Bioenergetic Pathways in the Sperm of an Under-Ice Spawning Fish, Burbot (Lota lota): The Role of Mitochondrial Respiration in a Varying Thermal Environment
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Broodstock, Spermiation, and Sperm Collection
2.2. Basal Solutions for Activation and Non-Activation Media
2.3. Reagents Used in the Experiment—Inhibitors and an Uncoupler
2.4. Motility Assessment
2.5. Sperm Concentration and Measurement of Oxygen Consumption Rate
2.6. Statistical Analyses
3. Results
3.1. Sperm Motility Parameters
3.2. Oxygen Consumption Rate (OCR)
4. Discussion
4.1. Influences of the Inhibitors and Uncoupler on Sperm Motility at Spawning Temperature
4.2. The Influence of Temperature on Sperm Motility
4.3. Oxygen Consumption Rate
4.4. Influences of Reagents (Inhibitors and Uncoupler) on OCR at Spawning Temperature
4.5. The Influence of Temperature on OCR
4.6. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McPhail, J.D.; Paragamian, V.L. Burbot biology and life history. In Burbot: Biology, Ecology and Management; Paragamian, V.L., Willis, D.W., Eds.; Fisheries Management Section of the American Fisheries Society: New York, NY, USA, 2000; Volume 128, pp. 11–23. [Google Scholar]
- Harrison, P.M.; Gutowsky, L.F.; Martins, E.G.; Patterson, D.A.; Cooke, S.J.; Power, M. Temporal plasticity in thermal-habitat selection of burbot Lota lota a diel-migrating winter-specialist. J. Fish Biol. 2016, 88, 2111–2129. [Google Scholar] [CrossRef]
- Lahnsteiner, F.; Mansour, N. The effect of temperature on sperm motility and enzymatic activity in brown trout Salmo trutta, burbot Lota lota and grayling Thymallus thymallus. J. Fish Biol. 2012, 81, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, R.S.; Galina, A.; Da-Silva, W.S. Cold acclimation increases mitochondrial oxidative capacity without inducing mitochondrial uncoupling in goldfish white skeletal muscle. Biol. Open 2013, 2, 82–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egginton, S.; Sidell, B.D. Thermal acclimation induces adaptive changes in subcellular structure of fish skeletal muscle. Am. J. Physiol. 1989, 256, R1–R9. [Google Scholar] [CrossRef]
- Lucassen, M.; Koschnick, N.; Eckerle, L.G.; Portner, H.O. Mitochondrial mechanisms of cold adaptation in cod (Gadus morhua L.) populations from different climatic zones. J. Exp. Biol. 2006, 209, 2462–2471. [Google Scholar] [CrossRef] [Green Version]
- Guderley, H. Metabolic responses to low temperature in fish muscle. Biol. Rev. Camb. Philos. Soc. 2004, 79, 409–427. [Google Scholar] [CrossRef] [PubMed]
