A Putative Lignin Copper Oxidase from Trichoderma reesei
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strain
2.2. Growth on Lignin
2.3. Characterization of Residual Lignin
2.4. Proteomic Analysis
2.5. Phylogenetic Study and Sequence Alignments
2.6. Production of TrLOx in Pichia pastoris
2.7. Protein Purification
2.8. Protein Characterization
2.9. Enzyme Activity
2.10. Steady-State Kinetics
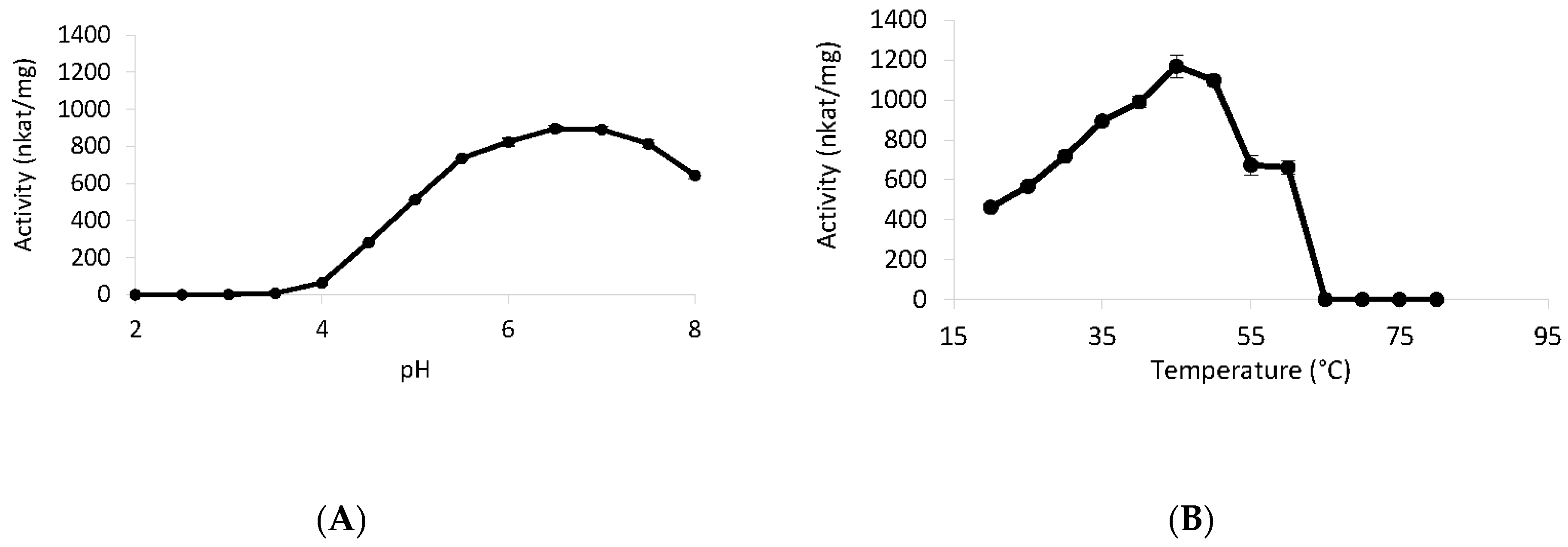
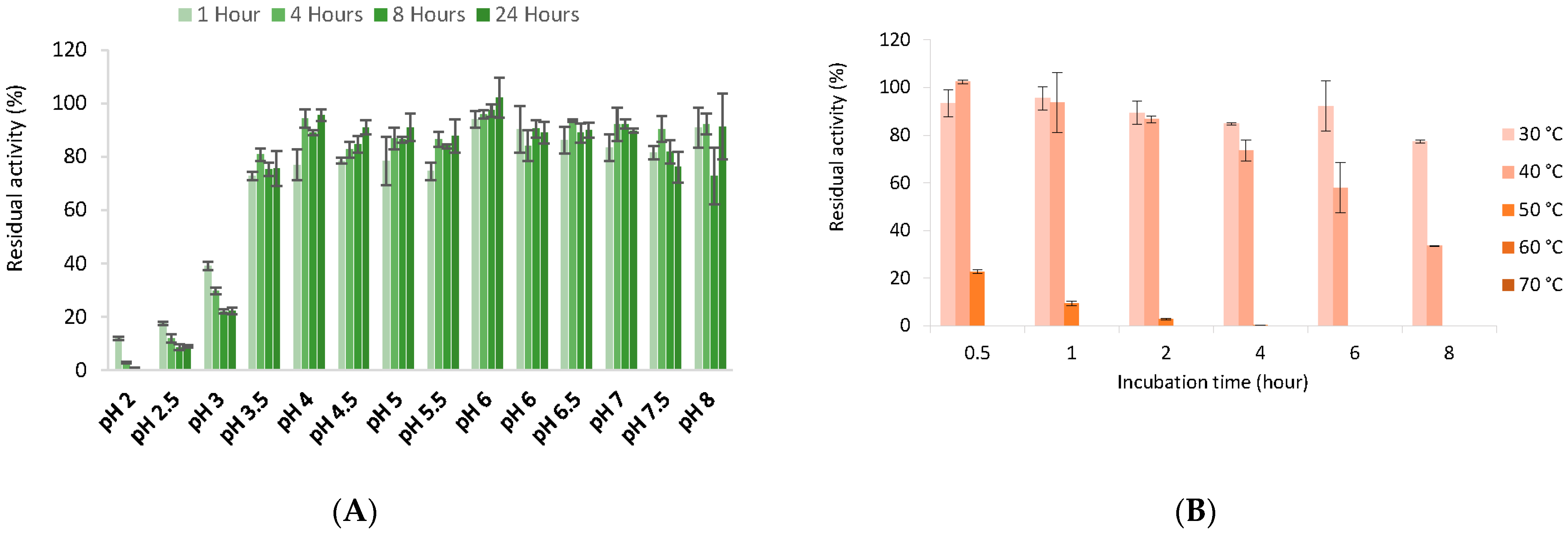
2.11. Temperature and pH Effect
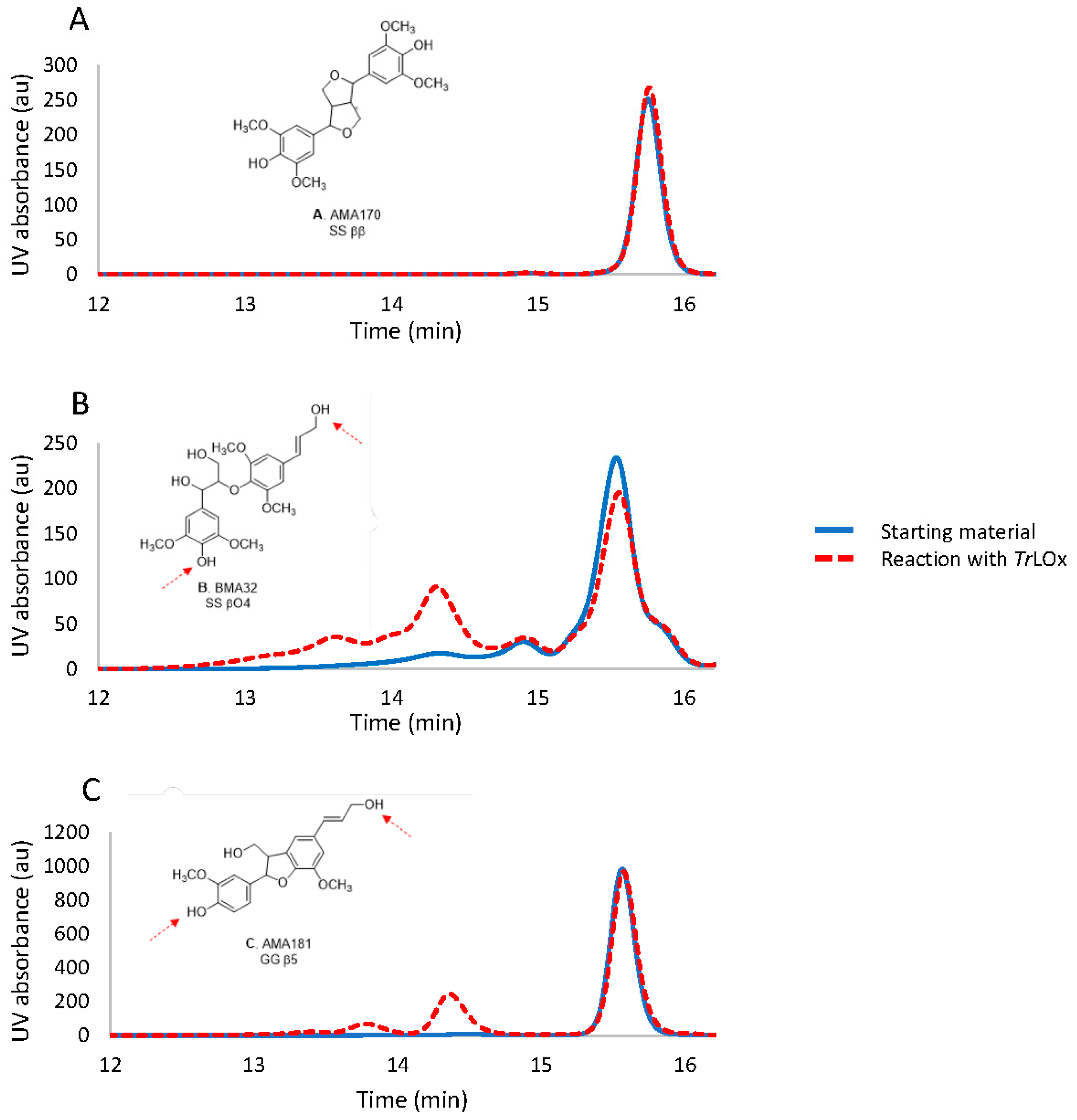
2.12. Activity of TrLOx on Lignin Derivatives
3. Results and Discussion
3.1. T. reesei Growth on Lignin
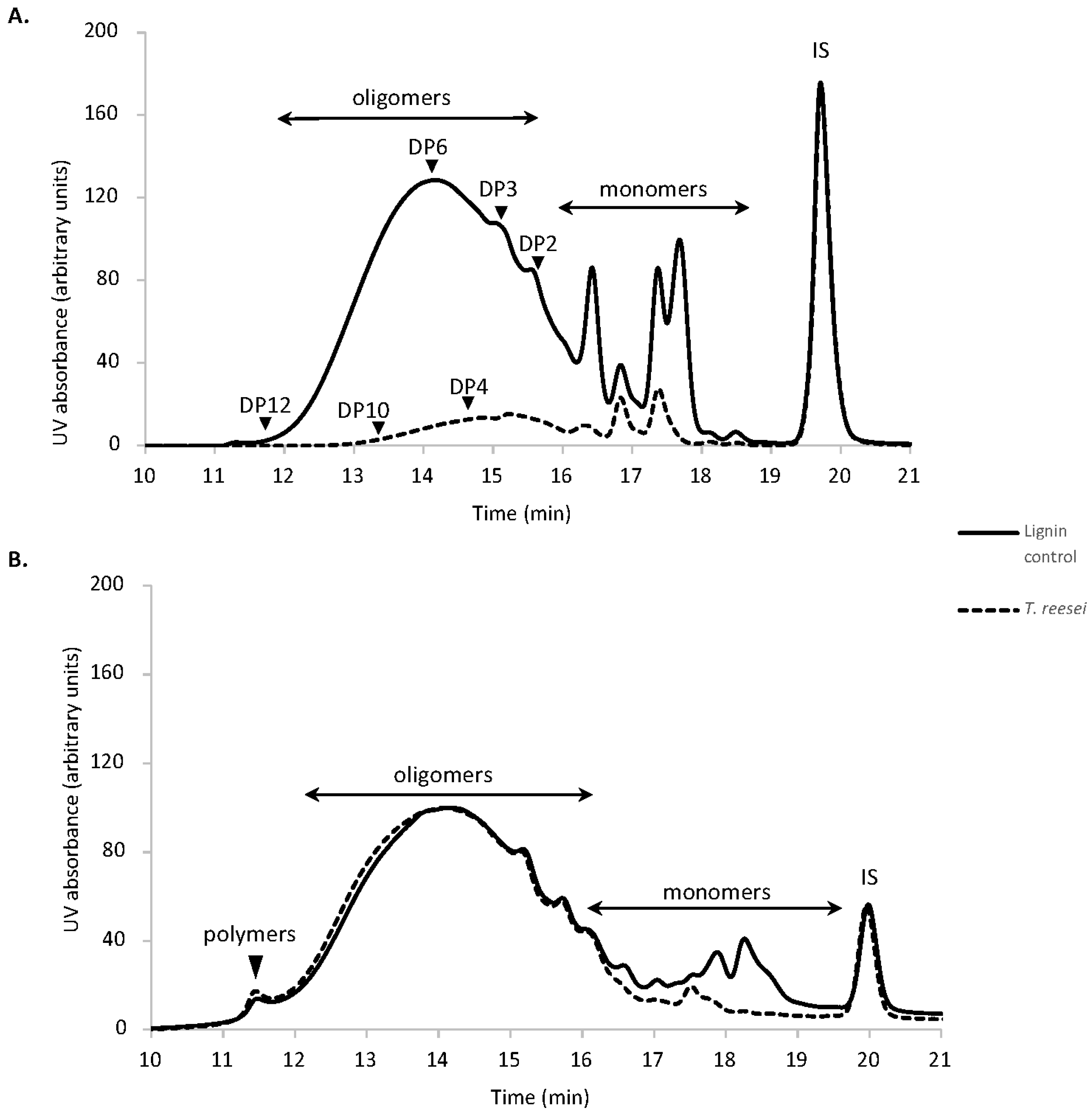
3.2. Characterization of Residual Lignin
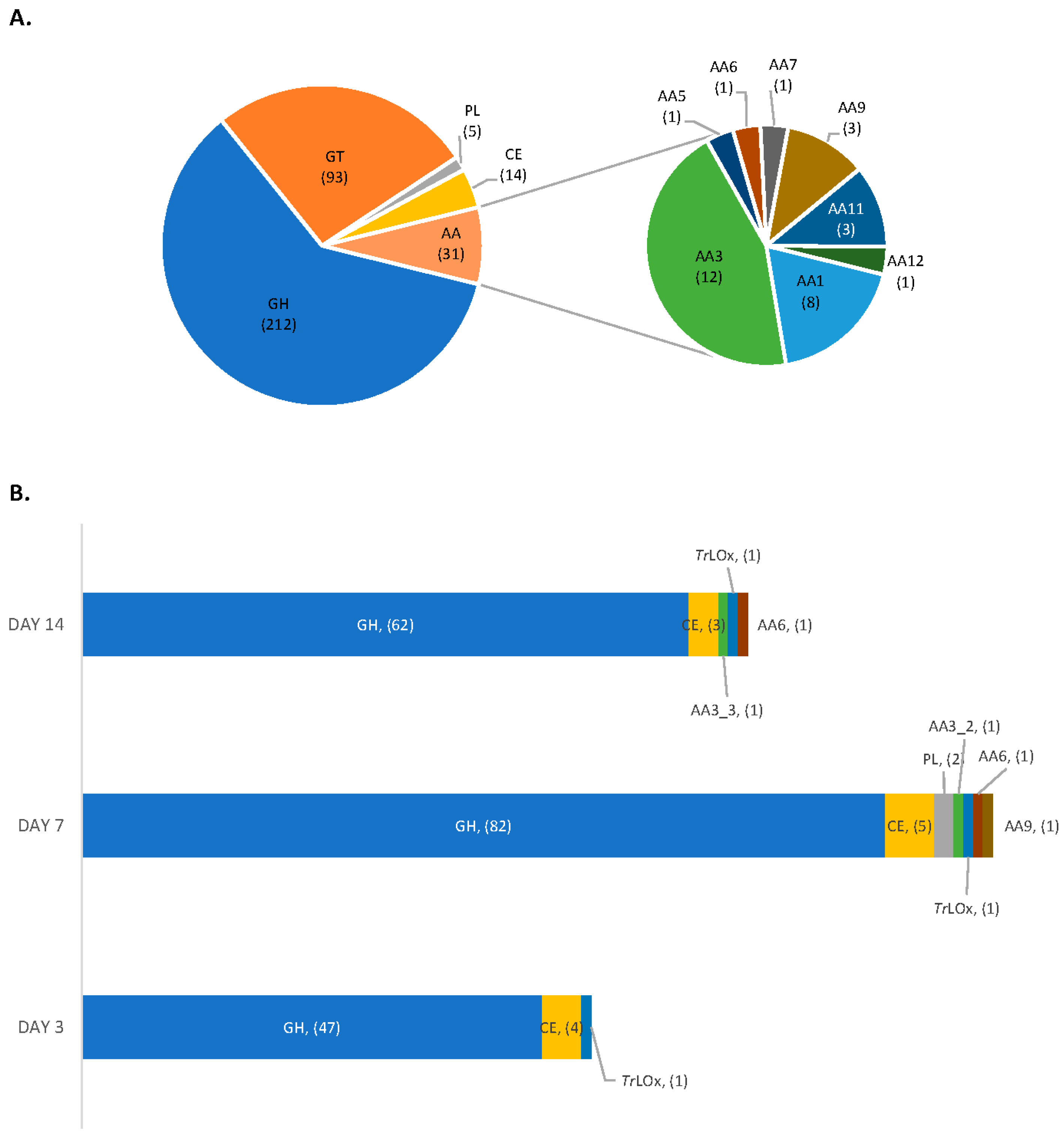
3.3. Proteomic Analysis
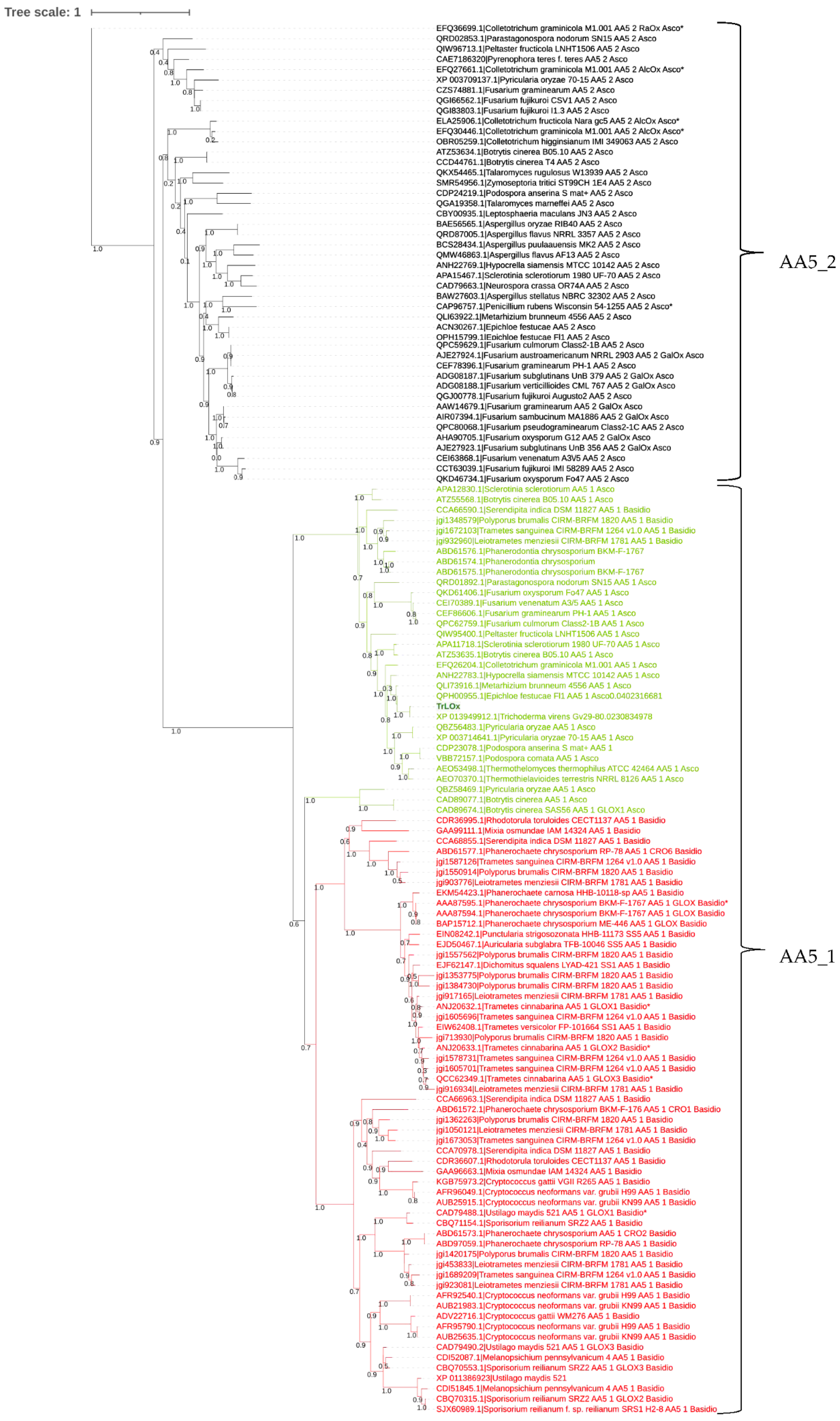
3.4. Phylogenetic Analysis
3.5. Production of Active TrLOx
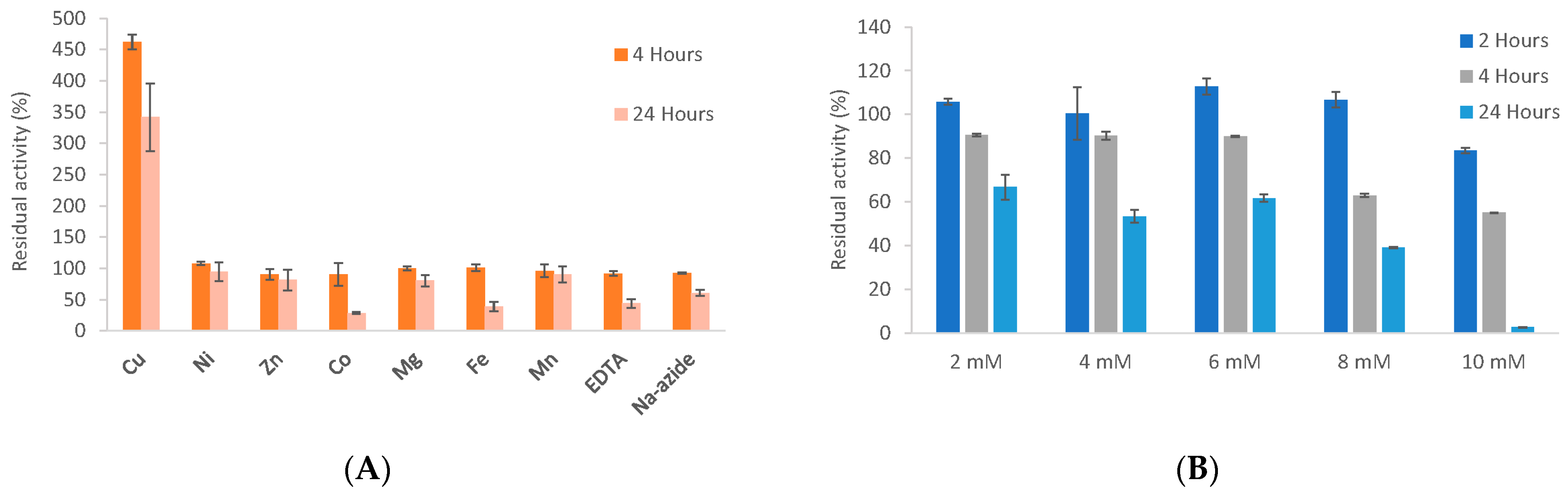
3.6. Biochemical Properties
3.7. Does TrLOx Modify Lignin?
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pollegioni, L.; Tonin, F.; Rosini, E. Lignin-degrading enzymes. FEBS J. 2015, 282, 1190–1213. [Google Scholar] [CrossRef]
- Berrin, J.-G.; Navarro, D.; Couturier, M.; Olivé, C.; Grisel, S.; Haon, M.; Taussac, S.; Lechat, C.; Courtecuisse, R.; Favel, A.; et al. Exploring the Natural Fungal Biodiversity of Tropical and Temperate Forests toward Improvement of Biomass Conversion. Appl. Environ. Microbiol. 2012, 78, 6483. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Li, Y. Fungal pretreatment of lignocellulosic biomass. Biotechnol. Adv. 2012, 30, 1447–1457. [Google Scholar] [CrossRef] [PubMed]
- Salvachúa, D.; Prieto, A.; Vaquero, M.E.; Martínez, Á.T.; Martínez, M.J. Sugar recoveries from wheat straw following treatments with the fungus Irpex lacteus. Bioresour. Technol. 2013, 131, 218–225. [Google Scholar] [CrossRef]
- Tuyen, V.D.; Cone, J.W.; Baars, J.J.P.; Sonnenberg, A.S.M.; Hendriks, W.H. Fungal strain and incubation period affect chemical composition and nutrient availability of wheat straw for rumen fermentation. Bioresour. Technol. 2012, 111, 336–342. [Google Scholar] [CrossRef]
- Tuck, C.O.; Pérez, E.; Horváth, I.T.; Sheldon, R.A.; Poliakoff, M. Valorization of Biomass: Deriving More Value from Waste. Science 2012, 337, 695. [Google Scholar] [CrossRef] [PubMed]
- de Vries, R.P.; Mäkelä, M.R. Genomic and Postgenomic Diversity of Fungal Plant Biomass Degradation Approaches. Trends Microbiol. 2020, 28, 487–499. [Google Scholar] [CrossRef]
- Hage, H.; Miyauchi, S.; Virágh, M.; Drula, E.; Min, B.; Chaduli, D.; Navarro, D.; Favel, A.; Norest, M.; Lesage-Meessen, L.; et al. Gene family expansions and transcriptome signatures uncover fungal adaptations to wood decay. Environ. Microbiol. 2021. early view. [Google Scholar] [CrossRef]
- Hage, H.; Rosso, M.-N. Evolution of Fungal Carbohydrate-Active Enzyme Portfolios and Adaptation to Plant Cell-Wall Polymers. J. Fungi 2021, 7, 185. [Google Scholar] [CrossRef]
- Zhou, S.; Raouche, S.; Grisel, S.; Sigoillot, J.; Herpoël-Gimbert, I. Efficient biomass pretreatment using the White-rot Fungus Polyporus Brumalis. Fungal Genom. Biol. 2017, 7, 1–6. [Google Scholar]
- Jurak, E.; Suzuki, H.; van Erven, G.; Gandier, J.A.; Wong, P.; Chan, K.; Ho, C.Y.; Gong, Y.; Tillier, E.; Rosso, M.N.; et al. Dynamics of the Phanerochaete carnosa transcriptome during growth on aspen and spruce. BMC Genom. 2018, 19, 815. [Google Scholar] [CrossRef]
- Daly, P.; López, S.C.; Peng, M.; Lancefield, C.S.; Purvine, S.O.; Kim, Y.M.; Zink, E.M.; Dohnalkova, A.; Singan, V.R.; Lipzen, A.; et al. Dichomitus squalens partially tailors its molecular responses to the composition of solid wood. Environ. Microbiol. 2018, 20, 4141–4156. [Google Scholar] [CrossRef] [PubMed]
- Arntzen, M.Ø.; Bengtsson, O.; Várnai, A.; Delogu, F.; Mathiesen, G.; Eijsink, V.G.H. Quantitative comparison of the biomass-degrading enzyme repertoires of five filamentous fungi. Sci. Rep. 2020, 10, 20267. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, S.; Hage, H.; Drula, E.; Lesage-Meessen, L.; Berrin, J.G.; Navarro, D.; Favel, A.; Chaduli, D.; Grisel, S.; Haon, M.; et al. Conserved white-rot enzymatic mechanism for wood decay in the Basidiomycota genus Pycnoporus. DNA Res. 2020, 27, dsaa011. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.M.; Ferreira Filho, E.X.; Moreira, L.R.S. An update on enzymatic cocktails for lignocellulose breakdown. J. Appl. Microbiol. 2018, 125, 632–645. [Google Scholar] [CrossRef]
