Phytochemicals and In Vitro Bioactivities of Aqueous Ethanolic Extracts from Common Vegetables in Thai Food
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Analyses
2.2. Antioxidant Activities
2.3. Enzyme- and Non-Enzyme Inhibitory Activities
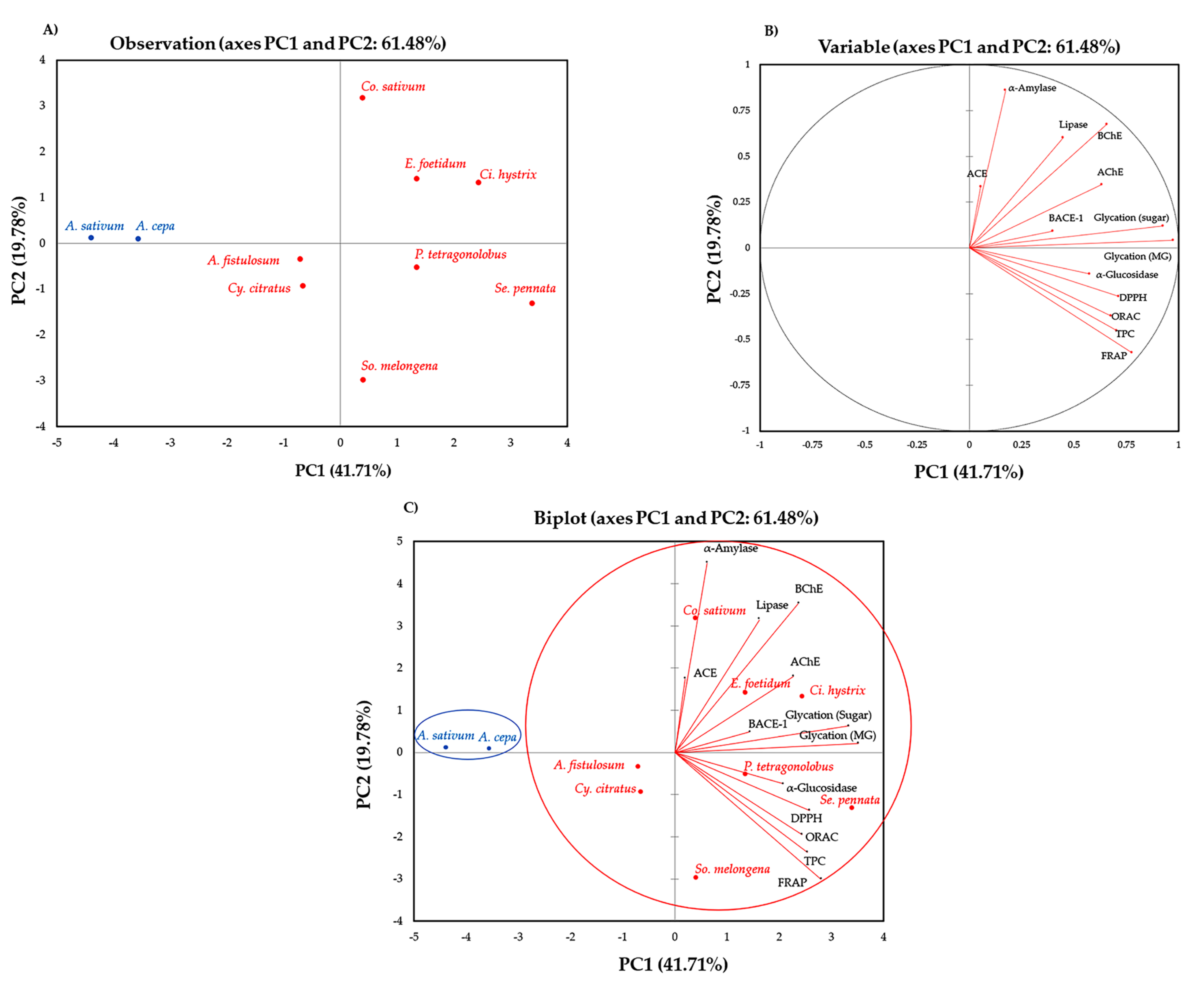
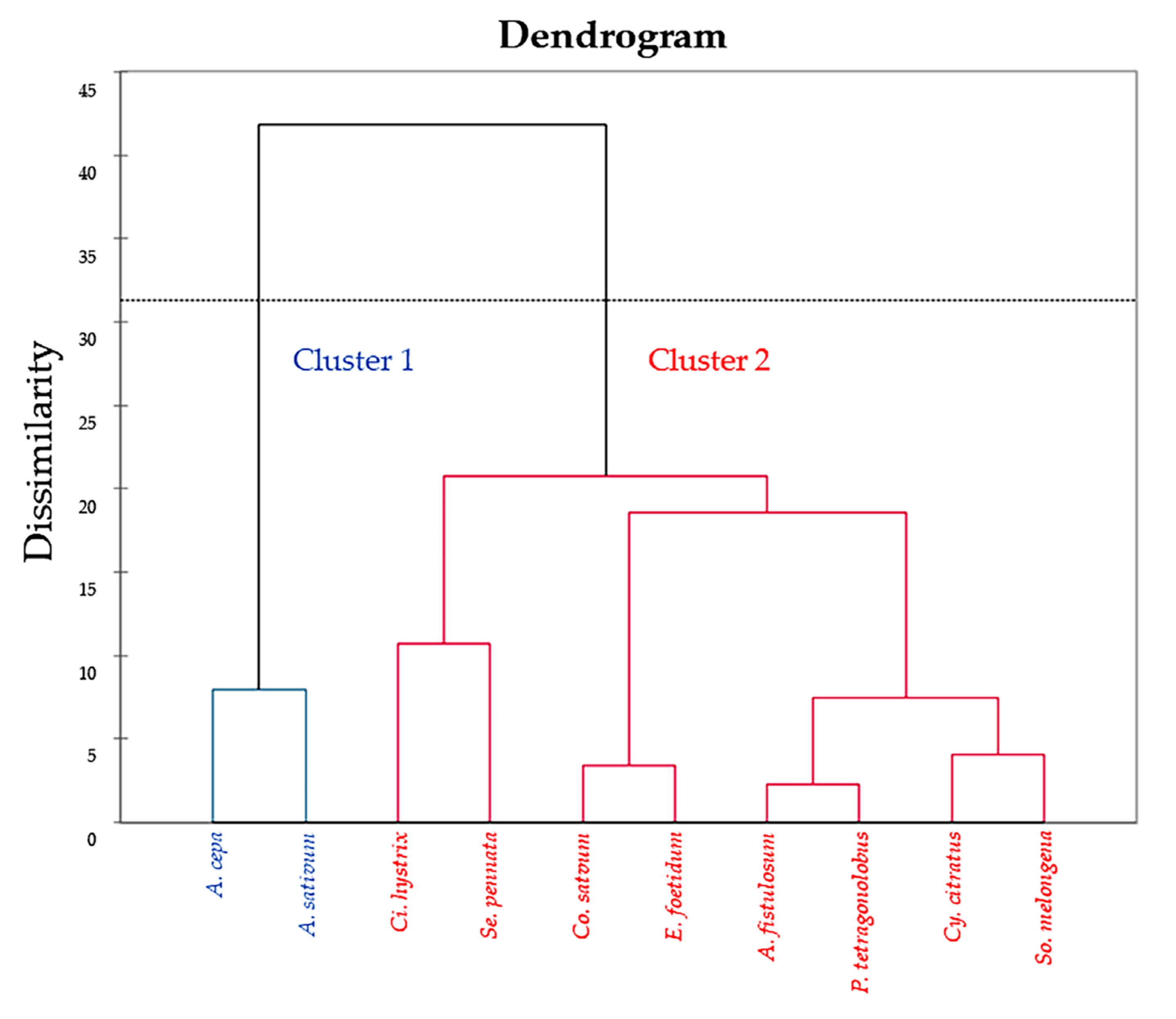
2.4. Correlation by Principal Component Analysis (PCA) and Hierarchical Cluster Analysis (HCA)
3. Discussion
4. Materials and Methods
4.1. Sample Collection, Preparation, and Extraction
4.2. Determination of Phenolics Profile and Total Phenolic Contents
4.3. Determination of Antioxidant Activities
4.4. Determination of Enzyme and Non-Enzyme Inhibitory Activities Using Spectrophotometric Techniques
4.5. Principal Component Analysis, Hierarchical Cluster Analysis, and Data Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE | Angiotensin-converting enzyme |
| AChE | Acetylcholinesterase |
| AD | Alzheimer’s disease |
| BACE-1 | β-secretase |
| BChE | Butyrylcholinesterase |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| DW | Dry weight |
| FRAP | Ferric reducing antioxidant power |
| HCA | Hierarchical cluster analysis |
| MG | Methylglyoxal |
| NCDs | Non-communicable diseases |
| ORAC | Oxygen radical absorbance capacity |
| PCA | Principal component analysis |
| TPCs | Total phenolic contents |
References
- World Health Organization (WHO). Global Status Report on Noncommunicable Disease 2014; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- World Health Organization (WHO). Fruit and Vegetables for Health: Report of the Joint FAO/WHO Workshop on Fruit and Vegetables for Health, Kobe, Japan, 1–3 September 2004; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Borgi, L.; Muraki, I.; Satija, A.; Willett, W.C.; Rimm, E.B.; Forman, J.P. Fruit and Vegetable Consumption and the Incidence of Hypertension in Three Prospective Cohort Studies. Hypertension 2016, 67, 288–293. [Google Scholar] [CrossRef] [Green Version]
- Chiavaroli, L.; Viguiliouk, E.; Nishi, S.K.; Blanco Mejia, S.; Rahelic, D.; Kahleova, H.; Salas-Salvado, J.; Kendall, C.W.; Sievenpiper, J.L. DASH Dietary Pattern and Cardiometabolic Outcomes: An Umbrella Review of Systematic Reviews and Meta-Analyses. Nutrients 2019, 11, 338. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, P.; Holland, T.M.; Wang, Y.; Bennett, D.A.; Morris, M.C. Association of Strawberries and Anthocyanidin Intake with Alzheimer’s Dementia Risk. Nutrients 2019, 11, 3060. [Google Scholar] [CrossRef] [Green Version]
