Oxytocin and Bone: Review and Perspectives
Abstract
:1. Introduction
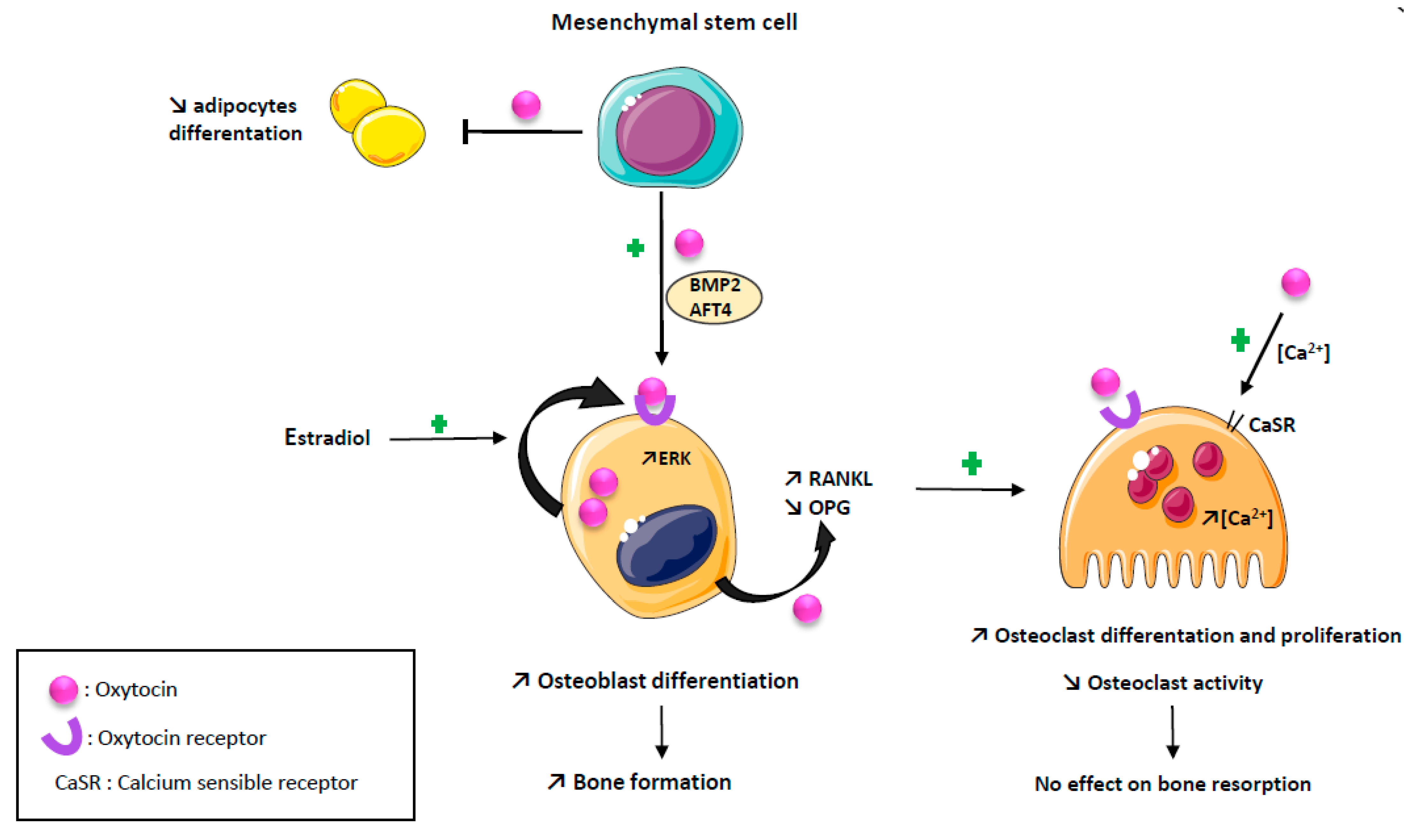
2. Oxytocin and Bone Cells
2.1. Oxytocin and Osteoblasts
2.2. Oxytocin and Osteoclasts
3. Oxytocin and Bone: Animal Studies
3.1. OT and Bone Cells
3.2. OT and Bone Microarchitecture
3.3. OT and Bone Marrow Adiposity
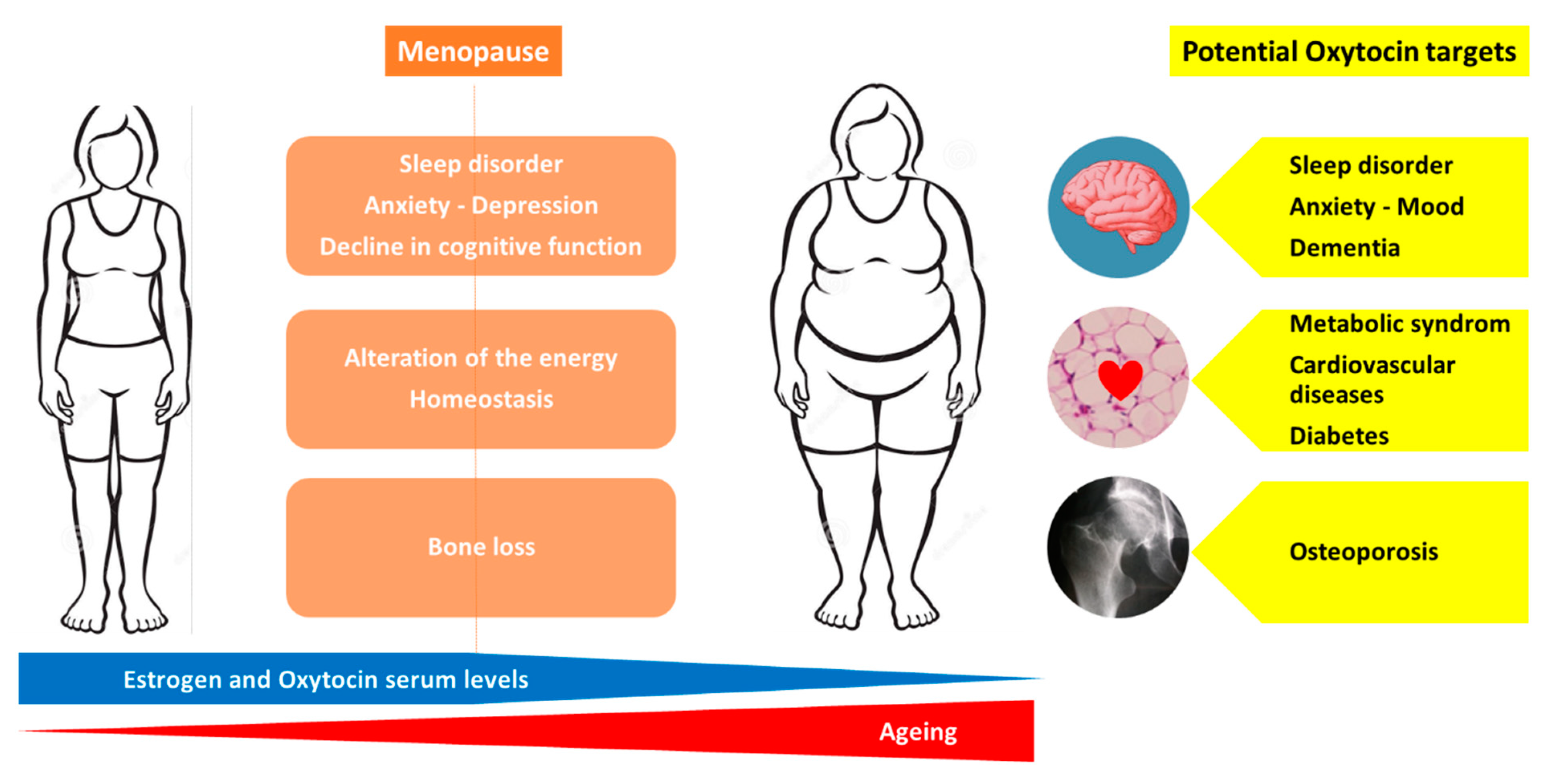
4. Oxytocin and Osteoporosis: Human Data
5. Oxytocin as a Therapeutic Agent: Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Carter, C.S.; Kenkel, W.M.; MacLean, E.L.; Wilson, S.R.; Perkeybile, A.M.; Yee, J.R.; Ferris, C.F.; Nazarloo, H.P.; Porges, S.W.; Davis, J.M.; et al. Is Oxytocin «Nature’s Medicine»? Pharmacol. Rev. 2020, 72, 829–861. [Google Scholar] [CrossRef]
- Elabd, S.; Sabry, I. Two Birds with One Stone: Possible Dual-Role of Oxytocin in the Treatment of Diabetes and Osteoporosis. Front. Endocrinol. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet Lond. Engl. 2019, 393, 364–376. [Google Scholar] [CrossRef]
- Leng, G.; Sabatier, N. Oxytocin—The Sweet Hormone? Trends Endocrinol. Metab. TEM 2017, 28, 365–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Tamma, R.; Yuen, T.; Colaianni, G.; Ji, Y.; Cuscito, C.; Bailey, J.; Dhawan, S.; Lu, P.; Calvano, C.D.; et al. Functions of vasopressin and oxytocin in bone mass regulation. Proc. Natl. Acad. Sci. USA 2016, 113, 164–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Benedetto, A.; Sun, L.; Zambonin, C.G.; Tamma, R.; Nico, B.; Calvano, C.D.; Colaianni, G.; Ji, Y.; Mori, G.; Grano, M.; et al. Osteoblast regulation via ligand-activated nuclear trafficking of the oxytocin receptor. Proc. Natl. Acad. Sci. USA 2014, 111, 16502–16507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosen, C.J. The Epidemiology and Pathogenesis of Osteoporosis. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dungan, K., Grossman, A., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Liu, J.; Curtis, E.; Cooper, C.; Harvey, N.C. State of the art in osteoporosis risk assessment and treatment. J. Endocrinol. Investig. 2019, 42, 1149–1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Copland, J.A.; Ives, K.L.; Simmons, D.J.; Soloff, M.S. Functional oxytocin receptors discovered in human osteoblasts. Endocrinology 1999, 140, 4371–4374. [Google Scholar] [CrossRef] [PubMed]
- Colucci, S.; Colaianni, G.; Mori, G.; Grano, M.; Zallone, A. Human osteoclasts express oxytocin receptor. Biochem. Biophys. Res. Commun. 2002, 297, 442–445. [Google Scholar] [CrossRef]
