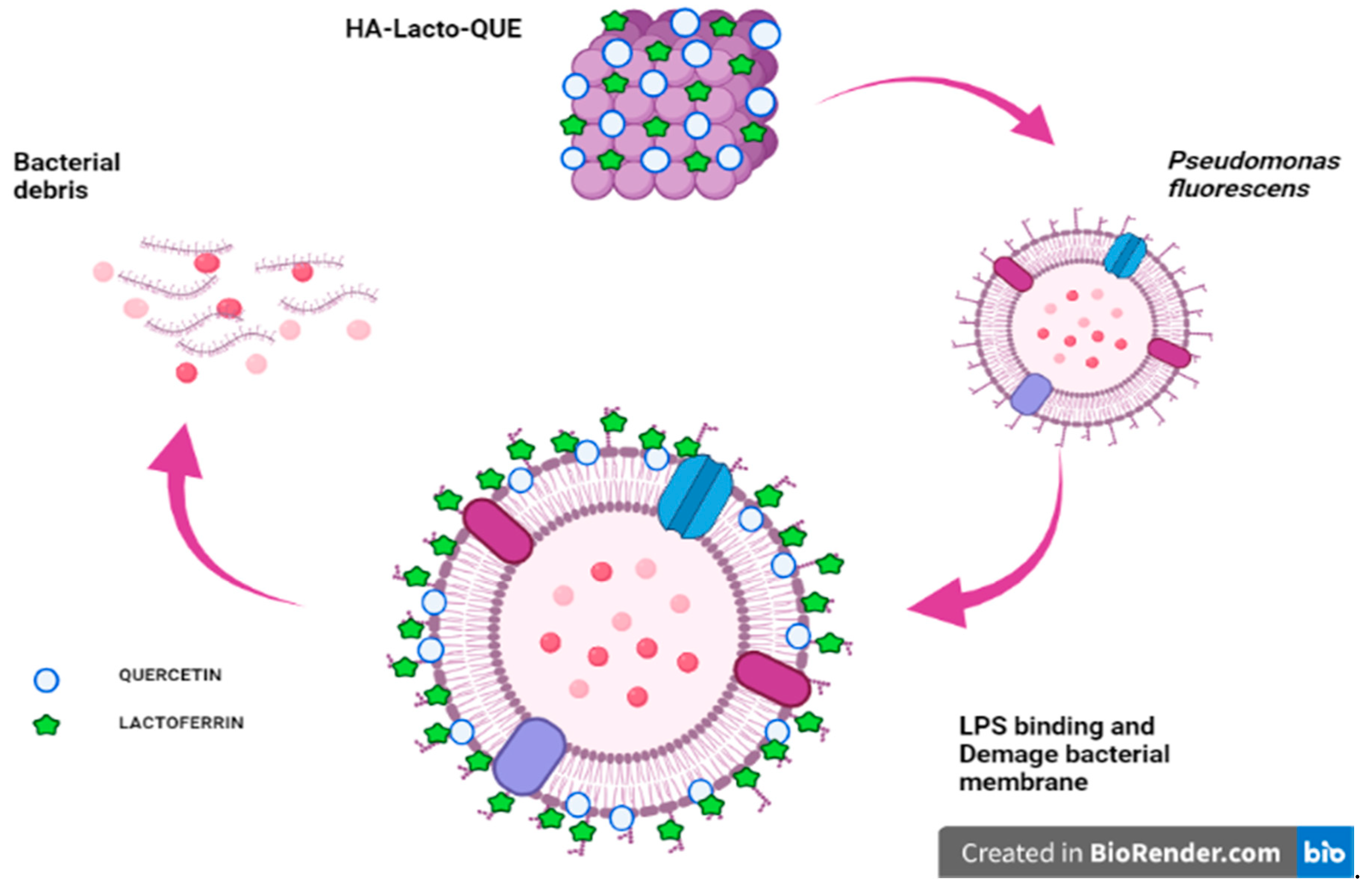
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Culture Conditions
4.2. Antimicrobial Compounds
4.3. Antimicrobial Activity
4.4. Biomimetic HA Nanocrystal Synthesis and Characterization
4.5. Evaluation of HA Absorption Capacity and Complex Preparation
4.6. Statistical Analysis for the Complex Synergy
4.7. Zeta Potential
4.8. SEM Image
4.9. In Vitro Infection Studies
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arnaut-Rollier, I.; De Zutter, L.; Van Hoof, J. Identities of the Pseudomonas spp. in flora from chilled chicken. Int. J. Food Microbiol. 1999, 48, 87–96. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Rahman, A.; Dung, N.T.; Huh, M.K.; Kang, S.C. In vitro inhibition of food spoilage and foodborne pathogenic bacteria by essential oil and leaf extracts of Magnolia liliflora Desr. J. Food Sci. 2008, 73, M314–M320. [Google Scholar] [CrossRef]
- de la Cruz Quiroz, R.; Rodriguez-Martinez, V.; Velazquez, G.; Perez, G.M.; Fagotti, F.; Welti-Chanes, J.; Torres, J.A. Residential Refrigerator Performance Based on Microbial Indicators of Ground Beef Preservation Assessed Using Predictive Microbiology Tools. Food Bioprocess. Technol. 2020, 13, 2172–2185. [Google Scholar] [CrossRef]
- Griffiths, M.I.W.; Laing, R.R.; Roy, D.; Mafu, A.A. Psychrotrophs in dairy products: Their effects and their control. Crit. Rev. Food Sci. Nutr. 1994, 34, 1–30. [Google Scholar]
- Tobiassen, R.O.; Stepaniak, L.; Sørhaug, T. Screening for differences in the proteolytic systems of Lactococcus, Lactobacillus and Propionibacterium. Eur. Food Res. Technol. 1997, 204, 273–278. [Google Scholar] [CrossRef]
