Platinum Recovery Techniques for a Circular Economy
Abstract
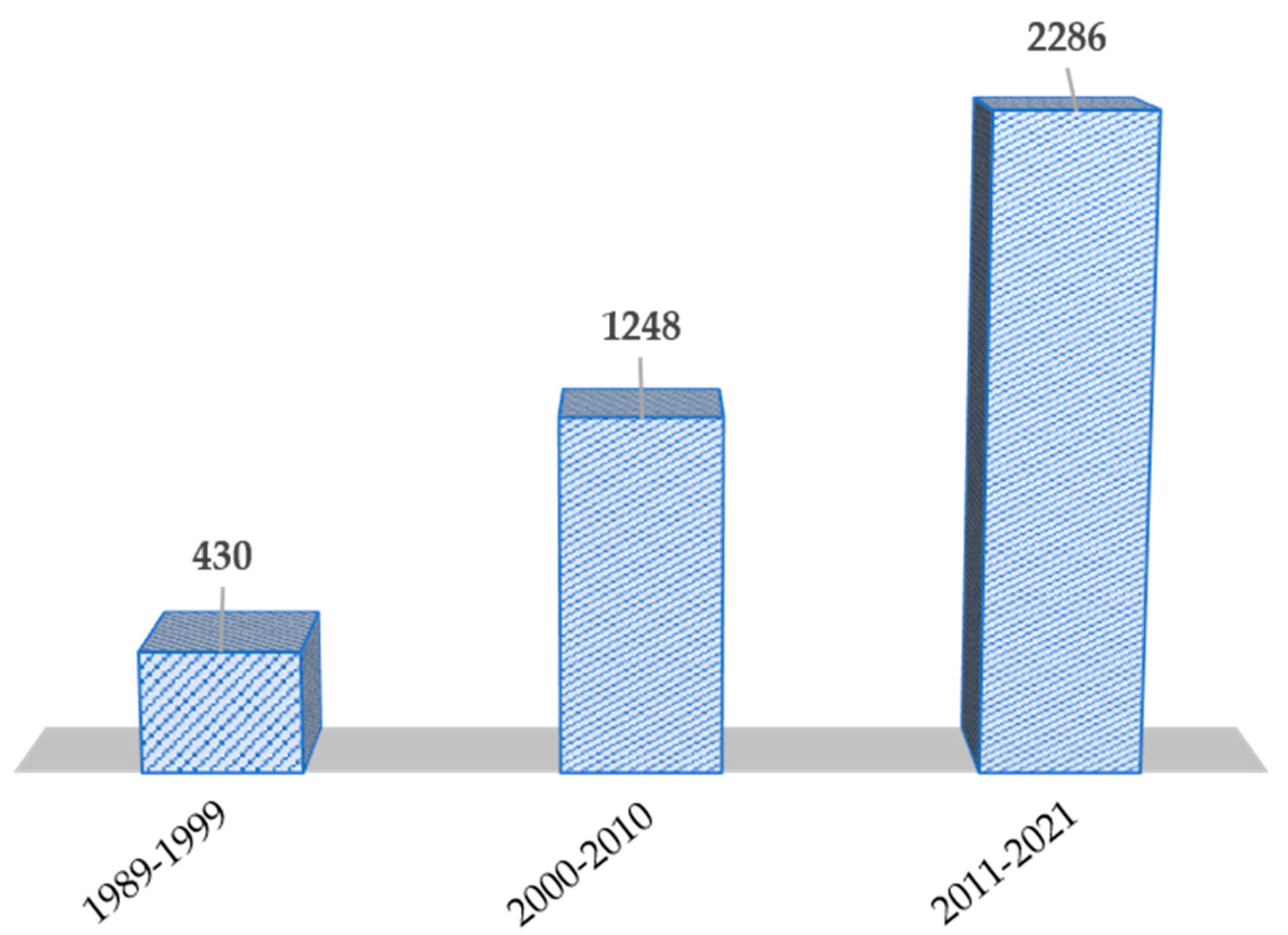
:1. Introduction
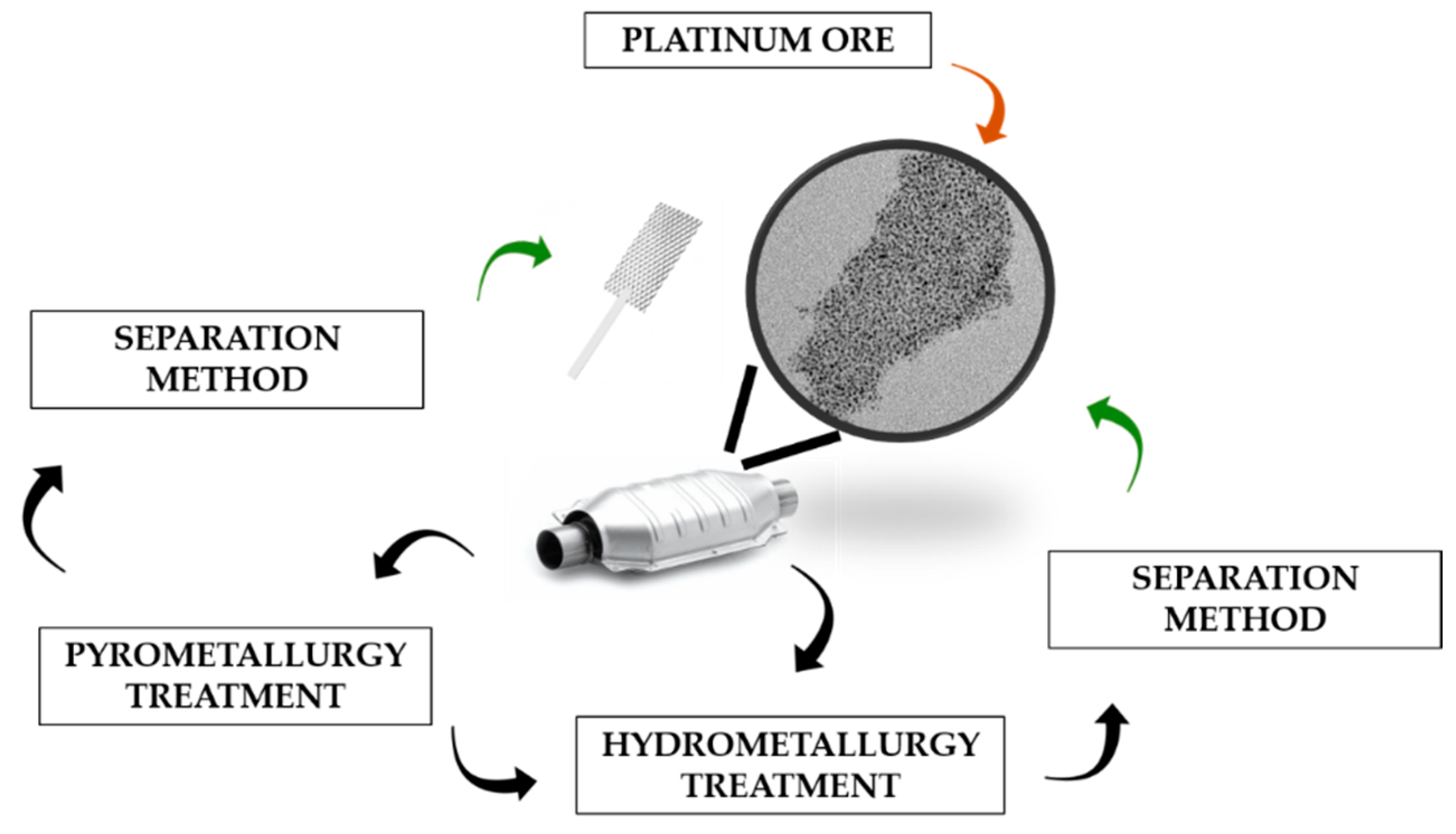
2. Recovery Technologies of Pt from Spent Catalysts
2.1. Conventional Technologies
2.1.1. Pyrometallurgical Recovery Process
2.1.2. Hydrometallurgical Recovery Process
2.2. Alternative and Novel Technologies
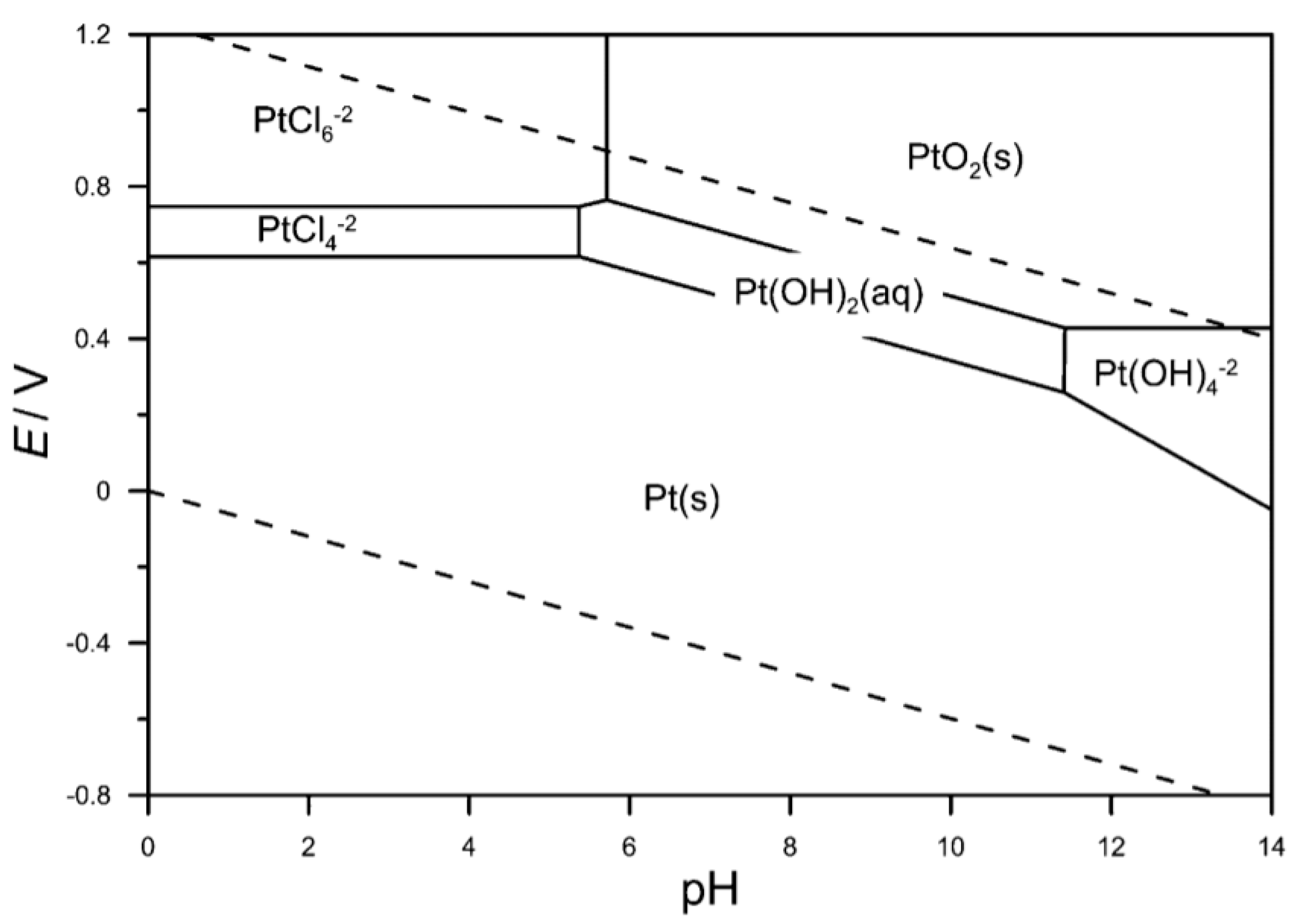
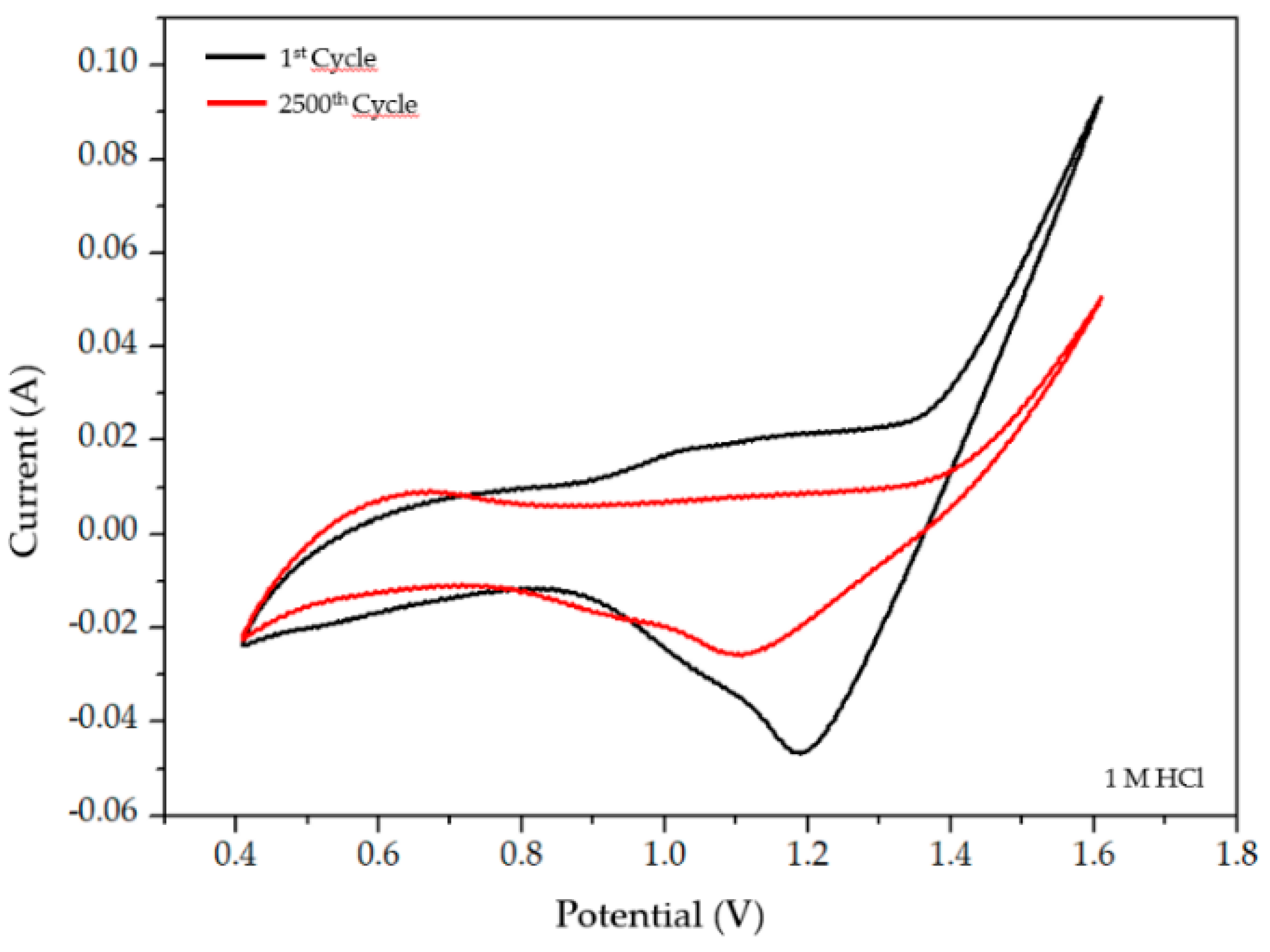
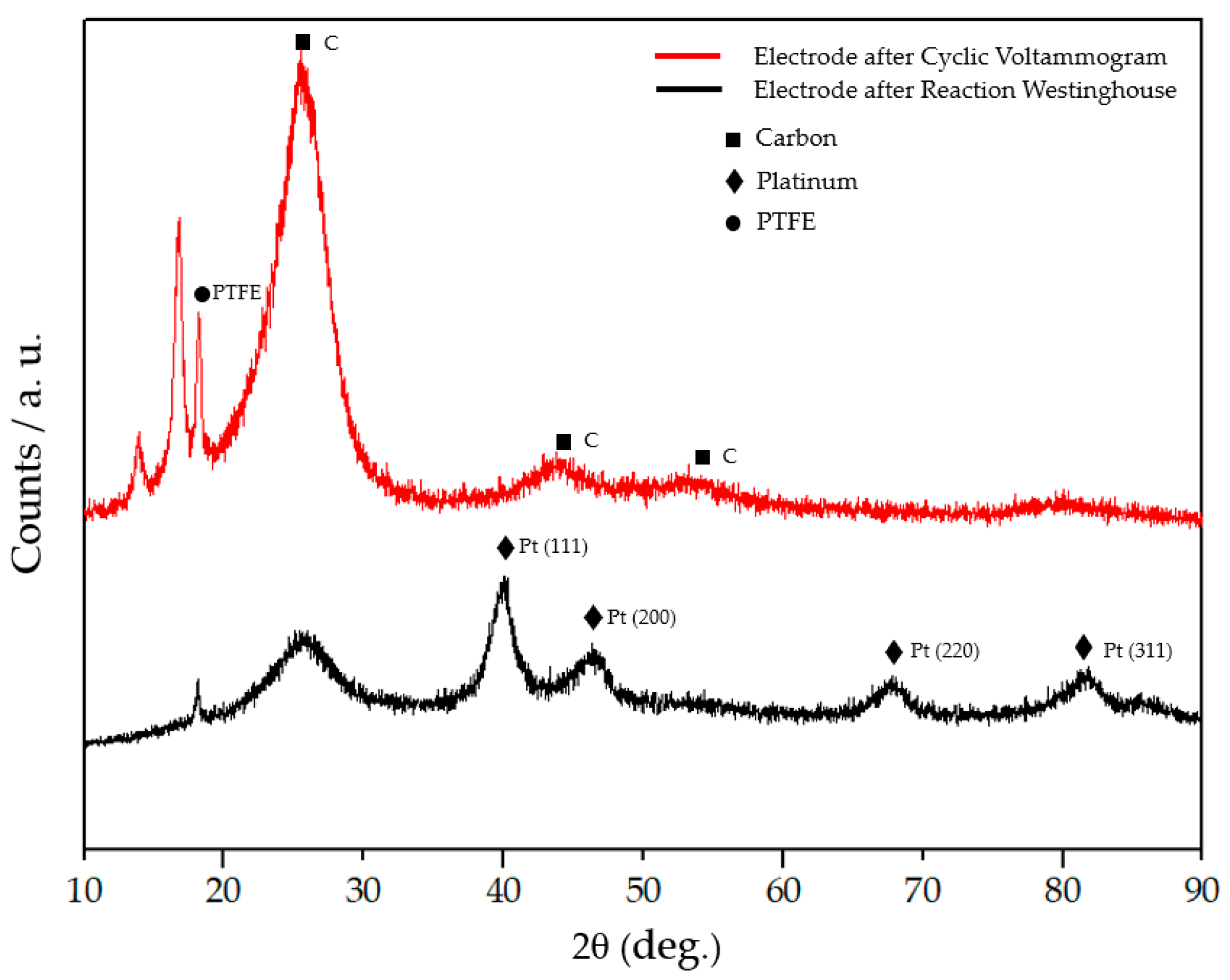
2.2.1. Selective Electrochemical Dissolution
2.2.2. Bioleaching
2.2.3. Other Techniques
3. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| VCCs | Vehicle catalytic converter |
| PEMFCs | Proton exchange membrane fuel cells |
| PGM | Platinum group metals |
| MEA | Membrane electrode assembly |
| TRL | Technology Readiness Level |
References
- Masson-Delmotte, V.; Zhai, P.; Pörtner, H.-O.; Roberts, D.; Skea, J.; Shukla, P.R.; Pirani, A.; Moufouma-Okia, W.; Péan, C.; Pidcock, R.; et al. Global Warming of 1.5 °C: An IPCC Special Report on the Impacts of Global Warming of 1.5 °C; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2020. [Google Scholar]
- European Commision. Europe’s Moment: Repair and Prepare for the Next Generation; European Commision: Brisel, Belgium, 2020. [Google Scholar]
- Anwar, R.; Iqbal, N.; Hanif, S.; Noor, T.; Shi, X.; Zaman, N.; Haider, D.; Rizvi, S.A.M.; Kannan, A.M. Mof-Derived Cupt/Nc Electrocatalyst for Oxygen Reduction Reaction. Catalysts 2020, 10, 799. [Google Scholar] [CrossRef]
- Zignani, S.C.; Baglio, V.; Sebastián, D.; Saccà, A.; Gatto, I.; Aricò, A.S. Towards Highly Performing and Stable PtNi Catalysts in Polymer Electrolyte Fuel Cells for Automotive Application. Materials 2017, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Brouzgou, A.; Song, S.; Liang, Z.X.; Tsiakaras, P. Non-Precious Electrocatalysts for Oxygen Reduction Reaction in Alkaline Media: Latest Achievements on Novel Carbon Materials. Catalysts 2016, 6, 159. [Google Scholar] [CrossRef]
- Alekseeva, E.; Stelmashuk, T.; Danilov, S.; Yang, P.; Levin, O. Bimetallic Cu/Pt Oxygen Reduction Reaction Catalyst for Fuel Cells Cathode Materials. Catalysts 2020, 10, 667. [Google Scholar] [CrossRef]
- Qin, C.; Wang, J.; Yang, D.; Li, B.; Zhang, C. Proton Exchange Membrane Fuel Cell Reversal: A Review. Catalysts 2016, 6, 197. [Google Scholar] [CrossRef]
- Drielsma, J.A.; Russell-Vaccari, A.J.; Drnek, T.; Brady, T.; Weihed, P.; Mistry, M.; Simbor, L.P. Mineral Resources in Life Cycle Impact Assessment—Defining the Path Forward. Int. J. Life Cycle Assess. 2016, 21, 85–105. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, K.D.; Wenzel, H.; Bangs, C.; Petavratzi, E.; Liu, G. Platinum Demand and Potential Bottlenecks in the Global Green Transition: A Dynamic Material Flow Analysis. Environ. Sci. Technol. 2019, 53, 11541–11551. [Google Scholar] [CrossRef]
- Grandell, L.; Lehtilä, A.; Kivinen, M.; Koljonen, T.; Kihlman, S.; Lauri, L.S. Role of Critical Metals in the Future Markets of Clean Energy Technologies. Renew. Energy 2016, 95, 53–62. [Google Scholar] [CrossRef]
- Gunn, G. Critical Metals Handbook; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- European Commission. Critical Raw Materials; European Commision Report; European Commision: Brisel, Belgium, 2019. [Google Scholar]
- Løvik, A.N.; Hagelüken, C.; Wäger, P. Improving Supply Security of Critical Metals: Current Developments and Research in the EU. Sustain. Mater. Technol. 2018, 15, 9–18. [Google Scholar] [CrossRef]
