Ngn3-Positive Cells Arise from Pancreatic Duct Cells
Abstract
1. Introduction
2. Results
2.1. Observation of Ngn3-Positive Cells in the Ligated Part of the Pancreas at 3, 5, 6, and 21 Days after Surgery
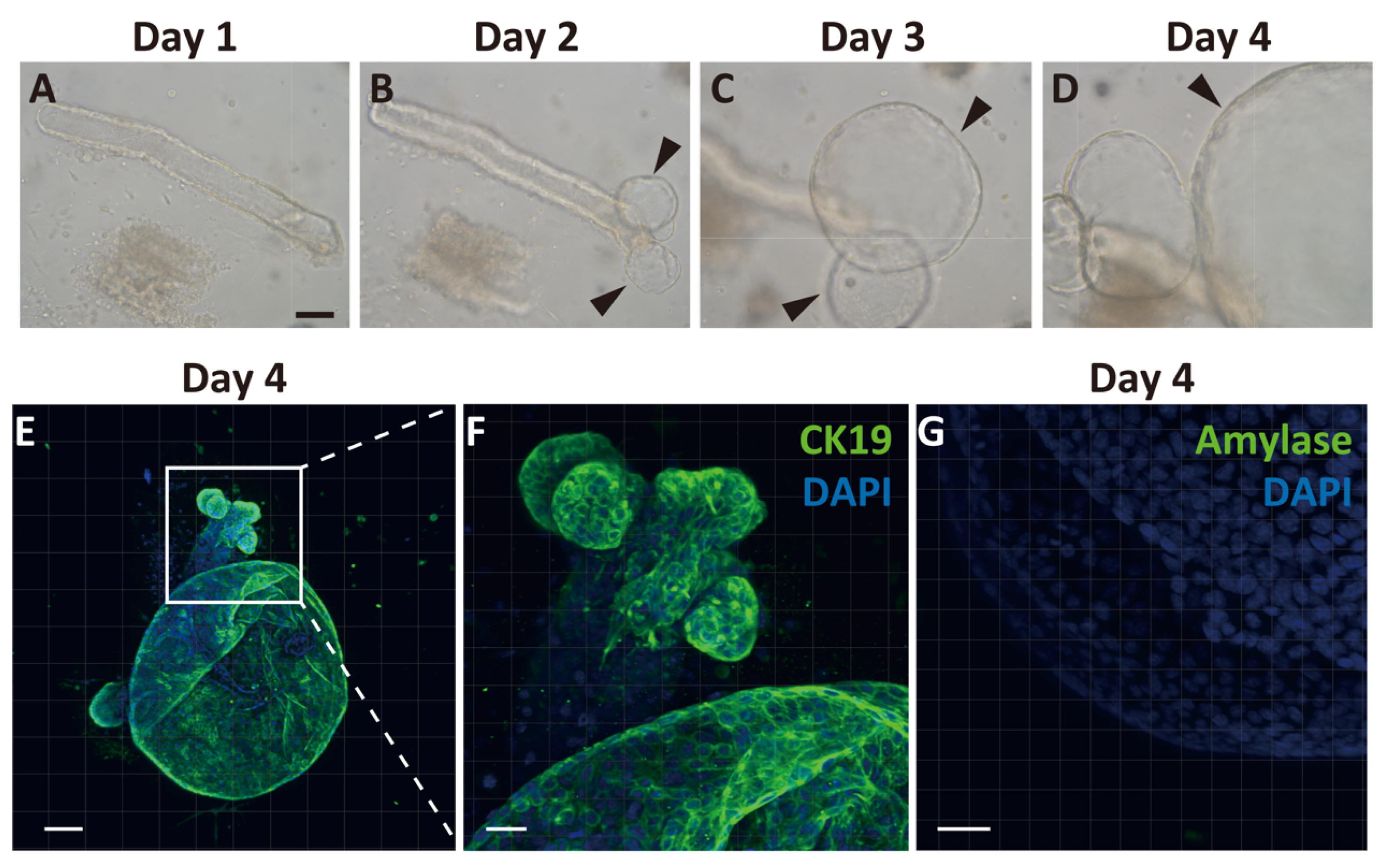
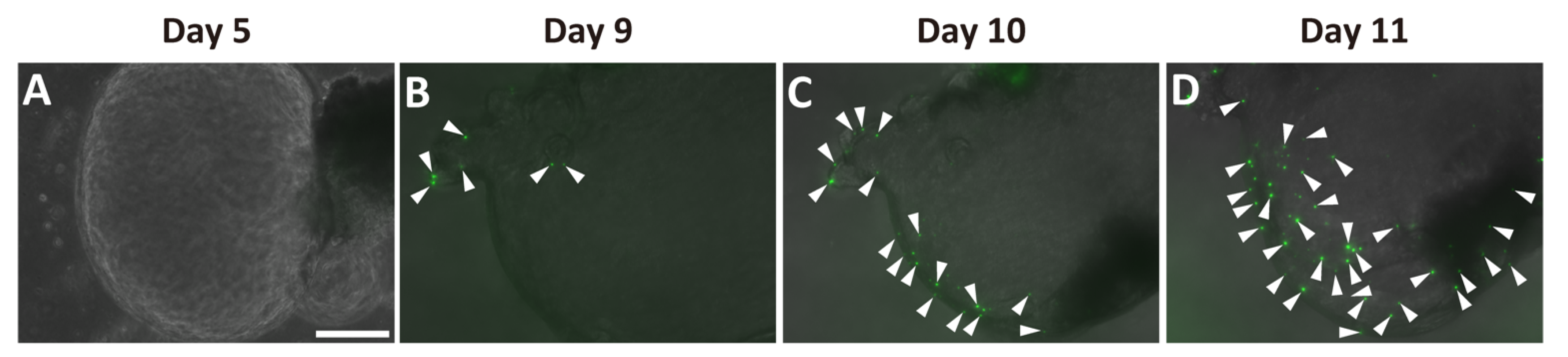
2.2. Generation of Pancreatic Ductal Organoids from Ngn3-GFP Mice
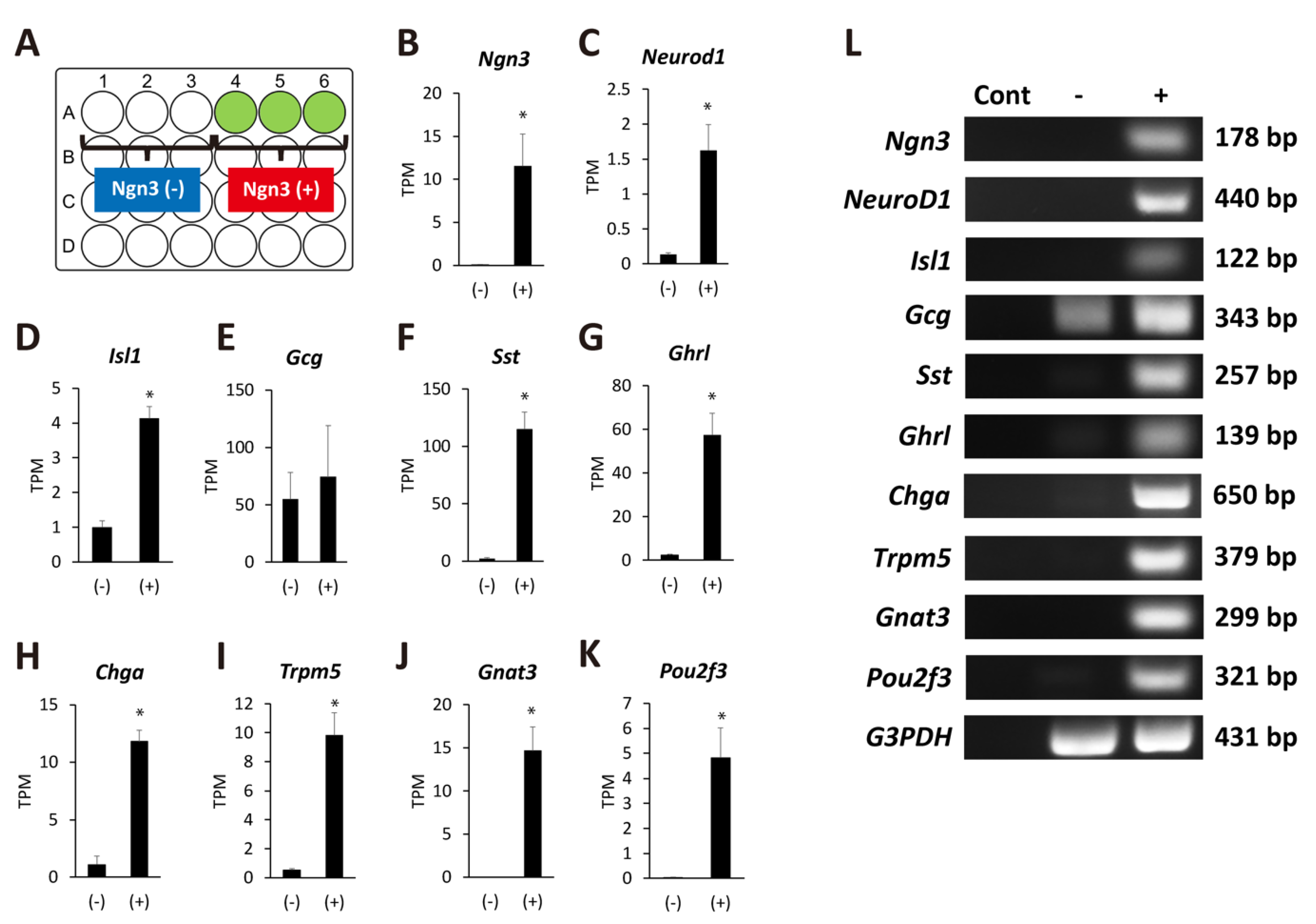
2.3. Ngn3-Positive Cells Can Differentiate into Endocrine Cells
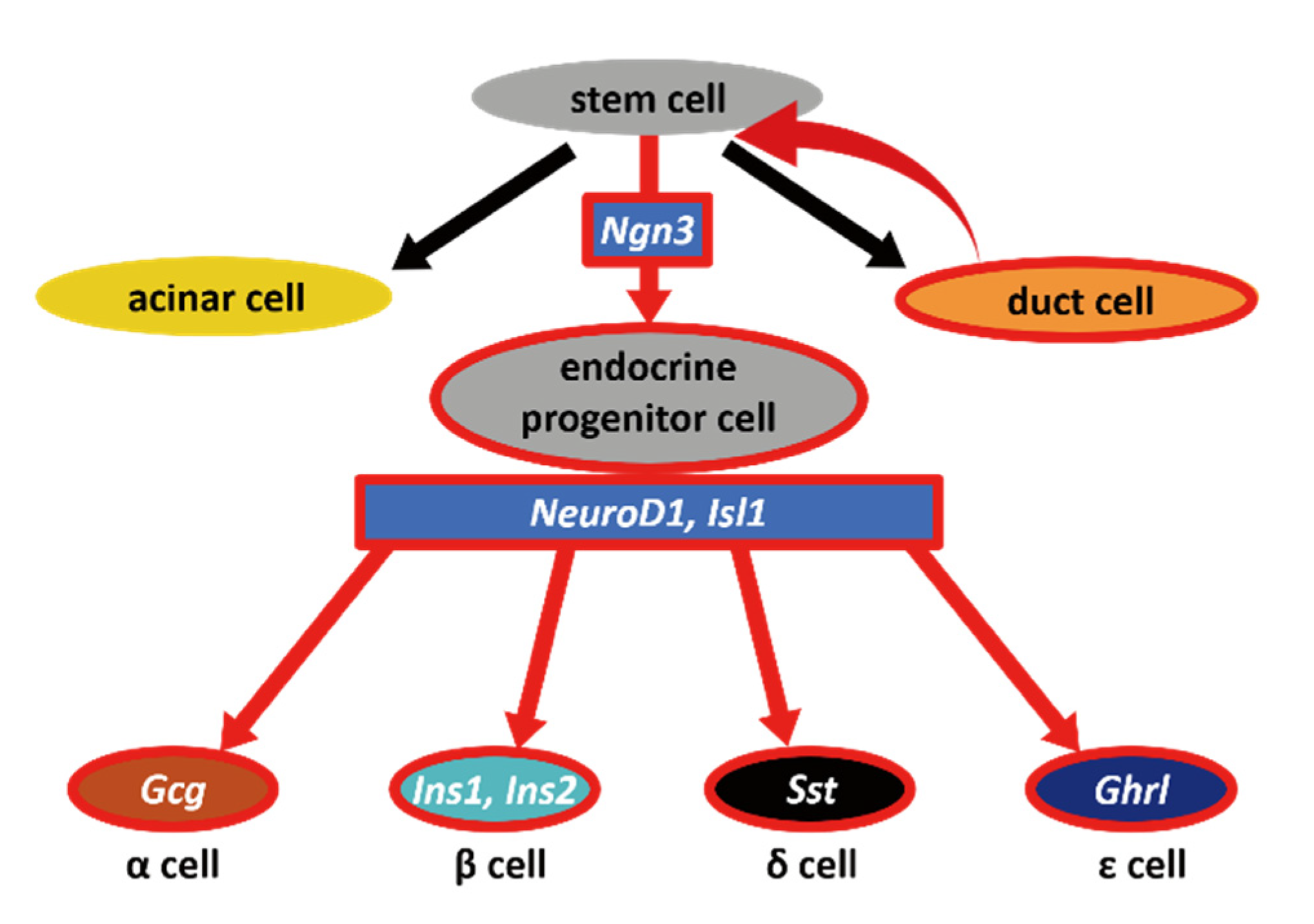
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. PDL after Cholyl-Lysyl-Fluorescein Injection
4.3. Immunohistochemistry
4.4. Organoid Culture
4.5. RNA-Seq Analysis
4.6. Statistical Analysis
4.7. Reverse Transcription (RT)-PCR
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, Q.; Melton, D.A. Pancreas regeneration. Nature 2018, 557, 351–358. [Google Scholar] [CrossRef]
- Loomans, C.J.M.; Williams Giuliani, N.; Balak, J.; Ringnalda, F.; van Gurp, L.; Huch, M.; Boj, S.F.; Sato, T.; Kester, L.; de Sousa Lopes, S.M.C.; et al. Expansion of adult human pancreatic tissue yields organoids harboring progenitor cells with endocrine differentiation potential. Stem Cell Rep. 2018, 10, 712–724. [Google Scholar] [CrossRef]
- Kopp, J.L.; Grompe, M.; Sander, M. Stem cells versus plasticity in liver and pancreas regeneration. Nat. Cell Biol. 2016, 18, 238–245. [Google Scholar] [CrossRef]
- Dor, Y.; Brown, J.; Martinez, O.I.; Melton, D.A. Adult pancreatic β-cells are formed by self-duplication rather than stem-cell differentiation. Nature 2004, 429, 41–46. [Google Scholar] [CrossRef]
- Matveyenko, A.V.; Veldhuis, J.D.; Butler, P.C. Adaptations in pulsatile insulin secretion, hepatic insulin clearance, and β-cell mass to age-related insulin resistance in rats. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E832–E841. [Google Scholar] [CrossRef]