- Kumar, H.; Franzetti, L.; Kaushal, A.; Kumar, D. Pseudomonas fluorescens: A potential food spoiler and challenges and advances in its detection. Ann. Microbiol. 2019, 69, 873–883. [Google Scholar] [CrossRef]
- Dogan, B.; Boor, K.J. Genetic diversity and spoilage potentials among Pseudomonas spp. isolated from fluid milk products and dairy processing plants. Appl. Environ. Microbiol. 2003, 69, 130–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, J.R.; Sanglay, G.C.; Sharma, M.; Solomon, M.B. Combining antimicrobials and hydrodynamic pressure processing for control of listeria monocytogenes in frankfurters. J. Muscle Foods 2007, 18, 1–18. [Google Scholar] [CrossRef]
- Samelis, J.; Sofos, J.N.; Kain, M.L.; Scanga, J.A.; Belk, K.E.; Smith, G.C. Organic acids and their salts as dipping solutions to control Listeria monocytogenes inoculated following processing of sliced pork bologna stored at 4 °C in vacuum packages. J. Food Prot. 2001, 64, 1722–1729. [Google Scholar] [CrossRef]
- Ibrahim Sallam, K. Antimicrobial and antioxidant effects of sodium acetate, sodium lactate, and sodium citrate in refrigerated sliced salmon. Food Control 2007, 18, 566–575. [Google Scholar] [CrossRef] [Green Version]
- Cavaliere, C.; Montone, A.M.I.; Aita, S.E.; Capparelli, R.; Cerrato, A.; Cuomo, P.; Laganà, A.; Montone, C.M.; Piovesana, S.; Capriotti, A.L. Production and characterization of medium-sized and short antioxidant peptides from soy flour-simulated gastrointestinal hydrolysate. Antioxidants 2021, 10, 734. [Google Scholar] [CrossRef]
- Cerrato, A.; Capriotti, A.L.; Capuano, F.; Cavaliere, C.; Montone, A.M.I.; Montone, C.M.; Piovesana, S.; Chiozzi, R.Z.; Laganà, A. Identification and antimicrobial activity of medium-sized and short peptides from yellowfin tuna (Thunnus albacares) simulated gastrointestinal digestion. Foods 2020, 9, 1185. [Google Scholar] [CrossRef]