- Crawford, R.H.; Carpenter, S.E.; Harmon, M.E. Communities of Filamentous Fungi and Yeast in Decomposing Logs of Pseudotsuga Menziesii. Mycologia 1990, 82, 759–765. [Google Scholar] [CrossRef]
- Fukasawa, Y.; Osono, T.; Takeda, H. Wood decomposing abilities of diverse lignicolous fungi on nondecayed and decayed beech wood. Mycologia 2011, 103, 474–482. [Google Scholar] [CrossRef]
- Kirk, T.K.; Farrell, R.L. Enzymatic “combustion”: The microbial degradation of lignin. Annu. Rev. Microbiol. 1987, 41, 465–505. [Google Scholar] [CrossRef]
- Cragg, S.M.; Beckham, G.T.; Bruce, N.C.; Bugg, T.D.H.; Distel, D.L.; Dupree, P.; Etxabe, A.G.; Goodell, B.S.; Jellison, J.; McGeehan, J.E.; et al. Lignocellulose degradation mechanisms across the Tree of Life. Curr. Opin. Chem. Biol. 2015, 29, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Worrall, J.J.; Anagnost, S.E.; Zabel, R.A. Comparison of wood decay among diverse lignicolous fungi. Mycologia 1997, 89, 199–219. [Google Scholar] [CrossRef]
- Nilsson, T.; Daniel, G. Chemistry and Microscopy of Wood Decay by Some Higher Ascomycetes. Holzforschung 1989, 43, 11–18. [Google Scholar] [CrossRef]
- Shary, S.; Ralph, S.A.; Hammel, K.E. New insights into the ligninolytic capability of a wood decay ascomycete. Appl. Environ. Microbiol. 2007, 73, 6691–6694. [Google Scholar] [CrossRef] [PubMed]
- Linley, E.; Denyer, S.P.; McDonnell, G.; Simons, C.; Maillard, J.Y. Use of hydrogen peroxide as a biocide: New consideration of its mechanisms of biocidal action. J. Antimicrob. Chemother. 2012, 67, 1589–1596. [Google Scholar] [CrossRef]
- Xie, N.; Chapeland-Leclerc, F.; Silar, P.; Ruprich-Robert, G. Systematic gene deletions evidences that laccases are involved in several stages of wood degradation in the filamentous fungus Podospora anserina. Environ. Microbiol. 2014, 16, 141–161. [Google Scholar] [CrossRef] [PubMed]
- Bourdais, A.; Bidard, F.; Zickler, D.; Berteaux-Lecellier, V.; Silar, P.; Espagne, E. Wood Utilization Is Dependent on Catalase Activities in the Filamentous Fungus Podospora anserina. PLoS ONE 2012, 7, e29820. [Google Scholar] [CrossRef] [PubMed]
- van Erven, G.; Kleijn, A.F.; Patyshakuliyeva, A.; Di Falco, M.; Tsang, A.; de Vries, R.P.; van Berkel, W.J.H.; Kabel, M.A. Evidence for ligninolytic activity of the ascomycete fungus Podospora anserina. Biotechnol. Biofuels 2020, 13, 75. [Google Scholar] [CrossRef] [PubMed]
- Dicko, M.; Ferrari, R.; Tangthirasunun, N.; Gautier, V.; Lalanne, C.; Lamari, F.; Silar, P. Lignin Degradation and Its Use in Signaling Development by the Coprophilous Ascomycete Podospora anserina. J. Fungi 2020, 6, 278. [Google Scholar] [CrossRef]
- Munk, L.; Sitarz, A.K.; Kalyani, D.C.; Mikkelsen, J.D.; Meyer, A.S. Can laccases catalyze bond cleavage in lignin? Biotechnol. Adv. 2015, 33, 13–24. [Google Scholar] [CrossRef]
- Otsuka, Y.; Sonoki, T.; Ikeda, S.; Kajita, S.; Nakamura, M.; Katayama, Y. Detection and characterization of a novel extracellular fungal enzyme that catalyzes the specific and hydrolytic cleavage of lignin guaiacylglycerol beta-aryl ether linkages. Eur. J. Biochem. 2003, 270, 2353–2362. [Google Scholar] [CrossRef]
- Whittaker, J.W. Free radical catalysis by galactose oxidase. Chem. Rev. 2003, 103, 2347–2364. [Google Scholar] [CrossRef]
- Hammel, K.E.; Mozuch, M.D.; Jensen, K.A., Jr.; Kersten, P.J. H2O2 recycling during oxidation of the arylglycerol beta-aryl ether lignin structure by lignin peroxidase and glyoxal oxidase. Biochemistry 1994, 33, 13349–13354. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, M.M.; Kersten, P.J.; Nakamura, N.; Sanders-Loehr, J.; Schweizer, E.S.; Whittaker, J.W. Glyoxal oxidase from Phanerochaete chrysosporium is a new radical-copper oxidase. J. Biol. Chem. 1996, 271, 681–687. [Google Scholar] [CrossRef]
- Takano, M.; Nakamura, M.; Yamaguchi, M. Glyoxal oxidase supplies hydrogen peroxide at hyphal tips and on hyphal wall to manganese peroxidase of white-rot fungus Phanerochaete crassa WD1694. J. Wood Sci. 2010, 56, 307–313. [Google Scholar] [CrossRef]