- Sorenson, R.L.; Brelje, T.C. Adaptation of islets of Langerhans to pregnancy: β-cell growth, enhanced insulin secretion and the role of lactogenic hormones. Horm. Metab. Res. 1997, 29, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Kloppel, G.; Lohr, M.; Habich, K.; Oberholzer, M.; Heitz, P.U. Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Surv. Synth. Pathol. Res. 1985, 4, 110–125. [Google Scholar]
- Bruning, J.C.; Winnay, J.; Bonner-Weir, S.; Taylor, S.I.; Accili, D.; Kahn, C.R. Development of a novel polygenic model of NIDDM in mice heterozygous for IR and IRS-1 null alleles. Cell 1997, 88, 561–572. [Google Scholar] [CrossRef]
- Teta, M.; Rankin, M.M.; Long, S.Y.; Stein, G.M.; Kushner, J.A. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev. Cell 2007, 12, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Lipsett, M.; Finegood, D.T. β-cell neogenesis during prolonged hyperglycemia in rats. Diabetes 2002, 51, 1834–1841. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, X.; D’Hoker, J.; Stange, G.; Bonne, S.; De Leu, N.; Xiao, X.; Van de Casteele, M.; Mellitzer, G.; Ling, Z.; Pipeleers, D.; et al. β cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 2008, 132, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Nir, T.; Melton, D.A.; Dor, Y. Recovery from diabetes in mice by. β cell regeneration. J. Clin. Invest. 2007, 117, 2553–2561. [Google Scholar] [CrossRef] [PubMed]
- Szkudelski, T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol. Res. 2001, 50, 537–546. [Google Scholar]
- Thorel, F.; Damond, N.; Chera, S.; Wiederkehr, A.; Thorens, B.; Meda, P.; Wollheim, C.B.; Herrera, P.L. Normal glucagon signaling and β-cell function after near-total α-cell ablation in adult mice. Diabetes 2011, 60, 2872–2882. [Google Scholar] [CrossRef] [PubMed]