- Verhoeckx, K.; Cotter, P.; López-Expósito, I.; Kleiveland, C.; Lea, T.; Mackie, A.; Requena, T.; Swiatecka, D.; Wichers, H. The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Tavassoli, M.; Sani, M.A.; Khezerlou, A.; Ehsani, A.; McClements, D.J. Multifunctional nanocomposite active packaging materials: Immobilization of quercetin, lactoferrin, and chitosan nanofiber particles in gelatin films. Food Hydrocoll. 2021, 118, 106747. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.; Zhou, C.; Cai, Y.; Tang, Y.; Sun, W.; Yao, H.; Zheng, T.; Chen, H.; Xiao, Y.; Shan, Z.; et al. Purification, characterization and antioxidant activities in vitro of polysaccharides from Amaranthus hybridus L. PeerJ 2020, 8, e9077. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Lim, T.T.; Tee, J.J. Antioxidant properties of several tropical fruits: A comparative study. Food Chem. 2007, 103, 1003–1008. [Google Scholar] [CrossRef]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine. Oxid. Med. Cell. Longev. 2020, 2020, 1–13. [Google Scholar] [CrossRef]
- Wang, S.; Yao, J.; Zhou, B.; Yang, J.; Chaudry, M.T.; Wang, M.; Xiao, F.; Li, Y.; Yin, W. Bacteriostatic effect of quercetin as an antibiotic alternative in vivo and its antibacterial mechanism in vitro. J. Food Prot. 2018, 81, 68–78. [Google Scholar] [CrossRef]
- Hendra, R.; Ahmad, S.; Sukari, A.; Shukor, M.Y.; Oskoueian, E. Flavonoid analyses and antimicrobial activity of various parts of Phaleria macrocarpa (Scheff. ) Boerl fruit. Int. J. Mol. Sci. 2011, 12, 3422–3431. [Google Scholar] [CrossRef] [Green Version]