- Ingermann, R. Energy metabolism and respiration in fish spermatozoa. In Fish Spermatology; Alpha Science International Ltda: Oxford, UK, 2008. [Google Scholar]
- Boryshpolets, S.; Dzyuba, B.; Drokin, S. Pre-spawning water temperature affects sperm respiration and reactivation parameters in male carps. Fish. Physiol. Biochem. 2009, 35, 661–668. [Google Scholar] [CrossRef]
- Rahi, D.; Dzyuba, B.; Xin, M.; Cheng, Y.; Dzyuba, V. Energy pathways associated with sustained spermatozoon motility in the endangered Siberian sturgeon Acipenser Baerii. J. Fish Biol. 2020, 97, 435–443. [Google Scholar] [CrossRef]
- Ingermann, R.L.; Robinson, M.L.; Cloud, J.G. Respiration of steelhead trout sperm: Sensitivity to pH and carbon dioxide. J. Fish Biol. 2003, 62, 13–23. [Google Scholar] [CrossRef]
- Wick, A.N.; Drury, D.R.; Nakada, H.I.; Wolfe, J.B. Localization of the primary metabolic block produced by 2-deoxyglucose. J. Biol. Chem. 1957, 224, 963–969. [Google Scholar] [CrossRef]
- Nelson, D.; Cox, M. Lehninger Principles of Biochemistry; WH Freeman: New York, NY, USA, 2008. [Google Scholar]
- Fei, M.J.; Yamashita, E.; Inoue, N.; Yao, M.; Yamaguchi, H.; Tsukihara, T.; Shinzawa-Itoh, K.; Nakashima, R.; Yoshikawa, S. X-ray structure of azide-bound fully oxidized cytochrome c oxidase from bovine heart at 2.9 Å resolution. Acta Crystallogr. Sect. D Biol. Crystallogr. 2000, 56, 529–535. [Google Scholar] [CrossRef] [Green Version]
- Hanstein, W.G. Uncoupling of oxidative phosphorylation. Trends Biochem. Sci. 1976, 1, 65–67. [Google Scholar] [CrossRef]
- Dadras, H.; Boryshpolets, S.; Golpour, A.; Policar, T.; Blecha, M.; Dzyuba, B. Effects of temperature on sperm motility of burbot Lota lota: Spontaneous activation and calcium dependency. J. Fish Biol. 2019, 95, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Vechtova, P.; Fussy, Z.; Sterba, J.; Linhartová, Z.; Rodina, M.; Tučková, V.; Gela, D.; Samarin, A.M.; Lebeda, I.; et al. Changes in Phenotypes and DNA Methylation of In Vitro Aging Sperm in Common Carp Cyprinus carpio. Int. J. Mol. Sci. 2021, 22, 5925. [Google Scholar] [CrossRef] [PubMed]
- Lusk, S. The status of the fish fauna in the Czech Republic. In Conservation of Endangered Freshwater Fish in Europe; Springer: Berlin/Heidelberg, Germany, 1996; pp. 89–98. [Google Scholar]
- Stapanian, M.A.; Paragamian, V.L.; Madenjian, C.P.; Jackson, J.R.; Lappalainen, J.; Evenson, M.J.; Neufeld, M.D. Worldwide status of burbot and conservation measures. Fish Fish. 2010, 11, 34–56. [Google Scholar] [CrossRef]