- Daou, M.; Yassine, B.; Wikee, S.; Record, E.; Duprat, F.; Bertrand, E.; Faulds, C.B. Pycnoporus cinnabarinus glyoxal oxidases display differential catalytic efficiencies on 5-hydroxymethylfurfural and its oxidized derivatives. Fungal Biol. Biotechnol. 2019, 6, 4. [Google Scholar] [CrossRef]
- Kersten, P.; Cullen, D. Copper radical oxidases and related extracellular oxidoreductases of wood-decay Agaricomycetes. Fungal Genet. Biol. 2014, 72, 124–130. [Google Scholar] [CrossRef]
- Daou, M.; Piumi, F.; Cullen, D.; Record, E.; Faulds, C.B. Heterologous Production and Characterization of Two Glyoxal Oxidases from Pycnoporus cinnabarinus. Appl. Environ. Microbiol. 2016, 82, 4867–4875. [Google Scholar] [CrossRef]
- Levasseur, A.; Drula, E.; Lombard, V.; Coutinho, P.M.; Henrissat, B. Expansion of the enzymatic repertoire of the CAZy database to integrate auxiliary redox enzymes. Biotechnol. Biofuels 2013, 6, 1–14. [Google Scholar] [CrossRef]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Urresti, S.; Lafond, M.; Johnston, E.M.; Derikvand, F.; Ciano, L.; Berrin, J.-G.; Henrissat, B.; Walton, P.H.; Davies, G.J.; et al. Structure-function characterization reveals new catalytic diversity in the galactose oxidase and glyoxal oxidase family. Nat. Commun. 2015, 6, 10197. [Google Scholar] [CrossRef]
- Mollerup, F.; Aumala, V.; Parikka, K.; Mathieu, Y.; Brumer, H.; Tenkanen, M.; Master, E. A family AA5_2 carbohydrate oxidase from Penicillium rubens displays functional overlap across the AA5 family. PLoS ONE 2019, 14, e0216546. [Google Scholar] [CrossRef] [PubMed]
- Kurek, B.; Kersten, P.J. Physiological regulation of glyoxal oxidase from Phanerochaete chrysosporium by peroxidase systems. Enzyme Microb. Technol. 1995, 17, 751–756. [Google Scholar] [CrossRef]
- Daou, M.; Farfan Soto, C.; Majira, A.; Cézard, L.; Cottyn, B.; Pion, F.; Navarro, D.; Oliveira Correia, L.; Drula, E.; Record, E.; et al. Fungal Treatment for the Valorization of Technical Soda Lignin. J. Fungi 2021, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, D.; Dorval, S. Cellulase production and ammonia metabolism in Trichoderma reesei on high levels of cellulose. Biotechnol. Bioeng. 1979, 21, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Sayers, E.W.; Agarwala, R.; Bolton, E.E.; Brister, J.R.; Canese, K.; Clark, K.; Connor, R.; Fiorini, N.; Funk, K.; Hefferon, T.; et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2018, 47, D23–D28. [Google Scholar] [CrossRef] [PubMed]
- Simeng, Z.; Sacha, G.; Isabelle, H.G.; Marie-Noëlle, R. A PCR-based method to quantify fungal growth during pretreatment of lignocellulosic biomass. J. Microbiol. Methods 2015, 115, 67–70. [Google Scholar] [CrossRef]
- Méchin, V.; Laluc, A.; Legée, F.; Cézard, L.; Denoue, D.; Barrière, Y.; Lapierre, C. Impact of the brown-midrib bm 5 mutation on maize lignins. J. Agric. Food Chem. 2014, 62, 5102–5107. [Google Scholar] [CrossRef]
- Grigoriev, I.V.; Nikitin, R.; Haridas, S.; Kuo, A.; Ohm, R.; Otillar, R.; Riley, R.; Salamov, A.; Zhao, X.; Korzeniewski, F.; et al. MycoCosm portal: Gearing up for 1000 fungal genomes. Nucleic Acids Res. 2013, 42, D699–D704. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. A simple method to control over-alignment in the MAFFT multiple sequence alignment program. Bioinformatics 2016, 32, 1933–1942. [Google Scholar] [CrossRef]
- Chang, J.M.; Di Tommaso, P.; Notredame, C. TCS: A new multiple sequence alignment reliability measure to estimate alignment accuracy and improve phylogenetic tree reconstruction. Mol. Biol. Evol. 2014, 31, 1625–1637. [Google Scholar] [CrossRef]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef]
- Lemoine, F.; Correia, D.; Lefort, V.; Doppelt-Azeroual, O.; Mareuil, F.; Cohen-Boulakia, S.; Gascuel, O. NGPhylogeny.fr: New generation phylogenetic services for non-specialists. Nucleic Acids Res. 2019, 47, W260–W265. [Google Scholar] [CrossRef]