- Plaper, A.; Golob, M.; Hafner, I.; Oblak, M.; Solmajer, T.; Jerala, R. Characterization of quercetin binding site on DNA gyrase. Biochem Biophys Res. Commun. 2003, 306, 530–536. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, J.H.; Cho, H.S.; Joo, S.W.; Cho, M.H.; Lee, J. Anti-biofilm activities of quercetin and tannic acid against Staphylococcus aureus. Biofouling 2013, 29, 491–499. [Google Scholar] [CrossRef]
- Yamauchi, K.; Tomita, M.; Giehl, T.J.; Ellison, R.T. Antibacterial activity of lactoferrin and a pepsin-derived lactoferrin peptide fragment. Infect. Immun. 1993, 61, 719–728. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Liu, Z.; Ma, Q.; He, Z.; Niu, Z.; Zhang, M.; Pan, L.; Qu, X.; Yu, J.; Niu, B. Studies on the Interaction between Three Small Flavonoid Molecules and Bovine Lactoferrin. Biomed. Res. Int. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Comalada, M.; Camuesco, D.; Sierra, S.; Ballester, I.; Xaus, J.; Gálvez, J.; Zarzuelo, A. In vivo quercitrin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-κB pathway. Eur. J. Immunol. 2005, 35, 584–592. [Google Scholar] [CrossRef]
- Min, Y.D.; Choi, C.H.; Bark, H.; Son, H.Y.; Park, H.H.; Lee, S.; Park, J.W.; Park, E.K.; Shin, H.I.; Kim, S.H. Quercetin inhibits expression of inflammatory cytokines through attenuation of NF-κB and p38 MAPK in HMC-1 human mast cell line. Inflamm. Res. 2007, 56, 210–215. [Google Scholar] [CrossRef]
- Chapple, D.S.; Hussain, R.; Joannou, C.L.; Hancock, R.E.W.; Odell, E.; Evans, R.W.; Siligardi, G. Structure and association of human lactoferrin peptides with Escherichia coli lipopolysaccharide. Antimicrob. Agents Chemother. 2004, 48, 2190–2198. [Google Scholar] [CrossRef] [Green Version]