- Blecha, M.; Dzyuba, B.; Boryshpolets, S.; Horokhovatskyi, Y.; Dadras, H.; Malinovskyi, O.; Sampels, S.; Policar, T. Spermatozoa quality and sperm lipid composition in intensively cultured and wild burbot (Lota lota). Anim. Reprod. Sci. 2018, 198, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Cott, P.A. Life History and Reproductive Ecology of a Mid-Winter Spawner: The Burbot (Lota lota). Ph.D. Thesis, Laurentian University, Sudbury, ON, Canada, 2013. [Google Scholar]
- Dadras, H.; Golpour, A.; Dzyuba, B.; Kristan, J.; Policar, T. Ultrastructural feature of spermatogenic cells and spermatozoon in cultured burbot Lota lota. Tissue Cell 2019, 61, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lahnsteiner, F.; Berger, B.; Weismann, T.; Patzner, R. Sperm motility and seminal fluid composition in the burbot, Lota lota. J. Appl. Ichthyol. 1997, 13, 113–119. [Google Scholar] [CrossRef]
- Żarski, D.; Kucharczyk, D.; Sasinowski, W.; Targońska, K.; Mamcarz, A. The influence of temperature on successful reproductions of Burbot, Lota lota (L.) under hatchery conditions. Pol. J. Nat. Sci. 2010, 25, 93–105. [Google Scholar] [CrossRef]
- Turner, E.; Montgomerie, R. Ovarian fluid enhances sperm movement in Arctic charr. J. Fish Biol. 2002, 60, 1570–1579. [Google Scholar] [CrossRef]
- Blecha, M.; Kristan, J.; Samarin, A.M.; Rodina, M.; Policar, T. Quality and quantity of pikeperch (Sander lucioperca) spermatozoa after varying cold water treatments. J. Appl. Ichthyol. 2015, 31, 75–78. [Google Scholar] [CrossRef]
- Nynca, J.; Dietrich, G.J.; Dobosz, S.; Grudniewska, J.; Ciereszko, A. Effect of cryopreservation on sperm motility parameters and fertilizing ability of brown trout semen. Aquaculture 2014, 433, 62–65. [Google Scholar] [CrossRef]
- Nynca, J.; Judycka, S.; Liszewska, E.; Dobosz, S.; Grudniewska, J.; Arai, K.; Fujimoto, T.; Ciereszko, A. Utility of different sugar extenders for cryopreservation and post-thaw storage of sperm from Salmonidae species. Aquaculture 2016, 464, 340–348. [Google Scholar] [CrossRef]
- Ruiz-Pesini, E.; Díez-Sánchez, C.; López-Pérez, M.J.; Enríquez, J.A. The role of the mitochondrion in sperm function: Is there a place for oxidative phosphorylation or is this a purely glycolytic process? Curr. Top. Dev. Biol. 2007, 77, 3–19. [Google Scholar] [PubMed]
