Recent Advances and Challenges in Nanodelivery Systems for Antimicrobial Peptides (AMPs)
Abstract
1. Introduction
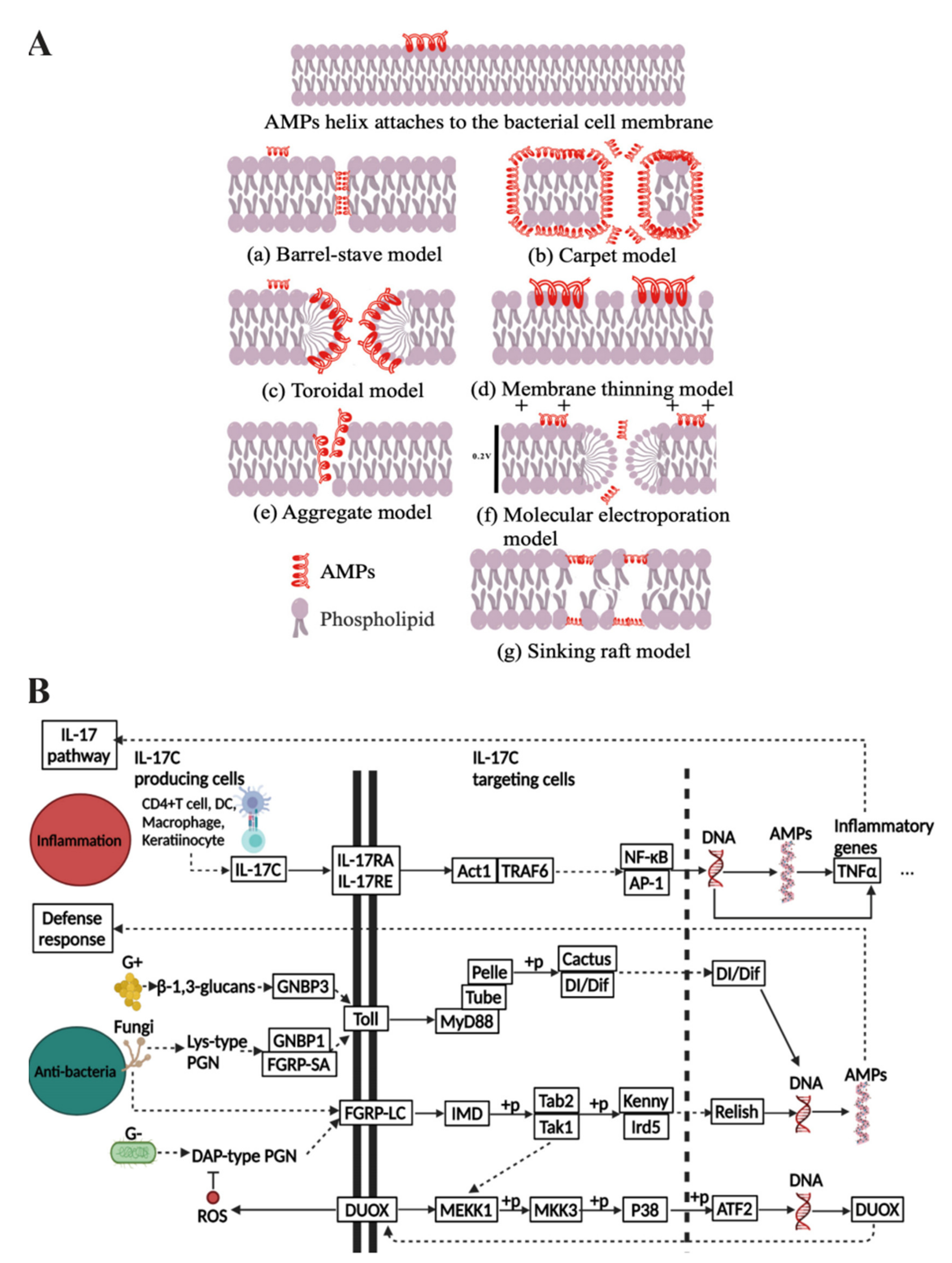
1.1. The Medical Value of AMPs and Their Mechanisms of Bioactivity
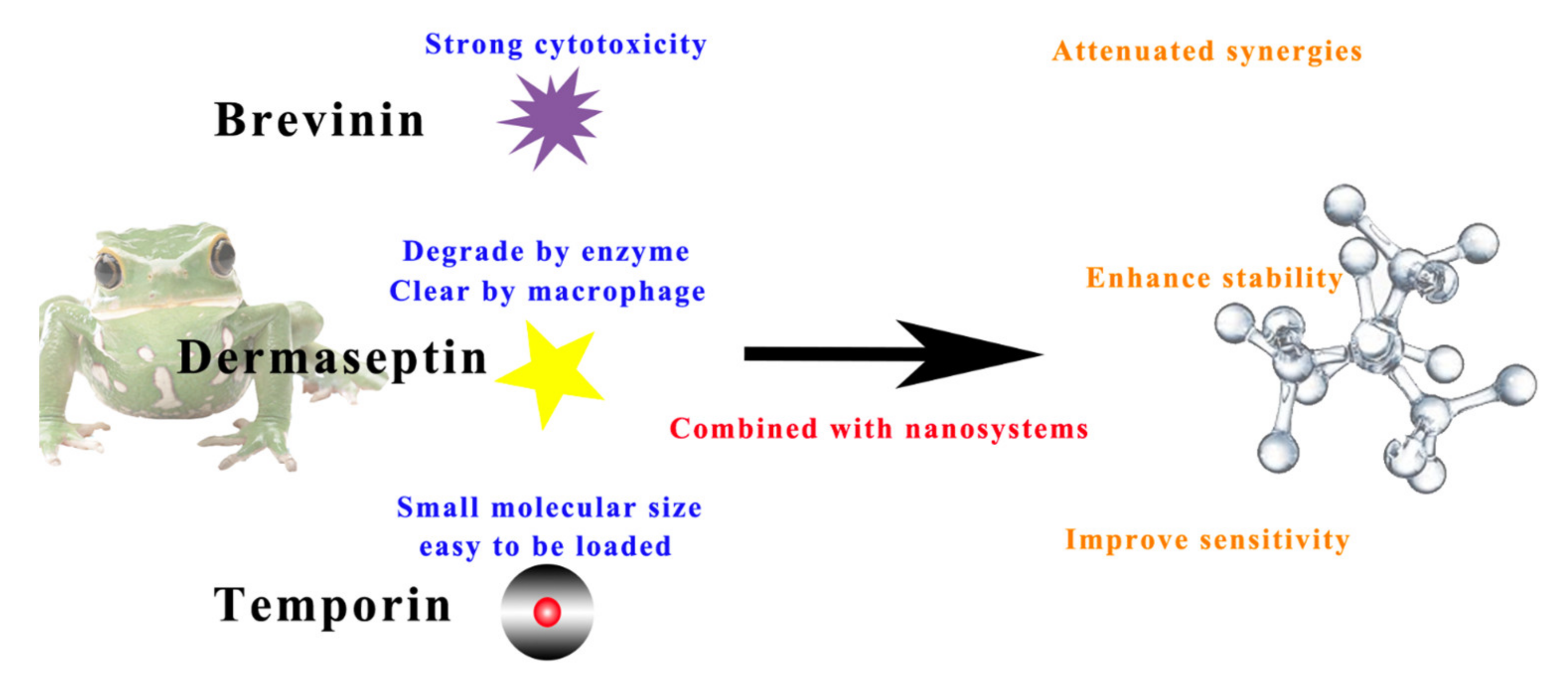
1.2. The Challenges of AMP Delivery and the Advantages of Combining with Nanosystems
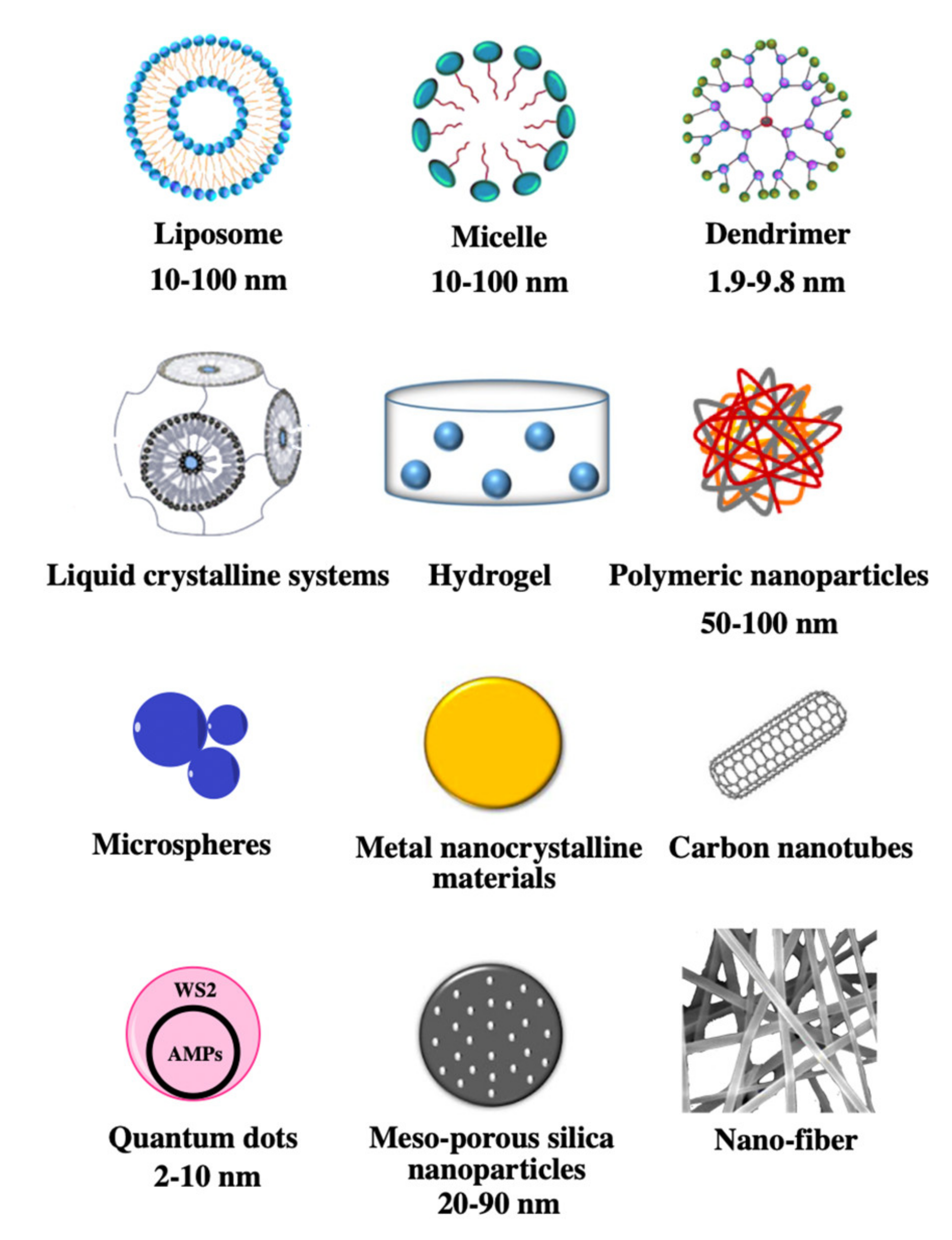
2. Advances in Nanosystems for AMPs Delivery
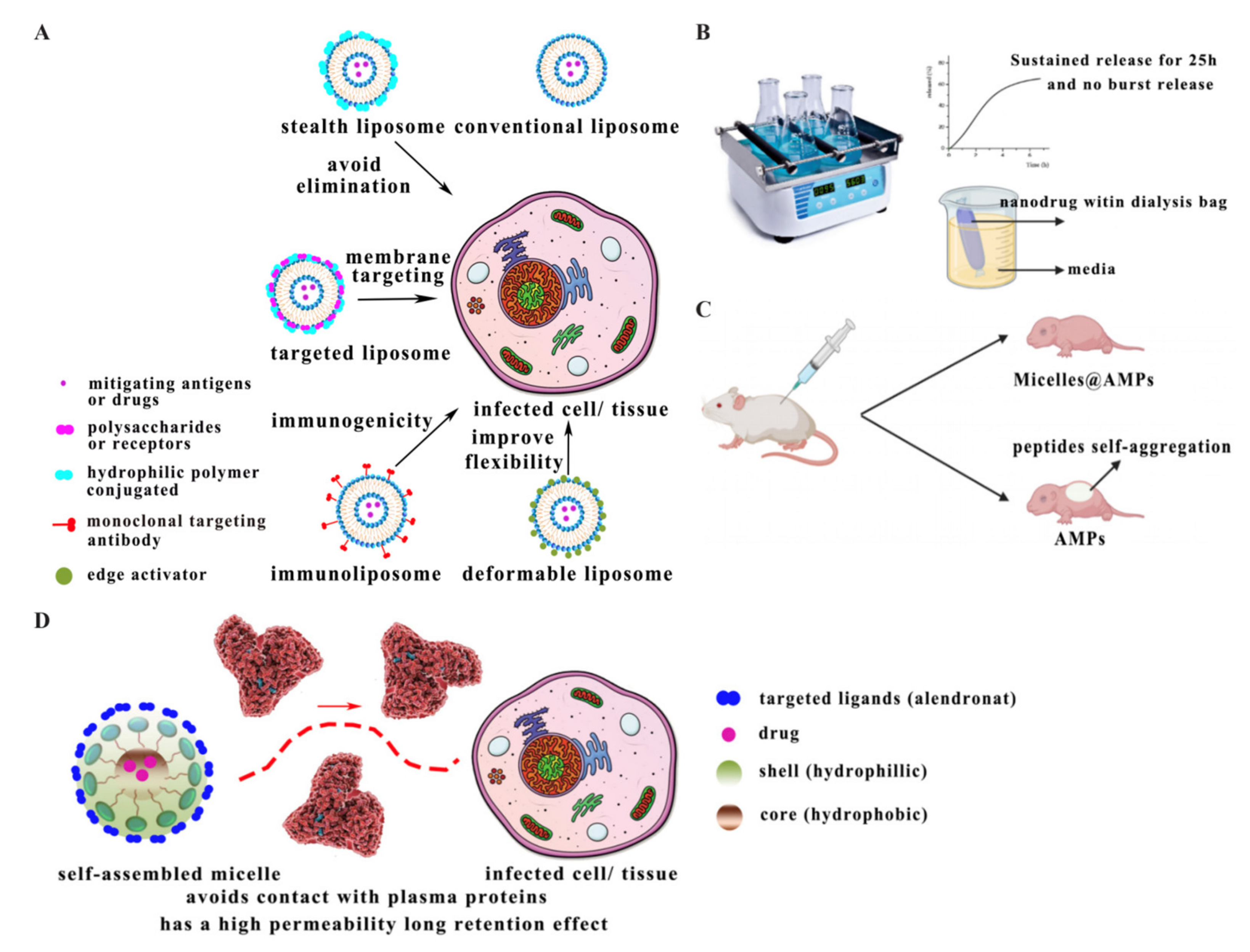
2.1. Liposomes
2.2. Micelles
2.3. Dendrimers
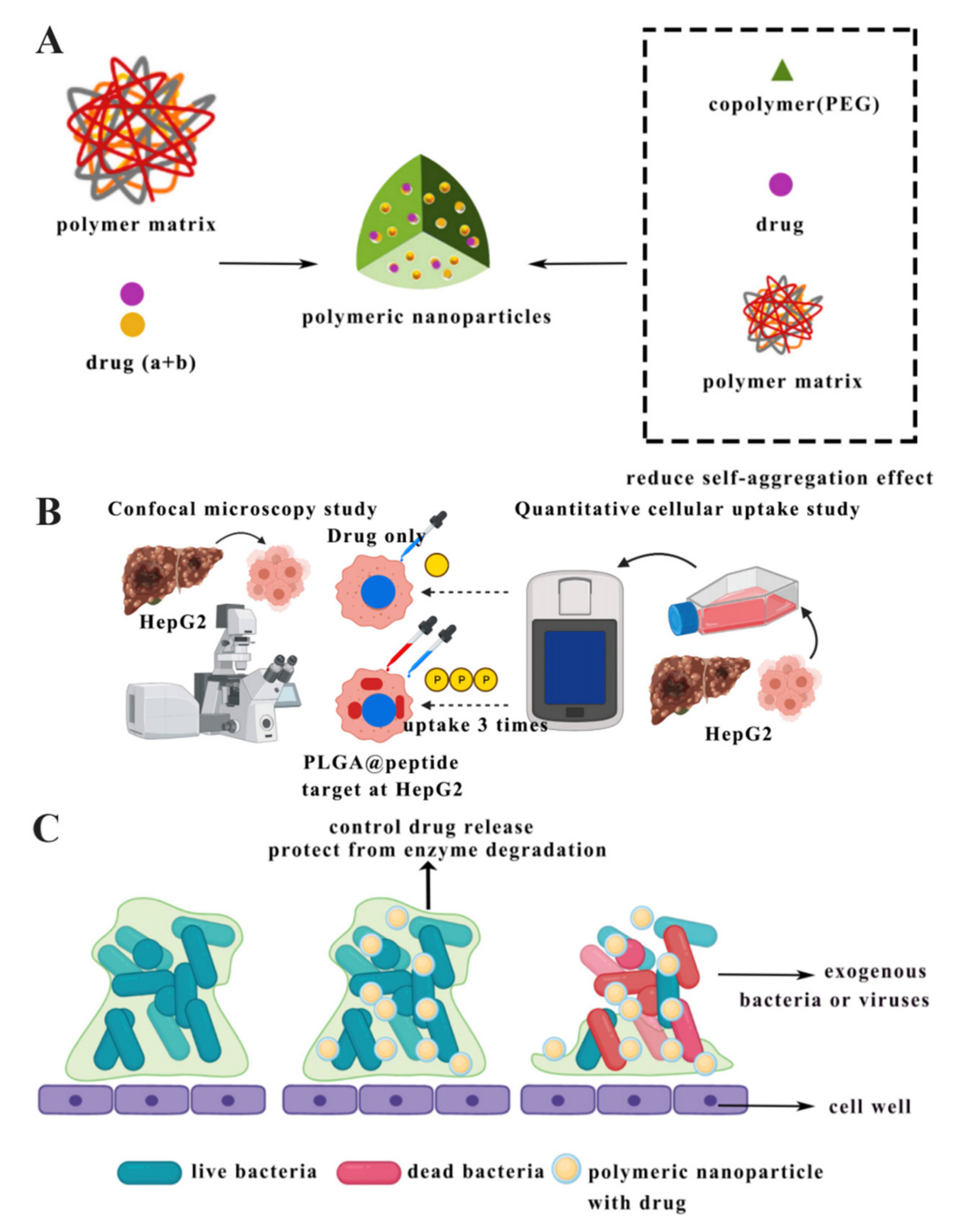
2.4. Polymeric Nanoparticles
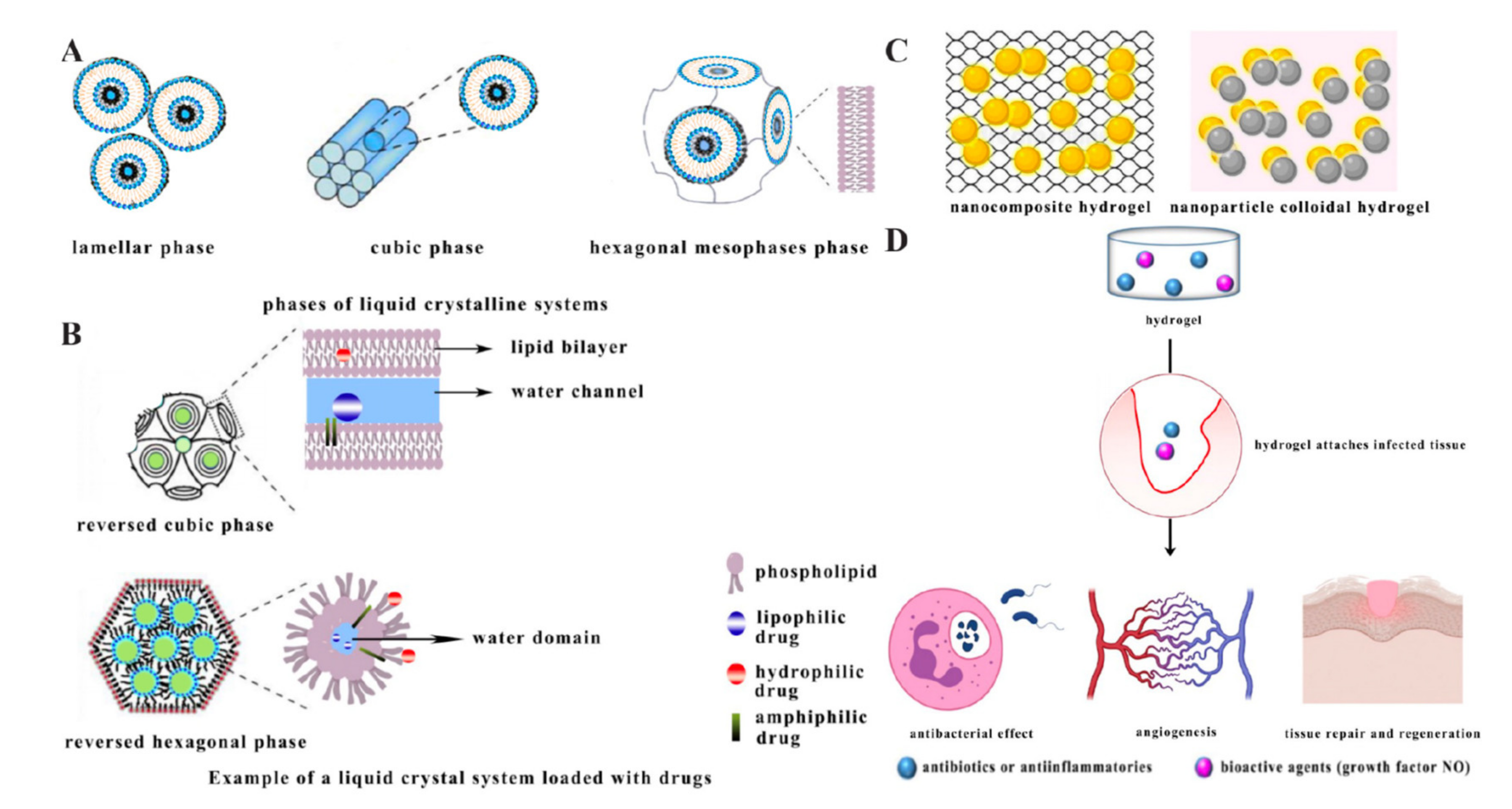
2.5. Liquid Crystalline Systems
2.6. Hydrogels
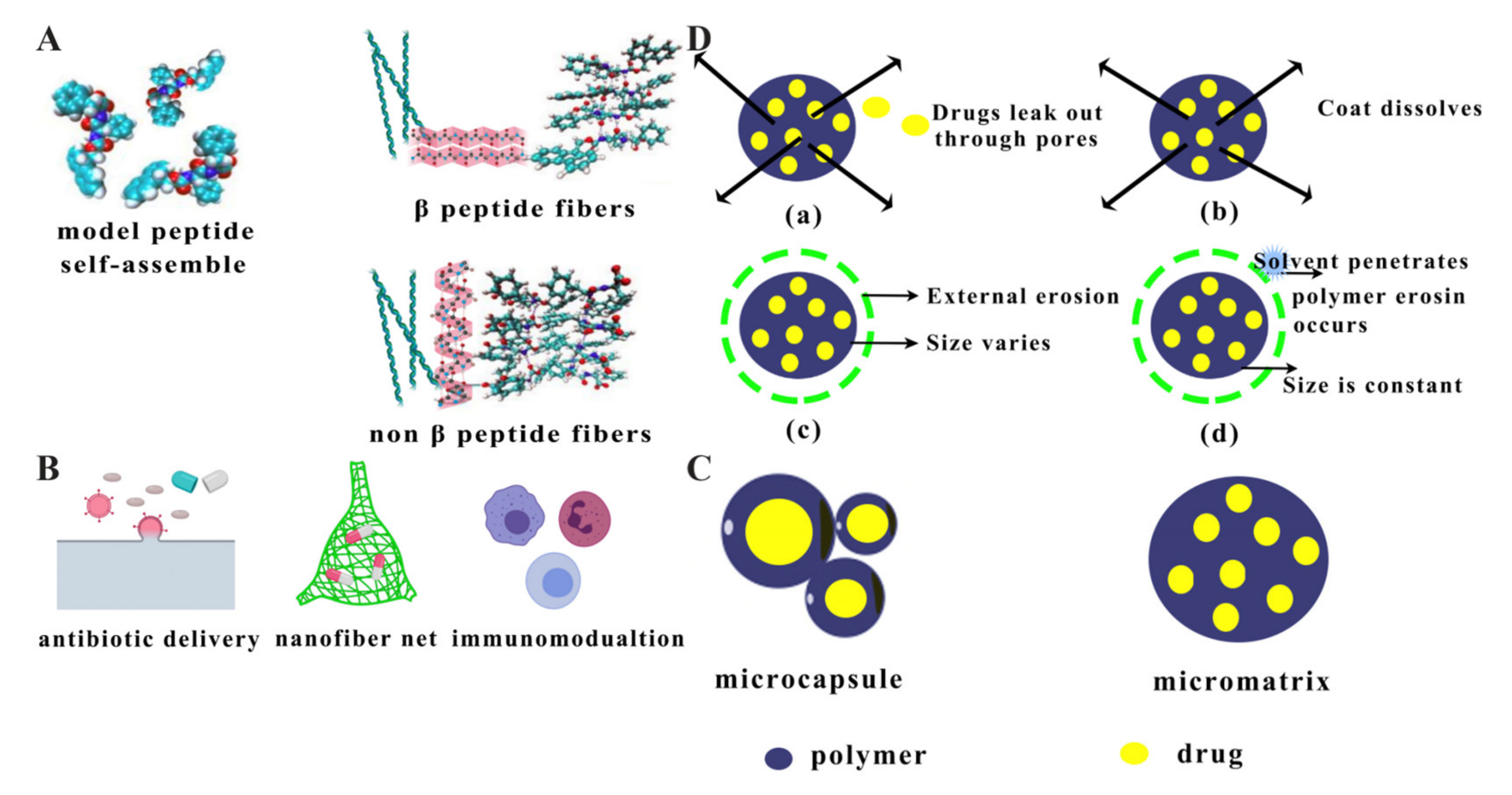
2.7. Nanofibres
2.8. Microspheres
2.9. Metal Nanocrystalline Materials
2.10. Mesoporous Silica Nanoparticles
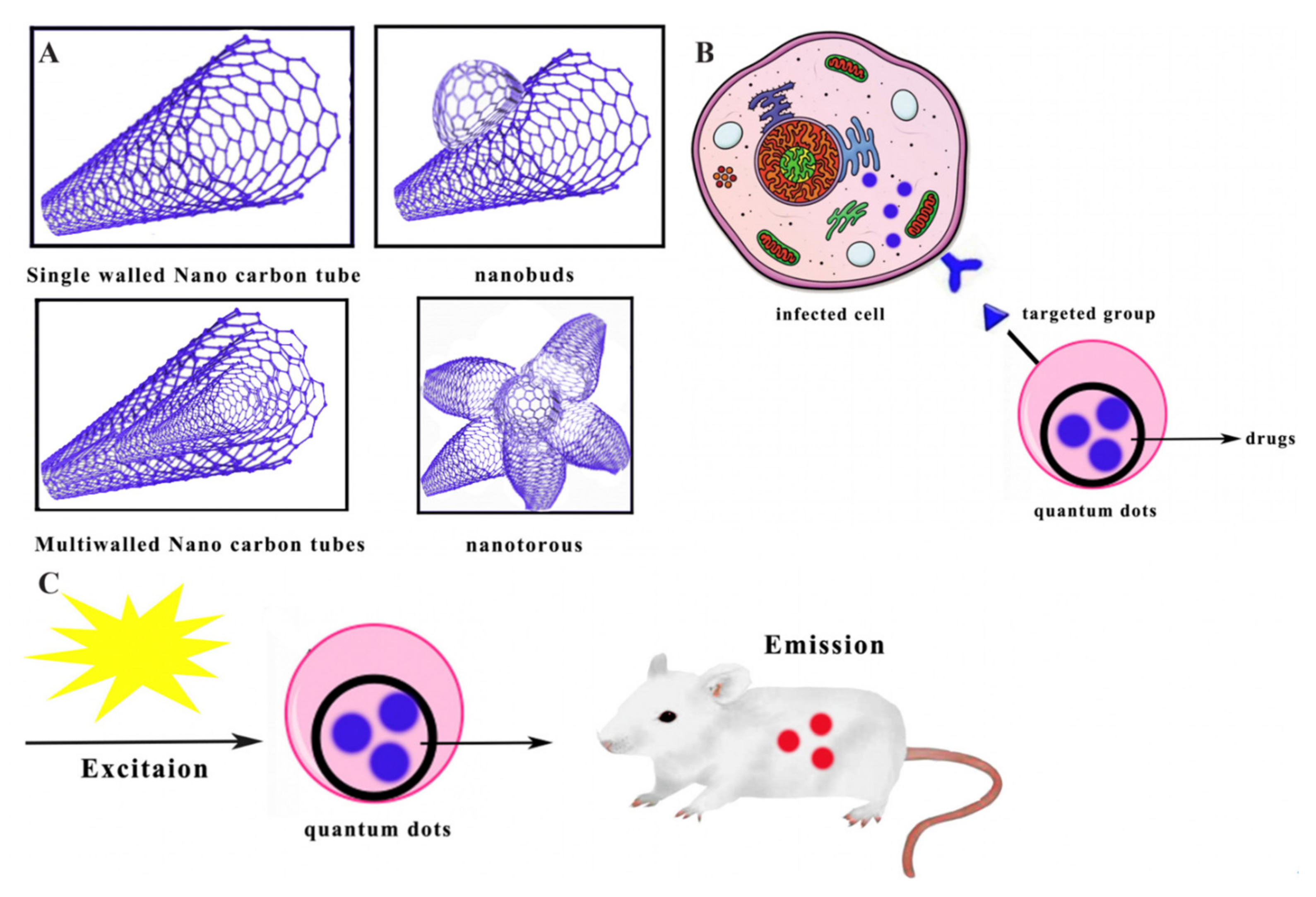
2.11. Carbon Nanotubes
2.12. Quantum Dots
3. Features and Applications for Constructing Nanodelivery Systems Loaded with AMPs
4. Conclusions and Challenges in Nanotechnology for AMP Delivery
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, X.; Kang, Y.; Pan, X.; Yu, J.; Ouyang, Q.; Luo, C. Studies of the drug resistance response of sensitive and drug-resistant strains in a microfluidic system. Integr. Biol. 2014, 6, 143–151. [Google Scholar] [CrossRef]
- Kaczor, A.A.; Polski, A.; Sobótka-Polska, K.; Pachuta-Stec, A.; Makarska-Bialokoz, M.; Pitucha, M. Novel antibacterial compounds and their drug targets-successes and challenges. Curr. Med. Chem. 2017, 24, 1948–1982. [Google Scholar] [CrossRef] [PubMed]
- Willyard, C. The drug-resistant bacteria that pose the greatest health threats. Nat. News 2017, 543, 15. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Sun, J.; Zhou, M.; Zhou, J.; Lao, X.; Zheng, H.; Xu, H. DRAMP: A comprehensive data repository of antimicrobial peptides. Sci. Rep. 2016, 6, 24482. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, C.; Liang, G.; Zhang, M.; Zheng, J. Engineering antimicrobial peptides with improved antimicrobial and hemolytic activities. J. Chem. Inf. Model. 2013, 53, 3280–3296. [Google Scholar] [CrossRef]
