Moringa Oleifera Seed Extract Concomitantly Supplemented with Chemotherapy Worsens Tumor Progression in Mice with Triple Negative Breast Cancer and Obesity
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Moringa Seed Extract Concentrate Does Not Alter Food Intake, Body Weight or Total Chemotherapy-Induced Weight Loss in Rag1null Female Mice with Diet-Induced Obesity and TNBC
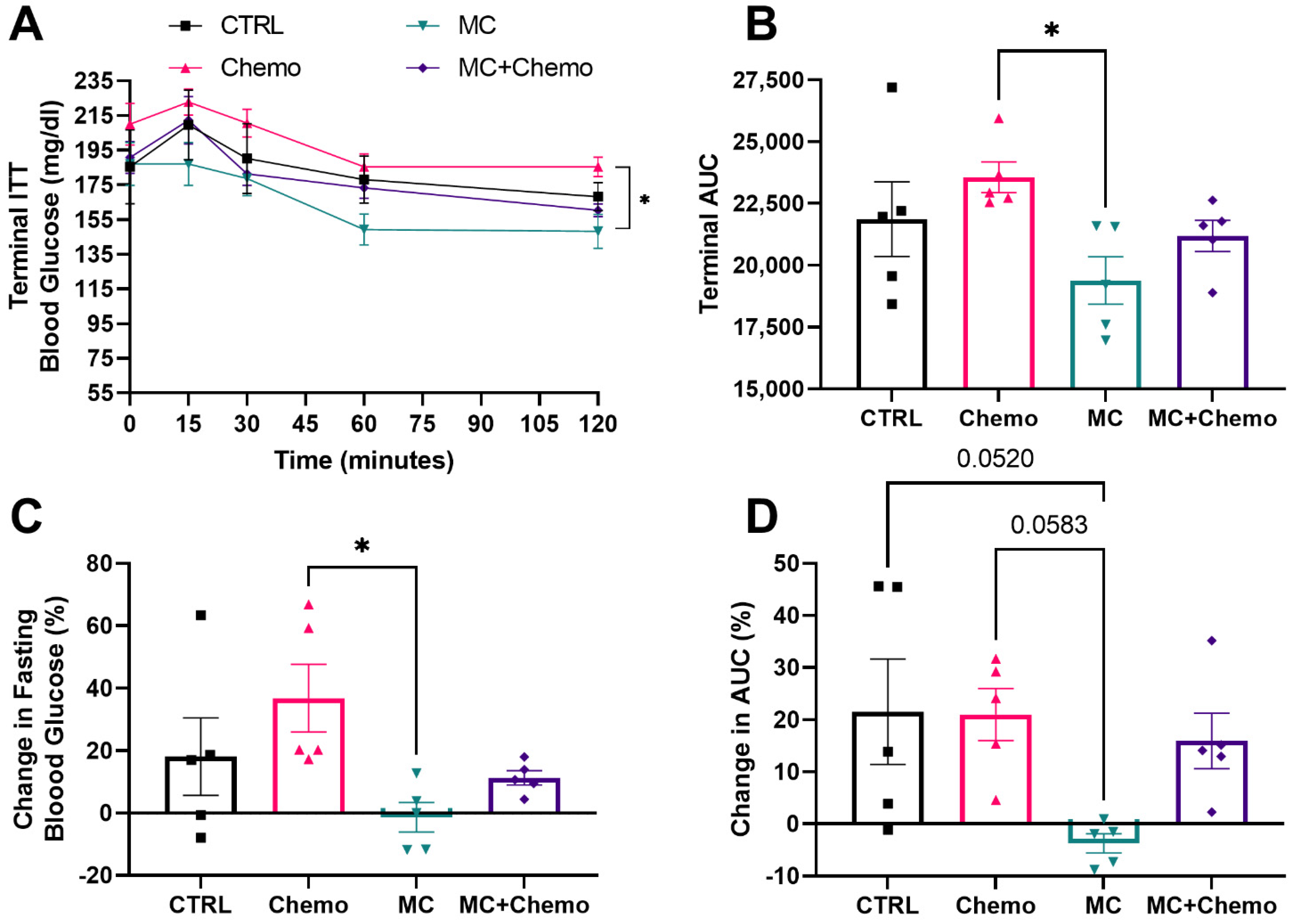
3.2. Moringa Seed Extract Concentrate Protects against High-Fat Diet- and Chemotherapy-Induced Increases in Fasting Glucose and Improves Insulin Sensitivity in Rag1null Female Mice with Diet-Induced Obesity and TNBC
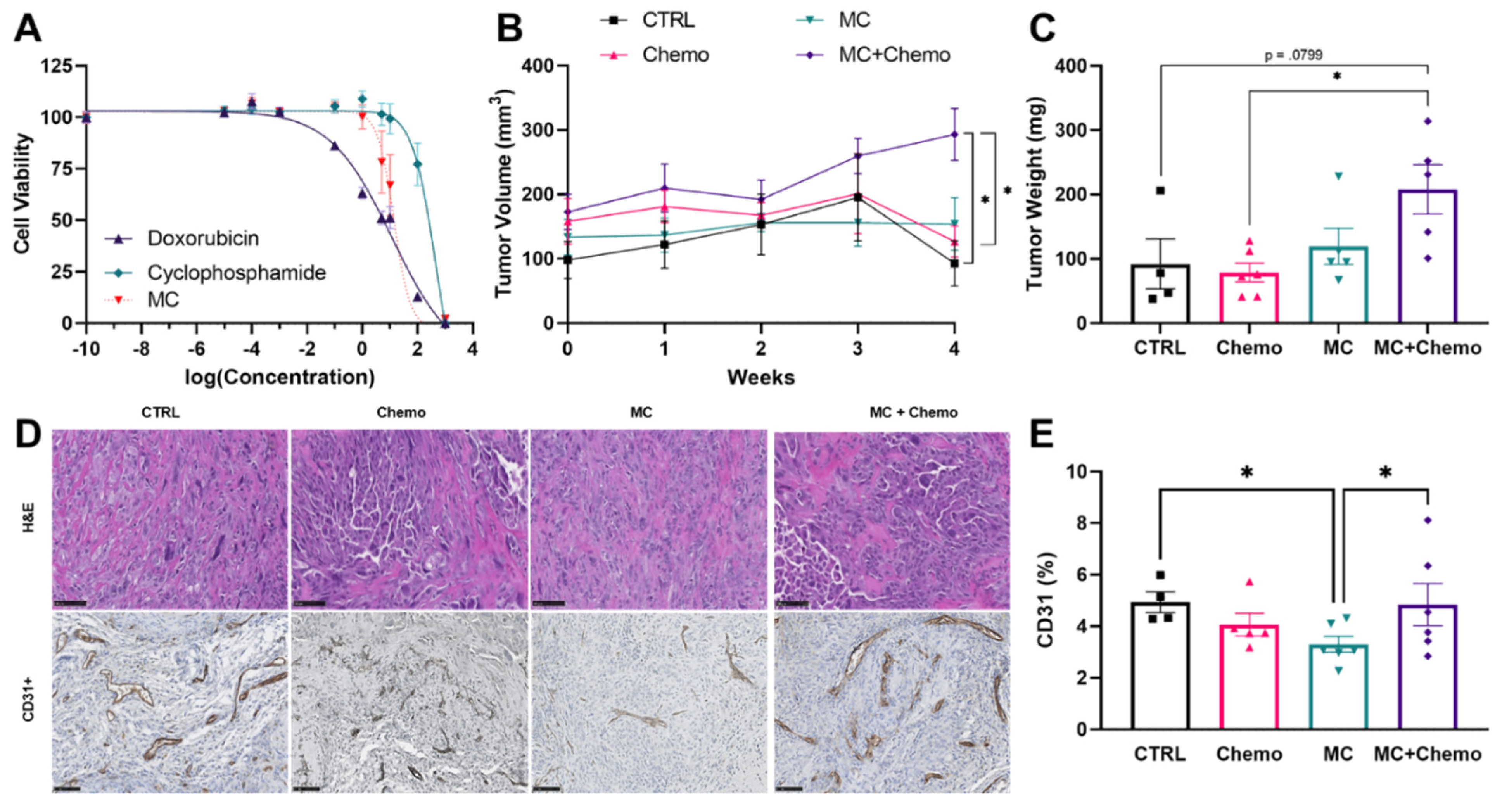
3.3. Moringa Seed Extract Concentrate Does Not Reduce Tumor Growth, Has a Negative Interaction with Chemotherapy and Reduces Tumor Angiogenesis in Rag1null Female Mice with Diet-Induced Obesity and TNBC
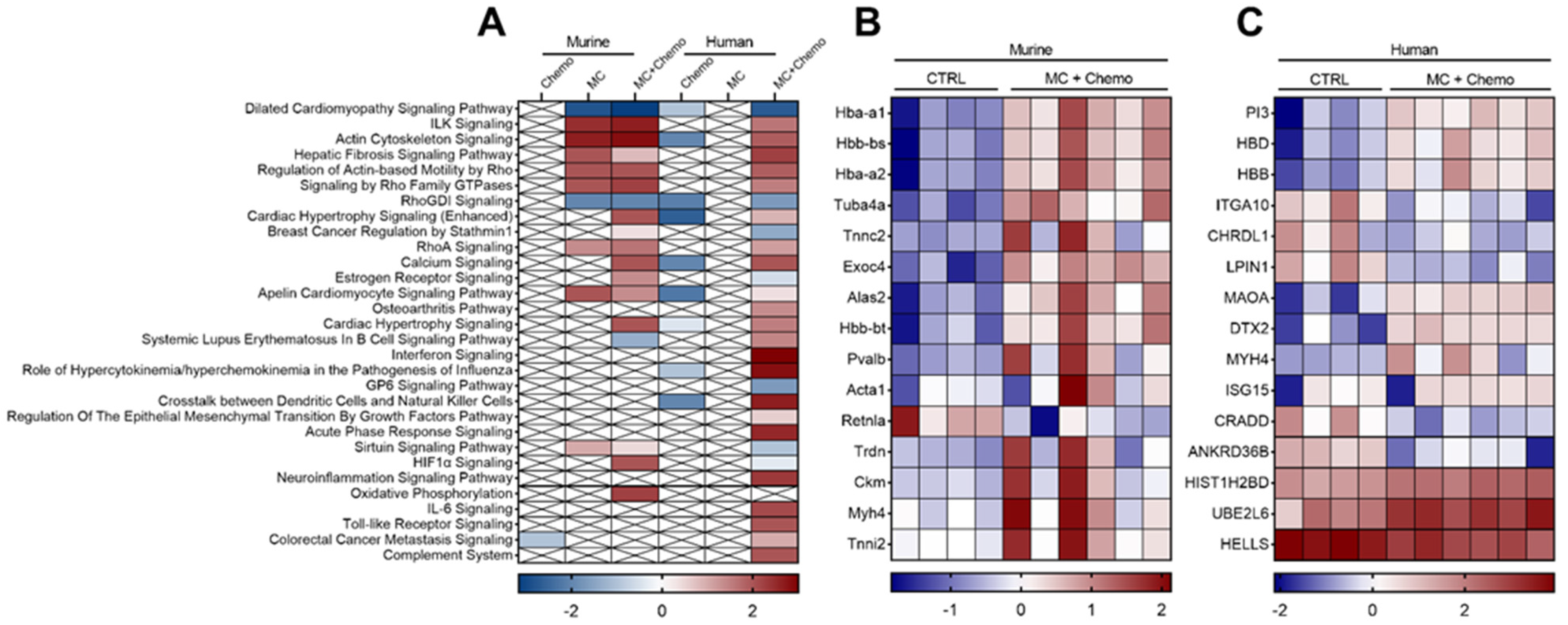
3.4. Moringa Seed Extract Concentrate Upregulates the Expression of Multiple Genes and Pathways Otherwise Downregulated by Chemotherapy in Tumors from Rag1null Female Mice with Diet-Induced Obesity and TNBC
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethical Review Statement
References
- Ryu, T.Y.; Park, J.; Scherer, P.E. Hyperglycemia as a risk factor for cancer progression. Diabetes Metab. J. 2014, 38, 330–336. [Google Scholar] [CrossRef]
- Sun, H.; Zou, J.; Chen, L.; Zu, X.; Wen, G.; Zhong, J. Triple-negative breast cancer and its association with obesity. Mol. Clin. Oncol. 2017, 7, 935–942. [Google Scholar] [CrossRef]
- Pierobon, M.; Frankenfeld, C.L. Obesity as a risk factor for triple-negative breast cancers: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2013, 137, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.A.; Kaklamani, V.G. Metabolic syndrome and triple-negative breast cancer: A new paradigm. Int. J. Breast Cancer 2012, 2012, 809291. [Google Scholar] [CrossRef] [PubMed]
- Blanquicett, C.; Roman, J.; Hart, C.M. Thiazolidinediones as anti-cancer agents. Cancer Ther. 2008, 6, 25–34. [Google Scholar] [PubMed]