- Storey, B.T. Mammalian sperm metabolism: Oxygen and sugar, friend and foe. Int J. Dev. Biol. 2008, 52, 427–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dreanno, C.; Cosson, J.; Suquet, M.; Seguin, F.; Dorange, G.; Billard, R. Nucleotide content, oxidative phosphorylation, morphology, and fertilizing capacity of turbot (Psetta maxima) spermatozoa during the motility period. Mol. Reprod. Dev. 1999, 53, 230–243. [Google Scholar] [CrossRef]
- Ingermann, R.L.; Schultz, C.L.; Kanuga, M.K.; Wilson-Leedy, J.G. Metabolism of motile zebrafish sperm. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2011, 158, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Perchec, G.; Jeulin, C.; Cosson, J.; Andre, F.; Billard, R. Relationship between sperm ATP content and motility of carp spermatozoa. J. Cell Sci. 1995, 108, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Bencic, D.; Krisfalusi, M.; Cloud, J.; Ingermann, R. Maintenance of steelhead trout (Oncorhynchus mykiss) sperm at different in vitro oxygen tensions alters ATP levels and cell functional characteristics. Fish. Physiol. Biochem. 1999, 21, 193–200. [Google Scholar] [CrossRef]
- Lahnsteiner, F.; Berger, B.; Weismann, T. Sperm metabolism of the telost fishes Chalcalburnus chalcoides and Oncorhynchus mykiss and its relation to motility and viability. J. Exp. Zool. 1999, 284, 454–465. [Google Scholar] [CrossRef]
- Mansour, N.; Lahnsteiner, F.; Berger, B. Metabolism of intratesticular spermatozoa of a tropical teleost fish (Clarias gariepinus). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2003, 135, 285–296. [Google Scholar] [CrossRef]
- Mukai, C.; Okuno, M. Glycolysis plays a major role for adenosine triphosphate supplementation in mouse sperm flagellar movement. Biol. Reprod. 2004, 71, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Nesci, S.; Spinaci, M.; Galeati, G.; Nerozzi, C.; Pagliarani, A.; Algieri, C.; Tamanini, C.; Bucci, D. Sperm function and mitochondrial activity: An insight on boar sperm metabolism. Theriogenology 2020, 144, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Lahnsteiner, F.; Patzner, R.A.; Weismann, T. Energy resources of spermatozoa of the rainbow trout Oncorhynchus mykiss (Pisces, Teleostei). Reprod. Nutr. Dev. 1993, 33, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Lahnsteiner, F.; Berger, B.; Weismann, T.; Patzner, R.A. Motility of spermatozoa of Alburnus alburnus (Cyprinidae) and its relationship to seminal plasma composition and sperm metabolism. Fish Physiol. Biochem. 1996, 15, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Lahnsteiner, F.; Patzner, R.; Weismann, T. Monosaccharides as energy resources during motility of spermatozoa in Leuciscus cephalus (Cyprinidae, Teleostei). Fish Physiol. Biochem. 1992, 10, 283–289. [Google Scholar] [CrossRef]