- Inada, A.; Nienaber, C.; Katsuta, H.; Fujitani, Y.; Levine, J.; Morita, R.; Sharma, A.; Bonner-Weir, S. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc. Natl. Acad. Sci. USA 2008, 105, 19915–19919. [Google Scholar] [CrossRef] [PubMed]
- Li, W.C.; Rukstalis, J.M.; Nishimura, W.; Tchipashvili, V.; Habener, J.F.; Sharma, A.; Bonner-Weir, S. Activation of pancreatic-duct-derived progenitor cells during pancreas regeneration in adult rats. J. Cell Sci. 2010, 123, 2792–2802. [Google Scholar] [CrossRef]
- Wang, R.N.; Kloppel, G.; Bouwens, L. Duct- to islet-cell differentiation and islet growth in the pancreas of duct-ligated adult rats. Diabetologia 1995, 38, 1405–1411. [Google Scholar] [CrossRef]
- Criscimanna, A.; Speicher, J.A.; Houshmand, G.; Shiota, C.; Prasadan, K.; Ji, B.; Logsdon, C.D.; Gittes, G.K.; Esni, F. Duct cells contribute to regeneration of endocrine and acinar cells following pancreatic damage in adult mice. Gastroenterology 2011, 141, 1451–1462.e6. [Google Scholar] [CrossRef] [PubMed]
- Huch, M.; Bonfanti, P.; Boj, S.F.; Sato, T.; Loomans, C.J.; van de Wetering, M.; Sojoodi, M.; Li, V.S.; Schuijers, J.; Gracanin, A.; et al. Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J. 2013, 32, 2708–2721. [Google Scholar] [CrossRef]
- Pan, F.C.; Bankaitis, E.D.; Boyer, D.; Xu, X.; Van de Casteele, M.; Magnuson, M.A.; Heimberg, H.; Wright, C.V. Spatiotemporal patterns of multipotentiality in Ptf1a-expressing cells during pancreas organogenesis and injury-induced facultative restoration. Development 2013, 140, 751–764. [Google Scholar] [CrossRef]
- Van de Casteele, M.; Leuckx, G.; Baeyens, L.; Cai, Y.; Yuchi, Y.; Coppens, V.; De Groef, S.; Eriksson, M.; Svensson, C.; Ahlgren, U.; et al. Neurogenin 3+ cells contribute to β-cell neogenesis and proliferation in injured adult mouse pancreas. Cell Death Dis. 2013, 4, e523. [Google Scholar] [CrossRef]