- Shafiei-Irannejad, V.; Samadi, N.; Salehi, R.; Yousefi, B.; Zarghami, N. New insights into antidiabetic drugs: Possible applications in cancer treatment. Chem. Biol. Drug Des. 2017, 90, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhu, J.; Prokop, L.J.; Murad, M.H. Pharmacologic Therapy of Diabetes and Overall Cancer Risk and Mortality: A Meta-Analysis of 265 Studies. Sci. Rep. 2015, 5, 10147. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.; Rios-Silva, M.; Huerta, M.; Cardenas, Y.; Bricio-Barrios, J.A.; Diaz-Reval, M.I.; Urzua, Z.; Huerta-Trujillo, M.; Lopez-Quezada, K.; Trujillo, X. Effects of Moringa oleifera leaf powder on metabolic syndrome induced in male Wistar rats: A preliminary study. J. Int. Med. Res. 2018, 46, 3327–3336. [Google Scholar] [CrossRef] [PubMed]
- Metwally, F.M.; Rashad, H.M.; Ahmed, H.H.; Mahmoud, A.A.; Raouf, E.R.A.; Abdalla, A.M. Molecular mechanisms of the anti-obesity potential effect of Moringa oleifera in the experimental model. Asian Pac. J. Trop. Biomed. 2017, 7, 214–221. [Google Scholar] [CrossRef]
- Jaja-Chimedza, A.; Zhang, L.; Wolff, K.; Graf, B.L.; Kuhn, P.; Moskal, K.; Carmouche, R.; Newman, S.; Salbaum, J.M.; Raskin, I. A dietary isothiocyanate-enriched moringa (Moringa oleifera) seed extract improves glucose tolerance in a high-fat-diet mouse model and modulates the gut microbiome. J. Funct. Foods 2018, 47, 376–385. [Google Scholar] [CrossRef]
- Waterman, C.; Rojas-Silva, P.; Tumer, T.B.; Kuhn, P.; Richard, A.J.; Wicks, S.; Stephens, J.M.; Wang, Z.; Mynatt, R.; Cefalu, W.; et al. Isothiocyanate-rich Moringa oleifera extract reduces weight gain, insulin resistance, and hepatic gluconeogenesis in mice. Mol. Nutr. Food Res. 2015, 59, 1013–1024. [Google Scholar] [CrossRef]
- Waterman, C.; Cheng, D.M.; Rojas-Silva, P.; Poulev, A.; Dreifus, J.; Lila, M.A.; Raskin, I. Stable, water extractable isothiocyanates from Moringa oleifera leaves attenuate inflammation in vitro. Phytochemistry 2014, 103, 114–122. [Google Scholar] [CrossRef]
- Vergara-Jimenez, M.; Almatrafi, M.M.; Fernandez, M.L. Bioactive Components in Moringa Oleifera Leaves Protect against Chronic Disease. Antioxidants 2017, 6, 91. [Google Scholar] [CrossRef]
