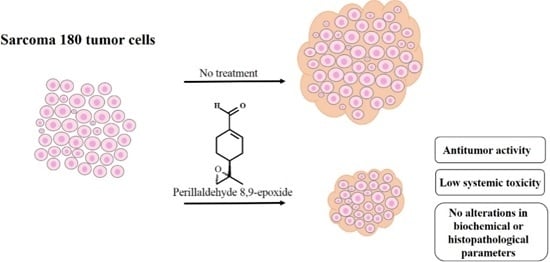
In Vivo Anti-Tumor Activity and Toxicological Evaluations of Perillaldehyde 8,9-Epoxide, a Derivative of Perillyl Alcohol
Abstract
:1. Introduction
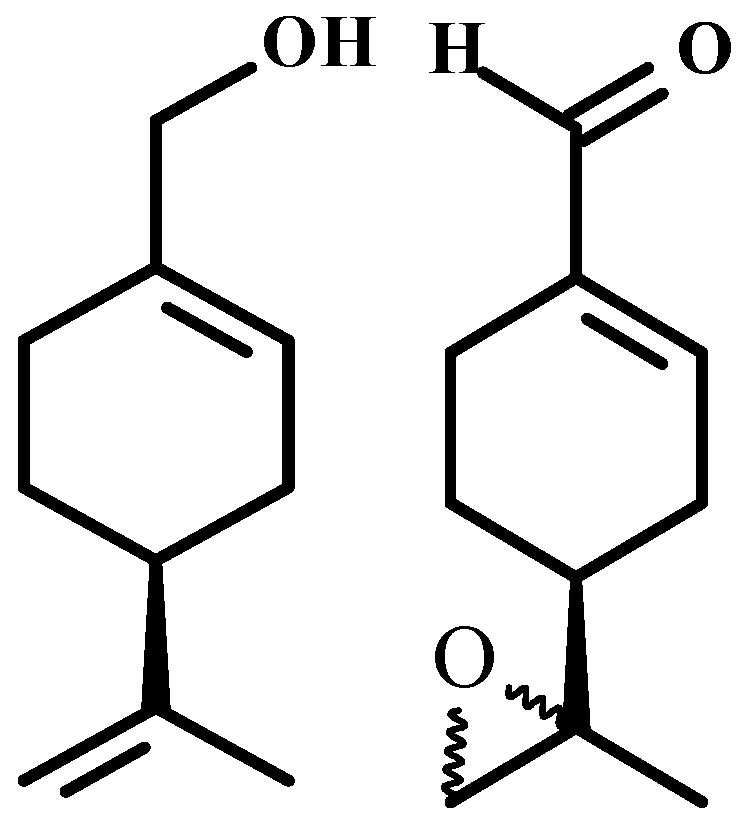
2. Results and Discussion
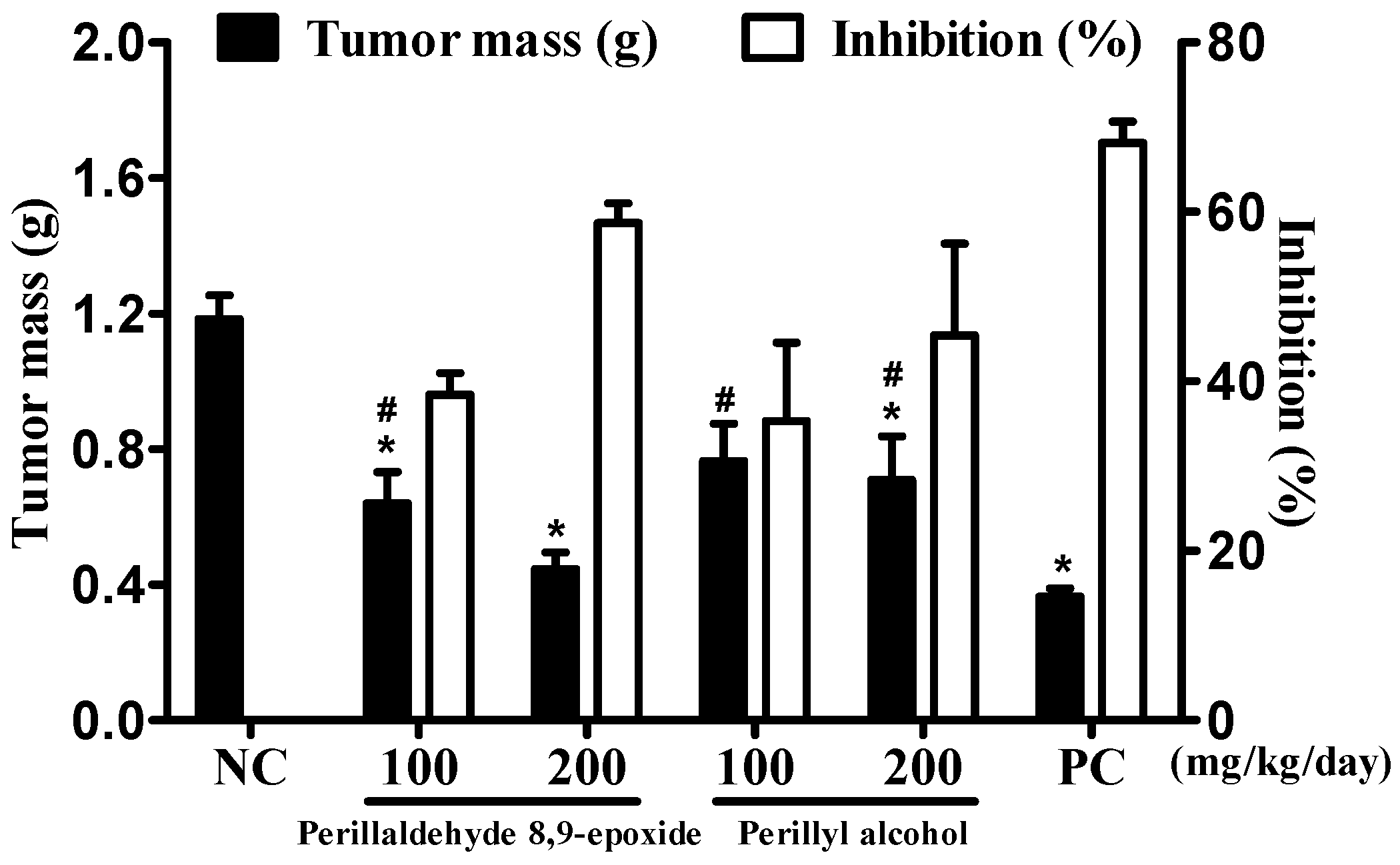
2.1. In Vivo Antitumor Evaluation of Perillaldehyde 8,9-Epoxide
2.2. Systemic Toxicological Evaluation
| Treatment | Dose (mg/kg/Day) | Variation Body in Mass (g) | Liver (g/100 g Body Mass) | Spleen (g/100 g Body Mass) | Kidney (g/100 g Body Mass) |
|---|---|---|---|---|---|
| Heathy mice | |||||
| Negative control (5% DMSO) | – | 0.14 ± 0.08 | 4.76 ± 0.13 | 0.36 ± 0.01 | 1.32 ± 0.05 |
| Positive control | 25 | −3.50 ± 0.66 * | 4.65 ± 0.21 | 0.19 ± 0.02 * | 1.13 ± 0.03 |
| Perillaldehyde | 100 | 0.90 ± 0.38 | 4.66 ± 0.09 | 0.25 ± 0.01 | 1.15 ± 0.06 |
| 8,9-epoxide | 200 | −0.71 ± 0.53 | 5.07 ± 0.13 | 0.37 ± 0.06 | 1.17 ± 0.19 |
| Mice with tumor S-180 | |||||
| Negative control (5% DMSO) | – | 0.61 ± 0.20 | 4.63 ± 0.09 | 0.49 ± 0.03 | 1.14 ± 0.02 |
| Positive control | 25 | −4.46 ± 0.83 # | 4.99 ± 0.16 | 0.33 ± 0.02 # | 1.15 ± 0.04 |
| Perillaldehyde | 100 | 1.11 ± 0.19 | 4.84 ± 0.07 | 0.52 ± 0.02 | 1.27 ± 0.03 |
| 8,9-epoxide | 200 | −1.58 ± 0.33 | 4.57 ± 0.09 | 0.49 ± 0.04 | 1.28 ± 0.03 |
| Drug | Dose (mg/kg/Day) | AST (U/L) | ALT (U/L) | Urea (mg/dL) | Creatinine (mg/dL) |
|---|---|---|---|---|---|
| Healthy mice | |||||
| Negative control (5% DMSO) | – | 82.5 ± 3.48 | 52.50 ± 4.80 | 64.75 ± 4.31 | 0.39 ± 0.01 |
| Positive control | 25 | 102.0 ± 15.06 | 47.25 ± 2.8 | 49.00 ± 5.86 | 0.37 ± 0.02 |
| Perillaldehyde | 100 | 100.5 ± 5.97 | 55.25 ± 6.26 | 54.25 ± 3.82 | 0.34 ± 0.01 |
| 8,9-epoxide | 200 | 78.75 ± 3.17 | 47.75 ± 4.64 | 41.00 ± 4.42 | 0.34 ± 0.01 |