- Holland, T.M.; Agarwal, P.; Wang, Y.; Leurgans, S.E.; Bennett, D.A.; Booth, S.L.; Morris, M.C. Dietary flavonols and risk of Alzheimer dementia. Neurology 2020, 94, e1749–e1756. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Singh, K.; Khedkar, R. Phytochemicals: Extraction process, safety assessment, toxicological evaluations, and regulatory issues. In Functional and Preservative Properties of Phytochemicals; Prakash, B., Ed.; Academic Press: Waltham, MA, USA, 2020; pp. 341–361. [Google Scholar]
- Halliwell, B.; Murcia, M.A.; Chirico, S.; Aruoma, O.I. Free radicals and antioxidants in food and in vivo: What they do and how they work. Crit. Rev. Food Sci. Nutr. 1995, 35, 7–20. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Xu, B. Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, R.H. Potential Mechanisms of Action of Dietary Phytochemicals for Cancer Prevention by Targeting Cellular Signaling Transduction Pathways. J. Agric. Food Chem. 2018, 66, 3260–3276. [Google Scholar] [CrossRef] [PubMed]
- Hinkaew, J.; Aursalung, A.; Sahasakul, Y.; Tangsuphoom, N.; Suttisansanee, U. A Comparison of the Nutritional and Biochemical Quality of Date Palm Fruits Obtained Using Different Planting Techniques. Molecules 2021, 26, 8. [Google Scholar] [CrossRef]
- Temviriyanukul, P.; Sritalahareuthai, V.; Jom, K.N.; Jongruaysup, B.; Tabtimsri, S.; Pruesapan, K.; Thangsiri, S.; Inthachat, W.; Siriwan, D.; Charoenkiatkul, S.; et al. Comparison of Phytochemicals, Antioxidant, and In Vitro Anti-Alzheimer Properties of Twenty-Seven Morus spp. Cultivated in Thailand. Molecules 2020, 25, 11. [Google Scholar] [CrossRef]
- Wannasaksri, W.; On-Nom, N.; Chupeerach, C.; Temviriyanukul, P.; Charoenkiatkul, S.; Suttisansanee, U. In Vitro Phytotherapeutic Properties of Aqueous Extracted Adenia viridiflora Craib. towards Civilization Diseases. Molecules 2021, 26, 4. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, J.; Bains, Y.; Guha, S.; Kahn, A.; Hall, D.; Bose, N.; Gugliucci, A.; Kapahi, P. The Role of Advanced Glycation End Products in Aging and Metabolic Diseases: Bridging Association and Causality. Cell Metab. 2018, 28, 337–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagai, R.; Shirakawa, J.; Ohno, R.; Moroishi, N.; Nagai, M. Inhibition of AGEs formation by natural products. Amino Acids 2014, 46, 261–266. [Google Scholar] [CrossRef]
- Verma, T.; Sinha, M.; Bansal, N.; Yadav, S.R.; Shah, K.A.-O.; Chauhan, N.A.-O. Plants Used as Antihypertensive. Nat. Prod. Bioprospect. 2021, 11, 155–184. [Google Scholar] [CrossRef] [PubMed]
- Geck, M.S.; Cristians, S.; Berger-González, M.; Casu, L.; Heinrich, M.; Leonti, M. Traditional Herbal Medicine in Mesoamerica: Toward Its Evidence Base for Improving Universal Health Coverage. Front. Pharmacol. 2020, 11, 1160. [Google Scholar] [CrossRef] [PubMed]