- Elabd, C.; Basillais, A.; Beaupied, H.; Breuil, V.; Wagner, N.; Scheideler, M.; Zaragosi, L.-E.; Massiéra, F.; Lemichez, E.; Trajanoski, Z.; et al. Oxytocin controls differentiation of human mesenchymal stem cells and reverses osteoporosis. Stem Cells 2008, 26, 2399–2407. [Google Scholar] [CrossRef] [Green Version]
- Tamma, R.; Colaianni, G.; Zhu, L.-L.; DiBenedetto, A.; Greco, G.; Montemurro, G.; Patano, N.; Strippoli, M.; Vergari, R.; Mancini, L.; et al. Oxytocin is an anabolic bone hormone. Proc. Natl. Acad. Sci. USA 2009, 106, 7149–7154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colaianni, G.; Sun, L.; Zaidi, M.; Zallone, A. Oxytocin and bone. Am. J. Physiol. Integr. Comp. Physiol. 2014, 307, R970–R977. [Google Scholar] [CrossRef]
- Richard, S.; Zingg, H.H. The human oxytocin gene promoter is regulated by estrogens. J. Biol. Chem. 1990, 265, 6098–6103. [Google Scholar] [CrossRef]
- Gimpl, G.; Fahrenholz, F. The Oxytocin Receptor System: Structure, Function, and Regulation. Physiol. Rev. 2001, 81, 629–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colaianni, G.; Di Benedetto, A.; Zhu, L.-L.; Tamma, R.; Li, J.; Greco, G.; Peng, Y.; Dell’Endice, S.; Zhu, G.; Cuscito, C.; et al. Regulated production of the pituitary hormone oxytocin from murine and human osteoblasts. Biochem. Biophys. Res. Commun. 2011, 411, 512–515. [Google Scholar] [CrossRef] [PubMed]
- Zallone, A. Direct and Indirect Estrogen Actions on Osteoblasts and Osteoclasts. Ann. N. Y. Acad. Sci. 2006, 1068, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, M.; New, M.I.; Blair, H.C.; Zallone, A.; Baliram, R.; Davies, T.F.; Cardozo, C.; Iqbal, J.; Sun, L.; Rosen, C.J.; et al. Actions of pituitary hormones beyond traditional targets. J. Endocrinol. 2018, 237, R83–R98. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, G.; Tamma, R.; Di Benedetto, A.; Yuen, T.; Sun, L.; Zaidi, M.; Zallone, A. The Oxytocin-Bone Axis. J. Neuroendocr. 2014, 26, 53–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beranger, G.E.; Pisani, D.F.; Castel, J.; Djedaini, M.; Battaglia, S.; Amiaud, J.; Boukhechba, F.; Ailhaud, G.; Michiels, J.-F.; Heymann, D.; et al. Oxytocin Reverses Ovariectomy-Induced Osteopenia and Body Fat Gain. Endocrinology 2014, 155, 1340–1352. [Google Scholar] [CrossRef] [Green Version]
- Moghazy, H.; Mahmoud, A.; ElBadre, H.; Aziz, H.O.A. Protective Effect of Oxytocin Against Bone Loss in a Female Rat Model of Osteoporosis. Rep. Biochem. Mol. Biol. 2020, 9, 147–155. [Google Scholar] [CrossRef]