- Bennett, R.N.; Mellon, F.A.; Foidl, N.; Pratt, J.H.; Dupont, M.S.; Perkins, L.; Kroon, P.A. Profiling glucosinolates and phenolics in vegetative and reproductive tissues of the multi-purpose trees Moringa oleifera L. (horseradish tree) and Moringa stenopetala L. J. Agric. Food Chem. 2003, 51, 3546–3553. [Google Scholar] [CrossRef] [PubMed]
- Bennour, N.; Mighri, H.; Bouhamda, T.; Mabrouk, M.; Apohan, E.; Yesilada, O.; Kucukbay, H.; Akrout, A. Moringa oleifera leaves: Could solvent and extraction method affect phenolic composition and bioactivities? Prep. Biochem. Biotechnol. 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tiloke, C.; Anand, K.; Gengan, R.M.; Chuturgoon, A.A. Moringa oleifera and their phytonanoparticles: Potential antiproliferative agents against cancer. Biomed. Pharm. 2018, 108, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, L.; Doriya, K.; Kumar, D.S. Moringa oleifera: A review on nutritive importance and its medicinal application. Food Sci. Hum. Wellness 2016, 5, 49–56. [Google Scholar] [CrossRef]
- Saini, R.K.; Sivanesan, I.; Keum, Y.-S. Phytochemicals of Moringa oleifera: A review of their nutritional, therapeutic and industrial significance. 3 Biotech 2016, 6, 203. [Google Scholar] [CrossRef]
- Jafarain, A.; Asghari, G.; Ghassami, E. Evaluation of cytotoxicity of Moringa oleifera Lam. callus and leaf extracts on Hela cells. Adv. Biomed. Res. 2014, 3, 194. [Google Scholar] [CrossRef] [PubMed]
- Sreelatha, S.; Jeyachitra, A.; Padma, P.R. Antiproliferation and induction of apoptosis by Moringa oleifera leaf extract on human cancer cells. Food Chem. Toxicol. 2011, 49, 1270–1275. [Google Scholar] [CrossRef] [PubMed]
- Al-Asmari, A.K.; Albalawi, S.M.; Athar, M.T.; Khan, A.Q.; Al-Shahrani, H.; Islam, M. Moringa oleifera as an Anti-Cancer Agent against Breast and Colorectal Cancer Cell Lines. PLoS ONE 2015, 10, e0135814. [Google Scholar] [CrossRef]
- Wisitpongpun, P.; Suphrom, N.; Potup, P.; Nuengchamnong, N.; Calder, P.C.; Usuwanthim, K. In Vitro Bioassay-Guided Identification of Anticancer Properties from Moringa oleifera Lam. Leaf against the MDA-MB-231 Cell Line. Pharmaceuticals 2020, 13, 464. [Google Scholar] [CrossRef]