- Sanlier, N.; Gencer, F. Role of spices in the treatment of diabetes mellitus: A minireview. Trends Food Sci. Technol. 2020, 99, 441–449. [Google Scholar] [CrossRef]
- Thomas, P.S.; Essien, E.E.; Ntuk, S.J.; Choudhary, M.I. Eryngium foetidum L. Essential Oils: Chemical Composition and Antioxidant Capacity. Medicines 2017, 4, 2. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.T.; Tsai, C.W.; Yao, H.T.; Lii, C.K.; Chen, H.W.; Wu, Y.L.; Chen, P.Y.; Liu, K.L. Suppressive effects of extracts from the aerial part of Coriandrum sativum L. on LPS-induced inflammatory responses in murine RAW 264.7 macrophages. J. Sci. Food Agric. 2010, 90, 1846–1854. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.Y.; Kim, D.S.; Kim, S.H.; Kim, H.K. Aqueous and ethanolic extracts of welsh onion, Allium fistulosum, attenuate high-fat diet-induced obesity. BMC Complement. Altern. Med. 2018, 18, 105. [Google Scholar] [CrossRef] [Green Version]
- Ried, K. Garlic lowers blood pressure in hypertensive subjects, improves arterial stiffness and gut microbiota: A review and meta-analysis. Exp. Ther. Med. 2020, 19, 1472–1478. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.T.; Liu, X.T.; Chen, Q.X.; Shi, Y. Lipase Inhibitors for Obesity: A Review. Biomed. Pharm. 2020, 128, 110314. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Menichini, F. Natural products as alpha-amylase and alpha-glucosidase inhibitors and their hypoglycaemic potential in the treatment of diabetes: An update. Mini Rev. Med. Chem. 2010, 10, 315–331. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Vlassara, H. Advanced glycation in health and disease: Role of the modern environment. Ann. N. Y. Acad. Sci. 2005, 1043, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Sihmar, S.; Jatain, A. A Study of Hierarchical Clustering Algorithms. In Proceedings of the 2015 2nd International Conference on Computing for Sustainable Global Development (INDIACom), New Delhi, India, 11–13 March 2015; Institute of Electrical and Electronics Engineers: Piscataway, NJ, USA, 2015; pp. 537–541. [Google Scholar]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic Content, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef] [Green Version]
- Nanasombat, S.; Teckchuen, N. Antimicrobial, antioxidant and anticancer activities of Thai local vegetables. J. Med. Plants Res. 2009, 3, 443–449. [Google Scholar]
- Ramli, S.; Bunrathep, S.; Tansaringkarn, T.; Ruangrungsi, N. Screening for Free Radical Scavenging Activity from Ethanolic Extract of Mimosaceous Plants Endemic to Thailand. J. Health Res. 2018, 22, 55–59. [Google Scholar]
- Chokthaweepanich, H.; Sriwicha, S.; Auvuchanon, A.; Supapvanich, S. Phytochemical Screening and Fruit Quality of Commercial Eggplants. Cast 2021, 21, 36–50. [Google Scholar]