- Latorre, D.; Puddu, P.; Valenti, P.; Gessani, S. Reciprocal interactions between lactoferrin and bacterial endotoxins and their role in the regulation of the immune response. Toxins 2010, 2, 54–68. [Google Scholar] [CrossRef] [Green Version]
- Balcão, V.M.; Costa, C.I.; Matos, C.M.; Moutinho, C.G.; Amorim, M.; Pintado, M.E.; Gomes, A.P.; Vila, M.M.; Teixeira, J.A. Nanoencapsulation of bovine lactoferrin for food and biopharmaceutical applications. Food Hydrocoll. 2013, 32, 425–431. [Google Scholar] [CrossRef] [Green Version]
- Celik, C.; Gencay, A.; Ocsoy, I. Can food and food supplements be deployed in the fight against the COVID 19 pandemic? Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129801. [Google Scholar] [CrossRef] [PubMed]
- Fulgione, A.; Ianniello, F.; Papaianni, M.; Contaldi, F.; Sgamma, T.; Giannini, C.; Pastore, S.; Velotta, R.; Ventura, B.D.; Roveri, N.; et al. Biomimetic hydroxyapatite nanocrystals are an active carrier for salmonella bacteriophages. Int. J. Nanomed. 2019, 14, 2219–2232, 102147/IJNS190188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roveri, N.; Iafisco, M. Biomimetic Nanostructured Apatitic Matrices for Drug Delivery. In Biomimetic Approaches for Biomaterials Development; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Malvano, F.; Montone, A.M.I.; Capparelli, R.; Capuano, F.; Albanese, D. Development of a Novel Active Edible Coating Containing Hydroxyapatite for Food Shelf-life Extension. Chem. Eng. Trans. 2021, 87, 25–30. [Google Scholar]