- Xie, J.; Luo, F.X.; Shi, C.Y.; Jiang, W.W.; Qian, Y.Y.; Yang, M.R.; Song, S.; Dai, T.Y.; Peng, L.; Gao, X.Y.; et al. Moringa oleifera Alkaloids Inhibited PC3 Cells Growth and Migration Through the COX-2 Mediated Wnt/beta-Catenin Signaling Pathway. Front. Pharmacol. 2020, 11, 523962. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, D.; Tavecchio, M.; Falcioni, C.; Frapolli, R.; Erba, E.; Iori, R.; Rollin, P.; Barillari, J.; Manzotti, C.; Morazzoni, P.; et al. The isothiocyanate produced from glucomoringin inhibits NF-kB and reduces myeloma growth in nude mice in vivo. Biochem. Pharmacol. 2010, 79, 1141–1148. [Google Scholar] [CrossRef]
- Langin, H.; Lefebvre, G.; Tresch-Bruneel, E.; Deley, M.-C.L.; Marliot, G.; Sakji, I.; Vanseymortier, M.; Reich, M.; Lartigau, E.; Bonneterre, J. Prevalence of herbal medicine (HM) use among breast cancer patients treated with chemotherapy, hormone therapy, or targeted therapy. J. Clin. Oncol. 2018, 36, e13108. [Google Scholar] [CrossRef]
- Morris, K.T.; Johnson, N.; Homer, L.; Walts, D. A comparison of complementary therapy use between breast cancer patients and patients with other primary tumor sites. Am. J. Surg. 2000, 179, 407–411. [Google Scholar] [CrossRef]
- Damery, S.; Gratus, C.; Grieve, R.; Warmington, S.; Jones, J.; Routledge, P.; Greenfield, S.; Dowswell, G.; Sherriff, J.; Wilson, S. The use of herbal medicines by people with cancer: A cross-sectional survey. Br. J. Cancer 2011, 104, 927–933. [Google Scholar] [CrossRef]
- Fasinu, P.S.; Rapp, G.K. Herbal Interaction With Chemotherapeutic Drugs-A Focus on Clinically Significant Findings. Front. Oncol. 2019, 9, 1356. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.N.; Deng, G.; Mao, J.J. Practical Application of “About Herbs” Website: Herbs and Dietary Supplement Use in Oncology Settings. Cancer J. 2019, 25, 357–366. [Google Scholar] [CrossRef]
- Berkovich, L.; Earon, G.; Ron, I.; Rimmon, A.; Vexler, A.; Lev-Ari, S. Moringa Oleifera aqueous leaf extract down-regulates nuclear factor-kappaB and increases cytotoxic effect of chemotherapy in pancreatic cancer cells. BMC Complement. Altern. Med. 2013, 13, 212. [Google Scholar] [CrossRef]
- Volpe, D.A.; Hamed, S.S.; Zhang, L.K. Use of different parameters and equations for calculation of IC(5)(0) values in efflux assays: Potential sources of variability in IC(5)(0) determination. AAPS J. 2014, 16, 172–180. [Google Scholar] [CrossRef]