- Dzyuba, B.; Bondarenko, O.; Fedorov, P.; Gazo, I.; Prokopchuk, G.; Cosson, J. Energetics of fish spermatozoa: The proven and the possible. Aquaculture 2017, 472, 60–72. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxidative Med. Cell. Longev. 2017, 8416763. [Google Scholar] [CrossRef]
- Simčič, T.; Jesenšek, D.; Brancelj, A. Effects of increased temperature on metabolic activity and oxidative stress in the first life stages of marble trout (Salmo marmoratus). Fish Physiol. Biochem. 2015, 41, 1005–1014. [Google Scholar] [CrossRef]
- Ashton, I. Understanding lipid oxidation in fish. In Safety and Quality Issues in Fish Processing; Elsevier; CRC Press: Washington, DC, USA, 2002; p. 507. [Google Scholar]
- Patterson, E.; Wall, R.; Fitzgerald, G.; Ross, R.; Stanton, C. Health implications of high dietary omega-6 polyunsaturated fatty acids. J. Nutr. Metab. 2012, 539426. [Google Scholar] [CrossRef]
- Korpela, R.; Seppo, L.; Laakso, J.; Lilja, J.; Karjala, K.; Lähteenmäki, T.; Solatunturi, E.; Vapaatalo, H.; Tikkanen, M.J. Dietary habits affect the susceptibility of low-density lipoprotein to oxidation. Eur. J. Clin. Nutr. 1999, 53, 802–807. [Google Scholar] [CrossRef] [Green Version]
- Dadras, H.; Dzyuba, B.; Cosson, J.; Golpour, A.; Siddique, M.A.M.; Linhart, O. Effect of water temperature on the physiology of fish spermatozoon function: A brief review. Aquac. Res. 2017, 48, 729–740. [Google Scholar] [CrossRef]
- Lahnsteiner, F. Spermatozoa of the teleost fish Perca fluviatilis (perch) have the ability to swim for more than two hours in saline solutions. Aquaculture 2011, 314, 221–224. [Google Scholar] [CrossRef]
- Vladiĉ, T.; Jätrvi, T. Sperm motility and fertilization time span in Atlantic salmon and brown trout—The effect of water temperature. J. Fish Biol. 1997, 50, 1088–1093. [Google Scholar]
- Alavi, S.M.; Cosson, J. Sperm motility in fishes. I. Effects of temperature and pH: A review. Cell Biol. Int. 2005, 29, 101–110. [Google Scholar] [CrossRef]
- Figueroa, E.; Lee-Estevez, M.; Valdebenito, I.; Watanabe, I.; Oliveira, R.P.S.; Romero, J.; Castillo, R.L.; Farías, J.G. Effects of cryopreservation on mitochondrial function and sperm quality in fish. Aquaculture 2019, 511. [Google Scholar] [CrossRef]