- Nocerino, N.; Fulgione, A.; Iannaccone, M.; Tomasetta, L.; Ianniello, F.; Martora, F.; Lelli, M.; Roveri, N.; Capuano, F.; Capparelli, R. Biological activity of lactoferrin-functionalized biomimetic hydroxyapatite nanocrystals. Int. J. Nanomed. 2014, 9, 1175–1184. [Google Scholar] [CrossRef] [Green Version]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The minimum inhibitory concentration of antibiotics: Methods, interpretation, clinical relevance. Pathogens 2021, 10, 165. [Google Scholar] [CrossRef]
- Bidaud, A.L.; Schwarz, P.; Herbreteau, G.; Dannaoui, E. Techniques for the assessment of in vitro and in vivo antifungal combinations. J. Fungi 2021, 7, 113. [Google Scholar] [CrossRef]
- Samimi, S.; Maghsoudnia, N.; Eftekhari, R.B.; Dorkoosh, F. Lipid-Based Nanoparticles for Drug Delivery Systems. In Characterization and Biology of Nanomaterials for Drug Delivery: Nanoscience and Nanotechnology in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Andreani, N.A.; Carraro, L.; Fasolato, L.; Balzan, S.; Lucchini, R.; Novelli, E.; Cardazzo, B. Characterisation of the thermostable protease AprX in strains of Pseudomonas fluorescens and impact on the shelf-life of dairy products: Preliminary results. Ital. J. Food Saf. 2016, 5, 6175. [Google Scholar] [CrossRef] [Green Version]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial Activity of Some Flavonoids and Organic Acids Widely Distributed in Plants. J. Clin. Med. 2019, 9, 109. [Google Scholar] [CrossRef] [Green Version]
- Fulgione, A.; Nocerino, N.; Iannaccone, M.; Roperto, S.; Capuano, F.; Roveri, N.; Lelli, M.; Crasto, A.; Calogero, A.; Pilloni, A.P.; et al. Lactoferrin adsorbed onto biomimetic hydroxyapatite nanocrystals controlling—In Vivo—The Helicobacter pylori infection. PLoS ONE 2016, 1, e0158646. [Google Scholar] [CrossRef] [Green Version]