- Jaja-Chimedza, A.; Graf, B.L.; Simmler, C.; Kim, Y.; Kuhn, P.; Pauli, G.F.; Raskin, I. Biochemical characterization and anti-inflammatory properties of an isothiocyanate-enriched moringa (Moringa oleifera) seed extract. PLoS ONE 2017, 12, e0182658. [Google Scholar] [CrossRef]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008, 2008, pdb.prot4986. [Google Scholar] [CrossRef]
- Trivers, K.F.; Lund, M.J.; Porter, P.L.; Liff, J.M.; Flagg, E.W.; Coates, R.J.; Eley, J.W. The epidemiology of triple-negative breast cancer, including race. Cancer Causes Control 2009, 20, 1071–1082. [Google Scholar] [CrossRef] [PubMed]
- Hursting, S.D.; Dunlap, S.M. Obesity, metabolic dysregulation, and cancer: A growing concern and an inflammatory (and microenvironmental) issue. Ann. N. Y. Acad. Sci. 2012, 1271, 82–87. [Google Scholar] [CrossRef]
- Hopkins, B.D.; Goncalves, M.D.; Cantley, L.C. Obesity and Cancer Mechanisms: Cancer Metabolism. J. Clin. Oncol. 2016, 34, 4277–4283. [Google Scholar] [CrossRef]
- Nijhawans, P.; Behl, T.; Bhardwaj, S. Angiogenesis in obesity. Biomed. Pharm. 2020, 126, 110103. [Google Scholar] [CrossRef]
- Ljungqvist, O. Insulin Resistance and Outcomes in Surgery. J. Clin. Endocrinol. Metab. 2010, 95, 4217–4219. [Google Scholar] [CrossRef] [PubMed]
- Westerink, N.L.; Nuver, J.; Lefrandt, J.D.; Vrieling, A.H.; Gietema, J.A.; Walenkamp, A.M. Cancer treatment induced metabolic syndrome: Improving outcome with lifestyle. Crit. Rev. Oncol. Hematol. 2016, 108, 128–136. [Google Scholar] [CrossRef]
- Kilany, O.E.; Abdelrazek, H.M.A.; Aldayel, T.S.; Abdo, S.; Mahmoud, M.M.A. Anti-obesity potential of Moringa olifera seed extract and lycopene on high fat diet induced obesity in male Sprauge Dawely rats. Saudi J. Biol. Sci. 2020, 27, 2733–2746. [Google Scholar] [CrossRef] [PubMed]
- Mapfumo, M.; Lembede, B.W.; Ndhlala, A.R.; Chivandi, E. Effect of crude Moringa oleifera Lam. seed extract on the blood markers of metabolic syndrome in high-fructose diet-fed growing Sprague-Dawley rats. J. Complement. Integr. Med. 2019, 17. [Google Scholar] [CrossRef]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Jayson, G.C.; Kerbel, R.; Ellis, L.M.; Harris, A.L. Antiangiogenic therapy in oncology: Current status and future directions. Lancet 2016, 388, 518–529. [Google Scholar] [CrossRef]