- Mookherjee, N.; Hancock, R. Cationic host defence peptides: Innate immune regulatory peptides as a novel approach for treating infections. Cell. Mol. Life Sci. 2007, 64, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Pletzer, D.; Mansour, S.C.; Hancock, R.E. Synergy between conventional antibiotics and anti-biofilm peptides in a murine, sub-cutaneous abscess model caused by recalcitrant ESKAPE pathogens. PLoS Pathog. 2018, 14, e1007084. [Google Scholar] [CrossRef]
- Vineeth Kumar, T.; Sanil, G. A review of the mechanism of action of amphibian antimicrobial peptides focusing on peptide-membrane interaction and membrane curvature. Curr. Protein Pept. Sci. 2017, 18, 1263–1272. [Google Scholar] [CrossRef]
- Holthausen, D.J.; Lee, S.H.; Kumar, V.T.; Bouvier, N.M.; Krammer, F.; Ellebedy, A.H.; Wrammert, J.; Lowen, A.C.; George, S.; Pillai, M.R. An amphibian host defense peptide is virucidal for human H1 hemagglutinin-bearing influenza viruses. Immunity 2017, 46, 587–595. [Google Scholar] [CrossRef]
- Lai, Y.; Gallo, R.L. AMPed up immunity: How antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009, 30, 131–141. [Google Scholar] [CrossRef]
- Lequin, O.; Bruston, F.; Convert, O.; Chassaing, G.; Nicolas, P. Helical structure of dermaseptin B2 in a membrane-mimetic environment. Biochemistry 2003, 42, 10311–10323. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, H.W.; Olah, G.A. Method of oriented circular dichroism. Biophys. J. 1990, 57, 797–806. [Google Scholar] [CrossRef]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919. [Google Scholar] [PubMed]
- Oren, Z.; Shai, Y. Mode of action of linear amphipathic α-helical antimicrobial peptides. Pept. Sci. 1998, 47, 451–463. [Google Scholar] [CrossRef]
- Huang, H.W.; Chen, F.-Y.; Lee, M.-T. Molecular mechanism of peptide-induced pores in membranes. Phys. Rev. Lett. 2004, 92, 198304. [Google Scholar] [CrossRef] [PubMed]