- Qiu, Y.; Tang, C.; Serrano-Sosa, M.; Hu, J.; Zhu, J.; Tang, G.; Huang, C.; Huang, M. Bone microarchitectural parameters can detect oxytocin induced changes prior to bone density on mitigating bone deterioration in rabbit osteoporosis model using micro-CT. BMC Musculoskelet. Disord. 2019, 20, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Beranger, G.; Djedaini, M.; Battaglia, S.; Roux, C.H.; Scheideler, M.; Heymann, D.; Amri, E.-Z.; Pisani, D.F. Oxytocin Reverses Osteoporosis in a Sex-Dependent Manner. Front. Endocrinol. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Lan, L.; Li, T.; Li, J.; Li, Y. The effect of oxytocin on osseointegration of titanium implant in ovariectomized rats. Connect. Tissue Res. 2016, 57, 220–225. [Google Scholar] [CrossRef]
- Akay, A.S.; Arısan, V.; Cevher, E.; Sessevmez, M.; Cam, B. Oxytocin-loaded sustained-release hydrogel graft provides accelerated bone formation: An experimental rat study. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2020, 38, 1676–1687. [Google Scholar] [CrossRef] [PubMed]
- During, A. Osteoporosis: A role for lipids. Biochimie 2020, 178, 49–55. [Google Scholar] [CrossRef]
- Amri, E.-Z.; Pisani, D.F. Control of bone and fat mass by oxytocin. Horm. Mol. Biol. Clin. Investig. 2016, 28, 95–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Paula, F.J.A.; Rosen, C.J. Marrow Adipocytes: Origin, Structure, and Function. Annu. Rev. Physiol. 2020, 82, 461–484. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.; Yao, J.; Wu, X.; Zhou, B.; Shao, H.; Hua, T.; Xiong, Z.; Tang, G. Longitudinal assessment of oxytocin efficacy on bone and bone marrow fat masses in a rabbit osteoporosis model through 3.0-T magnetic resonance spectroscopy and micro-CT. Osteoporos. Int. 2015, 26, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Lawson, E.A.; Donoho, D.A.; Blum, J.I.; Meenaghan, E.M.; Misra, M.; Herzog, D.B.; Sluss, P.M.; Miller, K.K.; Klibanski, A. Decreased Nocturnal Oxytocin Levels in Anorexia Nervosa Are Associated With Low Bone Mineral Density and Fat Mass. J. Clin. Psychiatry 2011, 72, 1546–1551. [Google Scholar] [CrossRef] [Green Version]
- Breuil, V.; Amri, E.-Z.; Panaia-Ferrari, P.; Testa, J.; Elabd, C.; Albert-Sabonnadière, C.; Roux, C.; Ailhaud, G.; Dani, C.; Carle, G.F.; et al. Oxytocin and bone remodelling: Relationships with neuropituitary hormones, bone status and body composition. Jt. Bone Spine 2011, 78, 611–615. [Google Scholar] [CrossRef]