- Wijaya, Y.A.; Widyadinata, D.; Irawaty, W.; Ayucitra, A. Fractionation of Phenolic Compounds from Kaffir Lime (Citrus Hystrix) Peel Extract and Evaluation of Antioxidant Activity. Reaktor 2017, 17, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Kawaguchi, K.; Mizuno, T.; Aida, K.; Uchino, K. Hesperidin as an Inhibitor of Lipases from Porcine Pancreas and Pseudomonas. Biosci. Biotechnol. Biochem. 1997, 61, 102–104. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Gonzalez, A.I.; Alvarez-Parrilla, E.; Díaz-Sánchez, Á.G.; de la Rosa, L.A.; Núñez-Gastélum, J.A.; Vazquez-Flores, A.A.; Gonzalez-Aguilar, G.A. In vitro Inhibition of Pancreatic Lipase by Polyphenols: A Kinetic, Fluorescence Spectroscopy and Molecular Docking Study. Food Technol. Biotechnol. 2017, 55, 519–530. [Google Scholar] [CrossRef]
- Bustos, A.-S.; Håkansson, A.; Linares-Pastén, J.A.; Penarrieta, J.M.; Nilsson, L. Interaction Between Phenolic Compounds and Lipase: The Influence of Solubility and Presence of Particles in the IC50 Value. J. Food Sci. 2018, 83, 2071–2076. [Google Scholar] [CrossRef] [PubMed]
- Aligita, W.; Susilawati, E.; Septiani, H.; Atsil, R. Antidiabetic Activity of Coriander (Coriandrum sativum L.) Leaves’ Ethanolic Extract. Int. J. Pharm. Phytopharm. Res. 2018, 8, 59–63. [Google Scholar]
- Narkhede, M.B. Evaluation of Alpha Amylase Inhibitory Potential of Four Traditional Culinary Leaves. Asian J. Pharm. Clin. Res. 2012, 5, 75–76. [Google Scholar]
- Oboh, G.; Agunloye, O.M.; Adefegha, S.A.; Akinyemi, A.J.; Ademiluyi, A.O. Caffeic and chlorogenic acids inhibit key enzymes linked to type 2 diabetes (in vitro): A comparative study. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 165–170. [Google Scholar] [CrossRef]
- Shori, A.B. Proteolytic activity, antioxidant, and α-Amylase inhibitory activity of yogurt enriched with coriander and cumin seeds. LWT Food Sci. Technol. 2020, 133, 109912. [Google Scholar] [CrossRef]
- Mechchate, H.; Es-Safi, I.; Amaghnouje, A.; Boukhira, S.; Alotaibi, A.A.; Al-Zharani, M.; Nasr, A.F.; Noman, M.O.; Conte, R.; Amal, E.H.; et al. Antioxidant, Anti-Inflammatory and Antidiabetic Proprieties of LC-MS/MS Identified Polyphenols from Coriander Seeds. Molecules 2021, 26, 487. [Google Scholar] [CrossRef] [PubMed]
- Hajlaoui, H.; Arraouadi, S.; Noumi, E.; Aouadi, K.; Adnan, M.; Khan, M.A.; Kadri, A.; Snoussi, M. Antimicrobial, Antioxidant, Anti-Acetylcholinesterase, Antidiabetic, and Pharmacokinetic Properties of Carum carvi L. and Coriandrum sativum L. Essential Oils Alone and in Combination. Molecules 2021, 26, 3625. [Google Scholar] [CrossRef]