- Guindon, S.; Gascuel, O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003, 52, 696–704. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL): An online tool for phylogenetic tree display and annotation. Bioinformatics 2007, 23, 127–128. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef] [PubMed]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J.E. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Martinez, D.; Berka, R.M.; Henrissat, B.; Saloheimo, M.; Arvas, M.; Baker, S.E.; Chapman, J.; Chertkov, O.; Coutinho, P.M.; Cullen, D.; et al. Genome sequencing and analysis of the biomass-degrading fungus Trichoderma reesei (syn. Hypocrea jecorina). Nat. Biotechnol. 2008, 26, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Haon, M.; Grisel, S.; Navarro, D.; Gruet, A.; Berrin, J.-G.; Bignon, C. Recombinant protein production facility for fungal biomass-degrading enzymes using the yeast Pichia pastoris. Front. Microbiol. 2015, 6, 1002. [Google Scholar] [CrossRef] [PubMed]
- Kersten, P.J. Glyoxal oxidase of Phanerochaete chrysosporium: Its characterization and activation by lignin peroxidase. Proc. Natl. Acad. Sci. USA 1990, 87, 2936–2940. [Google Scholar] [CrossRef] [PubMed]
- Paukner, R.; Staudigl, P.; Choosri, W.; Haltrich, D.; Leitner, C. Expression, purification, and characterization of galactose oxidase of Fusarium sambucinum in E. coli. Protein Expr. Purif. 2015, 108, 73–79. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bennati-Granier, C.; Garajova, S.; Champion, C.; Grisel, S.; Haon, M.; Zhou, S.; Fanuel, M.; Ropartz, D.; Rogniaux, H.; Gimbert, I.; et al. Substrate specificity and regioselectivity of fungal AA9 lytic polysaccharide monooxygenases secreted by Podospora anserina. Biotechnol. Biofuels 2015, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Jaufurally, A.S.; Teixeira, A.R.S.; Hollande, L.; Allais, F.; Ducrot, P.-H. Optimization of the Laccase-Catalyzed Synthesis of (±)-Syringaresinol and Study of its Thermal and Antiradical Activities. ChemistrySelect 2016, 1, 5165–5171. [Google Scholar] [CrossRef]
- Flourat, A.L.; Peru, A.A.M.; Haudrechy, A.; Renault, J.-H.; Allais, F. First Total Synthesis of (β-5)-(β-O-4) Dihydroxytrimer and Dihydrotrimer of Coniferyl Alcohol (G): Advanced Lignin Model Compounds. Front. Chem. 2019, 7, 5165–5171. [Google Scholar] [CrossRef] [PubMed]
- Liers, C.; Arnstadt, T.; Ullrich, R.; Hofrichter, M. Patterns of lignin degradation and oxidative enzyme secretion by different wood- and litter-colonizing basidiomycetes and ascomycetes grown on beech-wood. FEMS MicroBiol. Ecol. 2011, 78, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Liers, C.; Ullrich, R.; Steffen, K.T.; Hatakka, A.; Hofrichter, M. Mineralization of 14C-labelled synthetic lignin and extracellular enzyme activities of the wood-colonizing ascomycetes Xylaria hypoxylon and Xylaria polymorpha. Appl. MicroBiol. Biotechnol. 2006, 69, 573–579. [Google Scholar] [CrossRef]
- Pointing, S.B.; Parungao, M.M.; Hyde, K.D. Production of wood-decay enzymes, mass loss and lignin solubilization in wood by tropical Xylariaceae. Mycol. Res. 2003, 107 Pt 2, 231–235. [Google Scholar] [CrossRef]
- Regalado, V.; Rodriguez, A.; Perestelo, F.; Carnicero, A.; De La Fuente, G.; Falcon, M.A. Lignin Degradation and Modification by the Soil-Inhabiting Fungus Fusarium proliferatum. Appl. Environ. Microbiol. 1997, 63, 3716. [Google Scholar] [CrossRef] [PubMed]
- Bi, R.; Spadiut, O.; Brumer, H.; Henriksson, G. Isolation and identification of microorganisms from soil able to live on lignin as acarbon source and to produce enzymes which cleave the β-o-4 bond in a lignin model compound. Cellul. Chem Technol. 2012, 46, 227–242. [Google Scholar]
- Bi, R.; Huang, S.; Henriksson, G. Isolation of exceedingly low oxygen consuming fungal strains able to utilize lignin as carbon source. Cellul. Chem Technol. 2016, 50, 811–817. [Google Scholar]
- Bi, R.; Lawoko, M.; Henriksson, G. Phoma herbarum, a soil fungus able to grow on natural lignin and synthetic lignin (DHP) as sole carbon source and cause lignin degradation. J. Ind. Microbiol. Biotechnol. 2016, 43, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Neethu, K.; Rubeena, M.; Sajith, S.; Sreedevi, S.; Priji, P.; Unni, K.N.; Josh, M.K.S.; Jisha, V.N.; Pradeep, S.; Benjamin, S. A novel strain of Trichoderma viride shows complete lignocellulolytic activities. Adv. Biosci. Biotechnol. 2012, 3, 7. [Google Scholar] [CrossRef]
- Bohacz, J.; Korniłłowicz-Kowalska, T. Modification of post-industrial lignin by fungal strains of the genus Trichoderma isolated from different composting stages. J. Environ. Manag. 2020, 266, 110573. [Google Scholar] [CrossRef]
- Hammel, K.E.; Kalyanaraman, B.; Kirk, T.K. Substrate free radicals are intermediates in ligninase catalysis. Proc. Natl. Acad. Sci. USA 1986, 83, 3708. [Google Scholar] [CrossRef] [PubMed]