- Chintinne, M.; Stange, G.; Denys, B.; Ling, Z.; ’t Veld, P.; Pipeleers, D. Beta cell count instead of beta cell mass to assess and localize growth in beta cell population following pancreatic duct ligation in mice. PLoS ONE 2012, 7, e43959. [Google Scholar] [CrossRef]
- Kopp, J.L.; Dubois, C.L.; Schaffer, A.E.; Hao, E.; Shih, H.P.; Seymour, P.A.; Ma, J.; Sander, M. Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development 2011, 138, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Rankin, M.M.; Wilbur, C.J.; Rak, K.; Shields, E.J.; Granger, A.; Kushner, J.A. β-Cells are not generated in pancreatic duct ligation-induced injury in adult mice. Diabetes 2013, 62, 1634–1645. [Google Scholar] [CrossRef]
- Nakajima, C.; Kamimoto, K.; Miyajima, K.; Matsumoto, M.; Okazaki, Y.; Kobayashi-Hattori, K.; Shimizu, M.; Yamane, T.; Oishi, Y.; Iwatsuki, K. A method for identifying mouse pancreatic ducts. Tissue Eng. C Methods 2018, 24, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.X.; Li, K.; Archer, M.; Mehta, M.; Jamieson, E.; Charles, A.; Dickinson, J.E.; Matsumoto, M.; Morahan, G. Differentiation of Islet Progenitors Regulated by Nicotinamide into Transcriptome-Verified beta Cells That Ameliorate Diabetes. Stem Cells 2017, 35, 1341–1354. [Google Scholar] [CrossRef] [PubMed]
- Gradwohl, G.; Dierich, A.; LeMeur, M.; Guillemot, F. Neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc. Natl. Acad. Sci. USA 2000, 97, 1607–1611. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Watanabe, A.; Nakano, Y.; Matsumoto, M.; Okazaki, Y.; Miyajima, A. Reversible expansion of pancreatic islet progenitors derived from human induced pluripotent stem cells. Genes Cells 2020, 25, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Van de Casteele, M.; Leuckx, G.; Cai, Y.; Yuchi, Y.; Coppens, V.; De Groef, S.; Van Gassen, N.; Baeyens, L.; Heremans, Y.; Wright, C.V.; et al. Partial duct ligation: β-Cell proliferation and beyond. Diabetes 2014, 63, 2567–2577. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.P.; Kopp, J.L.; Sandhu, M.; Dubois, C.L.; Seymour, P.A.; Grapin-Botton, A.; Sander, M. A notch-dependent molecular circuitry initiates pancreatic endocrine and ductal cell differentiation. Development 2012, 139, 2488–2499. [Google Scholar] [CrossRef] [PubMed]
- Minami, K.; Okuno, M.; Miyawaki, K.; Okumachi, A.; Ishizaki, K.; Oyama, K.; Kawaguchi, M.; Ishizuka, N.; Iwanaga, T.; Seino, S. Lineage tracing and characterization of insulin-secreting cells generated from adult pancreatic acinar cells. Proc. Natl. Acad. Sci. USA 2005, 102, 15116–15121. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, Y.; Maitra, A.; Ghosh, B.; Zechner, U.; Argani, P.; Iacobuzio-Donahue, C.A.; Sriuranpong, V.; Iso, T.; Meszoely, I.M.; Wolfe, M.S.; et al. Notch mediates TGFα-induced changes in epithelial differentiation during pancreatic tumorigenesis. Cancer Cell 2003, 3, 565–576. [Google Scholar] [CrossRef]
- Miyatsuka, T.; Kaneto, H.; Shiraiwa, T.; Matsuoka, T.A.; Yamamoto, K.; Kato, K.; Nakamura, Y.; Akira, S.; Takeda, K.; Kajimoto, Y.; et al. Persistent expression of PDX-1 in the pancreas causes acinar-to-ductal metaplasia through Stat3 activation. Genes Dev. 2006, 20, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
- Rooman, I.; Heremans, Y.; Heimberg, H.; Bouwens, L. Modulation of rat pancreatic acinoductal transdifferentiation and expression of PDX-1 in vitro. Diabetologia 2000, 43, 907–914. [Google Scholar] [CrossRef]