- Lawson, E.; E Ackerman, K.; Estella, N.M.; Guereca, G.; Pierce, L.; Sluss, P.M.; Bouxsein, M.L.; Klibanski, A.; Misra, M. Nocturnal oxytocin secretion is lower in amenorrheic athletes than nonathletes and associated with bone microarchitecture and finite element analysis parameters. Eur. J. Endocrinol. 2013, 168, 457–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breuil, V.; Panaia-Ferrari, P.; Fontas, E.; Roux, C.; Kolta, S.; Eastell, R.; Ben Yahia, H.; Faure, S.; Gossiel, F.; Benhamou, C.-L.; et al. Oxytocin, a New Determinant of Bone Mineral Density in Post-Menopausal Women: Analysis of the OPUS Cohort. J. Clin. Endocrinol. Metab. 2014, 99, E634–E641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breuil, V.; Fontas, E.; Chapurlat, R.; Panaia-Ferrari, P.; Yahia, H.B.; Faure, S.; Euller-Ziegler, L.; Amri, E.Z.; Szulc, P. Oxytocin and bone status in men: Analysis of the MINOS cohort. Osteoporos. Int. 2015, 26, 2877–2882. [Google Scholar] [CrossRef] [PubMed]
- Maestrini, S.; Mele, C.; Mai, S.; Vietti, R.; Di Blasio, A.; Castello, L.M.; Surico, D.; Aimaretti, G.; Scacchi, M.; Marzullo, P. Plasma Oxytocin Concentration in Pre- and Postmenopausal Women: Its Relationship with Obesity, Body Composition and Metabolic Variables. Obes. Facts 2018, 11, 429–439. [Google Scholar] [CrossRef]
- Aulinas, A.; Guarda, F.J.; Yu, E.W.; Haines, M.S.; Asanza, E.; Silva, L.; Tritos, N.A.; Verbalis, J.; Miller, K.K.; Lawson, E.A. Lower Oxytocin Levels Are Associated with Lower Bone Mineral Density and Less Favorable Hip Geometry in Hypopituitary Men. Neuroendocrinology 2021, 111, 87–98. [Google Scholar] [CrossRef]
- Vanderschueren, D.; Laurent, M.; Claessens, F.; Gielen, E.; Lagerquist, M.; Vandenput, L.; Börjesson, A.E.; Ohlsson, C. Sex Steroid Actions in Male Bone. Endocr. Rev. 2014, 35, 906–960. [Google Scholar] [CrossRef]
- Emmanuelle, N.-E.; Marie-Cécile, V.; Florence, T.; Jean-Francois, A.; Françoise, L.; Coralie, F.; Alexia, V. Critical Role of Estrogens on Bone Homeostasis in Both Male and Female: From Physiology to Medical Implications. Int. J. Mol. Sci. 2021, 22, 1568. [Google Scholar] [PubMed]
- Hung, C.; Muñoz, M.; Shibli-Rahhal, A. Anorexia Nervosa and Osteoporosis. Calcif. Tissue Int. 2021, 5, 1–14. [Google Scholar] [CrossRef]
- Jankowski, M.; Broderick, T.L.; Gutkowska, J. The Role of Oxytocin in Cardiovascular Protection. Front. Psychol. 2020, 11. [Google Scholar] [CrossRef]
- Tampi, R.R.; Maksimowski, M.; Ahmed, M.; Tampi, D.J. Oxytocin for frontotemporal dementia: A systematic review. Ther. Adv. Psychopharmacol. 2016, 7, 48–53. [Google Scholar] [CrossRef] [Green Version]