- Ludtke, S.; He, K.; Huang, H. Membrane thinning caused by magainin 2. Biochemistry 1995, 34, 16764–16769. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Maier, E.; Benz, R.; Hancock, R.E. Mechanism of interaction of different classes of cationic antimicrobial peptides with planar bilayers and with the cytoplasmic membrane of Escherichia coli. Biochemistry 1999, 38, 7235–7242. [Google Scholar] [CrossRef]
- Miteva, M.; Andersson, M.; Karshikoff, A.; Otting, G. Molecular electroporation: A unifying concept for the description of membrane pore formation by antibacterial peptides, exemplified with NK-lysin. FEBS Lett. 1999, 462, 155–158. [Google Scholar] [CrossRef]
- Pokorny, A.; Almeida, P.F. Kinetics of dye efflux and lipid flip-flop induced by δ-lysin in phosphatidylcholine vesicles and the mechanism of graded release by amphipathic, α-helical peptides. Biochemistry 2004, 43, 8846–8857. [Google Scholar] [CrossRef]
- Tornesello, A.L.; Borrelli, A.; Buonaguro, L.; Buonaguro, F.M.; Tornesello, M.L. Antimicrobial peptides as anticancer agents: Functional properties and biological activities. Molecules 2020, 25, 2850. [Google Scholar] [CrossRef]
- Popovic, S.; Urbán, E.; Lukic, M.; Conlon, J.M. Peptides with antimicrobial and anti-inflammatory activities that have therapeutic potential for treatment of acne vulgaris. Peptides 2012, 34, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, F.A.; Modaresifar, K.; Azizian, S.; Niknejad, H. Induction of antimicrobial peptides secretion by IL-1β enhances human amniotic membrane for regenerative medicine. Sci. Rep. 2017, 7, 17022. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-K.; Kim, C.; Seo, C.H.; Park, Y. The therapeutic applications of antimicrobial peptides (AMPs): A patent review. J. Microbiol. 2017, 55, 1–12. [Google Scholar] [CrossRef] [PubMed]
- KEGG PATHWAY Database. Available online: https://www.genome.jp/kegg/pathway.html (accessed on 24 July 2021).