- Burlakovs, J.; Vincevica-Gaile, Z.; Krievans, M.; Jani, Y.; Horttanainen, M.; Pehme, K.M.; Dace, E.; Setyobudi, R.H.; Pilecka, J.; Denafas, G.; et al. Platinum Group Elements in Geosphere and Anthroposphere: Interplay among the Global Reserves, Urban Ores, Markets and Circular Economy. Minerals 2020, 10, 558. [Google Scholar] [CrossRef]
- Sahu, P.; Jena, M.S.; Mandre, N.R.; Venugopal, R. Platinum Group Elements Mineralogy, Beneficiation, and Extraction Practices–An Overview. Miner. Process. Extr. Metall. Rev. 2020, 1–14. [Google Scholar] [CrossRef]
- Glaister, B.J.; Mudd, G.M. The Environmental Costs of Platinum-PGM Mining and Sustainability: Is the Glass Half-Full or Half-Empty? Miner. Eng. 2010, 23, 438–450. [Google Scholar] [CrossRef]
- Hagelüken, C. Recycling the Platinum Group Metals: A European Perspective. Platin. Met. Rev. 2012, 56, 29–35. [Google Scholar] [CrossRef]
- Distribution of Platinum Group Metals Supply Worldwide in 2019, by Source. Available online: https://www.statista.com/statistics/602766/distribution-of-the-supply-of-platinum-group-metals-worldwide-by-source/ (accessed on 30 July 2021).
- Cui, J.; Zhang, L. Metallurgical Recovery of Metals from Electronic Waste: A Review. J. Hazard. Mater. 2008, 158, 228–256. [Google Scholar] [CrossRef] [PubMed]
- Jha, M.K.; Lee, J.C.; Kim, M.S.; Jeong, J.; Kim, B.S.; Kumar, V. Hydrometallurgical Recovery/Recycling of Platinum by the Leaching of Spent Catalysts: A Review. Hydrometallurgy 2013, 133, 23–32. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X. Progress in Recycling of Precious Metals in Automobile Catalysts. Xiyou Jinshu/Chin. J. Rare Met. 2013, 37, 1004–1015. [Google Scholar]
- Tansel, B.; Ying, W. Automotive Wastes. Water Environ. Res. 1995, 67, 507–517. [Google Scholar] [CrossRef]
- Xu, F.; Mu, S.; Pan, M. Recycling of Membrane Electrode Assembly of PEMFC by Acid Processing. Int. J. Hydrog. Energy 2010, 35, 2976–2979. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Elsaid, K.; Wilberforce, T.; Kamil, M.; Sayed, E.T.; Olabi, A. Environmental Aspects of Fuel Cells: A Review. Sci. Total Environ. 2021, 752, 141803. [Google Scholar] [CrossRef]
- Duclos, L.; Chattot, R.; Dubau, L.; Thivel, P.X.; Mandil, G.; Laforest, V.; Bolloli, M.; Vincent, R.; Svecova, L. Closing the Loop: Life Cycle Assessment and Optimization of a PEMFC Platinum-Based Catalyst Recycling Process. Green Chem. 2020, 22, 1919–1933. [Google Scholar] [CrossRef]
- Carmo, M.; Keeley, G.P.; Holtz, D.; Grube, T.; Robinius, M.; Müller, M.; Stolten, D. PEM Water Electrolysis: Innovative Approaches towards Catalyst Separation, Recovery and Recycling. Int. J. Hydrog. Energy 2019, 44, 3450–3455. [Google Scholar] [CrossRef]
- Dong, H.; Zhao, J.; Chen, J.; Wu, Y.; Li, B. Recovery of Platinum Group Metals from Spent Catalysts: A Review. Int. J. Miner. Process. 2015, 145, 108–113. [Google Scholar] [CrossRef]
- Padamata, S.K.; Yasinskiy, A.S.; Polyakov, P.V.; Pavlov, E.A.; Varyukhin, D.Y. Recovery of Noble Metals from Spent Catalysts: A Review. Metall. Mater. Trans. B Process. Metall. Mater. Process. Sci. 2020, 51, 2413–2435. [Google Scholar] [CrossRef]
- Peng, Z.; Li, Z.; Lin, X.; Tang, H.; Ye, L.; Ma, Y.; Rao, M.; Zhang, Y.; Li, G.; Jiang, T. Pyrometallurgical Recovery of Platinum Group Metals from Spent Catalysts. JOM 2017, 69, 1553–1562. [Google Scholar] [CrossRef]