- Diepart, C.; Verrax, J.; Calderon, P.B.; Feron, O.; Jordan, B.F.; Gallez, B. Comparison of methods for measuring oxygen consumption in tumor cells in vitro. Anal. Biochem. 2010, 396, 250–256. [Google Scholar] [CrossRef]
- Magdanz, V.; Boryshpolets, S.; Ridzewski, C.; Eckel, B.; Reinhardt, K. The motility-based swim-up technique separates bull sperm based on differences in metabolic rates and tail length. PLoS ONE 2019, 14, e0223576. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Wu, C.C.; Shiku, H.; Yasukawa, T.; Yokoo, M.; Ito-Sasaki, T.; Abe, H.; Hoshi, H.; Matsue, T. Oxygen consumption of cell suspension in a poly(dimethylsiloxane) (PDMS) microchannel estimated by scanning electrochemical microscopy. Analyst 2006, 131, 1006–1011. [Google Scholar] [CrossRef]
- Strovas, T.J.; McQuaide, S.C.; Anderson, J.B.; Nandakumar, V.; Kalyuzhnaya, M.G.; Burgess, L.W.; Holl, M.R.; Meldrum, D.R.; Lidstrom, M.E. Direct measurement of oxygen consumption rates from attached and unattached cells in a reversibly sealed, diffusionally isolated sample chamber. Adv. Biosci. Biotechnol. 2010, 5, 398–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christen, R.; Gatti, J.L.; Billard, R. Trout sperm motility. The transient movement of trout sperm is related to changes in the concentration of ATP following the activation of the flagellar movement. Eur. J. Biochem. 1987, 166, 667–671. [Google Scholar] [CrossRef]
- Billard, R.; Cosson, J.; Fierville, F.; Brun, R.; Rouault, T.; Williot, P. Motility analysis and energetics of the Siberian sturgeon Acipenser baerii spermatozoa. J. Appl. Ichthyol. 1999, 15, 199–203. [Google Scholar] [CrossRef]
- Inoda, T.; Ohtake, H.; Morisawa, M. Activation of respiration and initiation of motility in rainbow trout spermatozoa. Zool. Sci. 1988, 5, 939–945. [Google Scholar]
- Terner, C.; Korsh, G. The Oxidative Metabolism of Pyruvate, Acetate and Glucose in Isolated Fish Spermatozoa. J. Cell. Comp. Physiol. 1963, 62, 243–249. [Google Scholar] [CrossRef]
- Lahnsteiner, F.; Caberlotto, S. Motility of gilthead seabream Sparus aurata spermatozoa and its relation to temperature, energy metabolism and oxidative stress. Aquaculture 2012, 370–371, 76–83. [Google Scholar] [CrossRef]
- Lahnsteiner, F.; Berger, B.; Weismann, T.; Patzner, R. Determination of semen quality of the rainbow trout, Oncorhynchus mykiss, by sperm motility, seminal plasma parameters, and spermatozoal metabolism. Aquaculture 1998, 163, 163–181. [Google Scholar] [CrossRef]
- Lahnsteiner, F.; Mansour, N.; McNiven, M.A.; Richardson, G.F. Fatty acids of rainbow trout (Oncorhynchus mykiss) semen: Composition and effects on sperm functionality. Aquaculture 2009, 298, 118–124. [Google Scholar] [CrossRef]