| Mice with S-180 tumor cells | |||||
| Negative control (5% DMSO) | – | 181.2 ± 12.6 | 34.6 ± 1.5 | 55.20 ± 1.81 | 0.34 ± 0.02 |
| Positive control | 25 | 164.4 ± 10.0 | 42.8 ± 0.6 | 50.71 ± 2.18 | 0.28 ± 0.09 |
| Perillaldehyde | 100 | 178.0 ± 5.0 | 35.0 ± 2.2 | 43.0 ± 1.08 | 0.34 ± 0.01 |
| 8,9-epoxide | 200 | 208.3 ± 20.6 | 36.0 ± 3.5 | 47.80 ± 2.01 | 0.33 ± 0.02 |
| Treatment | Dose (mg/kg/Day) | Total Leukocytes (103 Cells/µL) | Differential Count of Leukocytes (%) | |||
|---|---|---|---|---|---|---|
| Eosinophil | Lymphocyte | Neutrophil | Monocyte | |||
| Healthy mice | ||||||
| Negative control (5% DMSO) | - | 7.78 ± 0.98 | 0 | 72.5 ± 2.08 | 25.8 ± 5.43 | 1.7 ± 0.64 |
| Positive control | 25 | 2.37 ± 0.77 * | 0 | 95.8 ± 0.70 * | 3.0 ± 0.70 * | 1.2 ± 0.70 |
| Perillaldehyde | 100 | 3.12 ± 0.38 * | 0 | 86.0 ± 1.68 * | 12.5 ± 1.08 * | 1.5 ± 0.86 |
| 8,9-epoxide | 200 | 2.62 ± 0.47 * | 0 | 89.5 ± 0.65 * | 9.2 ± 0.85 * | 1.3 ± 0.25 |
| Mice with S-180 tumor | ||||||
| Negative control (5% DMSO) | - | 11.20 ± 1.15 | 0 | 54.2 ± 2.61 | 44.6 ± 1.69 | 1.2 ± 0.58 |
| Positive control | 25 | 2.50 ± 0.32 # | 0 | 89.0 ± 0.70 # | 10.2 ± 0.54 # | 0.8 ± 0.37 |
| Perillaldehyde | 100 | 5.30 ± 0.37 # | 0 | 73.2 ± 2.17 # | 26.2 ± 5.92 # | 0.6 ± 0.40 |
| 8,9-epoxide | 200 | 6.50 ± 0.65 # | 0 | 80.6 ± 2.54 # | 18.6 ± 6.18 # | 0.8 ± 0.34 |
2.2.1. The Effect of Perillaldehyde 8,9-Epoxide on Biochemical Parameters
2.2.2. The Effect of Perillaldehyde 8,9-Epoxide on Hematological Parameters
2.2.3. Histopathological Analyses
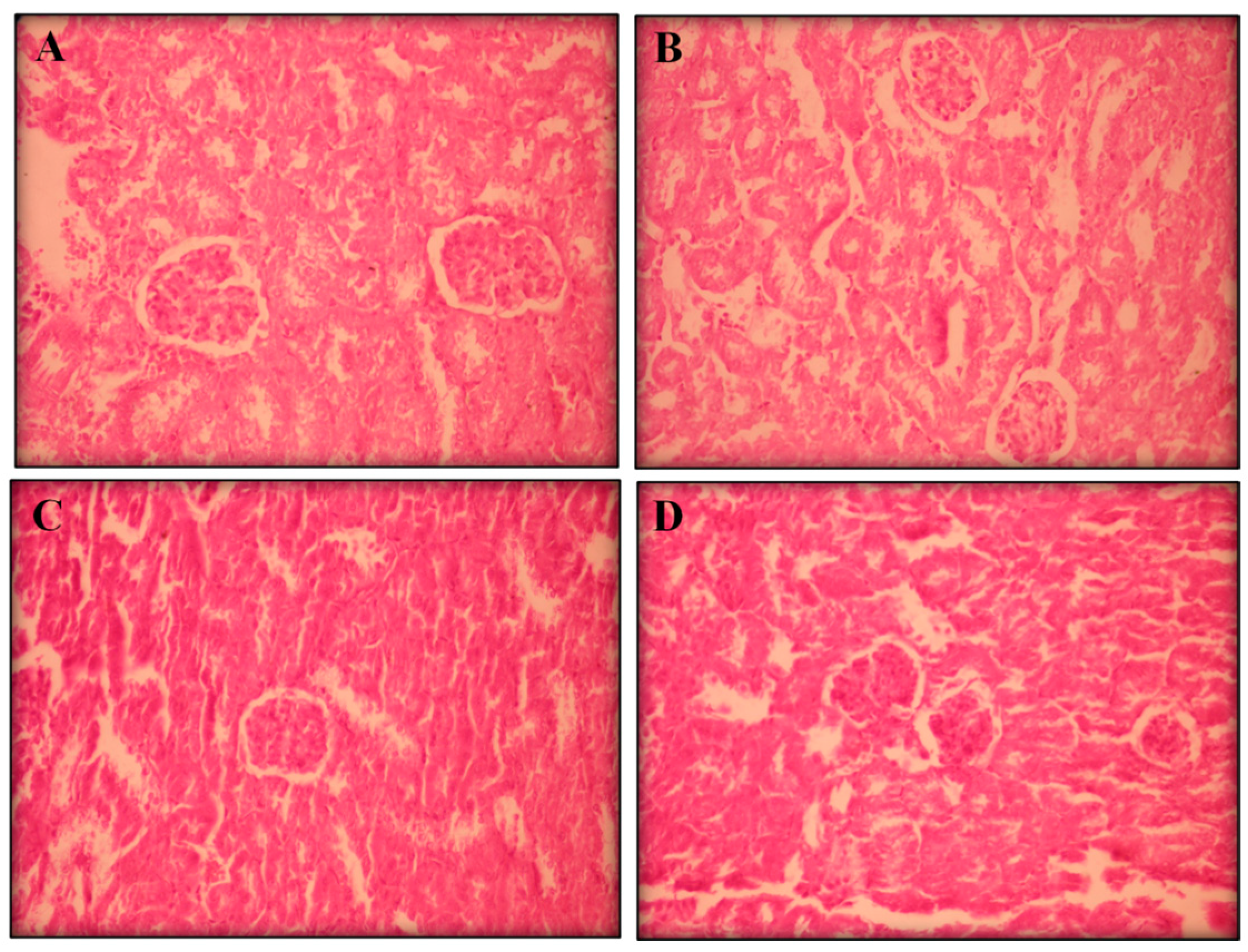
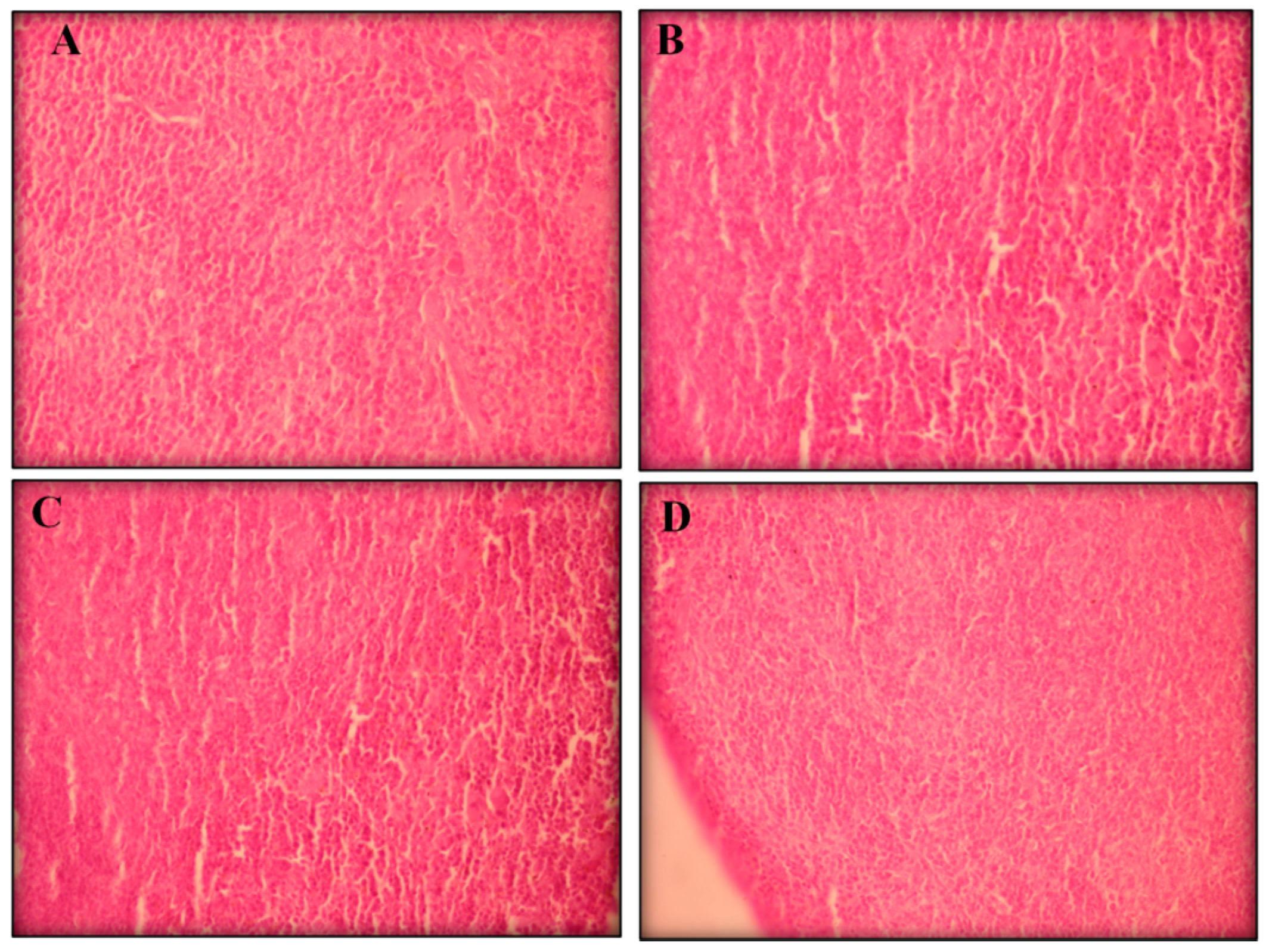
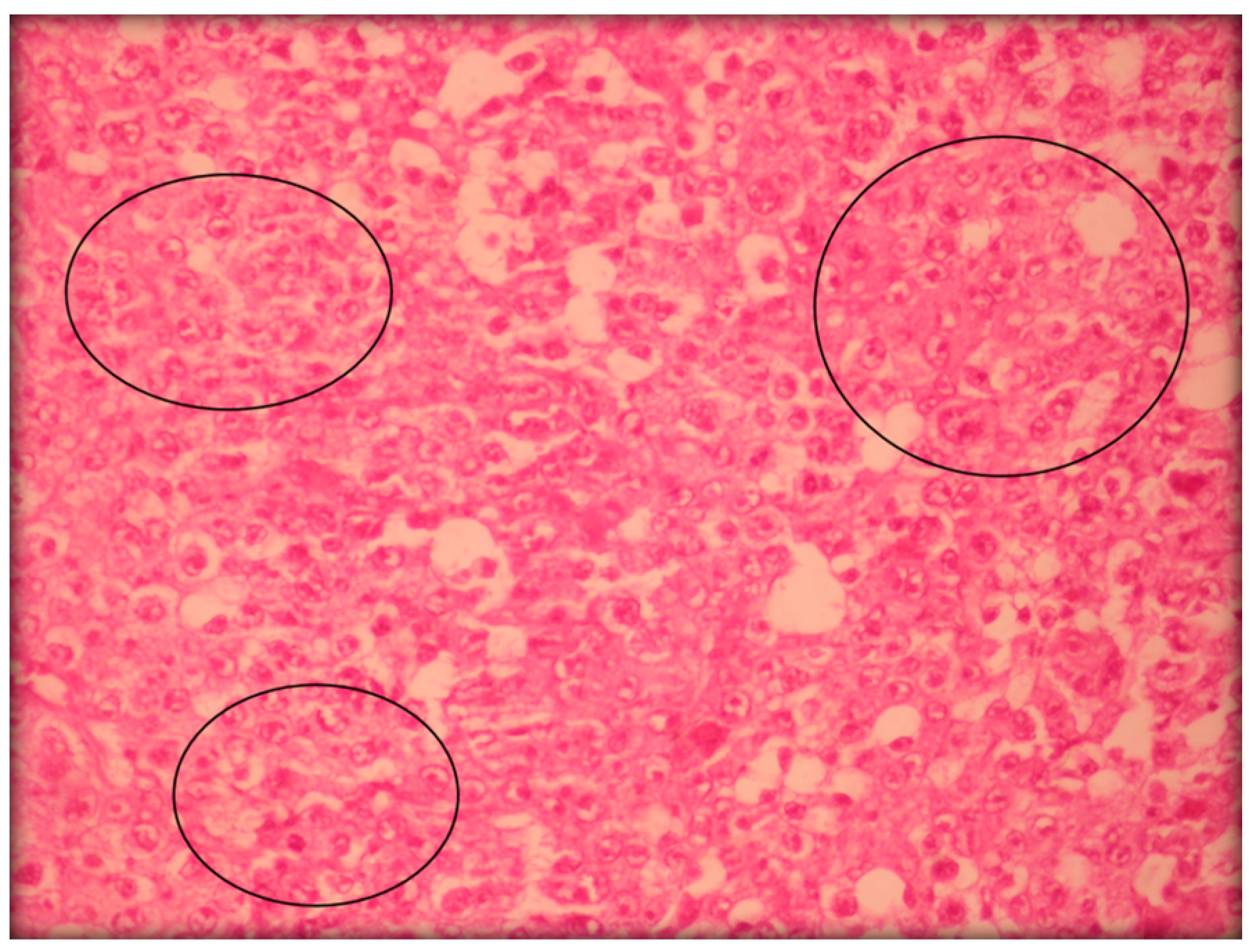
3. Experimental Section
3.1. Drug
3.2. Animals
3.3. In Vivo Antitumor Activity Assay
3.3.1. Determination of the Effect of Perillaldehyde 8,9-Epoxide on in Vivo Tumor Growth