- Kuroda, K.; Fukuda, T.; Krstic-Demonacos, M.; Demonacos, C.; Okumura, K.; Isogai, H.; Hayashi, M.; Saito, K.; Isogai, E. miR-663a regulates growth of colon cancer cells, after administration of antimicrobial peptides, by targeting CXCR4-p21 pathway. BMC Cancer 2017, 17, 33. [Google Scholar] [CrossRef] [PubMed]
- Ageitos, J.; Sánchez-Pérez, A.; Calo-Mata, P.; Villa, T. Antimicrobial peptides (AMPs): Ancient compounds that represent novel weapons in the fight against bacteria. Biochem. Pharmacol. 2017, 133, 117–138. [Google Scholar] [CrossRef]
- Nizet, V. Antimicrobial peptide resistance mechanisms of human bacterial pathogens. Curr. Issues Mol. Biol. 2006, 8, 11. [Google Scholar] [CrossRef]
- Navon-Venezia, S.; Feder, R.; Gaidukov, L.; Carmeli, Y.; Mor, A. Antibacterial properties of dermaseptin S4 derivatives with in vivo activity. Antimicrob. Agents Chemother. 2002, 46, 689–694. [Google Scholar] [CrossRef]
- Patel, A.; Patel, M.; Yang, X.; Mitra, A.K. Recent advances in protein and peptide drug delivery: A special emphasis on polymeric nanoparticles. Protein Pept. Lett. 2014, 21, 1102–1120. [Google Scholar] [CrossRef]
- Umerska, A.; Cassisa, V.; Bastiat, G.; Matougui, N.; Nehme, H.; Manero, F.; Eveillard, M.; Saulnier, P. Synergistic interactions between antimicrobial peptides derived from plectasin and lipid nanocapsules containing monolaurin as a cosurfactant against Staphylococcus aureus. Int. J. Nanomed. 2017, 12, 5687. [Google Scholar] [CrossRef]
- Ali, S.S.; Morsy, R.; El-Zawawy, N.A.; Fareed, M.F.; Bedaiwy, M.Y. Synthesized zinc peroxide nanoparticles (ZnO2-NPs): A novel antimicrobial, anti-elastase, anti-keratinase, and anti-inflammatory approach toward polymicrobial burn wounds. Int. J. Nanomed. 2017, 12, 6059. [Google Scholar] [CrossRef]
- Sun, L.; Zheng, C.; Webster, T.J. Self-assembled peptide nanomaterials for biomedical applications: Promises and pitfalls. Int. J. Nanomed. 2017, 12, 73. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.; Flórez, J.; Torres, R.; Urquiza, M.; Gutiérrez, J.; Guzmán, F.; Ortiz, C. Antimicrobial activity of a new synthetic peptide loaded in polylactic acid or poly (lactic-co-glycolic) acid nanoparticles against Pseudomonas aeruginosa, Escherichia coli O157: H7 and methicillin resistant Staphylococcus aureus (MRSA). Nanotechnology 2017, 28, 135102. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, Y.; Li, S.; Liu, M.; Qin, X.; Chen, X.; Lin, Y. Tetrahedral framework nucleic acids deliver antimicrobial peptides with improved effects and less susceptibility to bacterial degradation. Nano Lett. 2020, 20, 3602–3610. [Google Scholar] [CrossRef]
- Lalani, R.; Misra, A.; Amrutiya, J.; Patel, H.; Bhatt, P.; Patel, V. Challenges in dermal delivery of therapeutic antimicrobial protein and peptides. Curr. Drug Metab. 2017, 18, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Singh, S. Nanomaterials as non-viral siRNA delivery agents for cancer therapy. BioImpacts BI 2013, 3, 53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Jiang, A.M.; Ma, Z.Y.; Li, X.B.; Xiong, Y.Y.; Dou, J.F.; Wang, J.F. The synthetic antimicrobial peptide pexiganan and its nanoparticles (PNPs) exhibit the anti-helicobacter pylori activity in vitro and in vivo. Molecules 2015, 20, 3972–3985. [Google Scholar] [CrossRef]
- Harloff-Helleberg, S.; Nielsen, L.H.; Nielsen, H.M. Animal models for evaluation of oral delivery of biopharmaceuticals. J. Control. Release 2017, 268, 57–71. [Google Scholar] [CrossRef]
- Sadat, S.M.; Jahan, S.T.; Haddadi, A. Effects of size and surface charge of polymeric nanoparticles on in vitro and in vivo applications. J. Biomater. Nanobiotechnology 2016, 7, 91. [Google Scholar] [CrossRef]
- Faya, M.; Hazzah, H.A.; Omolo, C.A.; Agrawal, N.; Maji, R.; Walvekar, P.; Mocktar, C.; Nkambule, B.; Rambharose, S.; Albericio, F. Novel formulation of antimicrobial peptides enhances antimicrobial activity against methicillin-resistant Staphylococcus aureus (MRSA). Amino Acids 2020, 52, 1439–1457. [Google Scholar] [CrossRef]
- Kumar, P.; Pletzer, D.; Haney, E.F.; Rahanjam, N.; Cheng, J.T.; Yue, M.; Aljehani, W.; Hancock, R.E.; Kizhakkedathu, J.N.; Straus, S.K. Aurein-derived antimicrobial peptides formulated with pegylated phospholipid micelles to target methicillin-resistant Staphylococcus aureus skin infections. ACS Infect. Dis. 2018, 5, 443–453. [Google Scholar] [CrossRef]
- Scorciapino, M.A.; Serra, I.; Manzo, G.; Rinaldi, A.C. Antimicrobial dendrimeric peptides: Structure, activity and new therapeutic applications. Int. J. Mol. Sci. 2017, 18, 542. [Google Scholar] [CrossRef]
- Di-Wen, S.; Pan, G.-Z.; Hao, L.; Zhang, J.; Xue, Q.-Z.; Wang, P.; Yuan, Q.-Z. Improved antitumor activity of epirubicin-loaded CXCR4-targeted polymeric nanoparticles in liver cancers. Int. J. Pharm. 2016, 500, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Aida, K.L.; Kreling, P.F.; Caiaffa, K.S.; Calixto, G.M.F.; Chorilli, M.; Spolidorio, D.M.; Santos-Filho, N.A.; Cilli, E.M.; Duque, C. Antimicrobial peptide-loaded liquid crystalline precursor bioadhesive system for the prevention of dental caries. Int. J. Nanomed. 2018, 13, 3081. [Google Scholar] [CrossRef]
- Tang, C.; Miller, A.F.; Saiani, A. Peptide hydrogels as mucoadhesives for local drug delivery. Int. J. Pharm. 2014, 465, 427–435. [Google Scholar] [CrossRef]
- Edmans, J.G.; Murdoch, C.; Santocildes-Romero, M.E.; Hatton, P.V.; Colley, H.E.; Spain, S.G. Incorporation of lysozyme into a mucoadhesive electrospun patch for rapid protein delivery to the oral mucosa. Mater. Sci. Eng. C 2020, 112, 110917. [Google Scholar] [CrossRef]
- Li, Y.; Na, R.; Wang, X.; Liu, H.; Zhao, L.; Sun, X.; Ma, G.; Cui, F. Fabrication of antimicrobial peptide-loaded PLGA/chitosan composite microspheres for long-acting bacterial resistance. Molecules 2017, 22, 1637. [Google Scholar] [CrossRef]
- Rai, A.; Pinto, S.; Velho, T.R.; Ferreira, A.F.; Moita, C.; Trivedi, U.; Evangelista, M.; Comune, M.; Rumbaugh, K.P.; Simões, P.N. One-step synthesis of high-density peptide-conjugated gold nanoparticles with antimicrobial efficacy in a systemic infection model. Biomaterials 2016, 85, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Braun, K.; Pochert, A.; Lindén, M.; Davoudi, M.; Schmidtchen, A.; Nordström, R.; Malmsten, M. Membrane interactions of mesoporous silica nanoparticles as carriers of antimicrobial peptides. J. Colloid Interface Sci. 2016, 475, 161–170. [Google Scholar] [CrossRef]
- Chaudhari, A.A.; deb Nath, S.; Kate, K.; Dennis, V.; Singh, S.R.; Owen, D.R.; Palazzo, C.; Arnold, R.D.; Miller, M.E.; Pillai, S.R. A novel covalent approach to bio-conjugate silver coated single walled carbon nanotubes with antimicrobial peptide. J. Nanobiotechnol. 2016, 14, 58. [Google Scholar] [CrossRef] [PubMed]
- Mohid, S.A.; Bhunia, A. Combining Antimicrobial Peptides with Nanotechnology: An Emerging Field in Theranostics. Curr. Protein Pept. Sci. 2020, 21, 413–428. [Google Scholar] [CrossRef] [PubMed]
- Cantor, S.; Vargas, L.; Rojas, O.E.A.; Yarce, C.J.; Salamanca, C.H.; Oñate-Garzón, J. Evaluation of the antimicrobial activity of cationic peptides loaded in surface-modified nanoliposomes against foodborne bacteria. Int. J. Mol. Sci. 2019, 20, 680. [Google Scholar] [CrossRef]
- Park, S.-C.; Ko, C.; Hyeon, H.; Jang, M.-K.; Lee, D. Imaging and Targeted Antibacterial Therapy Using Chimeric Antimicrobial Peptide Micelles. ACS Appl. Mater. Interfaces 2020, 12, 54306–54315. [Google Scholar] [CrossRef] [PubMed]
- Donalisio, M.; Rusnati, M.; Civra, A.; Bugatti, A.; Allemand, D.; Pirri, G.; Giuliani, A.; Landolfo, S.; Lembo, D. Identification of a dendrimeric heparan sulfate-binding peptide that inhibits infectivity of genital types of human papillomaviruses. Antimicrob. Agents Chemother. 2010, 54, 4290–4299. [Google Scholar] [CrossRef]
- Pires, J.; Siriwardena, T.N.; Stach, M.; Tinguely, R.; Kasraian, S.; Luzzaro, F.; Leib, S.L.; Darbre, T.; Reymond, J.-L.; Endimiani, A. In vitro activity of the novel antimicrobial peptide dendrimer G3KL against multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2015, 59, 7915–7918. [Google Scholar] [CrossRef]
- Fernandez, J.; Acosta, G.; Pulido, D.; Malý, M.; Copa-Patiño, J.L.; Soliveri, J.; Royo, M.; Gómez, R.; Albericio, F.; Ortega, P. Carbosilane dendron–peptide nanoconjugates as antimicrobial agents. Mol. Pharm. 2019, 16, 2661–2674. [Google Scholar] [CrossRef]
- Piotrowska, U.; Oledzka, E.; Kamysz, W.; Białek, S.; Sobczak, M. The effect of polymer microstructure on encapsulation efficiency and release kinetics of Citropin 1.1 from the Poly (ε-caprolactone) microparticles. Nanomaterials 2018, 8, 482. [Google Scholar] [CrossRef]
- Gómez-Sequeda, N.; Ruiz, J.; Ortiz, C.; Urquiza, M.; Torres, R. Potent and Specific Antibacterial Activity against Escherichia coli O157: H7 and Methicillin Resistant Staphylococcus aureus (MRSA) of G17 and G19 Peptides Encapsulated into Poly-Lactic-Co-Glycolic Acid (PLGA) Nanoparticles. Antibiotics 2020, 9, 384. [Google Scholar] [CrossRef] [PubMed]
- Piras, A.M.; Maisetta, G.; Sandreschi, S.; Gazzarri, M.; Bartoli, C.; Grassi, L.; Esin, S.; Chiellini, F.; Batoni, G. Chitosan nanoparticles loaded with the antimicrobial peptide temporin B exert a long-term antibacterial activity in vitro against clinical isolates of Staphylococcus epidermidis. Front. Microbiol. 2015, 6, 372. [Google Scholar] [CrossRef]