- Promyos, N.; Temviriyanukul, P.; Suttisansanee, U. Investigation of Anthocyanidins and Anthocyanins for Targeting alpha-Glucosidase in Diabetes Mellitus. Prev. Nutr. Food Sci. 2020, 25, 263–271. [Google Scholar] [CrossRef]
- Hossain, U.; Das, A.K.; Ghosh, S.; Sil, P.C. An overview on the role of bioactive α-glucosidase inhibitors in ameliorating diabetic complications. Food Chem. Toxicol. 2020, 145, 111738. [Google Scholar] [CrossRef]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of alpha-glucosidase and alpha-amylase by flavonoids. J. Nutr. Sci. Vitam. 2006, 52, 149–153. [Google Scholar] [CrossRef] [Green Version]
- Brüll, V.; Burak, C.; Stoffel-Wagner, B.; Wolffram, S.; Nickenig, G.; Müller, C.; Langguth, P.; Alteheld, B.; Fimmers, R.; Naaf, S.; et al. Effects of a quercetin-rich onion skin extract on 24 h ambulatory blood pressure and endothelial function in overweight-to-obese patients with (pre-)hypertension: A randomised double-blinded placebo-controlled cross-over trial. Br. J. Nutr. 2015, 114, 1263–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrero, L.; Castillo, J.; Quiñones, M.; Garcia-Vallvé, S.; Arola, L.; Pujadas, G.; Muguerza, B. Inhibition of angiotensin-converting enzyme activity by flavonoids: Structure-activity relationship studies. PLoS ONE 2012, 7, e49493. [Google Scholar] [CrossRef] [Green Version]
- Bhullar, K.S.; Lassalle-Claux, G.; Touaibia, M.; Rupasinghe, H.P.V. Antihypertensive effect of caffeic acid and its analogs through dual renin–angiotensin–aldosterone system inhibition. Eur. J. Pharmacol. 2014, 730, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Kean, R.J.; Lamport, D.J.; Dodd, G.F.; Freeman, J.E.; Williams, C.M.; Ellis, J.A.; Butler, L.T.; Spencer, J.P. Chronic consumption of flavanone-rich orange juice is associated with cognitive benefits: An 8-wk, randomized, double-blind, placebo-controlled trial in healthy older adults. Am. J. Clin. Nutr. 2015, 101, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wang, Y.; Li, J.; Hua, L.; Han, B.; Zhang, Y.; Yang, X.; Zeng, Z.; Bai, H.; Yin, H.; et al. Effects of caffeic acid on learning deficits in a model of Alzheimer’s disease. Int. J. Mol. Med. 2016, 38, 869–875. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Youn, K.; Lim, G.; Lee, J.; Jun, M. In Silico Docking and In Vitro Approaches towards BACE1 and Cholinesterases Inhibitory Effect of Citrus Flavanones. Molecules 2018, 23, 1509. [Google Scholar] [CrossRef] [Green Version]
- Oboh, G.; Agunloye, O.M.; Akinyemi, A.J.; Ademiluyi, A.O.; Adefegha, S.A. Comparative study on the inhibitory effect of caffeic and chlorogenic acids on key enzymes linked to Alzheimer’s disease and some pro-oxidant induced oxidative stress in rats’ brain-in vitro. Neurochem. Res. 2013, 38, 413–419. [Google Scholar] [CrossRef]