3.3.2. Systemic Toxicological Assessment
Determination of the Effect of Perillaldehyde 8,9-Epoxide on Body and Organ Weight
Determination of the Effect of Perillaldehyde 8,9-Epoxide on Biochemical Parameters
Determination of the Effect of Perillaldehyde 8,9-Epoxide on Hematological Parameters
Histopathological Analyses
Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in Globocan 2012. Int. J. Cancer 2015, 136, 359–386. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Carvalho, A.A.; Andrade, L.N.; de Sousa, E.B.V.; de Sousa, D.P. Antitumor Phenylpropanoids Found in Essential Oils. Biomed. Res. Int. 2015, 1. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Yook, S.; Yhee, J.Y.; Yoon, H.Y.; Kim, M.G.; Ku, S.H.; Kim, S.H.; Park, J.H.; Jeong, J.H.; Kwon, I.C.; et al. Co-delivery of VEGF and Bcl-2 dual-targeted siRNA polymer using a single nanoparticle for synergistic anti-cancer effects in vivo. J. Control. Release 2015. [Google Scholar] [CrossRef] [PubMed]
- Karnati, H.K.; Yalagala, R.S.; Undi, R.; Pasupuleti, S.R.; Gutti, R.K. Therapeutic potential of siRNA and DNAzymes in cancer. Tumour Biol. 2014, 35, 9505–9521. [Google Scholar] [CrossRef] [PubMed]
- Gurnani, N.; Mehta, D.; Gupta, M.; Mehta, B.K. Natural Products: Source of Potential Drugs. Afr. J. Basic Appl. Sci. 2014, 6, 171–186. [Google Scholar]
- Lahlou, M. The Success of Natural Products in Drug Discovery. Pharmacol. Pharm. 2013, 4, 17–31. [Google Scholar] [CrossRef]
- Prakash, O.M.; Kumar, A.; Kumar, P. Anticancer potential of plants and natural products: A review. Am. J. Pharmacol. Sci. 2013, 6, 104–115. [Google Scholar] [CrossRef]
- De Sousa, D.P. Bioactive Essential Oils and Cancer, 1st ed.; Springer International Publishing: New York, NY, USA, 2015; p. 292. [Google Scholar]