- Havlik, T.; Orac, D.; Petranikova, M.; Miskufova, A.; Kukurugya, F.; Takacova, Z. Leaching of Copper and Tin from Used Printed Circuit Boards after Thermal Treatment. J. Hazard. Mater. 2010, 183, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, M.W.; Perino, E.; Ruiz, M.D.C. Gold Extraction by Chlorination Using a Pyrometallurgical Process. Miner. Eng. 2009, 22, 409–411. [Google Scholar] [CrossRef]
- Allen, R.J.; Foller, P.C.; Giallombardo, J. Two Step Method for Recovery of Dispersed Noble Metals. EU Patent Application No. 0492691A1, 1 July 1992. [Google Scholar]
- Kim, C.H.; Woo, S.I.; Jeon, S.H. Recovery of Platinum-Group Metals from Recycled Automotive Catalytic Converters by Carbochlorination. Ind. Eng. Chem. Res. 2000, 39, 1185–1192. [Google Scholar] [CrossRef]
- Horike, C.; Morita, K.; Okabe, T.H. Effective Dissolution of Platinum by Using Chloride Salts in Recovery Process. Metall. Mater. Trans. B Process. Metall. Mater. Process. Sci. 2012, 43, 1300–1307. [Google Scholar] [CrossRef] [Green Version]
- Murray, M.J.; Bicek, E.J. Recovery of Metals. U.S. Patent 3,021,209, 13 February 1962. [Google Scholar]
- Xu, S.Q.; Xu, L. Some Recovery Process for Precious Metals from Spent Automobile Catalyst. China Nat. Res. Recycl. 1998, 10, 7–11. [Google Scholar]
- Staszak, K. Chemical and Petrochemical Industry. Phys. Sci. Rev. 2018, 3, 1–28. [Google Scholar]
- Chiang, K.C.; Chen, K.L.; Chen, C.Y.; Huang, J.J.; Shen, Y.H.; Yeh, M.Y.; Wong, F.F. Recovery of Spent Alumina-Supported Platinum Catalyst and Reduction of Platinum Oxide via Plasma Sintering Technique. J. Taiwan Inst. Chem. Eng. 2011, 42, 158–165. [Google Scholar] [CrossRef]
- Bronshtein, I.; Feldman, Y.; Shilstein, S.; Wachtel, E.; Lubomirsky, I.; Kaplan, V. Efficient Chloride Salt Extraction of Platinum Group Metals from Spent Catalysts. J. Sustain. Metall. 2018, 4, 103–114. [Google Scholar] [CrossRef]
- Kayanuma, Y.; Okabe, T.H.; Maeda, M. Metal Vapor Treatment for Enhancing the Dissolution of Platinum Group Metals from Automotive Catalyst Scrap. Metall. Mater. Trans. B Process. Metall. Mater. Process. Sci. 2004, 35, 817–824. [Google Scholar] [CrossRef]
- Benson, M.; Bennett, C.R.; Harry, J.E.; Patel, M.K.; Cross, M. The Recovery Mechanism of Platinum Group Metals from Catalytic Converters in Spent Automotive Exhaust Systems. Resour. Conserv. Recycl. 2000, 31, 1–7. [Google Scholar] [CrossRef]
- Inoue, H.; Ezawa, N. Process of Recovering Platinum Group Metal. U.S. Patent 5,252,305, 12 October 1993. [Google Scholar]
- Hoffmann, J.E. Recovering Platinum-Group Metals from Gabbroic Rocks Metals from Auto Catalysts. JOM 1988, 40, 40–44. [Google Scholar] [CrossRef]
- Kolliopoulos, G.; Balomenos, E.; Giannopoulou, I.; Yakoumis, I.; Panias, D. Behavior of Platinum Group during Their Pyrometallurgical Recovery from Spent Automotive Catalysts. Open Access Libr. J. 2014, 1, 1–9. [Google Scholar] [CrossRef]
- He, X.; Li, Y.; Wu, X.; Zhoo, Y.; Weng, H.; Liu, W. Study on the Process of Enrichment Platinum Group Metals by Plasma Melting Technology. Precious Met. 2016, 37, 1–5. [Google Scholar]
- Mishra, R.K.; Redaly, R.G. Precious Metals 1986: Proceedings of the Tenth International Precious Metals Institute Conference; International Precious Metals Institute: Pensacola, FL, USA, 1986; pp. 217–231. [Google Scholar]