- Tocher, D.R. Metabolism and functions of lipids and fatty acids in teleost fish. Rev. Fish. Sci. 2003, 11, 107–184. [Google Scholar] [CrossRef]
- Figueroa, E.; Valdebenito, I.; Zepeda, A.B.; Figueroa, C.A.; Dumorné, K.; Castillo, R.L.; Farias, J.G. Effects of cryopreservation on mitochondria of fish spermatozoa. Rev. Aquac. 2017, 9, 76–87. [Google Scholar] [CrossRef]
- Psenicka, M.; Alavi, S.M.; Rodina, M.; Gela, D.; Nebesarova, J.; Linhart, O. Morphology and ultrastructure of Siberian sturgeon (Acipenser baerii) spermatozoa using scanning and transmission electron microscopy. Biol. Cell 2007, 99, 103–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benito, S.; Sánchez, A.; Unceta, N.; Andrade, F.; Aldámiz-Echevarria, L.; Goicolea, M.A.; Barrio, R.J. LC-QTOF-MS-based targeted metabolomics of arginine-creatine metabolic pathway-related compounds in plasma: Application to identify potential biomarkers in pediatric chronic kidney disease. Anal. Bioanal. Chem. 2016, 408, 747–760. [Google Scholar] [CrossRef]
- Brosnan, M.E.; Brosnan, J.T. Renal arginine metabolism. J. Nutr. 2004, 134, 2791S–2795S. [Google Scholar] [CrossRef] [Green Version]
- Simopoulos, A.P. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiessling, K.-H.; Kiessling, A. Selective utilization of fatty acids in rainbow trout (Oncorhynchus mykiss Walbaum) red muscle mitochondria. Can. J. Zool. 1993, 71, 248–251. [Google Scholar] [CrossRef]
- Bureau, B.; Kaushik, S.; Cho, C. Bioenergetics. In Fish Nutrition; Halver, J.E., Hardy, R.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 1–59. [Google Scholar]
- Gambino, R.; Piscitelli, J.; Ackattupathil, T.A.; Theriault, J.L.; Andrin, R.D.; Sanfilippo, M.L.; Etienne, M. Acidification of blood is superior to sodium fluoride alone as an inhibitor of glycolysis. Clin. Chem. 2009, 55, 1019–1021. [Google Scholar] [CrossRef] [Green Version]
- Gambino, R. Sodium fluoride: An ineffective inhibitor of glycolysis. Ann. Clin. Biochem. 2013, 50, 3–5. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, W.; Xue, X.; Zhang, Y.; Niu, R.; Li, X.; Li, B.; Wang, X.; Wang, J. Fluoride decreased the sperm ATP of mice through inhabiting mitochondrial respiration. Chemosphere 2016, 144, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Waring, W.; Evans, L.; Kirkpatrick, C. Glycolysis inhibitors negatively bias blood glucose measurements: Potential impact on the reported prevalence of diabetes mellitus. J. Clin. Pathol. 2007, 60, 820–923. [Google Scholar] [CrossRef] [Green Version]