- Thoppil, R.J.; Bishayee, A. Terpenoids as potential chemopreventive and therapeutic agents in liver cancer. World J. Hepatol. 2011, 3, 228–249. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.N.; Batista, J.S.; de Sousa, D.P. Spasmolytic activity of p-menthane esters. J. Med. Plant Res. 2011, 5, 6995–6999. [Google Scholar]
- Jayakumar, S.; Madankumar, A.; Asokkumar, S.; Raghunandhakumar, S.; Gokula, K.; Kamaraj, S.; Divya, M.G.; Devaki, T. Potential preventive effect of carvacrol against diethylnitrosamineinduced hepatocellular carcinoma in rats. Mol. Cell. Biochem. 2012, 360, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Jaafari, A.; Tilaoui, M.; Mouse, H.A.; M’barkI, L.A.; Aboufatima, I.R.; Chait, A.; Lepoivre, M.; Zyad, A. Comparative study of the antitumor effect of natural monoterpenes: Relationship to cell cycle analysis. Braz. J. Pharmacogn. 2012, 22, 534–540. [Google Scholar] [CrossRef]
- Deb, D.D.; Parimala, G.; Devi, S.; Chakraborty, T. Effect of thymol on peripheral blood mononuclear cell PBMC and acute promyelotic cancer cell line HL-60. Chem. Biol. Interact. 2011, 193, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Elegbede, J.A.; Elson, C.E.; Qureshi, A.; Tanner, M.A.; Gould, M.N. Inhibition of DMBA-induced mammary cancer by the monoterpene d-limonene. Carcinogenesis 1984, 5, 661–664. [Google Scholar] [CrossRef] [PubMed]
- Yeruva, L.; Pierre, K.J.; Elegbede, A.; Wang, R.C.; Carper, S.W. Perillyl alcohol and perillic acid induced cell cycle arrest and apoptosis in non-small cell lung cancer cells. Cancer Lett. 2007, 257, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Samaila, D.; Toy, B.J.; Wang Robert, C.; Elegbede, A. Monoterpenes enhanced the sensitivity of head and neck cancer cells to radiation treatment in vitro. Anticancer Res. 2004, 24, 3089–3095. [Google Scholar] [PubMed]