- Eksteen, J.J. A Mechanistic Model to Predict Matte Temperatures during the Smelting of UG2-Rich Blends of Platinum Group Metal Concentrates. Miner. Eng. 2011, 24, 676–687. [Google Scholar] [CrossRef]
- Van Schalkwyk, R.F.; Eksteen, J.J.; Akdogan, G. Leaching of Ni-Cu-Fe-S Converter Matte at Varying Iron Endpoints; Mineralogical Changes and Behaviour of Ir, Rh and Ru. Hydrometallurgy 2013, 136, 36–45. [Google Scholar] [CrossRef]
- You, G.; Fang, W.; Li, Q.; Ma, Y.; Yang, X.T.; Yang, H. Study on Enrichment Method of Platinum, Palladium and Rhodium in Spent Auto-Catalysts. Yejin Fenxi/Metall. Anal. 2016, 36, 7–11. [Google Scholar]
- Crundwell, F.; Moats, M.; Ramachandran, V.; Robinson, T.; Davenport, W.G. Extractive Metallurgy of Nickel, Cobalt and Platinum Group Metals; Elsevier: Amsterdam, The Netherland, 2011. [Google Scholar]
- Stoffner, F.; Hobbs, C. Process for the Production of a PGM-Enriched Alloy. U.S. Patent 10323302B2, 18 June 2019. [Google Scholar]
- De Aberasturi, D.J.; Pinedo, R.; De Larramendi, I.R.; De Larramendi, J.I.R.; Rojo, T. Recovery by Hydrometallurgical Extraction of the Platinum-Group Metals from Car Catalytic Converters. Miner. Eng. 2011, 24, 505–513. [Google Scholar] [CrossRef]
- Fornalczyk, A.; Saternus, M. Removal of platinum group metals fron the used autocatalytic converter. Metalurgija 2009, 48, 133–136. [Google Scholar]
- Angelidis, T.N. Development of a Laboratory Scale Hydrometallurgical Procedure for the Recovery of Pt and Rh from Spent Automotive Catalysts. Top. Catal. 2001, 16–17, 419–423. [Google Scholar] [CrossRef]
- Han, K.; Meng, X. Recovery of Platinum Group Metals and Rhenium from Materials Using Halogen Reagents. U.S. Patent 5,542,957, 6 August 1995. [Google Scholar]
- Yoo, J.S. Metal Recovery and Rejuvenation of Metal-Loaded Spent Catalysts. Catal. Today 1998, 44, 27–46. [Google Scholar] [CrossRef]
- Harjanto, S.; Cao, Y.; Shibayama, A.; Naitoh, I.; Nanami, T.; Kasahara, K.; Okumura, Y.; Liu, K.; Fujita, T. Leaching of Pt, Pd and Rh from Automotive Catalyst Residue in Various Chloride Based Solutions. Mater. Trans. 2006, 47, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Panda, R.; Jha, M.K.; Pathak, D.D. Commercial Processes for the Extraction of Platinum Group Metals (PGMs). In Rare Metal Technology; The Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2018; Volume F5, pp. 119–130. [Google Scholar]
- Saguru, C.; Ndlovu, S.; Moropeng, D. A Review of Recent Studies into Hydrometallurgical Methods for Recovering PGMs from Used Catalytic Converters. Hydrometallurgy 2018, 182, 44–56. [Google Scholar] [CrossRef]
- Massucci, M.; Clegg, S.L.; Brimblecombe, P. Equilibrium Partial Pressures, Thermodynamic Properties of Aqueous and Solid Phases, and Cl2 Production from Aqueous HCl and HNO3 and Their Mixtures. J. Phys. Chem. A 1999, 103, 4209–4226. [Google Scholar] [CrossRef]
- Tyson, D.R.; Bautista, R.G. Leaching Kinetics of Platinum and Palladium from Spent Automotive Catalysts. Sep. Sci. Technol. 1987, 22, 1149–1167. [Google Scholar] [CrossRef]
- Jafarifar, D.; Daryanavard, M.R.; Sheibani, S. Ultra Fast Microwave-Assisted Leaching for Recovery of Platinum from Spent Catalyst. Hydrometallurgy 2005, 78, 166–171. [Google Scholar] [CrossRef]
- Schreier, G.; Edtmaier, C. Separation of Ir, Pd and Rh from Secondary Pt Scrap by Precipitation and Calcination. Hydrometallurgy 2003, 68, 67–75. [Google Scholar] [CrossRef]
- Kizilaslan, E.; Aktaş, S.; Şeşen, M.K. Towards Environmentally Safe Recovery of Platinum from Scrap Automotive Catalytic Converters. Turk. J. Eng. Environ. Sci. 2009, 33, 83–90. [Google Scholar]