- Barapatre, A.; Jha, H. Degradation of alkali lignin by two ascomycetes and free radical scavenging activity of the products. Biocatal. Biotransform. 2017, 35, 269–286. [Google Scholar] [CrossRef]
- Bucher, V.V.C.; Pointing, S.B.; Hyde, K.D.; Reddy, C.A. Production of Wood Decay Enzymes, Loss of Mass, and Lignin Solubilization in Wood by Diverse Tropical Freshwater Fungi. Microb. Ecol. 2004, 48, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Koike, H.; Aerts, A.; LaButti, K.; Grigoriev, I.V.; Baker, S.E. Comparative Genomics Analysis of Trichoderma reesei Strains. Ind. Biotechnol. 2013, 9, 352–367. [Google Scholar] [CrossRef]
- Jourdier, E.; Baudry, L.; Poggi-Parodi, D.; Vicq, Y.; Koszul, R.; Margeot, A.; Marbouty, M.; Bidard, F. Proximity ligation scaffolding and comparison of two Trichoderma reesei strains genomes. Biotechnol. Biofuels 2017, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Angelova, M.B.; Pashova, S.B.; Spasova, B.K.; Vassilev, S.V.; Slokoska, L.S. Oxidative stress response of filamentous fungi induced by hydrogen peroxide and paraquat. Mycol. Res. 2005, 109, 150–158. [Google Scholar] [CrossRef]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.N.; Potter, S.C.; Finn, R.D.; et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef]
- Cavener, D.R. GMC oxidoreductases: A newly defined family of homologous proteins with diverse catalytic activities. J. Mol. Biol. 1992, 223, 811–814. [Google Scholar] [CrossRef]
- Venkatesagowda, B.; Dekker, R.F.H. Enzymatic demethylation of Kraft lignin for lignin-based phenol-formaldehyde resin applications. Biomass Convers. Biorefin. 2020, 10, 203–225. [Google Scholar] [CrossRef]
- Mathieu, Y.; Piumi, F.; Valli, R.; Aramburu, J.C.; Ferreira, P.; Faulds, C.B.; Record, E. Activities of Secreted Aryl Alcohol Quinone Oxidoreductases from Pycnoporus cinnabarinus Provide Insights into Fungal Degradation of Plant Biomass. Appl. Environ. Microbiol. 2016, 82, 2411–2423. [Google Scholar] [CrossRef]
- Ferreira, P.; Medina, M.; Guillén, F.; Martínez María, J.; Van Berkel Willem, J.H.; Martínez Ángel, T. Spectral and catalytic properties of aryl-alcohol oxidase, a fungal flavoenzyme acting on polyunsaturated alcohols. Biochem. J. 2005, 389, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Romero, E.; Ferreira, P.; Martínez, Á.T.; Martínez, M.J. New oxidase from Bjerkandera arthroconidial anamorph that oxidizes both phenolic and nonphenolic benzyl alcohols. Biochim. Biophys. Acta 2009, 1794, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Salvachúa, D.; Katahira, R.; Cleveland, N.S.; Khanna, P.; Resch, M.G.; Black, B.A.; Purvine, S.O.; Zink, E.M.; Prieto, A.; Martínez, M.J.; et al. Lignin depolymerization by fungal secretomes and a microbial sink. Green Chem. 2016, 18, 6046–6062. [Google Scholar] [CrossRef]
- Miyauchi, S.; Rancon, A.; Drula, E.; Hage, H.; Chaduli, D.; Favel, A.; Grisel, S.; Henrissat, B.; Herpoël-Gimbert, I.; Ruiz-Dueñas, F.J.; et al. Integrative visual omics of the white-rot fungus Polyporus brumalis exposes the biotechnological potential of its oxidative enzymes for delignifying raw plant biomass. Biotechnol. Biofuels 2018, 11, 201. [Google Scholar] [CrossRef]
- Challacombe, J.F.; Hesse, C.N.; Bramer, L.M.; McCue, L.A.; Lipton, M.; Purvine, S.; Nicora, C.; Gallegos-Graves, L.V.; Porras-Alfaro, A.; Kuske, C.R. Genomes and secretomes of Ascomycota fungi reveal diverse functions in plant biomass decomposition and pathogenesis. BMC Genom. 2019, 20, 976. [Google Scholar] [CrossRef]
- Gómez-Toribio, V.; García-Martín, A.B.; Martínez, M.J.; Martínez, Á.T.; Guillén, F. Induction of Extracellular Hydroxyl Radical Production by White-Rot Fungi through Quinone Redox Cycling. Appl. Environ. Microbiol. 2009, 75, 3944. [Google Scholar] [CrossRef]
- Brock, B.J.; Rieble, S.; Gold, M.H. Purification and Characterization of a 1,4-Benzoquinone Reductase from the Basidiomycete Phanerochaete chrysosporium. Appl. Environ. Microbiol. 1995, 61, 3076–3081. [Google Scholar] [CrossRef]
- Mori, T.; Koyama, G.; Kawagishi, H.; Hirai, H. Effects of Homologous Expression of 1,4-Benzoquinone Reductase and Homogentisate 1,2-Dioxygenase Genes on Wood Decay in Hyper-Lignin-Degrading Fungus Phanerochaete sordida YK-624. Curr. Microbiol. 2016, 73, 512–518. [Google Scholar] [CrossRef]