- Sonboli, A.; Esmaeili, M.A.; Gholipour, A.; Kanani, M. Composition, cytotoxicity and antioxidant activity of the essential oil of Dracocephalum surmandinum from Iran. Nat. Prod. Commun. 2010, 5, 341–344. [Google Scholar] [PubMed]
- Koyama, M.; Sowa, Y.; Hitomi, T.; Iizumi, Y.; Watanabe, M.; Taniguchi, T.; Ichikawa, M.; Sakai, T. Perillyl alcohol causes G1 arrest through p15INK4b and p21 WAF1/Cip1 induction. Oncol. Rep. 2013, 29, 779–784. [Google Scholar] [PubMed]
- Stark, M.J.; Burke, Y.D.; McKinzie, J.H.; Ayoubi, S.A.; Crowell, P.L. Chemotherapy of pancreatic cancer with the monoterpene perillyl alcohol. Cancer Lett. 1995, 96, 15–21. [Google Scholar] [CrossRef]
- Ripple, G.H.; Gould, M.N.; Arzoomanian, R.Z.; Alberti, D.; Feierabend, C.; Simon, K.; Binger, K.; Tutsch, K.D.; Pomplun, M.; Wahamaki, A.; et al. Phase I clinical and pharmacokinetic study of perillyl alcohol administered four times a day. Clin. Cancer Res. 2000, 6, 390–396. [Google Scholar] [PubMed]
- Meadows, S.M.; Mulkerin, D.; Berlin, J.; Bailey, H.; Kolesar, J.; Warren, D.; Thomas, J.P. Phase II trial of perillyl alcohol in patients with metastatic colorectal cancer. Int. J. Gastrointest. Cancer 2002, 32, 125–128. [Google Scholar] [CrossRef]
- Sobral, M.V.; Xavier, A.L.; Lima, T.C.; de Sousa, D.P. Antitumor activity of monoterpenes found in essential oils. Sci. World J. 2014. [Google Scholar] [CrossRef]
- Loutrari, H.; Skouridou, V.; Kolisis, F.N.; Roussos, C.; Papapetropoulos, A. Perillyl alcohol attenuates in vitro angiogenesis, modulates angiogenic factor production and inhibits cell proliferation and survival in endothelial and tumour cells. J. Pharmacol. Exp. Ther. 2004, 18, 30–32. [Google Scholar]