- Boge, L.; Bysell, H.; Ringstad, L.; Wennman, D.; Umerska, A.; Cassisa, V.; Eriksson, J.; Joly-Guillou, M.-L.; Edwards, K.; Andersson, M. Lipid-based liquid crystals as carriers for antimicrobial peptides: Phase behavior and antimicrobial effect. Langmuir 2016, 32, 4217–4228. [Google Scholar] [CrossRef] [PubMed]
- Antunes, L.; Faustino, G.; Mouro, C.; Vaz, J.; Gouveia, I.C. Bioactive microsphere-based coating for biomedical-textiles with encapsulated antimicrobial peptides (AMPs). Ciência Tecnol. Mater. 2014, 26, 118–125. [Google Scholar] [CrossRef]
- Ropero-Vega, J.; Ardila-Rosas, N.; Hernández, I.P.; Flórez-Castillo, J. Immobilization of Ib-M2 peptide on core@ shell nanostructures based on SPION nanoparticles and their antibacterial activity against Escherichia coli O157: H7. Appl. Surf. Sci. 2020, 515, 146045. [Google Scholar] [CrossRef]
- Mohid, S.A.; Ghorai, A.; Ilyas, H.; Mroue, K.H.; Narayanan, G.; Sarkar, A.; Ray, S.K.; Biswas, K.; Bera, A.K.; Malmsten, M. Application of tungsten disulfide quantum dot-conjugated antimicrobial peptides in bio-imaging and antimicrobial therapy. Colloids Surf. B Biointerfaces 2019, 176, 360–370. [Google Scholar] [CrossRef]
- Hossen, S.; Hossain, M.K.; Basher, M.; Mia, M.; Rahman, M.; Uddin, M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J. Adv. Res. 2019, 15, 1–18. [Google Scholar] [CrossRef]
- Clemente, I.; Bonechi, C.; Rodolfi, L.; Bacia-Verloop, M.; Rossi, C.; Ristori, S. Lipids from algal biomass provide new (nonlamellar) nanovectors with high carrier potentiality for natural antioxidants. Eur. J. Pharm. Biopharm. 2020, 158, 410–416. [Google Scholar] [CrossRef]
- Wang, W.; Lu, K.-J.; Yu, C.-H.; Huang, Q.-L.; Du, Y.-Z. Nano-drug delivery systems in wound treatment and skin regeneration. J. Nanobiotechnology 2019, 17, 82. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, S.B.; Idrus, R.B.H.; Hwei, N.M. Gelatin Microsphere for Cartilage Tissue Engineering: Current and Future Strategies. Polymers 2020, 12, 2404. [Google Scholar] [CrossRef]
- Ashfaq, U.A.; Riaz, M.; Yasmeen, E.; Yousaf, M.Z. Recent advances in nanoparticle-based targeted drug-delivery systems against cancer and role of tumor microenvironment. Crit. Rev. Ther. Drug Carr. Syst. 2017, 34. [Google Scholar] [CrossRef]
- Chen, J.; Cheng, D.; Li, J.; Wang, Y.; Guo, J.-X.; Chen, Z.-P.; Cai, B.-C.; Yang, T. Influence of lipid composition on the phase transition temperature of liposomes composed of both DPPC and HSPC. Drug Dev. Ind. Pharm. 2013, 39, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Brisaert, M.; Gabriels, M.; Matthijs, V.; Plaizier-Vercammen, J. Liposomes with tretinoin: A physical and chemical evaluation. J. Pharm. Biomed. Anal. 2001, 26, 909–917. [Google Scholar] [CrossRef]
- Friede, M.; Muller, S.; Briand, J.-P.; Van Regenmortel, M.H.; Schuber, F. Induction of immune response against a short synthetic peptide antigen coupled to small neutral liposomes containing monophosphoryl lipid A. Mol. Immunol. 1993, 30, 539–547. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Mirgorodskaya, A.B.; Kushnazarova, R.A.; Lukashenko, S.S.; Zakharova, L.Y. Self-assembly of mixed systems based on nonionic and carbamate-bearing cationic surfactants as a tool for fabrication of biocompatible nanocontainers. J. Mol. Liq. 2019, 292, 111407. [Google Scholar] [CrossRef]
- Gubernator, J.; Chwastek, G.; Korycińska, M.; Stasiuk, M.; Grynkiewicz, G.; Lewrick, F.; Süss, R.; Kozubek, A. The encapsulation of idarubicin within liposomes using the novel EDTA ion gradient method ensures improved drug retention in vitro and in vivo. J. Control. Release 2010, 146, 68–75. [Google Scholar] [CrossRef]
- Ran, R.; Middelberg, A.P.; Zhao, C.-X. Microfluidic synthesis of multifunctional liposomes for tumour targeting. Colloids Surf. B Biointerfaces 2016, 148, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Manjappa, A.S.; Chaudhari, K.R.; Venkataraju, M.P.; Dantuluri, P.; Nanda, B.; Sidda, C.; Sawant, K.K.; Murthy, R.S.R. Antibody derivatization and conjugation strategies: Application in preparation of stealth immunoliposome to target chemotherapeutics to tumor. J. Control. Release 2011, 150, 2–22. [Google Scholar] [CrossRef]
- Cevc, G. Rational design of new product candidates: The next generation of highly deformable bilayer vesicles for noninvasive, targeted therapy. J. Control. Release 2012, 160, 135–146. [Google Scholar] [CrossRef]
- Cajot, S.; Schol, D.; Danhier, F.; Préat, V.; Gillet De Pauw, M.C.; Jérôme, C. In vitro Investigations of Smart Drug Delivery Systems Based on Redox-S ensitive Cross-L inked Micelles. Macromol. Biosci. 2013, 13, 1661–1670. [Google Scholar] [CrossRef]
- Biswas, S.; Kumari, P.; Lakhani, P.M.; Ghosh, B. Recent advances in polymeric micelles for anti-cancer drug delivery. Eur. J. Pharm. Sci. 2016, 83, 184–202. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Mukai, H.; Saeki, T.; Ro, J.; Lin, Y.-C.; Nagai, S.E.; Lee, K.S.; Watanabe, J.; Ohtani, S.; Kim, S.B. A multi-national, randomised, open-label, parallel, phase III non-inferiority study comparing NK105 and paclitaxel in metastatic or recurrent breast cancer patients. Br. J. Cancer 2019, 120, 475–480. [Google Scholar] [CrossRef]
- Takahashi, C.; Akachi, Y.; Ogawa, N.; Moriguchi, K.; Asaka, T.; Tanemura, M.; Kawashima, Y.; Yamamoto, H. Morphological study of efficacy of clarithromycin-loaded nanocarriers for treatment of biofilm infection disease. Med. Mol. Morphol. 2017, 50, 9–16. [Google Scholar] [CrossRef]
- Shin, D.H.; Tam, Y.T.; Kwon, G.S. Polymeric micelle nanocarriers in cancer research. Front. Chem. Sci. Eng. 2016, 10, 348–359. [Google Scholar] [CrossRef]
- Khan, A.R.; Yang, X.; Fu, M.; Zhai, G. Recent progress of drug nanoformulations targeting to brain. J. Control. Release 2018, 291, 37–64. [Google Scholar] [CrossRef]
- Nisini, R.; Poerio, N.; Mariotti, S.; De Santis, F.; Fraziano, M. The multirole of liposomes in therapy and prevention of infectious diseases. Front. Immunol. 2018, 9, 155. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Laurent, R.; Majoral, J.-P. Characterization of dendrimers. Adv. Drug Deliv. Rev. 2005, 57, 2130–2146. [Google Scholar] [CrossRef]
- Serri, A.; Mahboubi, A.; Zarghi, A.; Moghimi, H.R. PAMAM-dendrimer enhanced antibacterial effect of vancomycin hydrochloride against gram-negative bacteria. J. Pharm. Pharm. Sci. 2019, 22, 10–21. [Google Scholar] [CrossRef]
- Majoros, I.J.; Williams, C.R.; Tomalia, D.A.; Baker, J.R., Jr. New dendrimers: Synthesis and characterization of POPAM−PAMAM hybrid dendrimers. Macromolecules 2008, 41, 8372–8379. [Google Scholar] [CrossRef]
- Dwivedi, D.K.; Singh, A.K. Dendrimers: A novel carrier system for drug delivery. J. Drug Deliv. Ther. 2014, 4, 1–6. [Google Scholar] [CrossRef]
- Siriwardena, T.N.; Lüscher, A.; Köhler, T.; van Delden, C.; Javor, S.; Reymond, J.L. Antimicrobial peptide dendrimer chimera. Helv. Chim. Acta 2019, 102, e1900034. [Google Scholar] [CrossRef]
- Bugno, J.; Hsu, H.-j.; Hong, S. Tweaking dendrimers and dendritic nanoparticles for controlled nano-bio interactions: Potential nanocarriers for improved cancer targeting. J. Drug Target. 2015, 23, 642–650. [Google Scholar] [CrossRef]
- Otto, D.P.; De Villiers, M.M. All-atomistic molecular dynamics (AA-MD) studies and pharmacokinetic performance of PAMAM-dendrimer-furosemide delivery systems. Int. J. Pharm. 2018, 547, 545–555. [Google Scholar] [CrossRef]
- Coppi, G.; Iannuccelli, V.; Leo, E.; Bernabei, M.T.; Cameroni, R. Chitosan-alginate microparticles as a protein carrier. Drug Dev. Ind. Pharm. 2001, 27, 393–400. [Google Scholar] [CrossRef]
- Franiak-Pietryga, I.; Ziemba, B.; Sikorska, H.; Jander, M.; Appelhans, D.; Bryszewska, M.; Borowiec, M. Neurotoxicity of poly (propylene imine) glycodendrimers. Drug Chem. Toxicol. 2020, 1–9. [Google Scholar] [CrossRef]
- Lee, J.-K.; Seo, C.H.; Luchian, T.; Park, Y. Antimicrobial peptide CMA3 derived from the CA-MA hybrid peptide: Antibacterial and anti-inflammatory activities with low cytotoxicity and mechanism of action in Escherichia coli. Antimicrob. Agents Chemother. 2016, 60, 495–506. [Google Scholar] [CrossRef]
- Apartsin, E.; Knauer, N.; Arkhipova, V.; Pashkina, E.; Aktanova, A.; Poletaeva, J.; Sánchez-Nieves, J.; de la Mata, F.J.; Gómez, R. pH-Sensitive Dendrimersomes of Hybrid Triazine-Carbosilane Dendritic Amphiphiles-Smart Vehicles for Drug Delivery. Nanomaterials 2020, 10, 1899. [Google Scholar] [CrossRef]
- Jebbawi, R.; Oukhrib, A.; Clement, E.; Blanzat, M.; Turrin, C.-O.; Caminade, A.-M.; Lacoste, E.; Fruchon, S.; Poupot, R. An anti-inflammatory poly (phosphorhydrazone) dendrimer capped with azabisphosphonate groups to treat psoriasis. Biomolecules 2020, 10, 949. [Google Scholar] [CrossRef] [PubMed]
- Stenström, P.; Manzanares, D.; Zhang, Y.; Ceña, V.; Malkoch, M. Evaluation of amino-functional polyester dendrimers based on Bis-MPA as nonviral vectors for siRNA delivery. Molecules 2018, 23, 2028. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Z.; Ding, L.; Dhumal, D.; Huang, A.Y.-T.; Kao, C.-L.; Peng, L. Poly (amidoamine)(PAMAM) dendrimers: Synthesis and biological applications. In Dendrimer Chemistry: Synthetic Approaches Towards Complex Architectures; The Royal Society of Chemistry: London, UK, 2020; pp. 85–113. [Google Scholar] [CrossRef]
- Kaur, D.; Jain, K.; Mehra, N.K.; Kesharwani, P.; Jain, N.K. A review on comparative study of PPI and PAMAM dendrimers. J. Nanoparticle Res. 2016, 18, 146. [Google Scholar] [CrossRef]