- Berrin, J.-G.; Rosso, M.-N.; Abou Hachem, M. Fungal secretomics to probe the biological functions of lytic polysaccharide monooxygenases. Carbohydr. Res. 2017, 448, 155–160. [Google Scholar] [CrossRef]
- Li, F.; Ma, F.; Zhao, H.; Zhang, S.; Wang, L.; Zhang, X.; Yu, H. A Lytic Polysaccharide Monooxygenase from a White-Rot Fungus Drives the Degradation of Lignin by a Versatile Peroxidase. Appl. Environ. Microbiol. 2019, 85, e02803-18. [Google Scholar] [CrossRef]
- Ni, H.; Li, M.; Li, F.; Wang, L.; Xie, S.; Zhang, X.; Yu, H. In-situ lignin drives lytic polysaccharide monooxygenases to enhance enzymatic saccharification. Int. J. Biol. Macromol. 2020, 161, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Adav, S.S.; Ravindran, A.; Chao, L.T.; Tan, L.; Singh, S.; Sze, S.K. Proteomic Analysis of pH and Strains Dependent Protein Secretion of Trichoderma reesei. J. Proteome Res. 2011, 10, 4579–4596. [Google Scholar] [CrossRef] [PubMed]
- Vanden Wymelenberg, A.; Sabat, G.; Mozuch, M.; Kersten, P.J.; Cullen, D.; Blanchette, R.A. Structure, organization, and transcriptional regulation of a family of copper radical oxidase genes in the lignin-degrading basidiomycete Phanerochaete chrysosporium. Appl. Environ. Microbiol. 2006, 72, 4871–4877. [Google Scholar] [CrossRef] [PubMed]
- Manavalan, A.; Adav, S.S.; Sze, S.K. iTRAQ-based quantitative secretome analysis of Phanerochaete chrysosporium. J. Proteom. 2011, 75, 642–654. [Google Scholar] [CrossRef]
- Adav, S.S.; Ravindran, A.; Sze, S.K. Quantitative proteomic analysis of lignocellulolytic enzymes by Phanerochaete chrysosporium on different lignocellulosic biomass. J. Proteom. 2012, 75, 1493–1504. [Google Scholar] [CrossRef]
- Crutcher, F.K.; Moran-Diez, M.E.; Krieger, I.V.; Kenerley, C.M. Effects on hyphal morphology and development by the putative copper radical oxidase glx1 in Trichoderma virens suggest a novel role as a cell wall associated enzyme. Fungal Genet. Biol. 2019, 131, 103245. [Google Scholar] [CrossRef]
- Oide, S.; Tanaka, Y.; Watanabe, A.; Inui, M. Carbohydrate-binding property of a cell wall integrity and stress response component (WSC) domain of an alcohol oxidase from the rice blast pathogen Pyricularia oryzae. Enzyme Microb. Technol. 2019, 125, 13–20. [Google Scholar] [CrossRef]
- Tong, S.-M.; Chen, Y.; Zhu, J.; Ying, S.-H.; Feng, M.-G. Subcellular localization of five singular WSC domain-containing proteins and their roles in Beauveria bassiana responses to stress cues and metal ions. Environ. MicroBiol. Rep. 2016, 8, 295–304. [Google Scholar] [CrossRef]
- Ito, N.; Phillips, S.E.; Yadav, K.D.; Knowles, P.F. Crystal structure of a free radical enzyme, galactose oxidase. J. Mol. Biol. 1994, 238, 794–814. [Google Scholar] [CrossRef]
- Mathieu, Y.; Offen, W.A.; Forget, S.M.; Ciano, L.; Viborg, A.H.; Blagova, E.; Henrissat, B.; Walton, P.H.; Davies, G.J.; Brumer, H. Discovery of a Fungal Copper Radical Oxidase with High Catalytic Efficiency toward 5-Hydroxymethylfurfural and Benzyl Alcohols for Bioprocessing. ACS Catal. 2020, 10, 3042–3058. [Google Scholar] [CrossRef]
- Gupta, R.; Jung, E.; Brunak, S. Prediction of N-glycosylation sites in human proteins. In Proceedings of the Pacific Symposium on Biocomputing 2004, Big Island, HI, USA, 6–10 January 2004; Volume 46, pp. 203–206. [Google Scholar]
- Steentoft, C.; Vakhrushev, S.Y.; Joshi, H.J.; Kong, Y.; Vester-Christensen, M.B.; Schjoldager, K.T.; Lavrsen, K.; Dabelsteen, S.; Pedersen, N.B.; Marcos-Silva, L.; et al. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J. 2013, 32, 1478–1488. [Google Scholar] [CrossRef]
- Baron, A.J.; Stevens, C.; Wilmot, C.; Seneviratne, K.D.; Blakeley, V.; Dooley, D.M.; Phillips, S.E.; Knowles, P.F.; McPherson, M.J. Structure and mechanism of galactose oxidase. The free radical site. J. Biol. Chem. 1994, 269, 25095–25105. [Google Scholar] [CrossRef]
- Andberg, M.; Mollerup, F.; Parikka, K.; Koutaniemi, S.; Boer, H.; Juvonen, M.; Master, E.; Tenkanen, M.; Kruus, K. A Novel Colletotrichum graminicola Raffinose Oxidase in the AA5 Family. Appl. Environ. Microbiol. 2017, 83, e01383-17. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Golightly, E.J.; Schneider, P.; Berka, R.M.; Brown, K.M.; Johnstone, J.A.; Baker, D.H.; Fuglsang, C.C.; Brown, S.H.; Svendsen, A.; et al. Expression and characterization of a recombinant Fusarium spp. galactose oxidase. Appl. Biochem. Biotechnol. 2000, 88, 23. [Google Scholar] [CrossRef]