- McKay, E.C.; Beck, J.S.; Khoo, S.K.; Dykema, K.J.; Cottingham, S.L.; E Winn, M.; Paulson, H.L.; Lieberman, A.P.; E Counts, S. Peri-Infarct Upregulation of the Oxytocin Receptor in Vascular Dementia. J. Neuropathol. Exp. Neurol. 2019, 78, 436–452. [Google Scholar] [CrossRef]
- McKay, E.C.; Counts, S.E. Oxytocin Receptor Signaling in Vascular Function and Stroke. Front. Neurosci. 2020, 14. [Google Scholar] [CrossRef] [PubMed]
- Abramova, O.; Zorkina, Y.; Ushakova, V.; Zubkov, E.; Morozova, A.; Chekhonin, V. The role of oxytocin and vasopressin dysfunction in cognitive impairment and mental disorders. Neuropeptides 2020, 83. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gruber, C.; Alewood, P.F.; Möller, A.; Muttenthaler, M. The oxytocin receptor signalling system and breast cancer: A critical review. Oncogene 2020, 39, 5917–5932. [Google Scholar] [CrossRef]
- Yang, F.; Li, N.; Gaman, M.-A.; Wang, N. Raloxifene has favorable effects on the lipid profile in women explaining its beneficial effect on cardiovascular risk: A meta-analysis of randomized controlled trials. Pharmacol. Res. 2021, 166. [Google Scholar] [CrossRef]
- Rozenberg, S.; Al-Daghri, N.; Aubertin-Leheudre, M.; Brandi, M.; Cano, A.; Collins, P.; Cooper, C.; Genazzani, A.R.; Hillard, T.; Kanis, J.A.; et al. Is there a role for menopausal hormone therapy in the management of postmenopausal osteoporosis? Osteoporos. Int. 2020, 31, 2271–2286. [Google Scholar] [CrossRef]
- Pinkerton, J.V. Hormone Therapy for Postmenopausal Women. N. Engl. J. Med. 2020, 382, 446–455. [Google Scholar] [CrossRef]
- Davis, S.R.; Lambrinoudaki, I.; Lumsden, M.; Mishra, G.D.; Pal, L.; Rees, M.; Santoro, N.; Simoncini, T. Menopause. Nat. Rev. Dis. Prim. 2015, 1. [Google Scholar] [CrossRef]
- Mancuso, P.; Bouchard, B. The Impact of Aging on Adipose Function and Adipokine Synthesis. Front. Endocrinol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Pugliese, G.; Barrea, L.; Laudisio, D.; Aprano, S.; Castellucci, B.; Framondi, L.; di Matteo, R.; Savastano, S.; Colao, A.; Muscogiur, G. Mediterranean diet as tool to manage obesity in menopause: A narrative review. Nutrition 2020, 79–80. [Google Scholar] [CrossRef] [PubMed]
- Ambikairajah, A.; Walsh, E.; Tabatabaei-Jafari, H.; Cherbuin, N. Fat mass changes during menopause: A metaanalysis. Am. J. Obstet. Gynecol. 2019, 221, 393–409.e50. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, E.; Collazo-Clavell, M.L.; Faubion, S.S. Weight Gain in Women at Midlife: A Concise Review of the Pathophysiology and Strategies for Management. Mayo Clin. Proc. 2017, 92, 1552–1558. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.; Stringhetta-Garcia, C.T.; Peres-Ueno, M.J.; Fernandes, F.; de Nicola, A.C.; Castoldi, R.C.; Ozaki, G.; Louzada, M.J.Q.; Chaves-Neto, A.H.; Ervolino, E.; et al. Oxytocin and bone quality in the femoral neck of rats in periestropause. Sci. Rep. 2020, 10, 7937. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Mora, J.J.; Cortés-Sierra, L.; García-Pérez, M.-Á.; Tarín, J.J.; Cano, A. Diet to Reduce the Metabolic Syndrome Associated with Menopause. The Logic for Olive Oil. Nutrients 2020, 12, 3184. [Google Scholar] [CrossRef]
- Lawson, E.A.; Olszewski, P.K.; Weller, A.; Blevins, J.E. The role of oxytocin in regulation of appetitive behaviour, body weight and glucose homeostasis. J. Neuroendocrinol. 2020, 32, e12805. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Lizneva, D.; Ji, Y.; Colaianni, G.; Hadelia, E.; Gumerova, A.; Ievleva, K.; Kuo, T.; Korkmaz, F.; Ryu, V.; et al. Oxytocin regulates body composition. Proc. Natl. Acad. Sci. USA 2019, 116, 26808–26815. [Google Scholar] [CrossRef] [PubMed]
- Horta, M.; Kaylor, K.; Feifel, D.; Ebner, N.C. Chronic oxytocin administration as a tool for investigation and treatment: A cross-disciplinary systematic review. Neurosci. Biobehav. Rev. 2020, 108, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Panaro, M.A.; Benameur, T.; Porro, C. Hypothalamic Neuropeptide Brain Protection: Focus on Oxytocin. J. Clin. Med. 2020, 9, 1534. [Google Scholar] [CrossRef] [PubMed]
- Chiodini, I.; Falchetti, A.; Merlotti, D.; Eller Vainicher, C.; Gennari, L. Updates in epidemiology, pathophysiology and management strategies of glucocorticoid-induced osteoporosis. Expert. Rev. Endocrinol. Metab. 2020, 15, 283–298. [Google Scholar]
- Romero-Díaz, C.; Duarte-Montero, D.; Gutiérrez-Romero, S.A.; Mendivil, C.O. Diabetes and Bone Fragility. Diabetes Ther. Res. Treat. Educ. Diabetes Relat. Disord. 2021, 12, 71–86. [Google Scholar] [CrossRef]
- Santopaolo, M.; Gu, Y.; Spinetti, G.; Madeddu, P. Bone marrow fat: Friend or foe in people with diabetes mellitus? Clin. Sci. Lond. Engl. 2020, 134, 1031–1048. [Google Scholar] [CrossRef]
- Compston, J. Management of glucocorticoid-induced osteoporosis: What is new? Int. J. Rheum. Dis. 2019, 22, 1595–1597. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, K.; Tarnowski, M. Bone Marrow Adipocytes-Role in Physiology and Various Nutritional Conditions in Human and Animal Models. Nutrients 2021, 13, 1412. [Google Scholar] [CrossRef]
- Napoli, N.; Chandran, M.; Pierroz, D.D.; Abrahamsen, B.; Schwartz, A.V.; Ferrari, S.L. Mechanisms of diabetes mellitus-induced bone fragility. Nat. Rev. Endocrinol. 2017, 13, 208–219. [Google Scholar] [CrossRef]