- Boas, U.; Christensen, J. Poly (lysine) dendrimers and other dendritic molecules from naturally occurring monomers. In Dendrimer Chemistry: Synthetic Approaches Towards Complex Architectures; The Royal Society of Chemistry: London, UK, 2020; pp. 58–84. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Nixon, L.S.; Hedstrand, D.M. The role of branch cell symmetry and other critical nanoscale design parameters in the determination of dendrimer encapsulation properties. Biomolecules 2020, 10, 642. [Google Scholar] [CrossRef]
- Ortega, P.; Sánchez-Nieves, J.; Cano, J.; Gómez, R.; de la Mata, F.J. Poly (carbosilane) dendrimers and other silicon-containing dendrimers. In Dendrimer Chemistry: Synthetic Approaches Towards Complex Architectures; The Royal Society of Chemistry: London, UK, 2020; pp. 114–145. [Google Scholar] [CrossRef]
- Caminade, A.-M. Poly (phosphorhydrazone) dendrimers and other phosphorus-containing dendrimers. In Dendrimer Chemistry: Synthetic Approaches Towards Complex Architectures; The Royal Society of Chemistry: London, UK, 2020; pp. 146–182. [Google Scholar] [CrossRef]
- Malkoch, M.; Garcia-Gallego, S. Bis-MPA dendrimers and other dendritic polyesters. In Dendrimer Chemistry: Synthetic Approaches Towards Complex Architectures; The Royal Society of Chemistry: London, UK, 2020; pp. 21–57. [Google Scholar] [CrossRef]
- Schito, A.M.; Alfei, S. Antibacterial activity of non-cytotoxic, amino acid-modified polycationic dendrimers against Pseudomonas aeruginosa and other non-fermenting gram-negative bacteria. Polymers 2020, 12, 1818. [Google Scholar] [CrossRef]
- Huang, H.; Li, J.; Liao, L.; Li, J.; Wu, L.; Dong, C.; Lai, P.; Liu, D. Poly (L-glutamic acid)-based star-block copolymers as pH-responsive nanocarriers for cationic drugs. Eur. Polym. J. 2012, 48, 696–704. [Google Scholar] [CrossRef]
- Hatano, K.; Matsuoka, K.; Terunuma, D. Carbosilane glycodendrimers. Chem. Soc. Rev. 2013, 42, 4574–4598. [Google Scholar] [CrossRef]
- Fruchon, S.; Caminade, A.-M.; Abadie, C.; Davignon, J.-L.; Combette, J.-M.; Turrin, C.-O.; Poupot, R. An azabisphosphonate-capped poly (phosphorhydrazone) dendrimer for the treatment of endotoxin-induced uveitis. Molecules 2013, 18, 9305–9316. [Google Scholar] [CrossRef]
- Ye, M.; Kim, S.; Park, K. Issues in long-term protein delivery using biodegradable microparticles. J. Control. Release 2010, 146, 241–260. [Google Scholar] [CrossRef]
- Yun, Y.H.; Goetz, D.J.; Yellen, P.; Chen, W. Hyaluronan microspheres for sustained gene delivery and site-specific targeting. Biomaterials 2004, 25, 147–157. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Garg, V.; Chawla, K.; Pawar, S.K. Nanotechnology controlled local drug delivery system for the treatment of periodontitisc. J. Adv. Med. Med. Res. 2018, 26, 1–17. [Google Scholar] [CrossRef]
- Ajun, W.; Yan, S.; Li, G.; Huili, L. Preparation of aspirin and probucol in combination loaded chitosan nanoparticles and in vitro release study. Carbohydr. Polym. 2009, 75, 566–574. [Google Scholar] [CrossRef]
- Calvo, P.; Remunan-Lopez, C.; Vila-Jato, J.L.; Alonso, M. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Zhang, L.; Chan, J.M.; Gu, F.X.; Rhee, J.-W.; Wang, A.Z.; Radovic-Moreno, A.F.; Alexis, F.; Langer, R.; Farokhzad, O.C. Self-assembled lipid−polymer hybrid nanoparticles: A robust drug delivery platform. ACS Nano 2008, 2, 1696–1702. [Google Scholar] [CrossRef]
- Cheow, W.S.; Hadinoto, K. Green amorphous nanoplex as a new supersaturating drug delivery system. Langmuir 2012, 28, 6265–6275. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhong, W.; Zhang, K.; Wang, D.; Hu, J.; Chan-Park, M.B. Biguanide-Derived Polymeric Nanoparticles Kill MRSA Biofilm and Suppress Infection in vivo. ACS Appl. Mater. Interfaces 2020, 12, 21231–21241. [Google Scholar] [CrossRef]
- Guo, C.; Wang, J.; Cao, F.; Lee, R.J.; Zhai, G. Lyotropic liquid crystal systems in drug delivery. Drug Discov. Today 2010, 15, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Phan, S.; Fong, W.-K.; Kirby, N.; Hanley, T.; Boyd, B.J. Evaluating the link between self-assembled mesophase structure and drug release. Int. J. Pharm. 2011, 421, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Guterres, S.S.; Alves, M.P.; Pohlmann, A.R. Polymeric nanoparticles, nanospheres and nanocapsules, for cutaneous applications. Drug Target Insights 2007, 2, 117739280700200002. [Google Scholar] [CrossRef]
- Milak, S.; Zimmer, A. Glycerol monooleate liquid crystalline phases used in drug delivery systems. Int. J. Pharm. 2015, 478, 569–587. [Google Scholar] [CrossRef] [PubMed]
- Mohammady, S.Z.; Pouzot, M.; Mezzenga, R. Oleoylethanolamide-based lyotropic liquid crystals as vehicles for delivery of amino acids in aqueous environment. Biophys. J. 2009, 96, 1537–1546. [Google Scholar] [CrossRef] [PubMed]
- Sagalowicz, L.; Leser, M.; Watzke, H.; Michel, M. Monoglyceride self-assembly structures as delivery vehicles. Trends Food Sci. Technol. 2006, 17, 204–214. [Google Scholar] [CrossRef]
- Boyd, B.J.; Whittaker, D.V.; Khoo, S.-M.; Davey, G. Hexosomes formed from glycerate surfactants—Formulation as a colloidal carrier for irinotecan. Int. J. Pharm. 2006, 318, 154–162. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Malinen, M.M.; Lauren, P.; Lou, Y.-R.; Kuisma, S.W.; Kanninen, L.; Lille, M.; Corlu, A.; GuGuen-Guillouzo, C.; Ikkala, O. Nanofibrillar cellulose hydrogel promotes three-dimensional liver cell culture. J. Control. Release 2012, 164, 291–298. [Google Scholar] [CrossRef]
- Anumolu, S.S.; Menjoge, A.R.; Deshmukh, M.; Gerecke, D.; Stein, S.; Laskin, J.; Sinko, P.J. Doxycycline hydrogels with reversible disulfide crosslinks for dermal wound healing of mustard injuries. Biomaterials 2011, 32, 1204–1217. [Google Scholar] [CrossRef]
- Da Silva, L.P.; Reis, R.L.; Correlo, V.M.; Marques, A.P. Hydrogel-based strategies to advance therapies for chronic skin wounds. Annu. Rev. Biomed. Eng. 2019, 21, 145–169. [Google Scholar] [CrossRef] [PubMed]
- Manconi, M.; Manca, M.L.; Caddeo, C.; Cencetti, C.; di Meo, C.; Zoratto, N.; Nacher, A.; Fadda, A.M.; Matricardi, P. Preparation of gellan-cholesterol nanohydrogels embedding baicalin and evaluation of their wound healing activity. Eur. J. Pharm. Biopharm. 2018, 127, 244–249. [Google Scholar] [CrossRef]
- Rice, J.J.; Martino, M.M.; De Laporte, L.; Tortelli, F.; Briquez, P.S.; Hubbell, J.A. Engineering the regenerative microenvironment with biomaterials. Adv. Healthc. Mater. 2013, 2, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Heunis, T.; Dicks, L. Nanofibers offer alternative ways to the treatment of skin infections. J. Biomed. Biotechnol. 2010, 2010, 510682. [Google Scholar] [CrossRef]
- Kumar, D.K.V.; Choi, S.H.; Washicosky, K.J.; Eimer, W.A.; Tucker, S.; Ghofrani, J.; Lefkowitz, A.; McColl, G.; Goldstein, L.E.; Tanzi, R.E. Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Sci. Transl. Med. 2016, 8, 340ra72. [Google Scholar] [CrossRef]
- Chairatana, P.; Nolan, E.M. Molecular basis for self-assembly of a human host-defense peptide that entraps bacterial pathogens. J. Am. Chem. Soc. 2014, 136, 13267–13276. [Google Scholar] [CrossRef] [PubMed]
- Zha, R.H.; Sur, S.; Stupp, S.I. Self-assembly of Cytotoxic Peptide Amphiphiles into Supramolecular Membranes for Cancer Therapy. Adv. Healthc. Mater. 2013, 2, 126–133. [Google Scholar] [CrossRef]
- Simonson, A.W.; Aronson, M.R.; Medina, S.H. Supramolecular Peptide Assemblies as Antimicrobial Scaffolds. Molecules 2020, 25, 2751. [Google Scholar] [CrossRef]
- Spitzer, P.; Condic, M.; Herrmann, M.; Oberstein, T.J.; Scharin-Mehlmann, M.; Gilbert, D.F.; Friedrich, O.; Grömer, T.; Kornhuber, J.; Lang, R. Amyloidogenic amyloid-β-peptide variants induce microbial agglutination and exert antimicrobial activity. Sci. Rep. 2016, 6, 32228. [Google Scholar] [CrossRef] [PubMed]
- Kandel, N.; Zheng, T.; Huo, Q.; Tatulian, S.A. Membrane binding and pore formation by a cytotoxic fragment of amyloid β peptide. J. Phys. Chem. B 2017, 121, 10293–10305. [Google Scholar] [CrossRef] [PubMed]
- Sancho-Vaello, E.; François, P.; Bonetti, E.-J.; Lilie, H.; Finger, S.; Gil-Ortiz, F.; Gil-Carton, D.; Zeth, K. Structural remodeling and oligomerization of human cathelicidin on membranes suggest fibril-like structures as active species. Sci. Rep. 2017, 7, 15371. [Google Scholar] [CrossRef]
- Wong, C.Y.; Al-Salami, H.; Dass, C.R. Microparticles, microcapsules and microspheres: A review of recent developments and prospects for oral delivery of insulin. Int. J. Pharm. 2018, 537, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, R.; Vaidhya, I. Novel drug delivery systems: An overview. Int. J. Pharm. Sci. Res. 2013, 4, 970. [Google Scholar] [CrossRef]
- Uyen, N.T.T.; Hamid, Z.A.A.; Tram, N.X.T.; Ahmad, N. Fabrication of alginate microspheres for drug delivery: A review. Int. J. Biol. Macromol. 2020, 153, 1035–1046. [Google Scholar] [CrossRef]
- Solanki, N. Microspheres an innovative approach in drug delivery system. MOJ Bioequivalence Bioavailab. 2018, 5, 56–58. [Google Scholar] [CrossRef][Green Version]
- Ramteke, K.; Jadhav, V.; Dhole, S. Microspheres: As carrieres used for novel drug delivery system. Iosrphr 2012, 2, 44–48. [Google Scholar] [CrossRef]
- Jamini, M.; Rawat, S. A review on microsphere. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 1223–1233. [Google Scholar]
- Davis, S.S.; Illum, L. Polymeric microspheres as drug carriers. Biomaterials 1988, 9, 111–115. [Google Scholar] [CrossRef]
- Prajapati, V.D.; Jani, G.K.; Kapadia, J.R. Current knowledge on biodegradable microspheres in drug delivery. Expert Opin. Drug Deliv. 2015, 12, 1283–1299. [Google Scholar] [CrossRef]