- Schutz, B.; Ruppert, A.L.; Strobel, O.; Lazarus, M.; Urade, Y.; Buchler, M.W.; Weihe, E. Distribution pattern and molecular signature of cholinergic tuft cells in human gastro-intestinal and pancreatic-biliary tract. Sci. Rep. 2019, 9, 17466. [Google Scholar] [CrossRef]
- Hofer, D.; Drenckhahn, D. Identification of the taste cell G-protein, α-gustducin, in brush cells of the rat pancreatic duct system. Histochem. Cell Biol. 1998, 110, 303–309. [Google Scholar] [CrossRef]
- Von Moltke, J.; Ji, M.; Liang, H.E.; Locksley, R.M. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 2016, 529, 221–225. [Google Scholar] [CrossRef]
- Gerbe, F.; Sidot, E.; Smyth, D.J.; Ohmoto, M.; Matsumoto, I.; Dardalhon, V.; Cesses, P.; Garnier, L.; Pouzolles, M.; Brulin, B.; et al. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 2016, 529, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Howitt, M.R.; Lavoie, S.; Michaud, M.; Blum, A.M.; Tran, S.V.; Weinstock, J.V.; Gallini, C.A.; Redding, K.; Margolskee, R.F.; Osborne, L.C.; et al. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science 2016, 351, 1329–1333. [Google Scholar] [CrossRef]
- Nadjsombati, M.S.; McGinty, J.W.; Lyons-Cohen, M.R.; Jaffe, J.B.; DiPeso, L.; Schneider, C.; Miller, C.N.; Pollack, J.L.; Nagana Gowda, G.A.; Fontana, M.F.; et al. Detection of succinate by intestinal tuft cells triggers a type 2 innate immune circuit. Immunity 2018, 49, 33–41.e7. [Google Scholar] [CrossRef]
- Kaneko, K.; Kamimoto, K.; Miyajima, A.; Itoh, T. Adaptive remodeling of the biliary architecture underlies liver homeostasis. Hepatology 2015, 61, 2056–2066. [Google Scholar] [CrossRef] [PubMed]
- Tanimizu, N.; Nishikawa, M.; Saito, H.; Tsujimura, T.; Miyajima, A. Isolation of hepatoblasts based on the expression of Dlk/Pref-1. J. Cell Sci. 2003, 116, 1775–1786. [Google Scholar] [CrossRef] [PubMed]