- Bredella, M.A.; Fazeli, P.K.; Daley, S.M.; Miller, K.K.; Rosen, C.J.; Klibanski, A.; Torriani, M. Marrow fat composition in anorexia nervosa. Bone 2014, 66, 199–204. [Google Scholar] [CrossRef] [Green Version]
- Fazeli, P.K.; Faje, A.T.; Bredella, M.A.; Polineni, S.; Russell, S.; Resulaj, M.; Rosen, C.J.; Klibanski, A. Changes in marrow adipose tissue with short-term changes in weight in premenopausal women with anorexia nervosa. Eur. J. Endocrinol. 2019, 180, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Hasselbalch, K.C.; Lanng, K.R.; Birkeland, M.; Sjögren, M. Potential shortcomings in current studies on the effect of intranasal oxytocin in Anorexia Nervosa and healthy controls—A systematic review and meta-analysis. Psychopharmacology 2020, 237, 2891–2903. [Google Scholar] [CrossRef]
- Finger, E.C.; MacKinley, J.; Blair, M.; Oliver, L.D.; Jesso, S.; Tartaglia, M.C.; Borrie, M.; Wells, J.; Dziobek, I.; Pasternak, S.; et al. Oxytocin for frontotemporal dementia: A randomized dose-finding study of safety and tolerability. Neurology 2015, 84, 174–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohan, S.; McCloskey, A.G.; McKillop, A.M.; Flatt, P.R.; Irwin, N.; Moffett, R.C. Development and characterisation of novel, enzymatically stable oxytocin analogues with beneficial antidiabetic effects in high fat fed mice. Biochim. Biophys. Acta Gen. Subj. 2021, 1865. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; Vaughan, B. In vitro comparison of liposomal drug delivery systems targeting the oxytocin receptor: A potential novel treatment for obstetric complications. Int. J. Nanomed. 2019, 14, 2191–2206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Refuerzo, J.S.; Leonard, F.; Bulayeva, N.; Gorenstein, D.; Chiossi, G.; Ontiveros, A.; Longo, M.; Godin, B. Uterus-targeted liposomes for preterm labor management: Studies in pregnant mice. Sci. Rep. 2016, 6, 34710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Masud, M.K.; Kaneti, Y.V.; Rewatkar, P.; Koradia, A.; Hossain, M.S.A.; Yamauchi, Y.; Popat, A.; Salomon, C. Extracellular Vesicle Nanoarchitectonics for Novel Drug Delivery Applications. Small 2021, e2102220. [Google Scholar] [CrossRef] [PubMed]
| References | Method | Population | Biological Parameters | Bone Measure | Fragility Fracture(n) | Main Result | Key Message |
|---|---|---|---|---|---|---|---|
| Elabd S. et al. 2008 [11] | Cross sectional study | 36 post-menopausal women:
| OT | BMD | NA | OT serum level 55% lower in OP (p = 0.005) | OT level is inversely correlate with the occurrence of OP. |
| Lawson E.A. et al. 2011 [30] | Cross sectional study | 36 women:
| Overnight OT secretion, leptin | BMD | NA | OT serum
| Decreased nocturnal OT levels is associated with low BMD and fat mass in anorexia nervosa. |
| Breuil V. et al. 2011 [31] | Cross sectional study | 36 post-menopausal women:
| OT, estradiol, testosterone, FSH, LH, SHGB, TSH, leptin, BTM | BMD | 52 | OT serum level:
| Low OT serum level is correlated to severe OP, independently of other factors associated with OP or known to regulate OT. |
| Lawson E.A. et al. 2013 [32] | Cross sectional study | 45 women:
| Overnight OT secretion | HR-pQCT (tibia, radius) | NA |
| Important role of oxytocin for variability in bone microarchitectural and strength parameters in amenorrheic athletes. |
| Breuil V. et al. 2014 [33] | Cross sectional study | OPUS cohort
| OT, leptin, estradiol, BTM | BMD | 313 | High OT level
| OT has a direct effect on bonec ell, independently of estradiol OT mediated action. |
| Breuil V. et al. 2015 [34] | Cross sectional study | MINOS cohort: 552 men Age 50–85 y
| OT, BTM | BMD | 60 |
| Unlike women, OT level is not associated to BMD or BTM level in > 50 years old men. |
| Maestrini S. et al. 2018 [35] | Cross sectional study | 109 women:
| OT | NA | NA | Lower OT serum level in
| Obesity and menopause are independent negative predictors of plasma oxytocin. |
| Aulinas A. et al. 2021 [36] | Cross sectional study | 37 Hypopituitary men:
| OT, vasopressin | BMD | NA | Positive association between
| Men with hypopituitarism and lower OT level showed lower BMD and less favorable hip geometry. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breuil, V.; Trojani, M.-C.; Ez-Zoubir, A. Oxytocin and Bone: Review and Perspectives. Int. J. Mol. Sci. 2021, 22, 8551. https://doi.org/10.3390/ijms22168551
Breuil V, Trojani M-C, Ez-Zoubir A. Oxytocin and Bone: Review and Perspectives. International Journal of Molecular Sciences. 2021; 22(16):8551. https://doi.org/10.3390/ijms22168551
Chicago/Turabian StyleBreuil, Véronique, Marie-Charlotte Trojani, and Amri Ez-Zoubir. 2021. "Oxytocin and Bone: Review and Perspectives" International Journal of Molecular Sciences 22, no. 16: 8551. https://doi.org/10.3390/ijms22168551
APA StyleBreuil, V., Trojani, M.-C., & Ez-Zoubir, A. (2021). Oxytocin and Bone: Review and Perspectives. International Journal of Molecular Sciences, 22(16), 8551. https://doi.org/10.3390/ijms22168551