- Szabó, E.E.; Adányi, N.; Váradi, M. Application of biosensor for monitoring galactose content. Biosens. Bioelectron. 1996, 11, 1051–1058. [Google Scholar] [CrossRef]
- Schumacher, D.; Vogel, J.; Lerche, U. Construction and applications of an enzyme electrode for determination of galactose and galactose-containing saccharides. Biosens. Bioelectron. 1994, 9, 85–89. [Google Scholar] [CrossRef]
- Knowles, P.F.; Brown, R.D.; Koenig, S.H.; Wang, S.; Scott, R.A.; McGuirl, M.A.; Brown, D.E.; Dooley, D.M. Spectroscopic Studies of the Active Site of Galactose Oxidase. Inorg. Chem. 1995, 34, 3895–3902. [Google Scholar] [CrossRef]
- Ribeaucourt, D.; Bissaro, B.; Guallar, V.; Yemloul, M.; Haon, M.; Grisel, S.; Alphand, V.; Brumer, H.; Lambert, F.; Berrin, J.-G.; et al. Comprehensive Insights into the Production of Long Chain Aliphatic Aldehydes Using a Copper-Radical Alcohol Oxidase as Biocatalyst. ACS Sustain. Chem. Eng. 2021, 9, 4411–4421. [Google Scholar] [CrossRef]
- Towne, V.; Will, M.; Oswald, B.; Zhao, Q. Complexities in horseradish peroxidase-catalyzed oxidation of dihydroxyphenoxazine derivatives: Appropriate ranges for pH values and hydrogen peroxide concentrations in quantitative analysis. Anal. Biochem. 2004, 334, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Mollerup, F.; Parikka, K.; Vuong, T.V.; Tenkanen, M.; Master, E. Influence of a family 29 carbohydrate binding module on the activity of galactose oxidase from Fusarium graminearum. Biochim. Biophys. Acta Gen. Subj. 2016, 1860, 354–362. [Google Scholar] [CrossRef]
- Rogers, M.S.; Baron, A.J.; McPherson, M.J.; Knowles, P.F.; Dooley, D.M. Galactose oxidase pro-sequence cleavage and cofactor assembly are self-processing reactions. J. Am. Chem. Soc. 2000, 122, 990–991. [Google Scholar] [CrossRef]
- Rogers, M.S.; Tyler, E.M.; Akyumani, N.; Kurtis, C.R.; Spooner, R.K.; Deacon, S.E.; Tamber, S.; Firbank, S.J.; Mahmoud, K.; Knowles, P.F.; et al. The stacking tryptophan of galactose oxidase: A second coordination sphere residue that has profound effects on tyrosyl radical behavior and enzyme catalysis. Biochemistry 2007, 46, 4606–4618. [Google Scholar] [CrossRef]
- Wohlschlager, L.; Csarman, F.; Zrilić, M.; Seiboth, B.; Ludwig, R. Comparative characterization of glyoxal oxidase from Phanerochaete chrysosporium expressed at high levels in Pichia pastoris and Trichoderma reesei. Enzyme Microb. Technol. 2021, 145, 109748. [Google Scholar] [CrossRef] [PubMed]
- Leonowicz, A.; Rogalski, J.; Jaszek, M.; Luterek, J.; Wojtas-Wasilewska, M.; Malarczyk, E.; Ginalska, G.; Fink-Boots, M.; Cho, N.-S. Cooperation of Fungal Laccase and Glucose 1-Oxidase in Transformation of Björkman Lignin and Some Phenolic Compounds. Holzforschung 1999, 53, 376–380. [Google Scholar] [CrossRef]
- Marzullo, L.; Cannio, R.; Giardina, P.; Santini, M.T.; Sannia, G. Veratryl Alcohol Oxidase from Pleurotus ostreatus Participates in Lignin Biodegradation and Prevents Polymerization of Laccase-oxidized Substrates (∗). J. Biol. Chem. 1995, 270, 3823–3827. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Fang, J.; Lu, X.; Gao, P.; Chen, J. Synergistic effects of cellobiose dehydrogenase and manganese-dependent peroxidases during lignin degradation. Chin. Sci. Bull. 2001, 46, 1956–1961. [Google Scholar] [CrossRef]
- Ai, M.-Q.; Wang, F.-F.; Zhang, Y.-Z.; Huang, F. Purification of pyranose oxidase from the white rot fungus Irpex lacteus and its cooperation with laccase in lignin degradation. Process. Biochem. 2014, 49, 2191–2198. [Google Scholar] [CrossRef]
- Majeke, B.M.; Collard, F.X.; Tyhoda, L.; Görgens, J.F. The synergistic application of quinone reductase and lignin peroxidase for the deconstruction of industrial (technical) lignins and analysis of the degraded lignin products. Bioresour. Technol. 2021, 319, 124152. [Google Scholar] [CrossRef]
- Jeon, J.-R.; Kim, E.-J.; Murugesan, K.; Park, H.-K.; Kim, Y.-M.; Kwon, J.-H.; Kim, W.-G.; Lee, J.-Y.; Chang, Y.-S. Laccase-catalysed polymeric dye synthesis from plant-derived phenols for potential application in hair dyeing: Enzymatic colourations driven by homo- or hetero-polymer synthesis. Microb. Biotechnol. 2010, 3, 324–335. [Google Scholar] [CrossRef]