- Burke, Y.D.; Stark, M.J.; Roach, M.S.L.; Sen, S.E.; Crowell, P.L. Inhibition of pancreatic cancer growth by the dietary isoprenoids farnesol and geraniol. Lipids 1997, 32, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Stayrook, K.R.; Mckinzie, J.H.; Barbhaiya, L.H.; Crowell, P.L. Effects of the antitumor agent perillyl alcohol on H-Ras vs. K-Ras farnesylation and signal transduction in pancreatic cells. Anticancer Res. 1998, 18, 823–828. [Google Scholar] [PubMed]
- Sundin, T.; Peffley, D.M.; Gauthier, D.; Hentosh, P. The isoprenoid perillyl alcohol inhibits telomerase activity in prostate cancer cells. Biochimie 2012, 94, 2639–2648. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.N.; Lima, T.C.; Amaral, R.G.; Pessoa, C.O.; Moraes Filho, M.O.; Soares, B.M.; Nascimento, L.G.; Carvalho, A.A.; de Sousa, D.P. Evaluation of the cytotoxicity of structurally correlated p-menthane derivatives. Molecules 2015, 20, 13264–13280. [Google Scholar] [CrossRef] [PubMed]
- Suffness, M.; Pezzuto, J.M. Methods in Plant Biochemistry: Assays for Bioactivity; Academic Press Inc.: London, UK, 1991; Volume 6, pp. 71–133. [Google Scholar]
- Da Fonseca, C.O.; Schwartsmann, G.; Fischer, J.; Nagel, J.; Futuro, D.; Quirico-Santos, T.; Gattass, C.R. Preliminary results from a phase I/II study of perillyl alcohol intranasal administration in adults with recurrent malignant gliomas. Surg. Neurol. 2008, 70, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T. Cancer therapy with natural products andmedicinal plants. Planta Medica 2010, 76, 1035–1036. [Google Scholar] [CrossRef] [PubMed]