- Wang, Z.; Dabrosin, C.; Yin, X.; Fuster, M.M.; Arreola, A.; Rathmell, W.K.; Generali, D.; Nagaraju, G.P.; El-Rayes, B.; Ribatti, D.; et al. Broad targeting of angiogenesis for cancer prevention and therapy. Semin. Cancer Biol. 2015, 35, S224–S243. [Google Scholar] [CrossRef] [PubMed]
- Kumar Gupta, S.; Kumar, B.; Srinivasan, B.P.; Nag, T.C.; Srivastava, S.; Saxena, R.; Aggarwal, A. Retinoprotective effects of Moringa oleifera via antioxidant, anti-inflammatory, and anti-angiogenic mechanisms in streptozotocin-induced diabetic rats. J. Ocul. Pharmacol. Ther. 2013, 29, 419–426. [Google Scholar] [CrossRef]
- Fitch, M.; Ray, S.; Zwetsloot, K.A.; Mowa, C. Moringa oleifera Whole Methanolic Leaf Extract Attenuates Levels of Angiogenic Factors in the Cervix of Preterm Labor Mice Models. FASEB J. 2016, 30, 921–927. [Google Scholar]
- Clayton, N.S.; Ridley, A.J. Targeting Rho GTPase Signaling Networks in Cancer. Front. Cell Dev. Biol. 2020, 8, 222. [Google Scholar] [CrossRef]
- Haga, R.B.; Ridley, A.J. Rho GTPases: Regulation and roles in cancer cell biology. Small GTPases 2016, 7, 207–221. [Google Scholar] [CrossRef]
- Hoang, M.V.; Whelan, M.C.; Senger, D.R. Rho activity critically and selectively regulates endothelial cell organization during angiogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 1874–1879. [Google Scholar] [CrossRef]
- Rundhaug, J.E. Matrix metalloproteinases and angiogenesis. J. Cell Mol. Med. 2005, 9, 267–285. [Google Scholar] [CrossRef]
- Vargas-Sanchez, K.; Garay-Jaramillo, E.; Gonzalez-Reyes, R.E. Effects of Moringa oleifera on Glycaemia and Insulin Levels: A Review of Animal and Human Studies. Nutrients 2019, 11, 2907. [Google Scholar] [CrossRef]
- Gallagher, E.J.; LeRoith, D. Hyperinsulinaemia in cancer. Nat. Rev. Cancer 2020, 20, 629–644. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.B.; Figueroa, A.; Pulido, E.G.; Campelo, R.G.; Aparicio, L.A. Potential role of sugar transporters in cancer and their relationship with anticancer therapy. Int. J. Endocrinol. 