- Shams, K.; Beiggy, M.R.; Shirazi, A.G. Platinum Recovery from a Spent Industrial Dehydrogenation Catalyst Using Cyanide Leaching Followed by Ion Exchange. Appl. Catal. A Gen. 2004, 258, 227–234. [Google Scholar] [CrossRef]
- Zanjani, A.; Baghalha, M. Factors Affecting Platinum Extraction from Used Reforming Catalysts in Iodine Solutions at Temperatures up to 95 °C. Hydrometallurgy 2009, 97, 119–125. [Google Scholar] [CrossRef]
- Torres, R.; Lapidus, G.T. Platinum, Palladium and Gold Leaching from Magnetite Ore, with Concentrated Chloride Solutions and Ozone. Hydrometallurgy 2016, 166, 185–194. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Riaño, S.; Aktan, E.; Deferm, C.; Fransaer, J.; Binnemans, K. Solvometallurgical Recovery of Platinum Group Metals from Spent Automotive Catalysts. ACS Sustain. Chem. Eng. 2021, 9, 337–350. [Google Scholar] [CrossRef]
- Sasaki, H.; Maeda, M. Zn-Vapor Pretreatment for Acid Leaching of Platinum Group Metals from Automotive Catalytic Converters. Hydrometallurgy 2014, 147–148, 59–67. [Google Scholar] [CrossRef]
- Suoranta, T.; Zugazua, O.; Niemelä, M.; Perämäki, P. Recovery of Palladium, Platinum, Rhodium and Ruthenium from Catalyst Materials Using Microwave-Assisted Leaching and Cloud Point Extraction. Hydrometallurgy 2015, 154, 56–62. [Google Scholar] [CrossRef]
- Sui, S.; Wang, X.; Zhou, X.; Su, Y.; Riffat, S.; Liu, C.-j. A Comprehensive Review of Pt Electrocatalysts for the Oxygen Reduction Reaction: Nanostructure, Activity, Mechanism and Carbon Support in PEM Fuel Cells. J. Mater. Chem. A 2017, 5, 1808–1825. [Google Scholar] [CrossRef]
- Wittstock, R.; Pehlken, A.; Wark, M. Challenges in Automotive Fuel Cells Recycling. Recycling 2016, 1, 343–364. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.; Nielsen, K.R.; Lund, P.B.; Simonsen, S.B.; Grahl-Madsen, L.; Andersen, S.M. Sustainable Platinum Recycling through Electrochemical Dissolution of Platinum Nanoparticles from Fuel Cell Electrodes. ChemElectroChem 2019, 6, 4471–4482. [Google Scholar] [CrossRef]
- Sugawara, Y.; Okayasu, T.; Yadav, A.P.; Nishikata, A.; Tsuru, T. Dissolution Mechanism of Platinum in Sulfuric Acid Solution. J. Electrochem. Soc. 2012, 159, F779. [Google Scholar] [CrossRef]
- Geiger, S.; Cherevko, S.; Mayrhofer, K.J.J. Dissolution of Platinum in Presence of Chloride Traces. Electrochim. Acta 2015, 179, 24–31. [Google Scholar] [CrossRef]
- Cherevko, S.; Kulyk, N.; Mayrhofer, K.J.J. Durability of Platinum-Based Fuel Cell Electrocatalysts: Dissolution of Bulk and Nanoscale Platinum. Nano Energy 2016, 29, 275–298. [Google Scholar] [CrossRef]
- Cherevko, S.; Keeley, G.P.; Geiger, S.; Zeradjanin, A.R.; Hodnik, N.; Kulyk, N.; Mayrhofer, K.J.J. Dissolution of Platinum in the Operational Range of Fuel Cells. ChemElectroChem 2015, 2, 1471–1478. [Google Scholar] [CrossRef] [Green Version]
- Topalov, A.A.; Katsounaros, I.; Auinger, M.; Cherevko, S.; Meier, J.C.; Klemm, S.O.; Mayrhofer, K.J.J. Dissolution of Platinum: Limits for the Deployment of Electrochemical Energy Conversion? Angew. Chem. Int. Ed. 2012, 51, 12613–12615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlišič, A.; Jovanovič, P.; Šelih, V.S.; Šala, M.; Hodnik, N.; Gaberšček, M. Platinum Dissolution and Redeposition from Pt/C Fuel Cell Electrocatalyst at Potential Cycling. J. Electrochem. Soc. 2018, 165, F3161–F3165. [Google Scholar] [CrossRef]