- El-Sayyad, H.I.; Ismail, M.F.; Shalaby, F.M.; Abou-El-Magd, R.F.; Gaur, R.L.; Fernando, A.; Raj, M.H.G.; Ouhtit, A. Histopathological effects of cisplatin, doxorubicin and 5-flurouracil (5-FU) on the liver of male albino rats. Int. J. Biol. Sci. 2009, 5, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Tayarani-Najaran, Z.; Talasaz-Firoozi, E.; Nasiri, R.; Jalali, N.; Hassanzadeh, M.K. Antiemetic activity of volatile oil from Mentha spicata and Mentha × piperita in chemotherapy-induced nausea and vomiting. Ecancemedicalscience 2013, 7, 290–297. [Google Scholar]
- Bezerra, D.P.; de Castro, F.O.; Alves, A.P.; Pessoa, C.; de Moraes, M.O.; Silveira, E.R.; Lima, M.A.; Elmiro, F.J.; de Alencar, N.M.; Mesquita, R.O. In vitro and in vivo antitumor effect of 5-FU combined with piplartine and piperine. J. Appl. Toxicol. 2008, 28, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Mincis, M. Gastroenterologia e Hepatologia—Diagnóstico e Tratamento, 4th ed.; Editora Casa Leitura Médica: São Paulo, Brazil, 2008; p. 1273. [Google Scholar]
- Cao, X.; Cai, R.; Ju, D.W.; Tao, Q.; Yu, Y.; Wang, J. Augmentation of hematopoiesis by fibroblast-mediated interleukin-6 gene therapy in mice with chemotherapy. J. Interferon Cytokine Res. 1998, 18, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Zamagni, C.; Martoni, A.; Cacciari, N.; Gentile, A.; Pannuti, F. The combination of paclitaxel and carboplatin as first-line chemotherapy in patients with stage III and stage IV ovarian cancer: A phase I-II study. Am. J. Clin. Oncol. 1998, 21, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Kido, F.; Abiko, T.; Kato, M. Spiroannulation by the [2,3]sigmatropic rearrangement via the cyclic allylsulfonium ylide. A stereoselective synthesis of (+)-acorenone B. J. Chem. Soc. Perkin Trans. 1992, 2, 229–233. [Google Scholar] [CrossRef]