- Kim, S.; Lee, H.; Kim, J.; Oliveira, F.; Souto, P.; Kim, H.; Nakamatsu, J. Laccase-mediated grafting of polyphenols onto cationized cotton fibers to impart UV protection and antioxidant activities. J. Appl. Polym. Sci. 2018, 135, 45801. [Google Scholar] [CrossRef]
- Zhang, A.; Meng, X.; Bao, C.; Zhang, Q. In situ synthesis of protein-loaded hydrogels via biocatalytic ATRP. Polym. Chem. 2020, 11, 1525–1532. [Google Scholar] [CrossRef]
| Substrate | Activity (nkat/mg) | |
|---|---|---|
| Phenolic | Syringyl alcohol | 0.44 ± 0.01 |
| Caffeic acid methyl ester | 0.24 ± 0.01 | |
| p-coumaric acid methyl ester | 0.18 ± 0.01 | |
| Vanillin | 0.12 ± 0.01 | |
| Acetovanillone | 0.10 ± 0.02 | |
| Homovanillic acid | 0.08 ± 0.01 | |
| Sinapic acid | 0.05 ± 0.001 | |
| Isovanillin | 0.05 ± 0.01 | |
| 4-Ethylphenol | 0.04 ± 0.01 | |
| Vanillyl alcohol | 0.03 ± 0.001 | |
| p-coumaryl alcohol | 0.02 ± 0.01 | |
| 4-vinylguaiacol | nd | |
| p-hydroxybenzaldehyde | nd | |
| Syringol | nd | |
| Syringaldehyde | nd | |
| p-coumaric acid | nd | |
| Syringic acid | nd | |
| Sinapic acid methyl ester | nd | |
| Homovanillyl alcohol | nd | |
| Eugenol | nd | |
| Vanillyl acetone | nd | |
| Ferulic acid | nd | |
| Ferulic acid methyl ester | nd | |
| Coniferyl alcohol | nd | |
| Guaiacol | nd | |
| 4-ethylguaiacol | nd | |
| Vanillic acid | nd | |
| Caffeic acid | nd | |
| Chlorogenic acid | nd | |
| Phenol | nd | |
| Carbohydrate | Xylose | 1.73 ± 1.31 |
| Galactose | 1.27 ± 0.37 | |
| Glucose | 0.64 ± 0.01 | |
| Raffinose | 0.44 ± 0.01 | |
| Cellobiose | nd | |
| Fructose | nd | |
| Maltose | nd | |
| Maltotriose | nd | |
| Furan | Furfuryl alcohol | 12.13 ± 0.29 |
| 5-Hydroxymethylfurfural | 1.30 ± 0.36 | |
| 5-Hydroxymethyl-2-furancarboxylic acid | 0.43 ± 0.02 | |
| 5-formylfuran-2-carboxylic Acid | 0.30 ± 0.08 | |
| Furfural | nd | |
| Furan-2,5-dicarbaldehyde | nd | |
| Alcohol | Veratryl alcohol | 5.49 ± 1.36 |
| 2-phenylethanol | 4.43 ± 0.22 | |
| 1-phenyl-3-propanol | 1.92 ± 0.04 | |
| Glycerol | 1.91 ± 0.09 | |
| Cinnamyl alcohol | 0.99 ± 0.01 | |
| 1-phenylethanol | 0.81 ± 0.06 | |
| Sorbitol | nd | |
| (±)-2-octanol | nd | |
| Methanol | nd | |
| Diethanolamine | nd | |
| Triethanolamine | nd | |
| Aldehyde, Ketone, Carboxylic acid | Dihydroxyacetone | 268.86 ± 5.71 |
| Methylglyoxal | 26.30 ± 1.15 | |
| Glyoxylic acid | 1.73 ± 0.16 | |
| Veratric acid | 0.93 ± 0.02 | |
| Formaldehyde | 0.76 ± 0.11 | |
| Glyceraldehyde | 0.64 ± 0.02 | |
| Glyoxal | 0.51 ± 0.03 | |
| Acetaldehyde | nd | |
| Veratraldehyde | nd | |
| Phenyl glyoxylic acid | nd | |
| Acetone | nd | |
| Acetophenone | nd | |
| Quinone | Benzoquinone (quinone) | nd |
| Aniline | Ortho-anisidine (aniline) | nd |
| Lactone | D-Xylono-1,4-lactone | nd |
| Substrate | Vmax (nkat/mg) | Km (mM) | Kcat (s−1) | Kcat/Km (s−1 mM−1) |
|---|---|---|---|---|
| Dihydroxyacetone | 2455.81 ± 56.25 | 24.16 ± 1.44 | 142.19 | 5.89 |
| Methylglyoxal | 46.84 ± 6.39 | 11.03 ± 2.84 | 1.08 | 0.10 |
| Furfuryl alcohol | 13.28 ± 1.56 | 6.72 ± 1.91 | 0.31 | 0.05 |
| 2-Phenylethanol | 11.23 ± 1.53 | 12.32 ± 5.18 | 0.26 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daou, M.; Bisotto, A.; Haon, M.; Oliveira Correia, L.; Cottyn, B.; Drula, E.; Garajová, S.; Bertrand, E.; Record, E.; Navarro, D.; et al. A Putative Lignin Copper Oxidase from Trichoderma reesei. J. Fungi 2021, 7, 643. https://doi.org/10.3390/jof7080643
Daou M, Bisotto A, Haon M, Oliveira Correia L, Cottyn B, Drula E, Garajová S, Bertrand E, Record E, Navarro D, et al. A Putative Lignin Copper Oxidase from Trichoderma reesei. Journal of Fungi. 2021; 7(8):643. https://doi.org/10.3390/jof7080643
Chicago/Turabian StyleDaou, Mariane, Alexandra Bisotto, Mireille Haon, Lydie Oliveira Correia, Betty Cottyn, Elodie Drula, Soňa Garajová, Emmanuel Bertrand, Eric Record, David Navarro, and et al. 2021. "A Putative Lignin Copper Oxidase from Trichoderma reesei" Journal of Fungi 7, no. 8: 643. https://doi.org/10.3390/jof7080643
APA StyleDaou, M., Bisotto, A., Haon, M., Oliveira Correia, L., Cottyn, B., Drula, E., Garajová, S., Bertrand, E., Record, E., Navarro, D., Raouche, S., Baumberger, S., & Faulds, C. B. (2021). A Putative Lignin Copper Oxidase from Trichoderma reesei. Journal of Fungi, 7(8), 643. https://doi.org/10.3390/jof7080643