- Jovanovič, P.; Petek, U.; Hodnik, N.; Ruiz-Zepeda, F.; Gatalo, M.; Šala, M.; Šelih, V.S.; Fellinger, T.P.; Gaberšček, M. Importance of Non-Intrinsic Platinum Dissolution in Pt/C Composite Fuel Cell Catalysts. Phys. Chem. Chem. Phys. 2017, 19, 21446–21452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, D.J.; Wang, X.; Smith, M.C.; More, K.L. Potentiostatic and Potential Cycling Dissolution of Polycrystalline Platinum and Platinum Nano-Particle Fuel Cell Catalysts. J. Electrochem. Soc. 2018, 165, F3178–F3190. [Google Scholar] [CrossRef] [Green Version]
- Topalov, A.A.; Cherevko, S.; Zeradjanin, A.R.; Meier, J.C.; Katsounaros, I.; Mayrhofer, K.J.J. Towards a Comprehensive Understanding of Platinum Dissolution in Acidic Media. Chem. Sci. 2014, 5, 631–638. [Google Scholar] [CrossRef] [Green Version]
- Pourbaix, M.; Zhang, H.; Pourbaix, A. Presentation of an Atlas of Chemical and Electrochemical Equilibria in the Presence of a Gaseous Phase. Mater. Sci. Forum 1997, 251–254, 143–148. [Google Scholar] [CrossRef]
- Latsuzbaia, R.; Negro, E.; Koper, G.J.M. Environmentally Friendly Carbon-Preserving Recovery of Noble Metals from Supported Fuel Cell Catalysts. ChemSusChem 2015, 8, 1926–1934. [Google Scholar] [CrossRef]
- Kanamura, S.; Yagyu, M. Electrochemical Dissolution of Platinum and Ruthenium from Membrane Electrode Assemblies of Polymer Electrolyte Fuel Cells. Mater. Trans. 2016, 57, 1972–1976. [Google Scholar] [CrossRef] [Green Version]
- Hodnik, N.; Baldizzone, C.; Polymeros, G.; Geiger, S.; Grote, J.P.; Cherevko, S.; Mingers, A.; Zeradjanin, A.; Mayrhofer, K.J.J. Platinum Recycling Going Green via Induced Surface Potential Alteration Enabling Fast and Efficient Dissolution. Nat. Commun. 2016, 7, 1–6. [Google Scholar] [CrossRef]
- Díaz-Abad, S.; Millán, M.; Rodrigo, M.A.; Lobato, J. Review of Anodic Catalysts for SO2 Depolarized Electrolysis for “Green Hydrogen” Production. Catalysts 2019, 9, 63. [Google Scholar] [CrossRef] [Green Version]
- Lobato, J.; Díaz-Abad, S.; Peláez, M.C.; Millán, M.; Rodrigo, M.A. Synthesis and Characterization of Pt on Novel Catalyst Supports for the H2 Production in the Westinghouse Cycle. Int. J. Hydrog. Energy 2020, 45, 25672–25680. [Google Scholar] [CrossRef]
- Valente, A.; Iribarren, D.; Dufour, J. End of Life of Fuel Cells and Hydrogen Products: From Technologies to Strategies. Int. J. Hydrog. Energy 2019, 44, 20965–20977. [Google Scholar] [CrossRef]
- Brandl, H.; Lehmann, S.; Faramarzi, M.A.; Martinelli, D. Biomobilization of Silver, Gold, and Platinum from Solid Waste Materials by HCN-Forming Microorganisms. Hydrometallurgy 2008, 94, 14–17. [Google Scholar] [CrossRef] [Green Version]
- Pham, V.A.; Ting, Y.P. Gold Bioleaching of Electronic Waste by Cyanogenic Bacteria and Its Enhancement with Bio-Oxidation. Adv. Mater. Res. 2009, 71–73, 661–664. [Google Scholar] [CrossRef]
- Ilyas, S.; Lee, J. chun Biometallurgical Recovery of Metals from Waste Electrical and Electronic Equipment: A Review. Chembioeng. Rev. 2014, 1, 148–169. [Google Scholar] [CrossRef]
- Bas, A.D.; Deveci, H.; Yazici, E.Y. Bioleaching of Copper from Low Grade Scrap TV Circuit Boards Using Mesophilic Bacteria. Hydrometallurgy 2013, 138, 65–70. [Google Scholar] [CrossRef]
- Natarajan, G.; Ting, Y.P. Pretreatment of E-Waste and Mutation of Alkali-Tolerant Cyanogenic Bacteria Promote Gold Biorecovery. Bioresour. Technol. 2014, 152, 80–85. [Google Scholar] [CrossRef]