2010, 2010, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Gao, X.; Mao, X.; Shi, Z.; Zhu, B.; Xie, L.; Di, S.; Jin, L. Knockdown of FOXO6 Inhibits Glycolysis and Reduces Cell Resistance to Paclitaxel in HCC Cells via PI3K/Akt Signaling Pathway. Onco Targets Ther. 2020, 13, 1545–1556. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.O.; Sanchez-Ramos, C.; Prieto-Arroyo, I.; Urbanek, P.; Steinbrenner, H.; Monsalve, M. Redox regulation of FoxO transcription factors. Redox Biol. 2015, 6, 51–72. [Google Scholar] [CrossRef]
- Ambrosone, C.B.; Zirpoli, G.R.; Hutson, A.D.; McCann, W.E.; McCann, S.E.; Barlow, W.E.; Kelly, K.M.; Cannioto, R.; Sucheston-Campbell, L.E.; Hershman, D.L.; et al. Dietary Supplement Use During Chemotherapy and Survival Outcomes of Patients With Breast Cancer Enrolled in a Cooperative Group Clinical Trial (SWOG S0221). J. Clin. Oncol. 2020, 38, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Wolf, C.; Rachow, T.; Ernst, T.; Hochhaus, A.; Zomorodbakhsch, B.; Foller, S.; Rengsberger, M.; Hartmann, M.; Hubner, J. Interactions in cancer treatment considering cancer therapy, concomitant medications, food, herbal medicine and other supplements. J. Cancer Res. Clin. Oncol. 2021, 1–13. [Google Scholar] [CrossRef]
- Zheng, C.C.; Hu, H.F.; Hong, P.; Zhang, Q.H.; Xu, W.W.; He, Q.Y.; Li, B. Significance of integrin-linked kinase (ILK) in tumorigenesis and its potential implication as a biomarker and therapeutic target for human cancer. Am. J. Cancer Res. 2019, 9, 186–197. [Google Scholar]
- Ponzetti, M.; Capulli, M.; Angelucci, A.; Ventura, L.; Monache, S.D.; Mercurio, C.; Calgani, A.; Sanita, P.; Teti, A.; Rucci, N. Non-conventional role of haemoglobin beta in breast malignancy. Br. J. Cancer 2017, 117, 994–1006. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Miyamoto, D.T.; Wittner, B.S.; Sullivan, J.P.; Aceto, N.; Jordan, N.V.; Yu, M.; Karabacak, N.M.; Comaills, V.; Morris, R.; et al. Expression of beta-globin by cancer cells promotes cell survival during blood-borne dissemination. Nat. Commun. 2017, 8, 14344. [Google Scholar] [CrossRef]
- Lyon (FR): International Agency for Research on Cancer. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Pharmaceuticals. Available online: https://www.ncbi.nlm.nih.gov/books/NBK304334/ (accessed on 8 July 2021).