- Chanput, W.; Peters, V.; Wichers, H. THP-1 and U937. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Scales, B.S.; Dickson, R.P.; Lipuma, J.J.; Huffnagle, G.B. Microbiology, genomics, and clinical significance of the Pseudomonas fluorescens species complex, an unappreciated colonizer of humans. Clin. Microbiol. Rev. 2014, 27, 927–948. [Google Scholar] [CrossRef] [Green Version]
- Berlutti, F.; Schippa, S.; Morea, C.; Sarli, S.; Perfetto, B.; Donnarumma, G.; Valenti, P. Lactoferrin downregulates pro-inflammatory cytokines upexpressed in intestinal epithelial cells infected with invasive or noninvasive Escherichia coli strains. Biochem. Cell Biol. 2006, 84, 351–357. [Google Scholar] [CrossRef]
- Choi, H.J.; Seo, C.H.; Park, S.H.; Yang, H.; Do, K.H.; Kim, J.; Kim, H.K.; Chung, D.H.; Ahn, J.H.; Moon, Y. Involvement of epidermal growth factor receptor-linked signaling responses in Pseudomonas fluorescens-infected alveolar epithelial cells. Infect. Immun. 2011, 79, 1998–2005. [Google Scholar] [CrossRef] [Green Version]
- Palazzo, B.; Walsh, D.; Iafisco, M.; Foresti, E.; Bertinetti, L.; Martra, G.; Bianchi, C.L.; Cappelletti, G.; Roveri, N. Amino acid synergetic effect on structure, morphology and surface properties of biomimetic apatite nanocrystals. Acta Biomater. 2009, 5, 1241–1252. [Google Scholar] [CrossRef]
- Papaianni, M.; Ricciardelli, A.; Casillo, A.; Corsaro, M.M.; Borbone, F.; Ventura, B.D.; Velotta, R.; Fulgione, A.; Woo, S.L.; Tutino, M.L.; et al. The union is strength: The synergic action of long fatty acids and a bacteriophage against xanthomonas campestris biofilm. Microorganisms 2021, 9, 60. [Google Scholar] [CrossRef]
- Rio, D.C.; Ares, M.; Hannon, G.J.; Nilsen, T.W. Purification of RNA Using TRIzol (TRI Reagent). Cold Spring Harb. Protoc. 2010, 2010. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