- Işıldar, A.; van de Vossenberg, J.; Rene, E.R.; van Hullebusch, E.D.; Lens, P.N.L. Two-Step Bioleaching of Copper and Gold from Discarded Printed Circuit Boards (PCB). Waste Manag. 2016, 57, 149–157. [Google Scholar] [CrossRef]
- Chi, T.D.; Lee, J.C.; Pandey, B.D.; Yoo, K.; Jeong, J. Bioleaching of Gold and Copper from Waste Mobile Phone PCBs by Using a Cyanogenic Bacterium. Proc. Miner. Eng. 2011, 24, 1219–1222. [Google Scholar] [CrossRef]
- Askeland, R.A.; Morrison, S.M. Cyanide Production by Pseudomonas Fluorescens and Pseudomonas Aeruginosa. Appl. Environ. Microbiol. 1983, 45, 1802–1807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karim, S.; Ting, Y.P. Recycling Pathways for Platinum Group Metals from Spent Automotive Catalyst: A Review on Conventional Approaches and Bio-Processes. Resour. Conserv. Recycl. 2021, 170, 105588. [Google Scholar] [CrossRef]
- Shin, D.; Park, J.; Jeong, J.; Kim, B.S. A Biological Cyanide Production and Accumulation System and the Recovery of Platinum-Group Metals from Spent Automotive Catalysts by Biogenic Cyanide. Hydrometallurgy 2015, 158, 10–18. [Google Scholar] [CrossRef]
- Karim, S.; Ting, Y.P. Ultrasound-Assisted Nitric Acid Pretreatment for Enhanced Biorecovery of Platinum Group Metals from Spent Automotive Catalyst. J. Clean. Prod. 2020, 255, 120199. [Google Scholar] [CrossRef]
- Trinh, H.B.; Lee, J.-c.; Suh, Y.-j.; Lee, J. A Review on the Recycling Processes of Spent Auto-Catalysts: Towards the Development of Sustainable Metallurgy. Waste Manag. 2020, 114, 148–165. [Google Scholar] [CrossRef] [PubMed]
- Taninouchi, Y.-K.; Okabe, T.H. Recovery of Platinum Group Metals from Spent Catalysts Using Iron Chloride Vapor Treatment. Metall. Mater. Trans. B Process. Metall. Mater. Process. Sci. 2018, 49, 1781–1793. [Google Scholar] [CrossRef]
- Taninouchi, Y.K.; Watanabe, T.; Okabe, T.H. Recovery of Platinum Group Metals from Spent Catalysts Using Electroless Nickel Plating and Magnetic Separation. Proc. Mater. Trans. 2017, 58, 410–419. [Google Scholar] [CrossRef] [Green Version]
- Martinez, A.M.; Osen, K.S.; Støre, A. Recovery of Platinum Group Metals from Secondary Sources by Selective Chlorination from Molten Salt Media. Proc. Miner. Met. Mater. Ser. 2020, 221–233. [Google Scholar]
- Ding, Y.; Zheng, H.; Zhang, S.; Liu, B.; Wu, B.; Jian, Z. Highly Efficient Recovery of Platinum, Palladium, and Rhodium from Spent Automotive Catalysts via Iron Melting Collection. Resour. Conserv. Recycl. 2020, 155, 104644. [Google Scholar] [CrossRef]
- Morcali, M.H. A New Approach to Recover Platinum-Group Metals from Spent Catalytic Converters via Iron Matte. Resour. Conserv. Recycl. 2020, 159, 104891. [Google Scholar] [CrossRef]
- Sasaki, H.; Maeda, M. Enhanced Dissolution of Pt from Pt-Zn Intermetallic Compounds and Underpotential Dissolution from Zn-Rich Alloys. J. Phys. Chem. C 2013, 117, 18457–18463. [Google Scholar] [CrossRef]
- Spooren, J.; Abo Atia, T. Combined Microwave Assisted Roasting and Leaching to Recover Platinum Group Metals from Spent Automotive Catalysts. Miner. Eng. 2020, 146, 106153. [Google Scholar] [CrossRef]
- Nicol, G.; Goosey, E.; Yildiz, D.S.; Loving, E.; Nguyen, V.T.; Riaño, S.; Yakoumis, I.; Martinez, A.M.; Siriwardana, A.; Unzurrunzaga, A.; et al. Platinum Group Metals Recovery Using Secondary Raw Materials (Platirus): Project Overview with a Focus on Processing Spent Autocatalyst. Johns. Matthey Technol. Rev. 2021, 65, 127–147. [Google Scholar]