- Horcajada, P.; Serre, C.; Vallet-Regí, M.; Sebban, M.; Taulelle, F.; Férey, G. Metal–organic frameworks as efficient materials for drug delivery. Angew. Chem. 2006, 118, 6120–6124. [Google Scholar] [CrossRef]
- Conde, J.; Doria, G.; Baptista, P. Noble metal nanoparticles applications in cancer. J. Drug Deliv. 2012, 26, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Noguez, C. Surface plasmons on metal nanoparticles: The influence of shape and physical environment. J. Phys. Chem. C 2007, 111, 3806–3819. [Google Scholar] [CrossRef]
- Wahajuddin, S.A. Superparamagnetic iron oxide nanoparticles: Magnetic nanoplatforms as drug carriers. Int. J. Nanomed. 2012, 7, 3445. [Google Scholar] [CrossRef]
- Kodama, R. Magnetic nanoparticles. J. Magn. Magn. Mater. 1999, 200, 359–372. [Google Scholar] [CrossRef]
- Iqbal, A.; Iqbal, K.; Li, B.; Gong, D.; Qin, W. Recent advances in iron nanoparticles: Preparation, properties, biological and environmental application. J. Nanosci. Nanotechnol. 2017, 17, 4386–4409. [Google Scholar] [CrossRef]
- Belenky, P.; Jonathan, D.Y.; Porter, C.B.; Cohen, N.R.; Lobritz, M.A.; Ferrante, T.; Jain, S.; Korry, B.J.; Schwarz, E.G.; Walker, G.C. Bactericidal antibiotics induce toxic metabolic perturbations that lead to cellular damage. Cell Rep. 2015, 13, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Rajchakit, U.; Sarojini, V. Recent developments in antimicrobial-peptide-conjugated gold nanoparticles. Bioconjugate Chem. 2017, 28, 2673–2686. [Google Scholar] [CrossRef]
- Zharov, V.P.; Mercer, K.E.; Galitovskaya, E.N.; Smeltzer, M.S. Photothermal nanotherapeutics and nanodiagnostics for selective killing of bacteria targeted with gold nanoparticles. Biophys. J. 2006, 90, 619–627. [Google Scholar] [CrossRef]
- Keren, S.; Zavaleta, C.; Cheng, Z.D.; de La Zerda, A.; Gheysens, O.; Gambhir, S. Noninvasive molecular imaging of small living subjects using Raman spectroscopy. Proc. Natl. Acad. Sci. USA 2008, 105, 5844–5849. [Google Scholar] [CrossRef]
- Durr, N.J.; Larson, T.; Smith, D.K.; Korgel, B.A.; Sokolov, K.; Ben-Yakar, A. Two-photon luminescence imaging of cancer cells using molecularly targeted gold nanorods. Nano Lett. 2007, 7, 941–945. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, W.; Tan, Y.; Ding, S. A label-free biosensor based on gold nanoshell monolayers for monitoring biomolecular interactions in diluted whole blood. Biosens. Bioelectron. 2008, 23, 1166–1170. [Google Scholar] [CrossRef]
- Chen, J.; Wang, D.; Xi, J.; Au, L.; Siekkinen, A.; Warsen, A.; Li, Z.-Y.; Zhang, H.; Xia, Y.; Li, X. Immuno gold nanocages with tailored optical properties for targeted photothermal destruction of cancer cells. Nano Lett. 2007, 7, 1318–1322. [Google Scholar] [CrossRef]
- Brühwiler, D. Postsynthetic functionalization of mesoporous silica. Nanoscale 2010, 2, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Watermann, A.; Brieger, J. Mesoporous silica nanoparticles as drug delivery vehicles in cancer. Nanomaterials 2017, 7, 189. [Google Scholar] [CrossRef] [PubMed]
- Nandiyanto, A.B.D.; Kim, S.-G.; Iskandar, F.; Okuyama, K. Synthesis of spherical mesoporous silica nanoparticles with nanometer-size controllable pores and outer diameters. Microporous Mesoporous Mater. 2009, 120, 447–453. [Google Scholar] [CrossRef]
- Asefa, T.; Tao, Z. Biocompatibility of mesoporous silica nanoparticles. Chem. Res. Toxicol. 2012, 25, 2265–2284. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Feng, N. Mesoporous silica nanoparticles: Synthesis, classification, drug loading, pharmacokinetics, biocompatibility, and application in drug delivery. Expert Opin. Drug Deliv. 2019, 16, 219–237. [Google Scholar] [CrossRef]
- Jia, L.; Shen, J.; Li, Z.; Zhang, D.; Zhang, Q.; Duan, C.; Liu, G.; Zheng, D.; Liu, Y.; Tian, X. Successfully tailoring the pore size of mesoporous silica nanoparticles: Exploitation of delivery systems for poorly water-soluble drugs. Int. J. Pharm. 2012, 439, 81–91. [Google Scholar] [CrossRef]
- Chen, B.; Quan, G.; Wang, Z.; Chen, J.; Wu, L.; Xu, Y.; Li, G.; Wu, C. Hollow mesoporous silicas as a drug solution delivery system for insoluble drugs. Powder Technol. 2013, 240, 48–53. [Google Scholar] [CrossRef]
- Mornet, S.; Lambert, O.; Duguet, E.; Brisson, A. The formation of supported lipid bilayers on silica nanoparticles revealed by cryoelectron microscopy. Nano Lett. 2005, 5, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Huh, S.; Wiench, J.W.; Yoo, J.-C.; Pruski, M.; Lin, V.S.-Y. Organic functionalization and morphology control of mesoporous silicas via a condensation synthesis method. Chem. Mater. 2003, 15, 4247–4256. [Google Scholar] [CrossRef]
- Ye, Q.; Chen, W.; Huang, H.; Tang, Y.; Wang, W.; Meng, F.; Wang, H.; Zheng, Y. Iron and zinc ions, potent weapons against multidrug-resistant bacteria. Appl. Microbiol. Biotechnol. 2020, 104, 5213–5227. [Google Scholar] [CrossRef] [PubMed]
- Jeffreys, A.J.; Wilson, V.; Thein, S.L. Individual-specific ‘fingerprints’ of human DNA. Nature 1985, 316, 76–79. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Z.; Zhang, Y. The application of carbon nanotubes in target drug delivery systems for cancer therapies. Nanoscale Res. Lett. 2011, 6, 555. [Google Scholar] [CrossRef]
- Li, Z.; de Barros, A.L.B.; Soares, D.C.F.; Moss, S.N.; Alisaraie, L. Functionalized single-walled carbon nanotubes: Cellular uptake, biodistribution and applications in drug delivery. Int. J. Pharm. 2017, 524, 41–54. [Google Scholar] [CrossRef]
- Wang, J.T.-W.; Al-Jamal, K.T. Functionalized carbon nanotubes: Revolution in brain delivery. Nanomedicine 2015, 10, 2639–2642. [Google Scholar] [CrossRef]
- Son, K.H.; Hong, J.H.; Lee, J.W. Carbon nanotubes as cancer therapeutic carriers and mediators. Int. J. Nanomed. 2016, 11, 5163. [Google Scholar] [CrossRef]
- Madani, S.Y.; Naderi, N.; Dissanayake, O.; Tan, A.; Seifalian, A.M. A new era of cancer treatment: Carbon nanotubes as drug delivery tools. Int. J. Nanomed. 2011, 6, 2963. [Google Scholar] [CrossRef]
- Kokel, A.; Torok, M. Recent advances in the development of antimicrobial peptides (AMPs): Attempts for sustainable medicine? Curr. Med. Chem. 2018, 25, 2503–2519. [Google Scholar] [CrossRef]
- Bai, Y.; Park, I.S.; Lee, S.J.; Bae, T.S.; Watari, F.; Uo, M.; Lee, M.H. Aqueous dispersion of surfactant-modified multiwalled carbon nanotubes and their application as an antibacterial agent. Carbon 2011, 49, 3663–3671. [Google Scholar] [CrossRef]
- Odom, T.W.; Huang, J.-L.; Kim, P.; Lieber, C.M. Atomic structure and electronic properties of single-walled carbon nanotubes. Nature 1998, 391, 62–64. [Google Scholar] [CrossRef]
- Popov, V.N. Carbon nanotubes: Properties and application. Mater. Sci. Eng. R Rep. 2004, 43, 61–102. [Google Scholar] [CrossRef]
- Endo, M.; Iijima, S.; Dresselhaus, M.S. Carbon Nanotubes; Elsevier: Oxford, UK, 1997. [Google Scholar]
- Hirlekar, R.; Yamagar, M.; Garse, H.; Vij, M.; Kadam, V. Carbon nanotubes and its applications: A review. Asian J. Pharm. Clin. Res. 2009, 2, 17–27. [Google Scholar]
- Walling, M.A.; Novak, J.A.; Shepard, J.R. Quantum dots for live cell and in vivo imaging. Int. J. Mol. Sci. 2009, 10, 441–491. [Google Scholar] [CrossRef]
- Matea, C.T.; Mocan, T.; Tabaran, F.; Pop, T.; Mosteanu, O.; Puia, C.; Iancu, C.; Mocan, L. Quantum dots in imaging, drug delivery and sensor applications. Int. J. Nanomed. 2017, 12, 5421. [Google Scholar] [CrossRef]
- Manna, S.; Ghosh, M.; Chakraborty, R.; Ghosh, S.; Mandal, S.M. A review on quantum dots: Synthesis to in-silico analysis as next generation antibacterial agents. Curr. Drug Targets 2019, 20, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Biswaro, L.S.; da Costa Sousa, M.G.; Rezende, T.; Dias, S.C.; Franco, O.L. Antimicrobial peptides and nanotechnology, recent advances and challenges. Front. Microbiol. 2018, 9, 855. [Google Scholar] [CrossRef] [PubMed]
- Storm, G.; Belliot, S.O.; Daemen, T.; Lasic, D.D. Surface modification of nanoparticles to oppose uptake by the mononuclear phagocyte system. Adv. Drug Deliv. Rev. 1995, 17, 31–48. [Google Scholar] [CrossRef]
- Lamprecht, A.; Ubrich, N.; Yamamoto, H.; Schäfer, U.; Takeuchi, H.; Maincent, P.; Kawashima, Y.; Lehr, C.-M. Biodegradable nanoparticles for targeted drug delivery in treatment of inflammatory bowel disease. J. Pharmacol. Exp. Ther. 2001, 299, 775–781. [Google Scholar] [PubMed]
- Liu, C.; Kou, Y.; Zhang, X.; Cheng, H.; Chen, X.; Mao, S. Strategies and industrial perspectives to improve oral absorption of biological macromolecules. Expert Opin. Drug Deliv. 2018, 15, 223–233. [Google Scholar] [CrossRef]
- Sneh-Edri, H.; Likhtenshtein, D.; Stepensky, D. Intracellular targeting of PLGA nanoparticles encapsulating antigenic peptide to the endoplasmic reticulum of dendritic cells and its effect on antigen cross-presentation in vitro. Mol. Pharm. 2011, 8, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-C.; Kim, Y.-M.; Lee, J.-K.; Kim, N.-H.; Kim, E.-J.; Heo, H.; Lee, M.-Y.; Lee, J.R.; Jang, M.-K. Targeting and synergistic action of an antifungal peptide in an antibiotic drug-delivery system. J. Control. Release 2017, 256, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.; Nascimento, J.; Romeu, A.; Estrada-López, E.; Pimentel, A. Penetration of antimicrobial peptides in a lung surfactant model. Colloids Surf. B Biointerfaces 2018, 167, 345–353. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46 Pt 1, 6387–6392. [Google Scholar]