- Broutier, L.; Andersson-Rolf, A.; Hindley, C.J.; Boj, S.F.; Clevers, H.; Koo, B.K.; Huch, M. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat. Protoc. 2016, 11, 1724–1743. [Google Scholar] [CrossRef] [PubMed]
- Iwatsuki, K.; Oda, M.; Sun, W.; Tanaka, S.; Ogawa, T.; Shiota, K. Molecular cloning and characterization of a new member of the rat placental prolactin (PRL) family, PRL-like protein H. Endocrinology 1998, 139, 4976–4983. [Google Scholar] [CrossRef] [PubMed][Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimura-Nakajima, C.; Sakaguchi, K.; Hatano, Y.; Matsumoto, M.; Okazaki, Y.; Tanaka, K.; Yamane, T.; Oishi, Y.; Kamimoto, K.; Iwatsuki, K. Ngn3-Positive Cells Arise from Pancreatic Duct Cells. Int. J. Mol. Sci. 2021, 22, 8548. https://doi.org/10.3390/ijms22168548
Kimura-Nakajima C, Sakaguchi K, Hatano Y, Matsumoto M, Okazaki Y, Tanaka K, Yamane T, Oishi Y, Kamimoto K, Iwatsuki K. Ngn3-Positive Cells Arise from Pancreatic Duct Cells. International Journal of Molecular Sciences. 2021; 22(16):8548. https://doi.org/10.3390/ijms22168548
Chicago/Turabian StyleKimura-Nakajima, Chiemi, Kousuke Sakaguchi, Yoshiko Hatano, Masahito Matsumoto, Yasushi Okazaki, Keisuke Tanaka, Takumi Yamane, Yuichi Oishi, Kenji Kamimoto, and Ken Iwatsuki. 2021. "Ngn3-Positive Cells Arise from Pancreatic Duct Cells" International Journal of Molecular Sciences 22, no. 16: 8548. https://doi.org/10.3390/ijms22168548
APA StyleKimura-Nakajima, C., Sakaguchi, K., Hatano, Y., Matsumoto, M., Okazaki, Y., Tanaka, K., Yamane, T., Oishi, Y., Kamimoto, K., & Iwatsuki, K. (2021). Ngn3-Positive Cells Arise from Pancreatic Duct Cells. International Journal of Molecular Sciences, 22(16), 8548. https://doi.org/10.3390/ijms22168548