- Chen, S.; Shen, S.; Cheng, Y.; Wang, H.; Lv, B.; Wang, F. Effect of O2, H2 and CO Pretreatments on Leaching Rh from Spent Auto-Catalysts with Acidic Sodium Chlorate Solution. Hydrometallurgy 2014, 144–145, 69–76. [Google Scholar] [CrossRef]
- Trinh, H.B.; Lee, J.-c.; Srivastava, R.R.; Kim, S. Total Recycling of All the Components from Spent Auto-Catalyst by NaOH Roasting-Assisted Hydrometallurgical Route. J. Hazard. Mater. 2019, 379, 120772. [Google Scholar] [CrossRef]
- Nogueira, C.A.; Paiva, A.P.; Costa, M.C.; Rosa da Costa, A.M. Leaching Efficiency and Kinetics of the Recovery of Palladium and Rhodium from a Spent Auto-Catalyst in HCl/CuCl2 Media. Environ. Technol. 2020, 41, 2293–2304. [Google Scholar] [CrossRef]
- Johnson Matthey. Pgm Refining. Available online: https://matthey.com/en/products-and-services/precious-metal-products/pgm-refining (accessed on 24 June 2021).
- Samotaev, N.; Antonov, A.; Tsarev, G.; Tietz, A. Effective Recycling of Spent Auto Catalytic Converters by Using Electrochlorination Method. In MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2018; Volume 207, p. 03024. [Google Scholar]
| Process | Technology | Pt Recovery Rate (%) | TRL | Energy Consumption | Waste Generated | Reference |
|---|---|---|---|---|---|---|
| Commercial Pt process. Any type of waste | Pyro-hydrometallurgy | 99.95 | 9 | ● | ● | [113] |
| Copper smelting collection process | Pyrometallurgy | 99 | 9 | ● | ● | [42] |
| Cyanide leaching of industrial catalyst | Hydrometallurgy | 85 | 5 | ● | ● | [65] |
| Zn-vapor pretreatment for acid leaching of VCCs | Hydrometallurgy | >95 | 3 | ● | ● | [69] |
| Chlorination assisted leching | Pyro-hydrometallurgy | 95.9 | 4 | ● | ● | [34] |
| Microwave assisted leaching | Hydrometallurgy | 91 | 4 | ● | ● | [70] |
| Electroless Nickel plating and magnetic separation | Physical concentration | 50–100 | 3 | ● | ● | [103] |
| Biogenic cyanide leaching | Biohydrometallurgy | 92.1 | 4 | ● | ● | [99] |
| Total VCC recycling by NaOH roasting-assisted hydrometallurgy | Hydrometallurgy | 97.5 | 5 | ● | ● | [111] |
| Microwave assisted roasting and leaching | Hydrometallurgy | 80–90 | 5 | ● | ● | [108] |
| Electrochemical dissolution of Pt nanoparticles | Selective electrochemical dissolution | 25–60 | 3 | ● | ● | [73] |
| Electrochlorination of VCCs | Electrochlorination | 97 | 6 | ● | ● | [114] |
| Induced surface potential alteration | Selective chemical dissolution | 100 | 4 | ● | ● | [86] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granados-Fernández, R.; Montiel, M.A.; Díaz-Abad, S.; Rodrigo, M.A.; Lobato, J. Platinum Recovery Techniques for a Circular Economy. Catalysts 2021, 11, 937. https://doi.org/10.3390/catal11080937
Granados-Fernández R, Montiel MA, Díaz-Abad S, Rodrigo MA, Lobato J. Platinum Recovery Techniques for a Circular Economy. Catalysts. 2021; 11(8):937. https://doi.org/10.3390/catal11080937
Chicago/Turabian StyleGranados-Fernández, Rafael, Miguel A. Montiel, Sergio Díaz-Abad, Manuel A. Rodrigo, and Justo Lobato. 2021. "Platinum Recovery Techniques for a Circular Economy" Catalysts 11, no. 8: 937. https://doi.org/10.3390/catal11080937
APA StyleGranados-Fernández, R., Montiel, M. A., Díaz-Abad, S., Rodrigo, M. A., & Lobato, J. (2021). Platinum Recovery Techniques for a Circular Economy. Catalysts, 11(8), 937. https://doi.org/10.3390/catal11080937