- Vaupel, P.; Kallinowski, F.; Okunieff, P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: A review. Cancer Res. 1989, 49, 6449–6465. [Google Scholar]
- Dong, P.; Zhou, Y.; He, W.; Hua, D. A strategy for enhanced antibacterial activity against Staphylococcus aureus by the assembly of alamethicin with a thermo-sensitive polymeric carrier. Chem. Commun. 2016, 52, 896–899. [Google Scholar] [CrossRef]
- Villiers, C.L.; Freitas, H.; Couderc, R.; Villiers, M.-B.; Marche, P.N. Analysis of the toxicity of gold nano particles on the immune system: Effect on dendritic cell functions. J. Nanoparticle Res. 2010, 12, 55–60. [Google Scholar] [CrossRef]
- Wen, H.; Li, Y. Redox sensitive nanoparticles with disulfide bond linked sheddable shell for intracellular drug delivery. Med. Chem. 2014, 4, 748–755. [Google Scholar] [CrossRef]
- Andresen, T.L.; Jensen, S.S.; Jørgensen, K. Advanced strategies in liposomal cancer therapy: Problems and prospects of active and tumor specific drug release. Prog. Lipid Res. 2005, 44, 68–97. [Google Scholar] [CrossRef]
- Hua, M.-Y.; Liu, H.-L.; Yang, H.-W.; Chen, P.-Y.; Tsai, R.-Y.; Huang, C.-Y.; Tseng, I.-C.; Lyu, L.-A.; Ma, C.-C.; Tang, H.-J. The effectiveness of a magnetic nanoparticle-based delivery system for BCNU in the treatment of gliomas. Biomaterials 2011, 32, 516–527. [Google Scholar] [CrossRef]
- Yang, G.; Liu, J.; Wu, Y.; Feng, L.; Liu, Z. Near-infrared-light responsive nanoscale drug delivery systems for cancer treatment. Coord. Chem. Rev. 2016, 320, 100–117. [Google Scholar] [CrossRef]
- Novković, M.; Simunić, J.; Bojović, V.; Tossi, A.; Juretić, D. DADP: The database of anuran defense peptides. Bioinformatics 2012, 28, 1406–1407. [Google Scholar] [CrossRef]
- Kumari, V.; Nagaraj, R. Structure-function studies on the amphibian peptide brevinin 1E: Translocating the cationic segment from the C-terminal end to a central position favors selective antibacterial activity. J. Pept. Res. 2001, 58, 433–441. [Google Scholar] [CrossRef]
- Van Zoggel, H.; Hamma-Kourbali, Y.; Galanth, C.; Ladram, A.; Nicolas, P.; Courty, J.; Amiche, M.; Delbé, J. Antitumor and angiostatic peptides from frog skin secretions. Amino Acids 2012, 42, 385–395. [Google Scholar] [CrossRef]
- Dass, C.R.; Choong, P.F. Carrier-mediated delivery of peptidic drugs for cancer therapy. Peptides 2006, 27, 3020–3028. [Google Scholar] [CrossRef]
- Medeiros, K.A.; Joanitti, G.A.; Silva, L.P. Chitosan nanoparticles for dermaseptin peptide delivery toward tumor cells in vitro. Anti-Cancer Drugs 2014, 25, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Argiolas, A.; Pisano, J.J. Isolation and characterization of two new peptides, mastoparan C and crabrolin, from the venom of the European hornet, Vespa crabro. J. Biol. Chem. 1984, 259, 10106–10111. [Google Scholar] [CrossRef]
- Wade, D.; Silberring, J.; Soliymani, R.; Heikkinen, S.; Kilpeläinen, I.; Lankinen, H.; Kuusela, P. Antibacterial activities of temporin A analogs. FEBS Lett. 2000, 479, 6–9. [Google Scholar] [CrossRef]
- Zhao, H.; Rinaldi, A.C.; Di Giulio, A.; Simmaco, M.; Kinnunen, P.K. Interactions of the antimicrobial peptides temporins with model biomembranes. Comparison of temporins B and L. Biochemistry 2002, 41, 4425–4436. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-N.; Xu, X.; Wen, N.; Song, R.; Meng, Q.; Guan, Y.; Cheng, S.; Cao, D.; Dong, Y.; Qie, J. A drug carrier for sustained zero-order release of peptide therapeutics. Sci. Rep. 2017, 7, 5524. [Google Scholar] [CrossRef]
- Piotrowska, U.; Oledzka, E.; Zgadzaj, A.; Bauer, M.; Sobczak, M. A novel delivery system for the controlled Release~of antimicrobial peptides: Citropin 1.1 and temporin A. Polymers 2018, 10, 489. [Google Scholar] [CrossRef] [PubMed]
- Al Shaer, D.; Al Musaimi, O.; Albericio, F.; de la Torre, B.G. 2019 FDA TIDES (peptides and oligonucleotides) harvest. Pharmaceuticals 2020, 13, 40. [Google Scholar] [CrossRef] [PubMed]
- Bray, B.L. Large-scale manufacture of peptide therapeutics by chemical synthesis. Nat. Rev. Drug Discov. 2003, 2, 587–593. [Google Scholar] [CrossRef]
- Da Costa, J.P.; Cova, M.; Ferreira, R.; Vitorino, R. Antimicrobial peptides: An alternative for innovative medicines? Appl. Microbiol. Biotechnol. 2015, 99, 2023–2040. [Google Scholar] [CrossRef]
- Zazo, H.; Colino, C.I.; Lanao, J.M. Current applications of nanoparticles in infectious diseases. J. Control. Release 2016, 224, 86–102. [Google Scholar] [CrossRef]
- Mohammadi-Samani, S.; Taghipour, B. PLGA micro and nanoparticles in delivery of peptides and proteins; problems and approaches. Pharm. Dev. Technol. 2015, 20, 385–393. [Google Scholar] [CrossRef]
- Gallarate, M.; Battaglia, L.; Peira, E.; Trotta, M. Peptide-loaded solid lipid nanoparticles prepared through coacervation technique. Int. J. Chem. Eng. 2011, 2011, 132435. [Google Scholar] [CrossRef]
- Beyth, N.; Houri-Haddad, Y.; Domb, A.; Khan, W.; Hazan, R. Alternative antimicrobial approach: Nano-antimicrobial materials. Evid.-Based Complement. Altern. Med. 2015, 2015, 246012. [Google Scholar] [CrossRef]
- Narang, A.S.; Varia, S. Role of tumor vascular architecture in drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 640–658. [Google Scholar] [CrossRef] [PubMed]
- Solaro, R.; Chiellini, F.; Battisti, A. Targeted delivery of protein drugs by nanocarriers. Materials 2010, 3, 1928–1980. [Google Scholar] [CrossRef]
- Cho, H.; Naskar, A.; Lee, S.; Kim, S.; Kim, K.-S. A New Surface Charge Neutralizing Nano-Adjuvant to Potentiate Polymyxins in Killing Mcr-1 Mediated Drug-Resistant Escherichia coli. Pharmaceutics 2021, 13, 250. [Google Scholar] [CrossRef] [PubMed]



| System | Liposome | Micelle | Dendrimer | |||||
|---|---|---|---|---|---|---|---|---|
| Phospholipid Micelles | PLGA-PEG Micelles | PLL | PAMAM | PPI | Carbosilane | |||
| Delivery mechanism | Passive delivery | Passive delivery | Passive delivery | Passive delivery | Passive delivery | Passive delivery | Targeted delivery | Passive delivery |
| Peptide | Synthetic peptide | Alyteserin-1c | Peptide 73 | HnMc | G3KL | SB056 | SB105 | AMP31 |
| Target | Methicillin-resistant Staphylococcus aureus (MRSA) | Listeria monocytogenes, E. coli | MRSA | S. aureus, P. aeruginosa, E. coli | A. baumannii, P. aeruginosa | Enterococcus faecalis, Staphylococcus epidermidis, S. aureus | HPV infection of 293TT cells | E. coli, S. aureus |
| Potential application | Bacterial infection | Bacterial infection | High-density infections | Bacterial infection | Bacterial infection | Bacterial infection | HPV infection | Bacterial infection |
| Reference | [40] | [52] | [41] | [53] | [54,55] | [42] | [54] | [56] |
| System | Polymeric Nanoparticle | Liquid Crystalline System | Hydrogel | Mesoporous Silica Nanoparticle | |||
|---|---|---|---|---|---|---|---|
| LPN | PLGA | Chitosan | Cubic Phase | Hexagonal Mesophases | |||
| Delivery mechanism | Passive delivery | Passive delivery | Passive delivery | Passive delivery | Passive delivery | Passive delivery | Passive delivery |
| Peptide | Citropin 1.1 | GAM019 | Temporin B | D1–23 | AP114, DPK-060, and LL-37 | Lysozyme | LL-37 |
| Target | MRSA | MRSA, E. coli | S. epidermidis | Streptococcus mutans | MRSA, E. coli | Streptococcus ratti | E. coli |
| Potential application | Bacterial infection | Bacterial infection | Bacterial infection | Bacterial infection | Bacterial infection | Oral infection | Bacterial infection |
| Reference | [57] | [58] | [59] | [44] | [60] | [46] | [49] |
| System | Microsphere | Metal Nanocrystalline Material | Carbon Nanotube | Quantum Dot | ||
|---|---|---|---|---|---|---|
| PLGA/Chitosan (Micromatrix) | Alginate/Chitosan (Microcapsule) | Gold Nanoparticles | SPIONs | WS2 | ||
| Delivery Mechanism | Passive delivery | Passive delivery | Passive delivery | Passive delivery | Passive delivery | Targeted delivery |
| Peptide | KSL-W | Dermicidin-1-L | Cecropin-melittin | Ib-M2 | APs | KG18 and VR18 |
| Target | Fusobacterium nucleatum | S. aureus, Klebsiella pneumoniae | S. aureus, E. coli | E. coli | Streptococcus pyogenes, E. coli | P. aeruginosa, Candida albicans |
| Potential application | Oral infection | Bacterial infection | Bacterial infection | Bacterial infection | Bacterial infection | Antimicrobial therapy and bioimaging. |
| Reference | [47] | [61] | [48] | [62] | [50] | [63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Z.; Ma, Q.; Chen, X.; Chen, T.; Ying, Y.; Xi, X.; Wang, L.; Ma, C.; Shaw, C.; Zhou, M. Recent Advances and Challenges in Nanodelivery Systems for Antimicrobial Peptides (AMPs). Antibiotics 2021, 10, 990. https://doi.org/10.3390/antibiotics10080990
Tang Z, Ma Q, Chen X, Chen T, Ying Y, Xi X, Wang L, Ma C, Shaw C, Zhou M. Recent Advances and Challenges in Nanodelivery Systems for Antimicrobial Peptides (AMPs). Antibiotics. 2021; 10(8):990. https://doi.org/10.3390/antibiotics10080990
Chicago/Turabian StyleTang, Ziyan, Quantao Ma, Xiaoling Chen, Tianbao Chen, Yuan Ying, Xinping Xi, Lei Wang, Chengbang Ma, Chris Shaw, and Mei Zhou. 2021. "Recent Advances and Challenges in Nanodelivery Systems for Antimicrobial Peptides (AMPs)" Antibiotics 10, no. 8: 990. https://doi.org/10.3390/antibiotics10080990
APA StyleTang, Z., Ma, Q., Chen, X., Chen, T., Ying, Y., Xi, X., Wang, L., Ma, C., Shaw, C., & Zhou, M. (2021). Recent Advances and Challenges in Nanodelivery Systems for Antimicrobial Peptides (AMPs). Antibiotics, 10(8), 990. https://doi.org/10.3390/antibiotics10080990