- Bezerra, D.P.; Castro, F.O.; Alves, A.P.; Pessoa, C.; Moraes, M.O.; Silveira, E.R.; Lima, M.A.S.; Elmiro, F.J.M.; Costa-Lotufo, L.V. In vivo growth-inhibition of sarcoma 180 by piplartine and peperine, two alkaloid amides from piper. Braz. J. Med. Biol. Res. 2006, 39, 801–807. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, L.N.; Amaral, R.G.; Dória, G.A.A.; Fonseca, C.S.; Da Silva, T.K.M.; Albuquerque Júnior, R.L.C.; Thomazzi, S.M.; Do Nascimento, L.G.; Carvalho, A.A.; De Sousa, D.P. In Vivo Anti-Tumor Activity and Toxicological Evaluations of Perillaldehyde 8,9-Epoxide, a Derivative of Perillyl Alcohol. Int. J. Mol. Sci. 2016, 17, 32. https://doi.org/10.3390/ijms17010032
Andrade LN, Amaral RG, Dória GAA, Fonseca CS, Da Silva TKM, Albuquerque Júnior RLC, Thomazzi SM, Do Nascimento LG, Carvalho AA, De Sousa DP. In Vivo Anti-Tumor Activity and Toxicological Evaluations of Perillaldehyde 8,9-Epoxide, a Derivative of Perillyl Alcohol. International Journal of Molecular Sciences. 2016; 17(1):32. https://doi.org/10.3390/ijms17010032
Chicago/Turabian StyleAndrade, Luciana Nalone, Ricardo Guimarães Amaral, Grace Anne Azevedo Dória, Cecília Santos Fonseca, Tayane Kayane Mariano Da Silva, Ricardo Luiz Cavalcante Albuquerque Júnior, Sara Maria Thomazzi, Lázaro Gomes Do Nascimento, Adriana Andrade Carvalho, and Damião Pergentino De Sousa. 2016. "In Vivo Anti-Tumor Activity and Toxicological Evaluations of Perillaldehyde 8,9-Epoxide, a Derivative of Perillyl Alcohol" International Journal of Molecular Sciences 17, no. 1: 32. https://doi.org/10.3390/ijms17010032
APA StyleAndrade, L. N., Amaral, R. G., Dória, G. A. A., Fonseca, C. S., Da Silva, T. K. M., Albuquerque Júnior, R. L. C., Thomazzi, S. M., Do Nascimento, L. G., Carvalho, A. A., & De Sousa, D. P. (2016). In Vivo Anti-Tumor Activity and Toxicological Evaluations of Perillaldehyde 8,9-Epoxide, a Derivative of Perillyl Alcohol. International Journal of Molecular Sciences, 17(1), 32. https://doi.org/10.3390/ijms17010032