- Shimmyo, Y.; Kihara, T.; Akaike, A.; Niidome, T.; Sugimoto, H. Flavonols and flavones as BACE-1 inhibitors: Structure-activity relationship in cell-free, cell-based and in silico studies reveal novel pharmacophore features. Biochim. Biophys. Acta 2008, 1780, 819–825. [Google Scholar] [CrossRef]
- Hunt, J.V.; Wolff, S.P. Oxidative glycation and free radical production: A causal mechanism of diabetic complications. Free Radic. Res. Commun. 1991, 12–13, 115–123. [Google Scholar] [CrossRef]
- Ronsisvalle, S.; Panarello, F.; Longhitano, G.; Siciliano, E.A.; Montenegro, L.; Panico, A. Natural Flavones and Flavonols: Relationships among Antioxidant Activity, Glycation, and Metalloproteinase Inhibition. Cosmetics 2020, 7, 71. [Google Scholar] [CrossRef]
- Sasaki, K.; Chiba, S.; Yoshizaki, F. Effect of natural flavonoids, stilbenes and caffeic acid oligomers on protein glycation. Biomed. Rep. 2014, 2, 628–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Lu, X.; Sun, Q.; Gao, J.; Ma, L.; Huang, J. Novel ACE Inhibitory Peptides Derived from Simulated Gastrointestinal Digestion in Vitro of Sesame (Sesamum indicum L.) Protein and Molecular Docking Study. Int. J. Mol. Sci. 2020, 21, 1059. [Google Scholar] [CrossRef] [Green Version]
- Srinuanchai, W.; Nooin, R.; Jarussophon, S.; Kasemwong, K.; Nuchuchua, O. Determination of gymnemic acid level in Gymnema inodorum leaves using multiple reaction monitoring mass spectrometry. J. Chem. Metrol. 2019, 13, 75–79. [Google Scholar] [CrossRef]
- Sripum, C.; Kukreja, R.K.; Charoenkiatkul, S.; Kriengsinyos, W.; Suttisansanee, U. The effect of storage conditions on antioxidant activities and total phenolic contents of parboiled germinated brown rice (Khao Dok Mali 105). Int. Food Res. J. 2016, 23, 1827–1831. [Google Scholar]
- Sripum, C.; Kukreja, R.K.; Charoenkiatkul, S.; Kriengsinyos, W.; Suttisansanee, U. The effect of extraction conditions on antioxidant activities and total phenolic contents of different processed Thai Jasmine rice. Int. Food Res. J. 2017, 24, 1644–1650. [Google Scholar]
- Vinson, J.A.; Howard, T.B. Inhibition of protein glycation and advanced glycation end products by ascorbic acid and other vitamins and nutrients. J. Nutr. Biochem. 1996, 7, 659–663. [Google Scholar] [CrossRef]
| Samples | Flavonoids (mg/100 g DW) | ||||||
|---|---|---|---|---|---|---|---|
| Quercetin | Kaempferol | Hesperidin | Luteolin | Apigenin | Delphinidin | Cyanidin | |
| A. cepa | 63.34 ± 1.01 b | ND | ND | ND | ND | ND | 13.35 ± 1.07 |
| A. fistulosum | ND | 47.97 ± 0.97 a | ND | ND | ND | ND | ND |