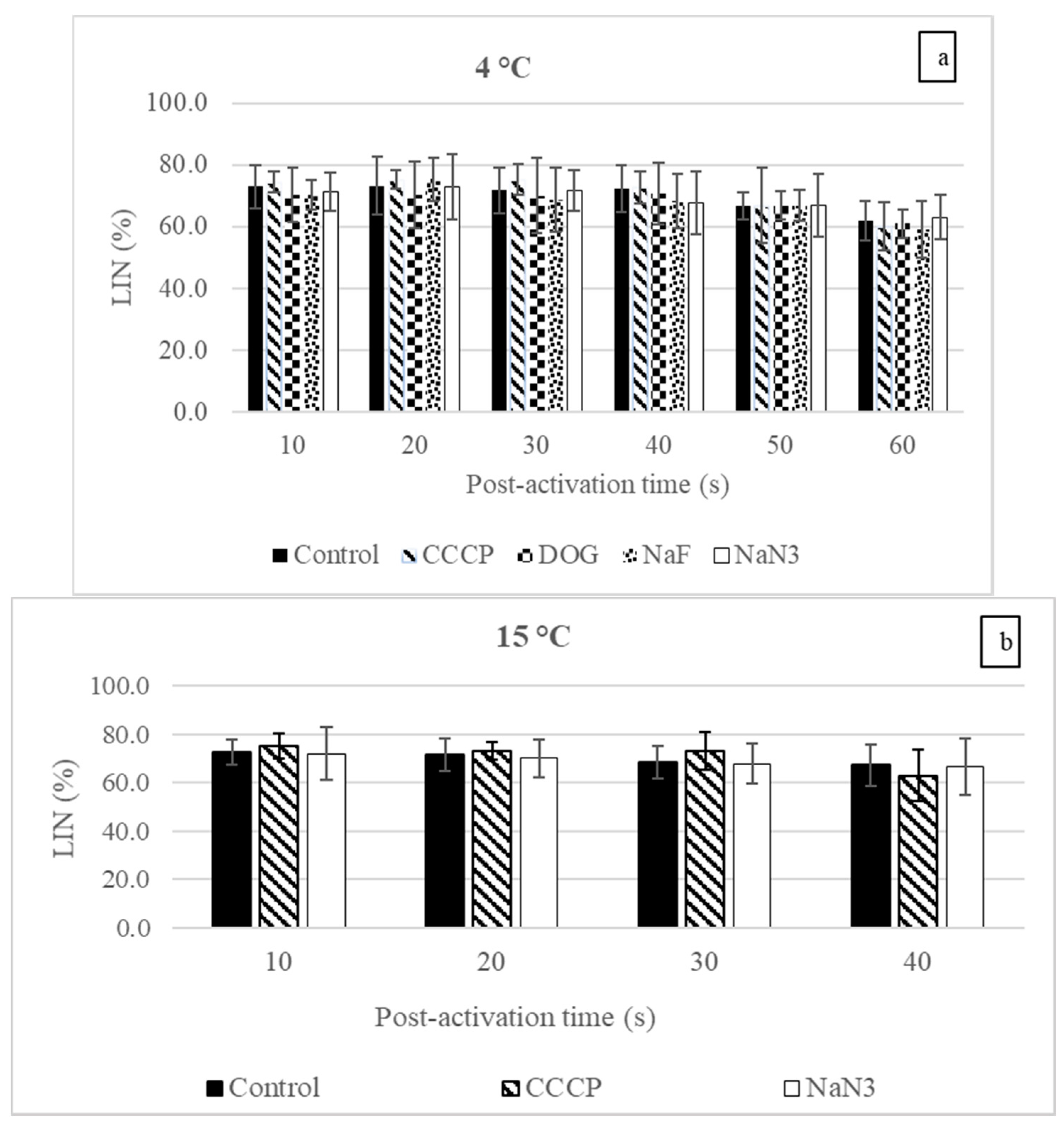
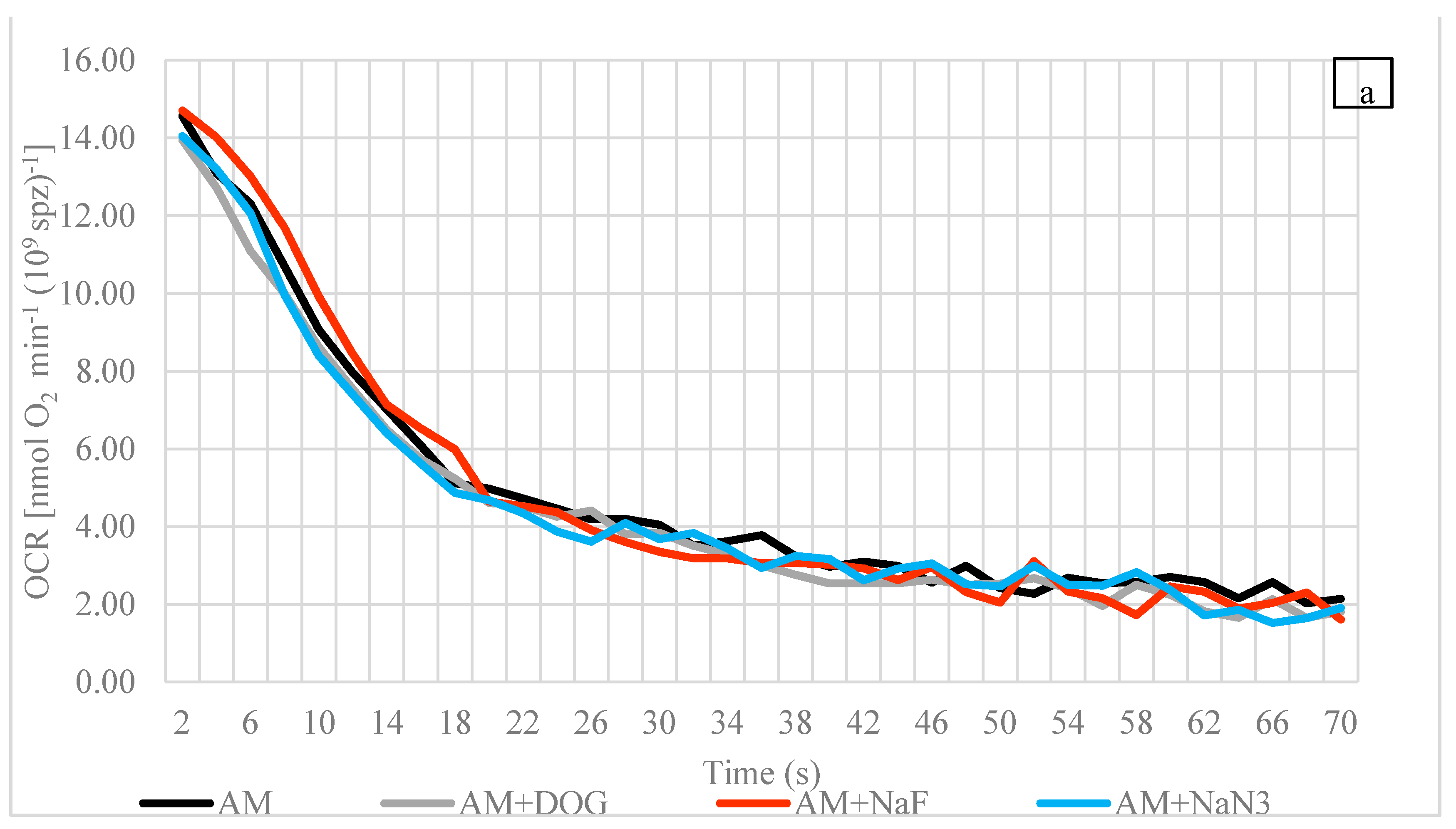
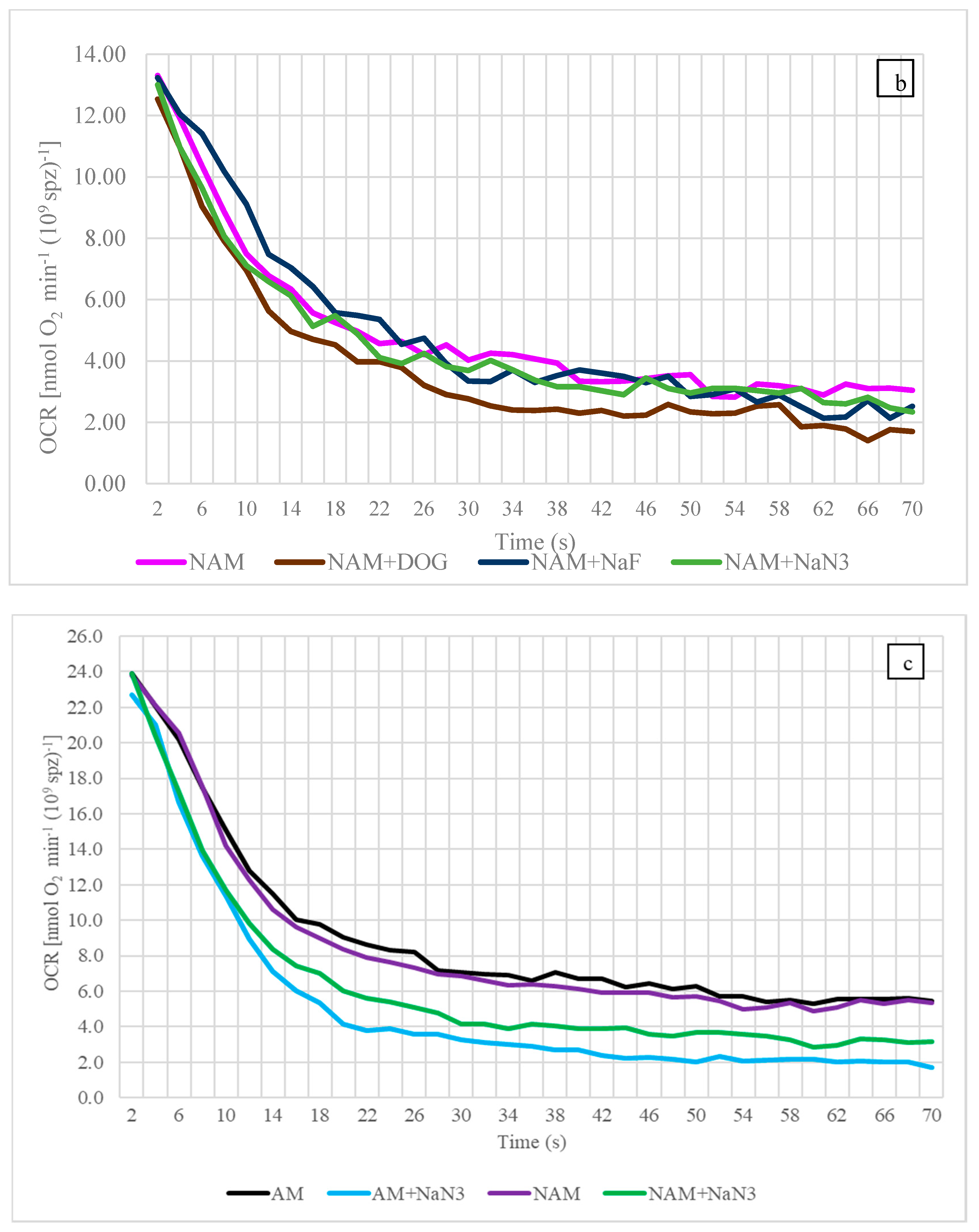
| Exposures | 4 °C (s) | 15 °C (s) |
|---|---|---|
| Control | 65.8 ± 5.1 a | 46.7 ± 1.8 b |
| CCCP | 63.0 ± 5.4 a | 45.6 ± 2.6 b |
| NaF | 67.1 ± 3.1 | - |
| DOG | 68.7 ± 4.2 | - |
| NaN3 | 68.2 ± 5.3 a | 47.0 ± 5.9 b |
| Exposures | 2 s | 30 s | 60 s |
|---|---|---|---|
| 4 °C | |||
| AM | 14.6 ± 3.3 a | 4.0 ± 0.8 a | 2.7 ± 1.0 a |
| AM + NaF | 14.7 ± 2.7 a | 3.3 ± 1.3 a | 2.6 ± 0.9 a |
| AM + DOG | 13.8 ± 2.3 a | 3.9 ± 1.9 a | 2.3 ± 0.6 a |
| AM + NaN3 | 14.0 ± 4.1 a | 3.7 ± 1.5 a | 2.4 ± 1.8 a |
| 4 °C | |||
| NAM | 13.3 ± 2.6 a | 4.0 ± 1.0 a | 3.0 ± 0.8 a |
| NAM + NaF | 13.2 ± 4.1 a | 3.3 ± 1.5 a | 2.5 ± 1.2 a |
| NAM + DOG | 12.5 ± 3.0 a | 2.8 ± 0.7 a | 1.9 ± 0.4 a |
| NAM + NaN3 | 13.0 ± 2.9 a | 3.7 ± 1.6 a | 3.1 ± 1.2 a |
| 15 °C | |||
| AM | 23.9 ± 5.2 a | 7.1 ± 1.4 a | 5.3 ± 0.8 a |
| AM + NaN3 | 22.7 ± 5.1 a | 3.2 ± 0.7 b | 2.1 ± 0.6 b |
| 15 °C | |||
| NAM | 23.5 ± 8.8 a | 6.5 ± 2.2 a | 4.9 ± 1.9 a |
| NAM + NaN3 | 23.9 ± 8.1 a | 4.2 ± 2.0 b | 2.9 ± 1.6 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahi, D.; Dzyuba, B.; Policar, T.; Malinovskyi, O.; Rodina, M.; Dzyuba, V. Bioenergetic Pathways in the Sperm of an Under-Ice Spawning Fish, Burbot (Lota lota): The Role of Mitochondrial Respiration in a Varying Thermal Environment. Biology 2021, 10, 739. https://doi.org/10.3390/biology10080739
Rahi D, Dzyuba B, Policar T, Malinovskyi O, Rodina M, Dzyuba V. Bioenergetic Pathways in the Sperm of an Under-Ice Spawning Fish, Burbot (Lota lota): The Role of Mitochondrial Respiration in a Varying Thermal Environment. Biology. 2021; 10(8):739. https://doi.org/10.3390/biology10080739
Chicago/Turabian StyleRahi, Deepali, Borys Dzyuba, Tomas Policar, Oleksandr Malinovskyi, Marek Rodina, and Viktoriya Dzyuba. 2021. "Bioenergetic Pathways in the Sperm of an Under-Ice Spawning Fish, Burbot (Lota lota): The Role of Mitochondrial Respiration in a Varying Thermal Environment" Biology 10, no. 8: 739. https://doi.org/10.3390/biology10080739
APA StyleRahi, D., Dzyuba, B., Policar, T., Malinovskyi, O., Rodina, M., & Dzyuba, V. (2021). Bioenergetic Pathways in the Sperm of an Under-Ice Spawning Fish, Burbot (Lota lota): The Role of Mitochondrial Respiration in a Varying Thermal Environment. Biology, 10(8), 739. https://doi.org/10.3390/biology10080739