- Showande, S.J.; Fakeye, T.O.; Kajula, M.; Hokkanen, J.; Tolonen, A. Potential inhibition of major human cytochrome P450 isoenzymes by selected tropical medicinal herbs-Implication for herb-drug interactions. Food Sci. Nutr. 2019, 7, 44–55. [Google Scholar] [CrossRef]
- Fantoukh, O.I.; Albadry, M.A.; Parveen, A.; Hawwal, M.F.; Majrashi, T.; Ali, Z.; Khan, S.I.; Chittiboyina, A.G.; Khan, I.A. Isolation, synthesis, and drug interaction potential of secondary metabolites derived from the leaves of miracle tree (Moringa oleifera) against CYP3A4 and CYP2D6 isozymes. Phytomedicine 2019, 60, 153010. [Google Scholar] [CrossRef] [PubMed]
- Helsby, N.; Yong, M.; Burns, K.; Findlay, M.; Porter, D. Cyclophosphamide bioactivation pharmacogenetics in breast cancer patients. Cancer Chemother. Pharmacol. 2021, 88, 533–542. [Google Scholar] [CrossRef]
- Huang, Z.; Waxman, D.J. Modulation of cyclophosphamide-based cytochrome P450 gene therapy using liver P450 inhibitors. Cancer Gene Ther. 2001, 8, 450–458. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Renaud, H.J.; Cui, J.Y.; Khan, M.; Klaassen, C.D. Tissue distribution and gender-divergent expression of 78 cytochrome P450 mRNAs in mice. Toxicol. Sci. 2011, 124, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Antona, C.; Ingelman-Sundberg, M. Cytochrome P450 pharmacogenetics and cancer. Oncogene 2006, 25, 1679–1691. [Google Scholar] [CrossRef]
- Sneha, S.; Baker, S.C.; Green, A.; Storr, S.; Aiyappa, R.; Martin, S.; Pors, K. Intratumoural Cytochrome P450 Expression in Breast Cancer: Impact on Standard of Care Treatment and New Efforts to Develop Tumour-Selective Therapies. Biomedicines 2021, 9, 290. [Google Scholar] [CrossRef]
- Afsharian, P.; Terelius, Y.; Hassan, Z.; Nilsson, C.; Lundgren, S.; Hassan, M. The effect of repeated administration of cyclophosphamide on cytochrome P450 2B in rats. Clin. Cancer Res. 2007, 13, 4218–4224. [Google Scholar] [CrossRef] [PubMed]
- Factors, F.A. Global Moringa Products Market Anticipates To Reach USD 8400 Million by 2026. Available online: https://www.fnfresearch.com/news/global-moringa-products-market-anticipates-to-reach-the (accessed on 8 July 2021).

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zunica, E.R.M.; Yang, S.; Coulter, A.; White, C.; Kirwan, J.P.; Gilmore, L.A. Moringa Oleifera Seed Extract Concomitantly Supplemented with Chemotherapy Worsens Tumor Progression in Mice with Triple Negative Breast Cancer and Obesity. Nutrients 2021, 13, 2923. https://doi.org/10.3390/nu13092923
Zunica ERM, Yang S, Coulter A, White C, Kirwan JP, Gilmore LA. Moringa Oleifera Seed Extract Concomitantly Supplemented with Chemotherapy Worsens Tumor Progression in Mice with Triple Negative Breast Cancer and Obesity. Nutrients. 2021; 13(9):2923. https://doi.org/10.3390/nu13092923
Chicago/Turabian StyleZunica, Elizabeth R. M., Shengping Yang, Ann Coulter, Christy White, John P. Kirwan, and Linda A. Gilmore. 2021. "Moringa Oleifera Seed Extract Concomitantly Supplemented with Chemotherapy Worsens Tumor Progression in Mice with Triple Negative Breast Cancer and Obesity" Nutrients 13, no. 9: 2923. https://doi.org/10.3390/nu13092923
APA StyleZunica, E. R. M., Yang, S., Coulter, A., White, C., Kirwan, J. P., & Gilmore, L. A. (2021). Moringa Oleifera Seed Extract Concomitantly Supplemented with Chemotherapy Worsens Tumor Progression in Mice with Triple Negative Breast Cancer and Obesity. Nutrients, 13(9), 2923. https://doi.org/10.3390/nu13092923