| A. sativum | ND | ND | ND | ND | ND | ND | ND |
| Ci. hystrix | 25.52 ± 0.36 c | ND | 453.47 ± 2.28 | ND | ND | ND | ND |
| Co. sativum | 166.16 ± 1.20 a | 4.44 ± 0.03 c | ND | ND | ND | ND | ND |
| Cy. citratus | ND | ND | ND | 4.57 ± 0.10 | ND | ND | ND |
| E. foetidum | ND | 22.09 ± 2.91 b | ND | ND | ND | ND | ND |
| P. tetragonolobus | ND | ND | ND | ND | ND | 15.77 ± 0.59 | 43.02 ± 0.87 * |
| Se. pennata | ND | ND | ND | ND | 3.46 ± 0.10 | ND | ND |
| So. melongena | ND | ND | ND | ND | ND | ND | ND |
| Samples | Phenolic Acids (mg/100 g DW) | ||||
|---|---|---|---|---|---|
| 4-Hydroxybenzoic Acid | Vanillic Acid | Caffeic Acid | p-Coumaric Acid | Ferulic Acid | |
| A. cepa | ND | ND | ND | ND | ND |
| A. fistulosum | ND | ND | ND | 7.12 ± 0.16 b | 23.13 ± 0.12 b |
| A. sativum | ND | ND | ND | ND | ND |
| Ci. hystrix | ND | ND | ND | ND | ND |
| Co. sativum | ND | 3.73 ± 0.04 | 23.81 ± 0.50 c | 5.20 ± 0.06 c | ND |
| Cy. citratus | ND | ND | 15.65 ± 0.28 d | 68.13 ± 1.09 a | 123.34 ± 2.82 a |
| E. foetidum | ND | ND | 52.69 ± 8.33 b | 2.63 ± 0.24 e | 1.77 ± 0.01 c |
| P. tetragonolobus | 10.80 ± 2.79 | 15.71 ± 0.02 * | 16.13 ± 5.37 d | 3.85 ± 0.44 d | ND |
| Se. pennata | ND | ND | 14.92 ± 0.09 d | ND | ND |
| So. melongena | ND | ND | 246.99 ± 10.55 a | 2.16 ± 0.04 e | ND |
| Sample | Total Phenolic Contents (mg GAE/g DW) | Antioxidant Activities (μmol TE/g DW) | ||
|---|---|---|---|---|
| DPPH Radical Scavenging Assay | FRAP Assay | ORAC Assay | ||
| A. cepa | 3.76 ± 0.07 f | 5.90 ± 0.17 f | 5.10 ± 0.05 i | 15.12 ± 0.46 g |
| A. fistulosum | 5.91 ± 0.11 e | 6.14 ± 0.25 f | 18.32 ± 0.08 g | 223.83 ± 11.46 c |
| A. sativum | 1.23 ± 0.02 i | 1.77 ± 0.01 g | 3.25 ± 0.08 j | 80.14 ± 1.51 f |
| Ci. hystrix | 12.98 ± 0.15 b | 6.14 ± 0.06 f | 38.12 ± 0.43 e | 418.32 ± 0.77 a |
| Co. sativum | 2.68 ± 0.07 h | 9.12 ± 0.14 e | 16.46 ± 0.29 h | 87.46 ± 3.96 f |
| Cy. citratus | 9.49 ± 0.28 d | 6.23 ± 0.51 f | 43.21 ± 1.62 c | 119.91 ± 4.00 e |
| E. foetidum | 4.15 ± 0.08 g | 16.46 ± 0.35 c | 27.39 ± 1.65 f | 199.99 ± 8.64 d |
| P. tetragonolobus | 5.41 ± 0.20 e | 21.58 ± 0.88 b | 40.92 ± 1.38 d | 211.83 ± 3.89 cd |
| Se. pennata | 15.33 ± 0.64 a | 33.06 ± 1.07 a | 62.33 ± 1.97 a | 266.11 ± 9.93 b |
| So. melongena | 10.07 ± 0.21 c | 12.07 ± 0.36 d | 57.15 ± 0.32 b | 415.92 ± 18.78 a |
| Samples | Enzyme Inhibitory Activities (%Inhibition) | ||||||
|---|---|---|---|---|---|---|---|
| Lipase | α-Amylase | α-Glucosidase | ACE | AChE | BChE | BACE-1 | |
| A. cepa | 12.86±0.37 h | 19.16±0.38 d | 8.09 ± 0.38 h | 91.31 ± 1.43 a | 8.32 ± 0.30 g | 8.44 ± 0.07 g | 35.22 ± 2.11 b |
| A. fistulosum | 33.76±0.21 f | 15.02±0.41 e | 40.82 ± 0.66 b | 60.01 ± 1.79 e | 21.31 ± 0.66 cd | 17.34 ± 1.16 f | 39.14 ± 1.04 a |