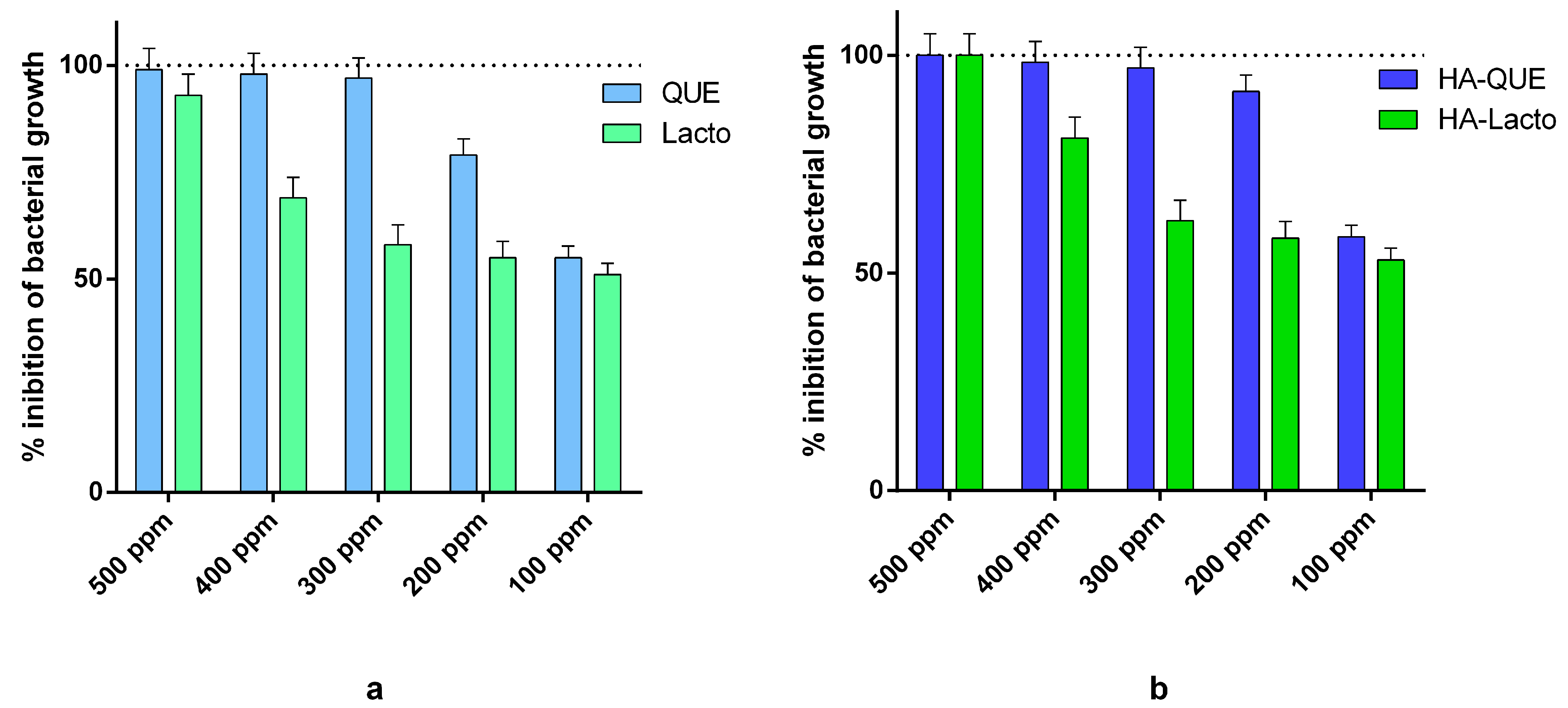
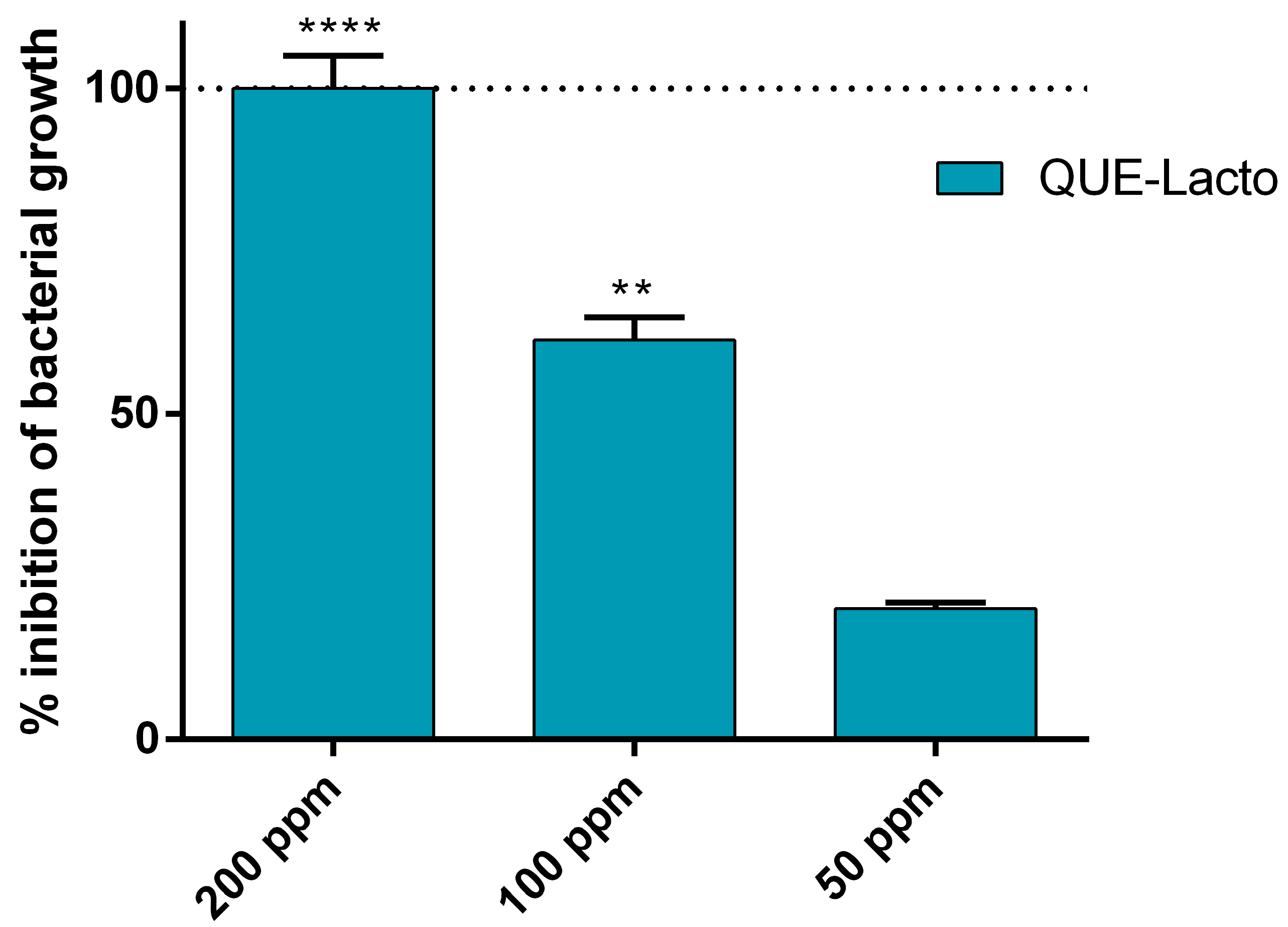
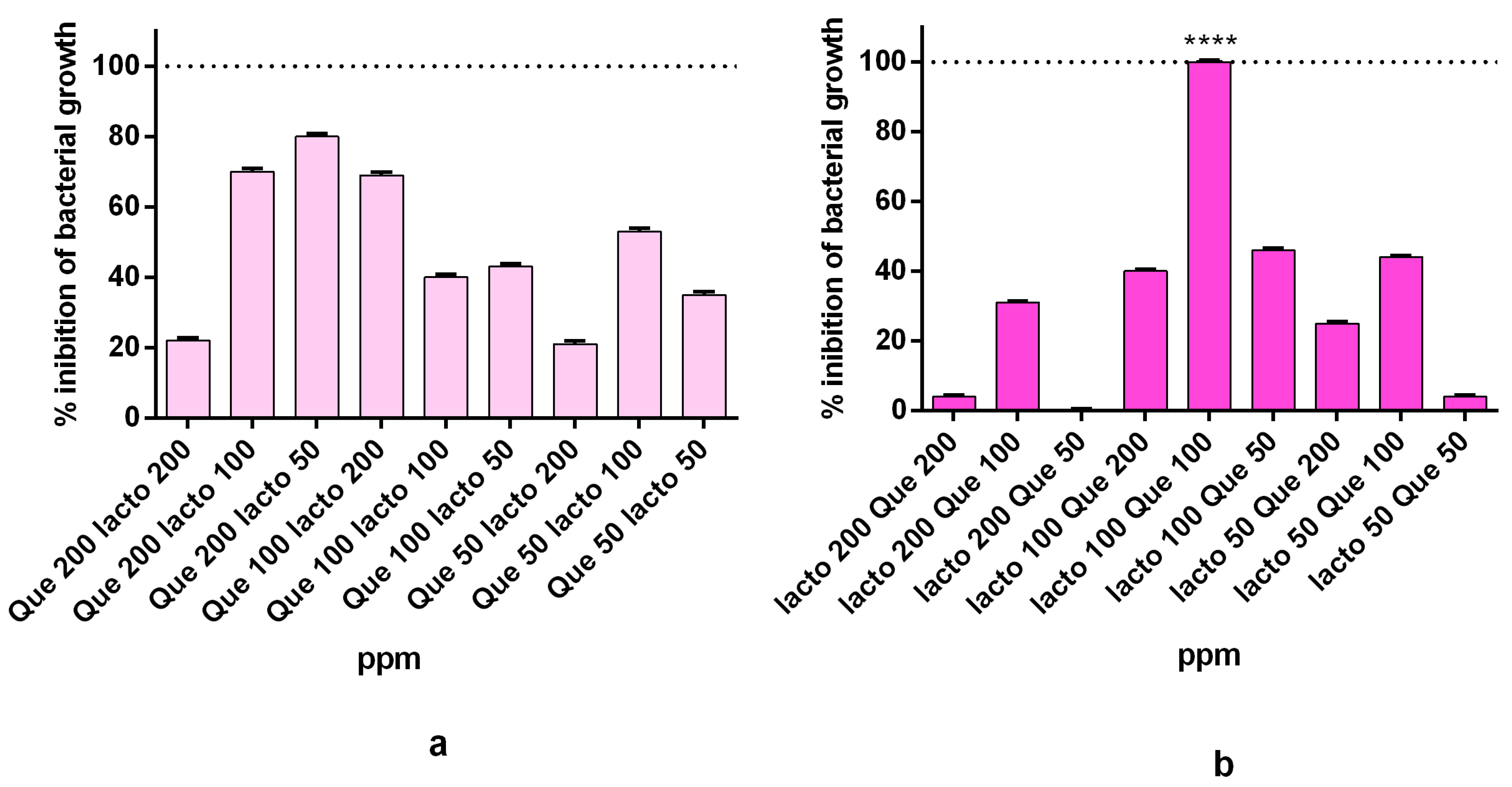
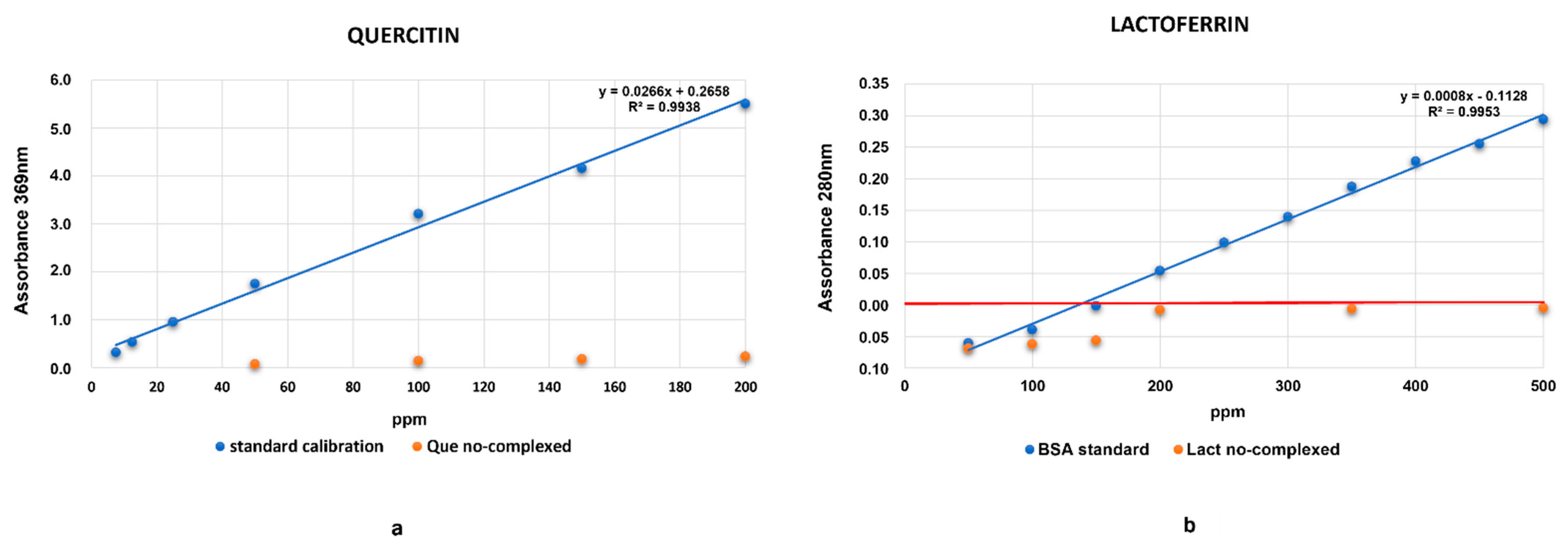
| Lacto 200 | Lacto 100 | Lacto 50 | |
| Que 100 | 0.83 ± 0.18 | 1.27 ± 0.39 | 0.89 ± 0.21 |
| Que 100 + HA | 1.77 ± 0.50 | 0.88 ± 0.35 | 1.51 ± 0.52 |
| Que 200 | Que 100 | Que 50 | |
| Lacto 100 | 1.67 ± 0.33 | 0.85 ± 0.30 | 2.19 ± 0.44 |
| Lacto 100 + HA | 2.91 ± 0.77 | 6.75 ± 2.67 | 2.09 ± 0.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montone, A.M.I.; Papaianni, M.; Malvano, F.; Capuano, F.; Capparelli, R.; Albanese, D. Lactoferrin, Quercetin, and Hydroxyapatite Act Synergistically against Pseudomonas fluorescens. Int. J. Mol. Sci. 2021, 22, 9247. https://doi.org/10.3390/ijms22179247
Montone AMI, Papaianni M, Malvano F, Capuano F, Capparelli R, Albanese D. Lactoferrin, Quercetin, and Hydroxyapatite Act Synergistically against Pseudomonas fluorescens. International Journal of Molecular Sciences. 2021; 22(17):9247. https://doi.org/10.3390/ijms22179247
Chicago/Turabian StyleMontone, Angela Michela Immacolata, Marina Papaianni, Francesca Malvano, Federico Capuano, Rosanna Capparelli, and Donatella Albanese. 2021. "Lactoferrin, Quercetin, and Hydroxyapatite Act Synergistically against Pseudomonas fluorescens" International Journal of Molecular Sciences 22, no. 17: 9247. https://doi.org/10.3390/ijms22179247
APA StyleMontone, A. M. I., Papaianni, M., Malvano, F., Capuano, F., Capparelli, R., & Albanese, D. (2021). Lactoferrin, Quercetin, and Hydroxyapatite Act Synergistically against Pseudomonas fluorescens. International Journal of Molecular Sciences, 22(17), 9247. https://doi.org/10.3390/ijms22179247