| A. sativum | 46.44±1.43 c | 4.11±0.30 i | ND | 76.15 ± 1.39 c | ND | 8.55 ± 0.20 g | ND |
| Ci. hystrix | 61.16±1.33 a | 26.62±0.32 c | 33.76 ± 1.60 d | 91.71 ± 2.11 a | 29.09 ± 1.07 b | 52.61 ± 1.60 a | 24.76 ± 1.44 e |
| Co. sativum | 55.76±1.40 b | 58.43±0.56 a | 18.55 ± 0.57 g | 70.66 ± 2.34 d | 22.34 ± 0.74 c | 40.09 ± 1.06 b | 28.00 ± 0.49 d |
| Cy. citratus | 39.15±2.29 e | 4.93±0.01 hi | 29.57 ± 0.44 f | 68.80 ± 0.74 d | 12.40 ± 0.70 f | 22.70 ± 0.35 e | 12.46 ± 1.28 h |
| E. foetidum | 43.28±0.85 d | 31.22±1.21 b | 38.36 ± 0.96 c | 68.53 ± 0.88 d | 58.57 ± 0.51 a | 32.85 ± 0.47 c | 20.39 ± 1.15 f |
| P. tetragonolobus | 39.15±2.29 e | 10.51±0.96 f | 64.03 ± 1.30 a | 76.80 ± 3.58 c | 28.18 ± 1.30 b | 23.63 ± 1.24 e | 30.98 ± 1.41 c |
| Se. pennata | 44.95±0.66 cd | 5.16±0.09 h | 19.78 ± 0.54 g | 87.29 ± 1.28 b | 20.22 ± 1.13 d | 28.12 ± 1.20 d | 36.39 ± 0.79 b |
| So. melongena | 20.85±0.52 g | 7.66±0.33 g | 32.03 ± 1.27 e | 50.35 ± 1.17 f | 16.59 ± 0.13 e | 3.65 ± 0.15 h | 15.53 ± 0.21 g |
| Samples | Anti-Glycation Reaction (%Inhibition) | |
|---|---|---|
| D-Glucose Induction | Methylglyoxal Induction | |
| A. cepa | 21.26 ± 1.78 f | 5.09 ± 0.04 i |
| A. fistulosum | 40.06 ± 1.11 e | 29.83 ± 2.03 h |
| A. sativum | 10.63 ± 0.45 g | 5.17 ± 1.18 i |
| Ci. hystrix | 52.30 ± 1.85 d | 63.79 ± 0.48 c |
| Co. sativum | 65.61 ± 0.73 b | 57.63 ± 0.65 b |
| Cy. citratus | 39.82 ± 0.65 e | 39.85 ± 1.73 g |
| E. foetidum | 61.40 ± 1.61 c | 50.53 ± 0.24 e |
| P. tetragonolobus | 50.96 ± 0.52 d | 53.13 ± 1.24 d |
| Se. pennata | 74.55 ± 0.71 a | 81.54 ± 2.68 a |
| So. melongena | 51.87 ± 1.06 d | 47.21 ± 0.69 f |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suttisansanee, U.; Thiyajai, P.; Chalermchaiwat, P.; Wongwathanarat, K.; Pruesapan, K.; Charoenkiatkul, S.; Temviriyanukul, P. Phytochemicals and In Vitro Bioactivities of Aqueous Ethanolic Extracts from Common Vegetables in Thai Food. Plants 2021, 10, 1563. https://doi.org/10.3390/plants10081563
Suttisansanee U, Thiyajai P, Chalermchaiwat P, Wongwathanarat K, Pruesapan K, Charoenkiatkul S, Temviriyanukul P. Phytochemicals and In Vitro Bioactivities of Aqueous Ethanolic Extracts from Common Vegetables in Thai Food. Plants. 2021; 10(8):1563. https://doi.org/10.3390/plants10081563
Chicago/Turabian StyleSuttisansanee, Uthaiwan, Parunya Thiyajai, Parisut Chalermchaiwat, Khanitha Wongwathanarat, Kanchana Pruesapan, Somsri Charoenkiatkul, and Piya Temviriyanukul. 2021. "Phytochemicals and In Vitro Bioactivities of Aqueous Ethanolic Extracts from Common Vegetables in Thai Food" Plants 10, no. 8: 1563. https://doi.org/10.3390/plants10081563